Abstract
Background
When exposed to smoking cues, nicotine dependent individuals activate brain regions overlapping with the default mode network (DMN), a network of regions involved in internally-focused cognition. The salience network (SN), which includes the dorsal anterior cingulate cortex (dACC), is thought to interact with the DMN and aids in directing attention toward salient internal or external stimuli. One possibility is that neurochemical variation in SN regions such as the dACC impact DMN reactivity to personally relevant stimuli such as smoking cues. This is consistent with emerging evidence suggesting an association between midline cortical glutamate (Glu) and activity in brain regions overlapping with the DMN.
Methods
In 18 nicotine-dependent individuals, we assessed the relationship between DMN activation to smoking relative to neutral cues using functional magnetic resonance imaging and dACC Glu as measured by magnetic resonance spectroscopy. This association also was tested in a replication sample of 14 nicotine-dependent participants.
Results
Not only was the DMN significantly less suppressed during smoking cue exposure, but also there was a positive association between DMN reactivity to smoking relative to neutral cues and dACC Glu (r = 0.56, p < 0.02). This finding was confirmed in the independent replication cohort (r = 0.64, p < 0.02).
Conclusions
The current findings confirm that the DMN is less suppressed when smokers view smoking relative to neutral cues, suggesting that smoking cues engage self-relevant processing. Furthermore, these results indicate that dACC Glu is associated with enhanced DMN engagement when nicotine-dependent individuals are exposed to self-relevant smoking cues.
Keywords: Nicotine, Default Mode Network, Glutamate, Dorsal Anterior Cingulate Cortex, fMRI
1. INTRODUCTION
Smoking cues are highly relevant for nicotine dependent individuals. For tobacco smokers, these cues have been associated with the rewarding effects of nicotine, signal the potential end of withdrawal symptoms, and have the ability to motivate continued drug use (Wileyto et al., 2004). Thus, it is unsurprising that cortical brain regions involved in self-relevant processing show enhanced activity when smokers view such cues. A meta-analysis showed that brain regions typically reactive to smoking cues include the medial prefrontal cortex (mPFC) extending into the rostral anterior cingulate cortex (rACC) and the precuneus (PrC) extending into the posterior cingulate cortex (PCC; Engelmann et al., 2012). It has been pointed out that this pattern of activation overlaps with the default mode network (DMN; Claus et al., 2013; Janes et al., 2015a, 2015b), which is a commonly studied network of brain regions involved in self-referential processing.
While DMN activity is associated with cognitive processes involving an internal focus such as self-reference, when recalling one’s past, and planning one’s personal future (Schacter et al., 2007; Spreng et al., 2009, 2010), the DMN is typically less active during cognitive tasks that require an external focus (McKiernan et al., 2003; Raichle et al., 2001; Raichle and Snyder, 2007). Some evidence suggests that cortical midline glutamate (Glu) may play a role in regulating DMN suppression during externally focused cognition (Hu et al., 2013). Specifically, a high level of Glu in the PCC/precuneus is associated with reduced task-related DMN suppression (Hu et al., 2013). Further, dorsal anterior cingulate (dACC) Glu may modulate task-related activity in regions of the DMN during cognitive control (Falkenberg et al., 2012). This concept is consistent with nicotine dependence research as the smoking cessation therapy varenicline not only facilitated DMN suppression during externally focused cognition, but also reduced dACC Glu (Wheelock et al., 2014). The relationship between dACC Glu and DMN activity is particularly relevant considering the fact that the dACC is a primary hub of the salience network (SN), which is thought to guide task-specific suppression or engagement of the DMN by identifying the most relevant internal or extrapersonal stimuli (Menon and Uddin, 2010; Seeley et al., 2007). For instance, SN brain regions, which include the dACC and insula, co-activate with the DMN during tasks requiring an internal focus such as autobiographical planning (Spreng et al., 2010), and these regions are strongly implicated in smoking cue-reactivity (Brody et al., 2007; Engelmann et al., 2012; Janes et al., 2010, 2015a), craving (Brewer et al., 2013), and dependence severity (Courtney et al., 2014).
It is still unclear whether DMN reactivity to smoking cues is related to the level of dACC Glu. Such a finding would support the role of dACC Glu in the self-referential processing of smoking cues, which may influence the impact these cues have on behavior. We hypothesized a positive association between dACC Glu and DMN activity during smoking cue-reactivity due to the self-relevant nature of these stimuli. This conjecture is supported by the prior work linking heightened cortical midline Glu and disrupted suppression of DMN regions during tasks requiring external focus (Hu et al., 2013; Wheelock et al., 2014).
Additionally, structural integrity of the salience network impacts DMN function (Bonnelle et al., 2012), which begs the question of whether neurochemical variation in the salience network also impacts DMN function. To address this question we measured dACC Glu using magnetic resonance spectroscopy (MRS) and brain reactivity to smoking cues using functional magnetic resonance imaging (fMRI) in two previously published independent samples of nicotine dependent individuals (Janes et al., 2015a, 2015b).
