Abstract
Introduction
Infiltration of cancers by T cells is associated with improved patient survival and response to immune therapies; however, optimal approaches to induce T cell infiltration of tumors are not known. This study was designed to assess whether topical treatment of melanoma metastases with the TLR7 agonist imiquimod plus administration of a multipeptide cancer vaccine will improve immune cell infiltration of melanoma metastases.
Patients and methods
Eligible patients were immunized with a vaccine comprised of 12 melanoma peptides and a tetanus toxoid-derived helper peptide, and imiquimod was applied topically to metastatic tumors daily. Adverse events were recorded, and effects on the tumor microenvironment were evaluated from sequential tumor biopsies. T cell responses were assessed by IFNγ ELIspot assay and T cell tetramer staining. Patient tumors were evaluated for immune cell infiltration, cytokine and chemokine production, and gene expression.
Results and conclusions
Four eligible patients were enrolled, and administration of imiquimod and vaccination were well tolerated. Circulating T cell responses to the vaccine was detected by ex vivo ELIspot assay in 3 of 4 patients. Treatment of metastases with imiquimod induced immune cell infiltration and favorable gene signatures in the patients with circulating T cell responses. This study supports further study of topical imiquimod combined with vaccines or other immune therapies for the treatment of melanoma.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-016-1880-z) contains supplementary material, which is available to authorized users.
Keywords: Immunotherapy, Human, Cytotoxic T lymphocytes, Melanoma, Tumor vaccines, TLR agonists
Introduction
Melanoma metastases lacking T cells often fail to respond to immune therapies and are associated with shorter overall patient survival [1–7]. Improving the trafficking and retention of effector T cells in tumors may beneficially impact patient survival. The TLR7 agonist, imiquimod, has induced regression of basal and squamous cell carcinomas [8] associated with CD8+ T cell infiltration [9].
Furthermore, imiquimod enhanced IFNγ production and effector function of tumor infiltrating T cells, with effector T cells producing more granzyme and perforin [9]. Imiquimod also induced E-selectin expression by tumor vasculature endothelium, and ICAM-1 expression on dermal endothelial cells, which may support an influx of skin homing T cells [9, 10]. Imiquimod can induce direct apoptosis of melanoma cells, which may support antigen presentation [11]. In murine studies, imiquimod inhibited melanoma development by promoting plasmacytoid DC recruitment, triggering cytotoxic functions and type 1 IFN responses, and suppressing tumor vascularization [12–14]. Therefore, imiquimod may modulate the TME to support infiltration by T cells induced by vaccination or other T cell-based therapies.
Imiquimod is supplied in a cream for topical application to selected skin sites. The site of application determines where its effect will be realized. Imiquimod has been administered at the site of vaccination, as a vaccine adjuvant, to support DC activation at that site and to increase systemic immune responses to the vaccines [15–17]. In those 3 studies, one showed that imiquimod-treated skin had increased mononuclear cell infiltrates and DC activation [16]; another that imiquimod may have increased immunogenicity [17]; however, the third study found that imiquimod at the vaccine site did not improve the immune response to a viral vaccine [15]. On the other hand, imiquimod has also been administered topically to skin metastases of melanoma. This has induced significant regressions of treated lesions in some patients, suggesting anti-melanoma activity as monotherapy [18–20]. That clinical activity may be mediated by induction of antitumor immune responses and by supporting T cell infiltration. We have hypothesized that topical treatment of melanoma metastases with imiquimod may support infiltration with T cells induced by concurrent melanoma vaccines. To our knowledge, topical treatment of tumors with imiquimod has not been evaluated in combination with vaccination in patients with melanoma. This study was initiated to obtain pilot data on the safety of topical imiquimod for the treatment of melanoma and to address whether imiquimod treatment in conjunction with vaccination would promote T cell recruitment into tumors.
Methods
Study design
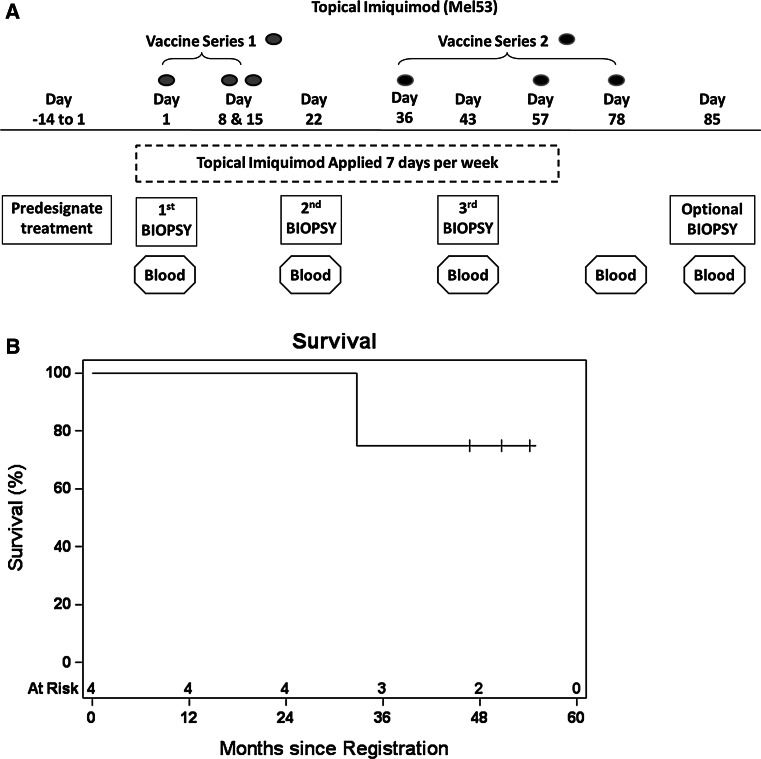
This was designed as a two cohort, nonrandomized, open-label pilot study testing the combination treatment of topical imiquimod of skin metastases plus vaccination with a multipeptide vaccine. The study was designed to assess the safety and to obtain preliminary data on the biological effect of topical imiquimod, with or without the vaccine. The sample size and stopping rules were based on testing that the probability of developing a dose-limiting toxicity from a null rate of 0.33 against the alternative rate of 0.05. Patients were studied following informed consent, with Institutional Review Board approval (IRB# 15168) and Food and Drug Administration review (IND #12191). The Mel53 trial was registered with ClinicalTrials.gov (NCT01264731) and was performed at the University of Virginia. The schema is shown in Fig. 1a.
Fig. 1.
Protocol schema for the Mel53 clinical trial (a). Graph depicting survival probability for trial patients (b)
Vaccine composition and administration
Each vaccine contained a mixture of 12 class I MHC-restricted melanoma-derived peptides (12MP, 100 mcg each) and a class II MHC-restricted tetanus toxoid-derived helper peptide (200 mcg) [21, 22], listed in Supplemental Table 1, emulsified 1:1 (v/v) in 1 ml Montanide ISA-51 VG adjuvant (Seppic, Inc, Paris, France), referred to as MELITAC 12.1. The vaccine was administered subcutaneously (1 ml) and intradermally (1 ml), in the same extremity on each visit, at or near the same site, within a 2-cm margin. Vaccines were administered in two treatment cycles: day 1, day 8, day 15 for cycle one, then day 36, day 57, day 78 for cycle two.
Lesion selection for treatment
Lesions for biopsy and imiquimod treatment were selected prospectively at the time of enrollment (day −14 to day 1) and were based on clinical standards, clinician’s judgment, and patient’s informed consent. The number of lesions treated depended on the availability and size of the lesions.
Imiquimod treatment
5 % imiquimod cream (3M, Maplewood, MN) was purchased commercially and was applied by the patient topically once a day, 7 days per week to selected superficial skin metastases, for 12 weeks beginning day 1. Each single-use imiquimod packet contained 250 mg of cream (12.5 mg imiquimod) and could be used for a surface area of up to 20 cm2.
Tumor biopsies
Biopsies (incisional, excisional, or core biopsies, and fine needle aspiration biopsies) of cutaneous metastatic melanoma were obtained on day 1 (baseline, no treatment), day 22 (after 3 weeks of imiquimod treatment, and 1 week after the third vaccine), and day 43 (after 6 weeks of imiquimod, and 1 week after the 4th vaccine). Additional biopsies on day 85 were optional in patients with more than the minimal tumor requirements. Each biopsy was divided into three portions and allocated at the bedside within 5 min of excision, with half saved in medium for evaluation of short-term explant cell cultures, one-fourth in formalin, and one-fourth frozen in Tissue-Tek optimum cutting temperature (O.C.T.) medium (VWR, Radnor, PA).
Trial enrollment
The study was designed as a 2-cohort study, with patients in cohort 1 to receive vaccine plus imiquimod, and patients in cohort 2 to receive imiquimod only. Cohort 1 consisted of patients who were eligible for the vaccine based on HLA type and eligibility criteria, and cohort 2 consisted of patients who were not eligible for the vaccine based upon HLA type yet met all other criteria. Patients were enrolled only to cohort 1, and slow enrollment led to halting enrollment early. Target enrollment was 14 eligible patients for cohort 1, based upon safety assessment and assessment of primary immunologic endpoints.
Eligibility criteria
Patients were eligible if they had histologically or cytologically proven Stage IIIB-IV melanoma (7th edition American Joint Committee on Cancer), with skin metastases, and were age 18 years or older. Additionally, patients needed adequate cutaneous melanoma tissue available in 1–4 lesions to provide a sample, at least 0.1 cm3, at each of 3 biopsy time points, with at least one lesion amenable to topical imiquimod application. Participants could have distant or visceral disease in addition to the cutaneous metastases. Eligibility for cohort 1 also included expression of HLA-A1, HLA-A2, HLA-A3, or HLA-A11. Exclusion criteria included: pregnancy; cytotoxic chemotherapy, interferon, or radiation within the preceding 4 weeks; known or suspected allergies to vaccine components; multiple brain metastases; and use of steroids or Class III-IV heart disease.
Primary endpoints
(1) Safety of vaccine and topical imiquimod treatment, (2) Changes in levels of intratumoral immune cells after imiquimod treatment. Secondary endpoints included: reactivity of intratumoral T cells to peptides in the vaccine, and clinical regression of imiquimod-treated lesions.
Adverse events
Were recorded for all patients using National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events, v4.03.
IFNγ ELIspot assay
Circulating immune responses were assessed by IFNγ ELIspot assay, using reported methods [22, 23]. Briefly, assays were conducted on PBMCs directly ex vivo after cryopreservation. A patient was considered to have a response to vaccination (yes/no) if the following criteria were met: the ratio of T cell response to the pooled 12MP peptides relative to T cell response to the maximum of the negative controls was greater than 2, and the spots counted for the pooled experimental peptides were at least 20 (per 105 CD8+ cells) greater than the number of spots for the negative control [24]. If the maximum of the negative controls for a given sample was zero, a meaningful fold increase could not be calculated; in those cases, the minimum detectable value among all similar assays was used as the negative control for that sample to enable defining a T cell response. For this study 0.5 spots per 105 was the minimum value. Interassay coefficients of variation (CVs) were calculated for normal donor PBMC responses to a pool of 32 peptides from CMV, Epstein–Barr virus, and influenza proteins (CEF peptides, Proimmune, Oxford, United Kingdom) [25] testing high and low responders in each assay. For a high-responder donor (mean 244 spots per 100,000 CD8+ cells), CVs were 15 %. For a low-responder donor (mean 25 spots per 100,000 CD8+ cells), CVs were 3 %.
Evaluation of tumor infiltrating T cells
Portions of each tumor biopsy, in Iscove’s DMEM (Mediatech, Manassas, VA) supplemented with 20 % FBS, pen/strep (1:100), l-glutamine (1:100, Invitrogen, Carlsbad, CA), 2-mercaptoethanol (1.75 μl/500 ml, Bio-Rad, Hercules, CA), fungizone (1:100, Invitrogen), and gentamicin (1:200, MP Biomedicals, Santa Ana, CA), were shipped in a refrigerated container overnight for morning delivery from Charlottesville, VA to Boston, MA. T cells from melanoma lesion explant cultures were grown with IL-2 (100 I.U./ml, National Cancer Institute, Frederick, MD) and IL-15 (10 ng/ml, Peprotech, Rocky Hill, NJ) for 1–2 weeks as previously described [26]. T cells were surface stained with antibodies and tetramers detailed in Supplemental Table 2, as previously described [22, 27], before flow cytometry analysis. For analysis of regulatory T cells (Tregs), cells were surface stained followed by intranuclear staining for Foxp3 (Supplemental Table 2) using the Foxp3/Transcription Factor Staining Buffer Set (eBioscience, San Diego, CA) per manufacturer’s protocol. Samples were run on FACSCanto instruments, and data were analyzed using FACSDiva software (V5.1, BD Biosciences).
Chemokine and cytokine quantification
Total protein from tumor biopsies was isolated using total protein extraction reagent (Thermo Scientific, Waltham, MA), complete protease inhibitor (Roche, Indianapolis, IN), and a cold Dounce homogenizer. Samples were disrupted by sonication, centrifuged to remove residual debris, filtered through a 1.2-μm Gelman 4190 syringe filter, and assayed for protein concentration using a NanoDrop ND1000. Indicated cytokines and chemokines were measured from biopsy samples using cytokine multiplex kits (EMD Millipore Corporation, Billerica, MA) and quantified against calibration curves from recombinant protein standards using a Bio-Plex array reader (Bio-Rad).