2. MATERIALS AND METHODS
2.1 Participants
Data were evaluated in two independent samples of individuals who participated in all study elements at McLean Hospital’s Imaging Center. The primary analysis was conducted using data from our prior work where nicotine dependent individuals were exposed to smoking vs. neutral cues (Janes et al., 2015a), while our replication cohort was exposed to smoking vs. neutral cues within the context of a working memory task (Janes et al., 2015b). Our primary cohort was comprised of 18 nicotine-dependent individuals aged 31.4 ± 1.4 years (10 women / 8 men), while our replication cohort was comprised of 14 nicotine-dependent individuals aged 26 ± 1.22 years (8 women / 6 men). Both cohorts were moderately nicotine dependent as indicated by the Fagerstrom test of nicotine dependence and reported smoking ≥10 cig/day over the last 6 months. Smoking status was confirmed by expired carbon monoxide immediately upon entering the study (CO: Micro Smokerlyzer II, Bedfont Scientific Instruments, Kent UK). For full demographics see table 1.
Table 1.
Demographic information for the primary and replication cohorts. Significant differences between groups are indicated with an asterisk.
| Characteristic | Primary Cohort N=18 |
Replication Cohort N=14 |
Group Comparison |
|---|---|---|---|
| Age (Years) | 31.4 ± 1.4 | 26 ± 1.22 | t = 2.3, p < 0.04* |
| Education (years) | 14.6 ± 0.54 | 15.6 ± 0.58 | t = 1.33, p > 0.18 |
| FTND | 5.33 ± 0.323 | 6.43 ± 0.25 | t = 2.6, p < 0.02* |
| Average Cig/Day | 12.7 ± 0.82 | 15.2 ± 1.0 | t = 1.9, p > 0.06 |
| Pack-Year | 8.5 ± 1.02 | 7.1 ± 1.3 | t = 0.88, p > 0.38 |
| Expired CO on arrival |
19.3 ± 2.6 | 2.89 ± 3.7 | t = 2.2, p > 0.04* |
| Expired CO Prescan |
21.6 ± 2.2 | 28.6 ± 3.5 | t = 1.8, p > 0.08 |
| Expired CO Postscan |
17.2 ± 1.6 | 19.7 ± 2.2 | t = 0.98, p > 0.36 |
| Subjective Smoking vs. Neutral Craving |
1.3 ± 0.32 | 1.95 ± 0.35 | t = 1.4, p > 0.18 |
The structured clinical interview for DSM IV-TR was used to exclude participants with the following conditions: organic mental disorder, bipolar disorder, and schizophrenia spectrum disorder. Participants also were excluded for a substance use disorder other than nicotine dependence, current depressive episode, psychotropic drug use, pregnancy, and were required to have a zero blood alcohol level as measured by a breath sample (Alco-Sensor IV, Intoximeters, St Louis, MO). All participants gave verbal and written consent prior to participating in any study procedures and this research was approved by the Partners Human Research Committee, which is the institutional review board of Partners Healthcare hospitals.
2.2 Functional Neuroimaging
To standardize the time since a cigarette was last smoked relative to all study procedures, all participants in both cohorts smoked one of their own cigarettes after signing the informed consent approximately 1.5 hours prior to MR scanning. Expired carbon monoxide was measured immediately before and after scanning. Subjective craving to smoking and neutral images also was assessed after scanning by asking participants to rate how much craving they experienced when viewing each image on a five-point scale.
Acquisition parameters were identical between cohorts. Imaging was completed on a Siemens Trio 3T (2.89T) scanner (Erlangen, Germany) using a 32-channel head coil. Multiecho multi-planar rapidly acquired gradient echostructural images were acquired with the following parameters (TR = 2.1 s, TE = 3.3 ms, slices = 128, matrix = 256 × 256, flip angle 7 degrees, resolution = 1.0 × 1.0 × 1.33 mm). Task-related fMRI was collected using a gradient echo echoplanar sequence with the following parameters (TR = 2 s, TE = 30 ms, flip angle = 75 degrees, slices = 37, distance factor 10%, voxel size = 3.5 mm isotropic and a GRAPPA acceleration factor of 2).
2.3 Magnetic Resonance Spectroscopy
Magnetic Resonance Spectroscopy also was identical between cohorts and was collected following procedures outlined in our prior work (Janes et al., 2013). Proton spectra were acquired using a modified J-resolved PRESS protocol (two-dimensional (2D)-JPRESS). A single 2 × 2 × 3 cm voxel was placed in the dACC using the high-resolution T1-weighted anatomical images. Shimming of the magnetic field within the prescribed voxel was done automatically using an automated shimming routine. Automated optimization also included water suppression power, carrier frequency, tip angles, and coil tuning. The 2D-JPRESS sequence collected 22 echo time (TE)-stepped spectra with the TE ranging from 30 to 350 ms in 15 ms increments. Acquisition parameters were: TR: 2 s, acquisition bandwidth = 67 Hz, spectral bandwith = 2 kHz, readout duration = 512 ms, NEX = 16/TE-step.