Enumeration of immune subsets in melanoma tumors
Immune cells were identified in formalin-fixed paraffin-embedded sections of tumor metastases by IHC staining with antibodies to CD8 (Dako, Carpinteria, CA, USA), and CD45 (Dako), followed by 3,3′-Diaminobenzidine (Vector Labs, Burlingame, CA) and hematoxylin staining or Azure B (Poly Scientific, Bay Shore, NY). Stained slides were imaged at 20× using the Leica SCN400 slide scanner. Immune cells were enumerated using Digital Image Hub Tissue IA software (Leica Biosystems, Buffalo Grove, IL) on the entire available tumor sections excluding edges and tissue folds, and cell counts were normalized per mm2. Automated cell counts were verified by manually counting by eye, when the cells were at a reasonable density, to audit the automated counts.
Gene expression
Fine needle aspirations from melanoma metastases were lysed directly in QIAzol Lysis Reagent (Qiagen, Hilden, Germany), and RNA was extracted according to the manufacturer’s protocol. RNA was amplified using the Ambion WT expression kit (Life Technologies, Carlsbad, CA). Fragmented single-stranded sense DNA were terminally labeled and hybridized to the Human GeneChip 1.0 ST array and stained on a Genechip Fluidics Station 450 (Affymetrix, Santa Clara, CA) according to the manufacturer’s protocol. Arrays were scanned on a GeneChip Scanner 3000-7G (Affymetrix). Data were analyzed using Expression Console and Transcriptome Analysis Console Software; differentially expressed genes were identified by paired ANOVA test with a significance cutoff p ≤ 0.05 and fold change (linear) of < −2 or >2 (Affymetrix).
Statistics
Sample sizes were deemed too small to statistically model changes in ELIspot, cytokine, and cell enumeration studies. Kaplan–Meier survival estimates were calculated using SAS 9.4 (SAS Institute, Cary, NC).
Results
Trial enrollment
A total of 4 eligible patients were enrolled to cohort 1 of the study from February 2011 through March 2012. Enrollment was discontinued early due to slow accrual because of competing therapeutic options. Clinical characteristics of the patients are listed in Supplemental Table 3. All had recurrent melanoma with multiple in-transit metastases.
Toxicity assessment
There were no dose-limiting toxicities or grade 3 or higher treatment-related adverse events. Study-related grade 1–2 adverse events included vaccine site reactions, systemic symptoms including chills, fever, fatigue, flu-like symptoms, rash at imiquimod application sites, and biopsy site wound complications (detailed in Supplemental Table 4).
Clinical outcome
Patients had all evident metastases excised as part of the study procedures; therefore, clinical responses beyond week 6 were not evaluable. Three enrolled patients are currently living; two with metastatic disease, and one without evidence of disease since treatment; one patient has died from disease (median follow-up 4.05 years, 75 % survival, Fig. 1b, Supplemental Table 3).
Vaccine induced T cell responses in circulation and in melanoma metastases
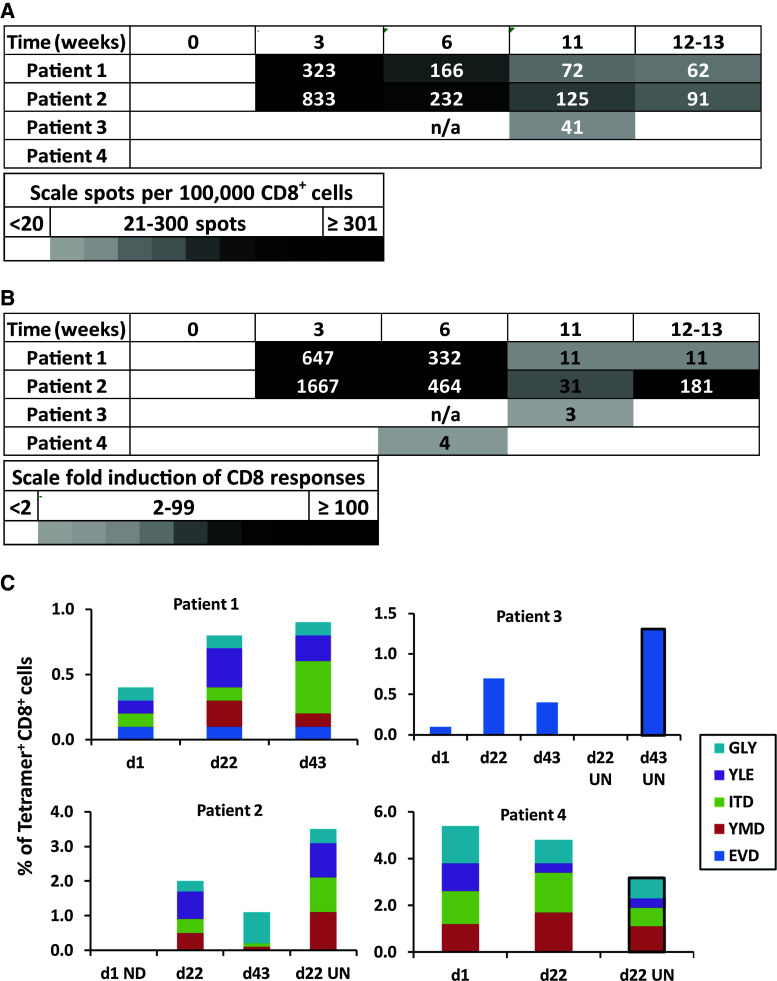
Circulating T cell responses to vaccination was measured by ELIspot assay. CD8+ IFNγ responses to the pooled 12MP were detected ex vivo in 3 of 4 patients (75 %, patients 1–3), two of which were evident by week 3 (day 22, patients 1, 2), as shown in Fig. 2a, b. To assess whether T cells reactive to the vaccines infiltrated melanoma metastases, peptide-reactive T cells in tumor metastases were assessed by tetramer staining after short-term culture. Pretreatment tumor was not evaluable for patient 2. Tetramer positive CD8+ cells in imiquimod-treated tumors increased by day 22 in both evaluable patients who had circulating T cell responses detected by ELIspot (patients 1,3; Fig. 2c), but did not increase in patient 4, who did not develop T cell circulating response to vaccine, but interestingly had a high proportion of peptide-reactive T cells in the tumor at baseline. Several tumors that were not treated with imiquimod were evaluated for patients 2, 3, and 4 (Fig. 2c, “UN”); these suggest a favorable effect of vaccination alone.
Fig. 2.
Patients receiving 12 MP vaccination generate tumor-reactive T cell responses. Heat map depicting the number of CD8+ cells (per 105 cells) producing IFNγ after stimulation with 12 pooled melanoma peptides, after subtracting negative control values (a), and the fold induction of IFNγ CD8 responses, relative to negative controls (b). A response required increases by at least a twofold and at least 20 spots per 100,000 CD8+ T cells. c Graphs depicting the percent of MHC-tetramer+CD8+ cells from patient tumors at day 1 (untreated), day 22 and day 43 (imiquimod treated), and day 22 and day 43 untreated tumor controls denoted UN. D1 untreated tumor controls were unevaluable for patient 2. When available, tumors not treated with imiquimod were assessed and are marked “UN” with black border added around the bars
T cell activation in melanoma metastases
To characterize T cell function and phenotype, we evaluated the expression of activation markers (CD25, CD69), chemokine receptors (CCR4, CCR6, CCR7, CCR9, CXCR3), homing receptors (cutaneous leukocyte antigen [CLA] and α4β7 integrin), and markers of differentiation (CD161 (Th17, Tc17), Foxp3) on tumor infiltrating CD3+ cells after short-term culture with IL-2 and IL-15. Pretreatment tumor controls (day 1) were unevaluable for patient 2. Among CD3+ cells for the patients 1, 3, and 4, increases were observed (from day 1 to day 22) in the percentage of CD161+ cells, an increase that persisted in patients 1 and 3 until day 43 (data not available for patient 4). Increased expression of CCR9 was observed from CD3+ cells from patient 3 and persisted to day 43 (Supplemental Figure 1).
Cytokines and chemokines detected in melanoma metastases
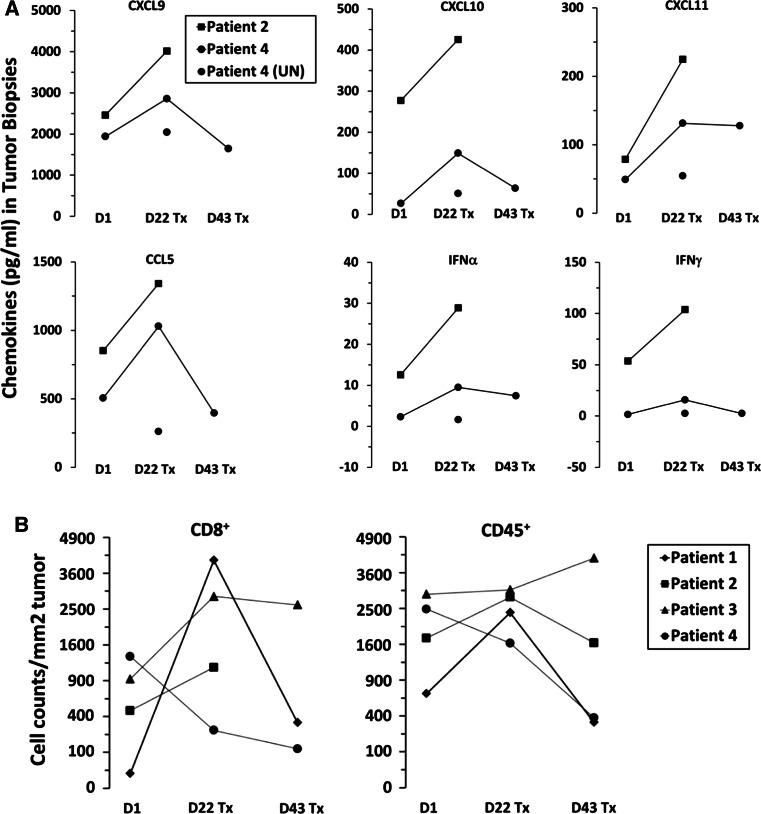
Metastases were evaluated for expression of cytokines (IFNα, IFNγ, IL-10, IL-12, TGFβ) and chemokines associated with immune cell recruitment (CXCL9, CXCL10, CXCL11, CCL21, CCL22) by multiplex assay to assess whether imiquimod treatment plus vaccine increases their production. Sufficient tumor samples were evaluable only for patients 2 and 4; in both, there were increases in CXCL9, CXCL10, CXCL11, CCL5, IFNα, and IFNγ on day 22 post-initiation of vaccine and imiquimod treatment (Fig. 3a).
Fig. 3.
Vaccination and imiquimod treatment promote chemokine generation from tumors and immune cell infiltration of tumors 22 days post-treatment. Tumor samples were evaluated at day 1 (pretreatment), day 22, and day 43 post-imiquimod treatment (Tx). a Graphs depict the amounts of CXCL9, CXCL10, CXCL11 (top row), CCL5, IFNγ, and IFNγ (bottom row) (pg/ml) detected in tumor samples by luminex assay. b Graphs depict the numbers of CD8+ (left) and CD45+ (right) cells infiltrating tumor per mm2 of tumor sections
Enumeration of CD8+ and CD45+ cell infiltrates in tumors
To assess immune cell infiltrates in tumors, CD8+ and CD45+ cells were enumerated per mm2 of histologic sections. For patients 1–3, CD8+ cells and CD45+ cells increased from day 1 to day 22; in patient 4, who did not have increased circulating T cell responses to vaccination (Fig. 2), both CD8+ and CD45+ cell densities decreased from day 1 to day 22, and decreased further to day 43 (Fig. 3b). Increases in CD8+ and CD45+ cell infiltrates often declined from day 22 to day 43, despite continued vaccination and application of imiquimod.
Gene expression of melanoma metastases
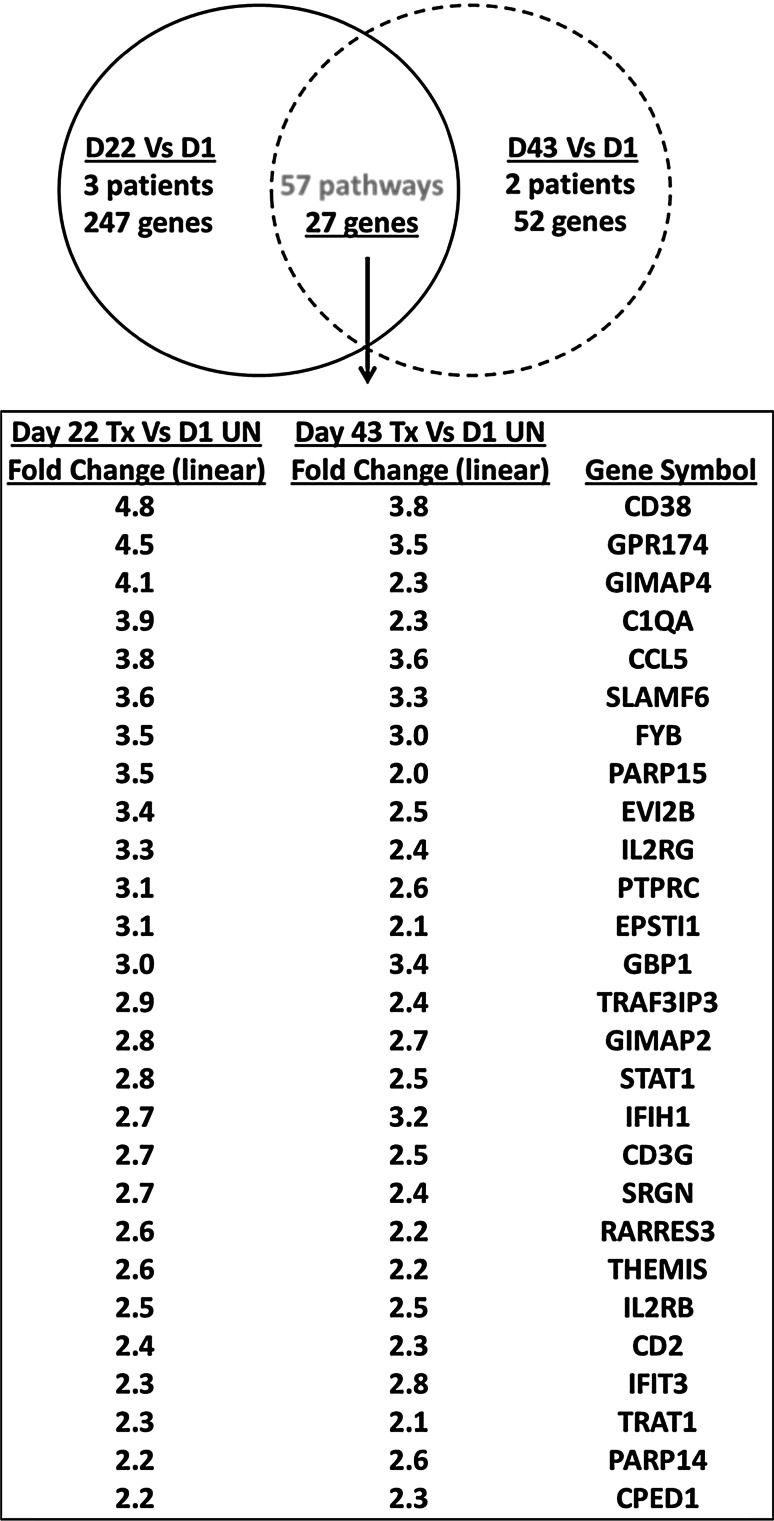
Melanoma metastases from patients 1–3 were also evaluated for changes in gene expression profiles from days 1 to 22 (n = 3) and days 1 to 43 (n = 2). Principal component analysis suggested that treatment with topical imiquimod plus vaccine had modest effects on the tumor samples, which otherwise clustered according to patient (Supplemental Figure 2). Gene expression in imiquimod-treated tumor samples (day 22 and day 43) was compared, in a paired analysis, to pretreated tumors (day 1). Changes in expression of 247 genes were observed from day 1 to day 22 (Supplemental Tables 5 & 6), and changes in expression of 52 genes were observed from day 1 to day 43 (Supplemental Tables 7 & 8). In these two datasets, 27 gene expression changes were in common, and all were upregulations from day 1 (Fig. 4). These changes represented 56 common pathways, which were changed in both day 22 and day 43 imiquimod-treated samples when compared to pretreatment tumors, for which the 25 most upregulated are shown in Table 1. The upregulated pathways and genes included adaptive immunity (TCR signaling, IFNγ signaling, IL-2 signaling), and innate immunity (TLR signaling, and complement activation).
Fig. 4.
Vaccination and imiquimod treatment induce favorable gene signatures for cancer rejection from patient tumors. Gene targets that were upregulated in common in patient tumors for the following two comparisons: day 22 and day 43 post-imiquimod treatment vs day 1 untreated tumors. Tumor samples from 3 patients were evaluable at day 22 and 2 patients at day 43, and comparisons were done in a paired fashion
Table 1.
Top 25 gene expression pathways increased in common on day 22 (n = 3) and on day 43 (n = 2), vs day 1
| Pathway | Day 22 VS D1 | Day 43 VS D1 | ||
|---|---|---|---|---|
| # | Genes with increased expression d22 vs dl | # | Genes with increased expression d43 vs d1 | |
| Allograft rejection | 17 | CXCL9,CXCL11,C3,CXCL12,STAT1,CXCL13,C2,HLA-DRB3,HLA-DRA,HLA-DMB,HLA-DRB1,HLA-DPA1,HLA-DRB4,CD80,HLA-DMA,HLA-DPB1,CD86 | 1 | STAT1 |
| Regulation of toll-like receptor signaling pathway | 12 | CXCL11,CXCL9,CXCL10,CD86,TLR7,PIK3CG,CCL5,STAT1,CD80,TLR4,CD14,CD180 | 3 | CCL4,CCL5,STAT1 |
| Toll-like receptor signaling pathway | 11 | CXCL11,CXCL9,CXCL10,CD86,TLR7,PIK3CG,CCL5,STAT1,CD80,TLR4,CD14 | 3 | CCL4,CCL5,STAT1 |
| Complement and coagulation cascades | 9 | C1QB,C1QA,C1S,C2,C3,SERPING1,C3AR1,CFH,SERPINA1 | 1 | C1QA |
| Type II interferon signaling (IFNG) | 9 | STAT1,GBP1,PSMB9,OAS1,CXCL10,CYBB,IFIT2,EIF2AK2,CXCL9 | 2 | STAT1,GBP1 |
| Interferon alpha/beta signaling | 7 | STAT1,IFITM1,IFIT2,IFIT3,IFIT1,XAF1,IFI6 | 2 | STAT1,IFIT3 |
| TCR signaling pathway | 7 | FYB,ITK,CD3E,CD3D,CD3G,CD4,TNFRSF9 | 4 | CBLB,FYB,CD3G,ICOS |
| GPCRs, class A rhodopsin-like | 6 | C3AR1,CMKLR1,PTGER4,P2RY14,GPR174,GPR65 | 2 | GPR174,GPR171 |
| Human complement system | 6 | CFH,C2,C3,SELL,SELPLG,CFB | 1 | LRP2 |
| Complement activation, classical pathway | 5 | C1QA,C1QB,C1S,C2,C3 | 1 | C1QA |
| TSLP signaling pathway | 5 | STAT1,STAT4,IL7R,HCK,LYN | 1 | STAT1 |
| Cell surface interactions at the vascular wall | 4 | SELPLG,CD48,CD2,INPP5D | 1 | CD2 |
| IL-2 signaling pathway | 4 | STAT1,IL2RG,IL2RB,NMI | 3 | STAT1,IL2RG,IL2RB |
| Inflammatory response pathway | 4 | IL2RG,IL2RB,CD86,CD80 | 2 | IL2RG,IL2RB |
| Nuclear receptors meta-pathway | 4 | ALOX5AP,BIRC3,SRGN,SERPINA1 | 1 | SRGN |
| EGF/EGFR signaling pathway | 3 | STAT1,PLSCR1,INPP5D | 2 | STAT1,CBLB |
| Glucocorticoid receptor pathway | 3 | ALOX5AP,BIRC3,SRGN | 1 | SRGN |
The CCL5 gene was upregulated after imiquimod treatment, which may support recruitment of cytotoxic cells and other T cells [6]. Further evidence of T cell/NK cell recruitment is suggested through upregulation of: IL2RG, IL2RB, CD3G, CD2, SLAMF6, CD38, TRAT1, FYB, GIMAP2, IFIH1, PTPRC, GPR174, THEMIS, and TRAF3IP3 genes (Fig. 4). Collectively, the gene profiles support the observed increases in immune cell infiltrates and suggest that T cells and NK cells may be activated in patient tumors post-imiquimod treatment, and may be of a Th1 phenotype since there is evidence of IFNγ signaling (Fig. 4).
However, the increased gene signatures and increased T cell infiltration into tumors evident at day 22 were less prominent on day 43, despite continued imiquimod application and vaccination. This raises the possibility that immune regulatory processes may be induced after 3 weeks. To explore this possibility, changes in gene expression from day 22 to day 43 were assessed. This analysis identified increases in 10 genes (DSP, TRNAU2, RN5S19, SERPINB5, RNU1-10P, IRAK3, CLCA2, ALDH1A1) and decreases in 2 (RNU6-1052P, TMEM232), which represent increases in several pathways, including tryptophan metabolism, fatty acid omega oxidation, micro-RNA-targeted genes in lymphocytes, IL-1 signaling, apoptotic execution phase, and regulation of TLR signaling (Supplemental Tables 9 & 10).
Discussion
This pilot study was performed to evaluate the safety and immunogenicity of administering imiquimod directly to melanoma metastases, in combination with vaccination at a distant site. The hypothesis underlying this study is that there may be benefit in modulating the TME to support T cell infiltration in combination with vaccination or other approaches to expand circulating antigen-specific T cells. The study is limited by small patient numbers, but provides provocative findings supporting our hypothesis. Both imiquimod treatment and the vaccines were well tolerated, with treatment-related adverse events limited to grade 1 and grade 2 events that match those observed in prior studies [21, 23]. Imiquimod is not approved for use in topical treatment of melanoma metastases, but its off-label use has been reported in that setting [18, 28, 29], with beneficial control of treated lesions in some patients. Cancer vaccines have a very favorable safety profile [30], so the present study is consistent with prior work supporting safety of vaccines plus imiquimod in combination.
Evaluation of the melanoma metastases in patients treated with vaccine plus topical imiquimod revealed several consistent findings across multiple analyses. In the patients who developed T cell responses to vaccine in blood, there were increases in the chemokines CXCL9, CXCL10, CXCL11, CCL5, and the cytokines IFNγ, and IFNα at day 22 in treated tumors (Fig. 3a), findings that were also supported by significant increases in gene expression for CXCL9-11 and CCL5 by day 22, which persisted to day 43 for CCL5 (Fig. 4, Supplemental Tables 5 & 7). These chemokines have been associated with homing of CD8+ T cells to melanoma metastases and appear critical for T cell-mediated tumor control [6]. Consistent with those findings, there were increases in total and vaccine-specific CD8+ T cell infiltrates (Figs. 2c, 3b) for those patients who had circulating T cell responses to the vaccine (Fig. 2a, b). Interestingly, the patient who did not develop a T cell response to vaccination did not have increased T cell infiltration (Fig. 3b). This is only one patient, but the finding is consistent with the hypothesis that imiquimod treatment of melanoma metastases may favorably modulate the TME, but may require antigen-specific T cells in circulation to mediate increases in tumor-reactive T cells in the tumors.
This study was originally designed to include a cohort treated with imiquimod only, as control for the combination of vaccine plus topical imiquimod; however, no patients enrolled to that cohort. However, we have completed another study of vaccination plus intratumoral interferon-gamma, in which tumors were biopsied before vaccination and after 3 weeks of vaccines (and before interferon-gamma treatment). In that study, effects of vaccine alone on the tumor microenvironment were minimal, and there was no significant increase in T cell infiltration with vaccines alone (manuscript submitted). That experience provides a nonrandomized control for the effects of vaccine alone, and support the hypothesis that imiquimod application to the tumors supports T cell infiltration and immune activation that is not otherwise attributable to vaccine alone.
Interestingly, many of the changes in the TME that are evident by day 22 are no longer evident by day 43, despite continued treatment. This raises interesting questions about whether immune regulatory processes intervene to limit the immunoprotective changes that happen by day 22. Some clues are suggested by differences in the gene expression profiles between day 22 and day 43. Genes upregulated day 22 (vs day 1) but not day 43 (vs day 1) include: CXCL9-11, which recruit CD8+ T cells and Th1 CD4+ T cells, CXCL12 and CXCL13, which have key roles in induction of tertiary lymphoid structures [31], granzymes A and K (GZMA and GZMK), T cell genes CD3E, CD3D, and CD4, markers of DC activation CD80 and CD86, the T cell activation marker CD69, myeloid markers CD14 and CD163, the costimulatory molecules CD137 (TNFRSF9) and IL7R, and the homing receptor molecule vascular cell adhesion molecule 1 (VCAM1), and kynureninase (KYNU). These suggest a gene signature supporting T cell homing, DC activation, and T cell co-stimulation. On the other hand, genes upregulated day 43 (vs day 1) but not day 22 (vs day 1) include IDO1, as well as CD27, ICOS, and CD122. It may be significant that IDO1 is upregulated at day 43, but not day 22, whereas kynureninase, which degrades kynurenine, downstream of IDO1, is upregulated at day 22 but not day 43. Thus, one postulated mechanism for the findings is that IFNγ signaling leads to a secondary upregulation of IDO1 [32], which in turn may limit the effect of imiquimod and vaccine in the TME. This should be evaluated in a larger dataset but if it does explain the phenomenon, then it raises the possibility that the beneficial effects of imiquimod on the TME, with or without vaccination, may be extended by combining with an IDO1 inhibitor after several weeks. The persistent expression of CD27 and CD122 at day 43 raises the possibility that combination with an agonistic antibody for CD27 or IL-15 may support expansion of T cells in the TME during continued treatment with imiquimod.
Genes upregulated between day 22 and day 43 also suggest potential mechanisms for downregulation of immune signatures and infiltration after day 22. Among 10 genes upregulated by day 43, the most upregulated (5.3×, Supplemental Table 9) was desmoplakin (DSP), which we have recently identified as one of several desmosomal proteins associated with the lack of protective immune signatures in melanoma and ovarian cancer (manuscript submitted). Its upregulation after day 22 suggests that its expression may be modulated by imiquimod and vaccination. Also upregulated is IRAK3 (2.2×), which is expressed by myeloid cells and has been reported to regulate TLR signaling [33–35], and to mediate cancer-associated dysfunction of human monocytes [36]. ALDH1A1 expression also increased and has been associated with cancer stem cells, including in melanoma [37]. The role of other identified genes deserves further exploration, especially to test whether their downregulation may support immune-mediated tumor control.
Previous studies indicate that three common themes are associated with cancer rejection: activation of IFN-stimulated genes toward a Th1 cell polarization, such as through the IFNy-STAT1-IRF1-IFN-stimulated gene pathway; recruitment of cytotoxic cells through production of chemokine ligands, such as, CXCL9-10, and CCL5; and activation of the immune-effector function genes such as granzymes, perforins, granulysin, and caspases [38]. The gene array data highlight IFNγ-stimulated genes as dominant upregulated genes in treated tumors (Supplemental Tables 5 & 7). Further, the IHC data and flow cytometry from this study suggest that imiquimod treatment plus vaccine increases T cells in tumors. The gene expression data for day 22 tumors also revealed increased granzyme A (2.5-fold) and granzyme K (5.6-fold). Also, there was increased serglycin (SRGN) at both time points. Serglycin is associated with secretory granules containing granzymes and perforins. This is consistent with increases in granzyme genes in basal cell cancers after imiquimod treatment, though the basal cell carcinoma studies were conducted over a shorter time course [14]. A limitation of this study is the small number of patients evaluated. However, despite this limitation, the findings suggest that imiquimod plus vaccine may support a tumor rejection phenotype, supporting further investigation of imiquimod and/or other TLR agonists in combination with vaccines or other therapies that may increase circulating tumor-reactive T cells.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank the Biorepository and Tissue Research Facility for technical assistance with assays, and Dr. Stefan Bekiranov for advising on gene array analysis. We appreciate the work of Patrice Neese, Carmel Nail, and Kathleen Haden for administering vaccines and managing patient toxicities, and to Cheryl Murphy Chase for RNA preparation from tumor biopsies. Appreciation also goes to clinical research coordinators Kristy Scott, Emily Allred, and Alex Carney, and we thank Eugene Butcher for the ACT-1 alpha4beta7 antibody.
Funding
Support for this work was provided by the University of Virginia Cancer Center Support Grant (NIH/National Cancer Institute P30 CA44579: Clinical Trials Office, Biorepository and Tissue Research Facility, Flow Cytometry Core, Biomolecular Core Facility, Biostatistics Shared Resource and pilot projects funding). Additional philanthropic support was provided by George and Linda Suddock and by Alice and Bill Goodwin and the Commonwealth Foundation for Cancer Research. Support was also provided by the Rebecca Clary Harris Fellowship (Ileana S Mauldin) and the University of Virginia Cancer Training Grant T32 CA009109 (Ileana S Mauldin), a Melanoma Research Alliance Young Investigator Award (David Mullins) and United States Public Health Service R01 CA134799 (David Mullins), and NIH/National Cancer Institute grant K25 CA181638 (Nolan A Wages).
Abbreviations
- 12MP
12 Class I MHC-restricted melanoma peptides
- CCL
C-C motif chemokine ligand (applies to CCL5, CCL21, CCL22)
- CCR
C-C motif chemokine receptor (applies to CCR4, CCR6, CCR7, CCR9)
- CV
Coefficient of variation
- CXCL
C-X-C motif chemokine ligand 9 (applies to CXCL9, CXCL10, CXCL11, CXCL12, and CXCL13)
- CXCR3
Chemokine (C-X-C motif) receptor 3
- mcg
Micrograms
- NCI
National Cancer Institute
- Tc17
T cytotoxic, type 17
- Th1
T helper, type 1
- Th17
T helper, type 17
- TME
Tumor microenvironment
Compliance with ethical standards
Conflict of interest
Craig Slingluff is an inventor of several peptides included in the vaccine that was administered during the clinical trials studied within this paper. The University of Virginia Licensing and Ventures Group holds the patents for those peptides, which have been licensed through the Ludwig Institute for Cancer Research to Glaxo Smith Kline. He also has relationships with several commercial interests related to this work, including Immatics (member, Scientific Advisory Board), Polynoma (principal investigator for MAVIS cancer vaccine trial), Glaxo Smith Kline (recipient of grant support for a clinical trial), but funds from those relationships go to the University of Virginia, not to Dr. Slingluff personally. The remaining authors have nothing to disclose or competing interests in association with this study.
Footnotes
This paper is published together with doi:10.1007/s00262-016-1881-y.
References
- 1.Weiss G, Grosh WW, Chianese-Bullock KA, Zhao Y, Liu H, Slingluff CL, Marincola FM, Wang E. Molecular insights on the peripheral and intra-tumoral effects of systemic high dose rIL-2 (Aldesleukin) administration for the treatment of metastatic melanoma. Clin Cancer Res. 2011;17:7440–7450. doi: 10.1158/1078-0432.CCR-11-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gough M, Crittenden M, Thanarajasingam U, Sanchez-Perez L, Thompson J, Jevremovic D, Vile R. Gene therapy to manipulate effector T cell trafficking to tumors for immunotherapy. J Immunol. 2005;174:5766–5773. doi: 10.4049/jimmunol.174.9.5766. [DOI] [PubMed] [Google Scholar]
- 3.Wu R, Forget MA, Chacon J, Bernatchez C, Haymaker C, Chen JQ, Hwu P, Radvanyi LG. Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer J. 2012;18:160–175. doi: 10.1097/PPO.0b013e31824d4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea S, Dengel LT, Patterson JW, Slingluff CL., Jr Immunotype and immunohistologic characteristics of tumor infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72:1070–1080. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong M, Puaux AL, Huang C, et al. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res. 2011;71:6997–7009. doi: 10.1158/0008-5472.CAN-11-1466. [DOI] [PubMed] [Google Scholar]
- 6.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. Chemokine expression in melanoma metastases associated with CD8 + T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schon M, Bong AB, Drewniok C, et al. Tumor-selective induction of apoptosis and the small-molecule immune response modifier imiquimod. J Natl Cancer Inst. 2003;95:1138–1149. doi: 10.1093/jnci/djg016. [DOI] [PubMed] [Google Scholar]
- 9.Huang SJ, Hijnen D, Murphy GF, et al. Imiquimod enhances IFN-gamma production and effector function of T cells infiltrating human squamous cell carcinomas of the skin. J Investig Dermatol. 2009;129:2676–2685. doi: 10.1038/jid.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urosevic M, Maier T, Benninghoff B, Slade H, Burg G, Dummer R. Mechanisms underlying imiquimod-induced regression of basal cell carcinoma in vivo. Arch Dermatol. 2003;139:1325–1332. doi: 10.1001/archderm.139.10.1325. [DOI] [PubMed] [Google Scholar]
- 11.Schon MP, Wienrich BG, Drewniok C, Bong AB, Eberle J, Geilen CC, Gollnick H, Schon M. Death receptor-independent apoptosis in malignant melanoma induced by the small-molecule immune response modifier imiquimod. J Investig Dermatol. 2004;122:1266–1276. doi: 10.1111/j.0022-202X.2004.22528.x. [DOI] [PubMed] [Google Scholar]
- 12.Aspord C, Tramcourt L, Leloup C, Molens JP, Leccia MT, Charles J, Plumas J. Imiquimod inhibits melanoma development by promoting pDC cytotoxic functions and impeding tumor vascularization. J Investig Dermatol. 2014;134:2551–2561. doi: 10.1038/jid.2014.194. [DOI] [PubMed] [Google Scholar]
- 13.Drobits B, Holcmann M, Amberg N, Swiecki M, Grundtner R, Hammer M, Colonna M, Sibilia M. Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. J Clin Investig. 2012;122:575–585. doi: 10.1172/JCI61034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panelli MC, Stashower ME, Slade HB et al Sequential gene profiling of basal cell carcinomas treated with imiquimod in a placebo-controlled study defines the requirements for tissue rejection. Genome Biol. 2007;8:R8. doi: 10.1186/gb-2007-8-1-r8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roukens AH, Vossen AC, Boland GJ, Verduyn W, van Dissel JT, Visser LG. Intradermal hepatitis B vaccination in non-responders after topical application of imiquimod (Aldara) Vaccine. 2010;28:4288–4293. doi: 10.1016/j.vaccine.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Adams S, O’Neill DW, Nonaka D, et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol. 2008;181:776–784. doi: 10.4049/jimmunol.181.1.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shackleton M, Davis ID, Hopkins W, et al. The impact of imiquimod, a Toll-like receptor-7 ligand (TLR7L), on the immunogenicity of melanoma peptide vaccination with adjuvant Flt3 ligand. Cancer Immun. 2004;4:9. [PubMed] [Google Scholar]
- 18.Turza K, Dengel L, Harris RC, Patterson JW, White K, Grosh WW, Slingluff CL., Jr Effectiveness of imiquimod limited to dermal melanoma metastases, with simultaneous resistance of subcutaneous metastasis. J Cutan Pathol. 2010;37:94–98. doi: 10.1111/j.1600-0560.2009.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lonsdale-Eccles AA, Morgan JM, Nagarajan S, Cruickshank DJ. Successful treatment of vulval melanoma in situ with topical 5% imiquimod cream. Br J Dermatol. 2006;155:215–217. doi: 10.1111/j.1365-2133.2006.07297.x. [DOI] [PubMed] [Google Scholar]
- 20.Bong AB, Bonnekoh B, Franke I, Schon MP, Ulrich J, Gollnick H. Imiquimod, a topical immune response modifier, in the treatment of cutaneous metastases of malignant melanoma. Dermatology. 2002;205:135–138. doi: 10.1159/000063904. [DOI] [PubMed] [Google Scholar]
- 21.Slingluff CL, Jr, Petroni GR, Chianese-Bullock KA, et al. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res. 2007;13:6386–6395. doi: 10.1158/1078-0432.CCR-07-0486. [DOI] [PubMed] [Google Scholar]
- 22.Slingluff CL, Jr, Petroni GR, Olson WC, et al. Effect of GM-CSF on circulating CD8+ and CD4+ T cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin Cancer Res. 2009;15:7036–7044. doi: 10.1158/1078-0432.CCR-09-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slingluff CL, Jr, Petroni GR, Chianese-Bullock KA, Smolkin ME, Ross MI, Haas NB, von Mehren M, Grosh WW. Randomized multicenter trial of the effects of melanoma-associated helper peptides and cyclophosphamide on the immunogenicity of a multipeptide melanoma vaccine. J Clin Oncol. 2011;29:2924–2932. doi: 10.1200/JCO.2010.33.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slingluff CL, Jr, Petroni GR, Olson WC, et al. A randomized pilot trial testing the safety and immunologic effects of a MAGE-A3 protein plus AS15 immunostimulant administered into muscle or into dermal/subcutaneous sites. Cancer Immunol Immunother. 2016;65:25–36. doi: 10.1007/s00262-015-1770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Currier JR, Kuta EG, Turk E, Earhart LB, Loomis-Price L, Janetzki S, Ferrari G, Birx DL, Cox JH. A panel of MHC class I restricted viral peptides for use as a quality control for vaccine trial ELISPOT assays. J Immunol Methods. 2002;260:157–172. doi: 10.1016/S0022-1759(01)00535-X. [DOI] [PubMed] [Google Scholar]
- 26.Clark RA, Chong BF, Mirchandani N, Yamanaka K, Murphy GF, Dowgiert RK, Kupper TS. A novel method for the isolation of skin resident T cells from normal and diseased human skin. J Investig Dermatol. 2006;126:1059–1070. doi: 10.1038/sj.jid.5700199. [DOI] [PubMed] [Google Scholar]
- 27.Salerno EP, Shea SM, Olson WC, Petroni GR, Smolkin ME, McSkimming C, Chianese-Bullock KA, Slingluff CL., Jr Activation, dysfunction and retention of T cells in vaccine sites after injection of incomplete Freund’s adjuvant, with or without peptide. Cancer Immunol Immunother. 2013;62:1149–1159. doi: 10.1007/s00262-013-1435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green DS, Bodman-Smith MD, Dalgleish AG, Fischer MD. Phase I/II study of topical imiquimod and intralesional interleukin-2 in the treatment of accessible metastases in malignant melanoma. Br J Dermatol. 2007;156:337–345. doi: 10.1111/j.1365-2133.2006.07664.x. [DOI] [PubMed] [Google Scholar]
- 29.Redondo P, del Olmo J, de Lopez-Diaz CA, Inoges S, Marquina M, Melero I, Bendandi M. Imiquimod enhances the systemic immunity attained by local cryosurgery destruction of melanoma lesions. J Investig Dermatol. 2007;127:1673–1680. doi: 10.1038/sj.jid.5700777. [DOI] [PubMed] [Google Scholar]
- 30.Rahma OE, Gammoh E, Simon RM, Khleif SN. Is the “3 + 3” dose-escalation phase I clinical trial design suitable for therapeutic cancer vaccine development? A recommendation for alternative design. Clin Cancer Res. 2014;20:4758–4767. doi: 10.1158/1078-0432.CCR-13-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messina JL, Fenstermacher DA, Eschrich S, et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci Rep. 2012 doi: 10.1038/srep00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/S0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 34.Escoll P, del Fresno C, Garcia L, Valles G, Lendinez MJ, Arnalich F, Lopez-Collazo E. Rapid up-regulation of IRAK-M expression following a second endotoxin challenge in human monocytes and in monocytes isolated from septic patients. Biochem Biophys Res Commun. 2003;311:465–472. doi: 10.1016/j.bbrc.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 35.van ‘t Veer C, van den Pangaart PS, van Zoelen MA, de Kruif M, Birjmohun RS, Stroes ES, de Vos AF, van der Poll T. Induction of IRAK-M is associated with lipopolysaccharide tolerance in a human endotoxemia model. J Immunol. 2007;179:7110–7120. doi: 10.4049/jimmunol.179.10.7110. [DOI] [PubMed] [Google Scholar]
- 36.del Fresno C, Otero K, Gomez-Garcia L, et al. Tumor cells deactivate human monocytes by up-regulating IL-1 receptor associated kinase-M expression via CD44 and TLR4. J Immunol. 2005;174:3032–3040. doi: 10.4049/jimmunol.174.5.3032. [DOI] [PubMed] [Google Scholar]
- 37.Luo Y, Dallaglio K, Chen Y, et al. ALDH1A isozymes are markers of human melanoma stem cells and potential therapeutic targets. Stem Cells. 2012;30:2100–2113. doi: 10.1002/stem.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11–26. doi: 10.1016/j.immuni.2013.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.