2.4 MRS Processing and Analysis
To quantify Glu with the JPRESS data, the 22 TE-stepped free-induction decay was zero-filled out to 64 points, Gaussian-filtered and Fourier transformed. Using our previously published methods, every J-resolved spectral extraction (64 in total) within the 67 Hz bandwith was fitted with LCModel and its theoretically-correct template, which is optimized GAMMA-simulated J-resolved basis sets modeled for 2.89 T (Friedman et al., 2013; Henry et al., 2011; Jensen et al., 2009). All spectral data were modeled for 2.89 T field strength (123.05 MHz) to match that of our TRIO scanner. The integrated area under the 2D surface for each metabolite was calculated by summing the raw peak areas across all 64 J-resolved extractions. Glutamate metabolites were expressed as ratios of total creatine (Cr).
2.5 Image Segmentation/voxel Tissue Analysis
As in our prior work (Janes et al., 2013), all image segmentation was performed using FSL (FMRIB Software Library; Analysis Group, FMRIB; Oxford, UK). The FSL segmentation tool was used to automatically segment cortical gray matter (GM), white matter (WM), and cerebral spinal fluid (CSF). These segmented images were evaluated using an automated voxel coregistration and partial-volume analysis in-house program written in C-code. Subsequently, the volumetric tissue contribution for each oblique dACC voxel was determined and volumetric contributions of total GM, WM, and CSF calculated.
2.6 fMRI and Smoking Cues
Evaluating brain reactivity to smoking > neutral cues was done in the context of one of two studies involving either the traditional cue reactivity task or working memory for smoking cues. These tasks are explained in detail in our prior work (Janes et al., 2015a, 2015b). In both tasks, the same smoking and neutral cues were used. Smoking images included smoking-related content such as people smoking, people holding cigarettes, or cigarettes alone. Neutral images were matched for content in that they involved people, hands, or objects such as pens or paintbrushes. In the traditional cue-reactivity task, presented to our primary cohort, target images were also presented to ensure participants were awake and attending to the task. Targets were pictures of animals and participants were asked to press a button when a target appeared. This data only was used to ensure participants were attending to the task. Our primary cohort was shown 60 smoking, 60 neutral and 12 target images divided evenly across 5 blocks lasting 5 m and 18 s each. Images were presented for 4 s in a pseudorandom order with no more than 2 of the same picture type occurring in a row as performed in our prior work (Janes et al., 2010). Images were divided by a jittered inter-trial-interval ranging from 6 – 14s in intervals of 2 s with a 10 s average across block. During this inter-trial interval, participants in the primary cohort were shown a white fixation cross on a black screen. The replication cohort was exposed to smoking cues in the context of the delay-match-to-sample working memory task (LoPresti et al., 2008; Schon et al., 2004). For the purposes of the present study only the “sample” portion of this task was analyzed, as this corresponds most closely to the traditional cue-reactivity task. During the sample period, participants were shown either a smoking or neutral image for 2 s that they would have to match to a subsequent image following a 10 s delay. Thus, the sample period allows for the smoking > neutral contrast to be conducted without involving the working memory component involved in the other task elements and closely resembles the traditional cue reactivity task. To insure participants were attending, only trials where participants performed accurately were included in the analysis. The majority of trials were included, as accuracy ranged from 0.92 ± 0.01 – 0.97 ± 0.07 depending on trial type.
2.7 fMRI Pre-Processing
Tools from the fMRI of the brain (FMRIB) software Library (FSL; http://www.fmrib.ox.ac.uk/fsl) were used to process all fMRI data. For all analyses, the first 5 volumes were removed to allow for signal stabilization. Functional data were then pre-processed including motion correction, brain extraction, slice timing correction, spatial smoothing with a Gaussian kernel for a full-width half-maximum 6 mm, and a high-pass temporal filter with Gaussian-weighted least-squares straight-line fitting with 100 s. Each individual participant’s data was registered to the MNI152 2 mm3 standard space template (Montreal Neurological Institute, Montreal, QC, Canada). For task-related data, an in-house program used in our prior work (Janes et al., 2015a, 2015b) was used to detect and adjust for artifacts generated by intensity spiking.
2.8 Cue-Reactivity
For both the traditional cue-reactivity and working memory tasks, the first-level analysis was conducted on each of the participant’s individual task runs separately and all task-related regressors were convolved with the gamma hemodynamic response function. Confound regressors representing motion were also included in the model. Contrasts were conducted between the smoking and neutral image conditions. For the working memory task these contrasts were conducted during the sample period of the delay-match-to-sample task. In both instances, these lower level individual runs were combined in the second level to generate the average brain reactivity for each individual participant. For the primary cohort we conducted a group level mixed effects (FLAME) whole brain analysis for the smoking vs. neutral contrast. Multiple comparisons were corrected to p < 0.05 using a cluster-based threshold across the entire brain Z – 2.3. This whole brain activation to the smoking vs. neutral contrast was compared to the DMN region of interest (ROI) defined by Smith et al. (2009, Fig.1) by calculating the cross correlation between these maps using the FSL command fslcc. To more directly evaluate how the DMN responds to smoking > neutral cues, beta-weights from the DMN ROI were extracted using FSL’s featquery from each individual. The Pearson’s correlation coefficient was then calculated between DMN beta-weights and dACC Glu/Cr for each cohort to assess the association between these measures.
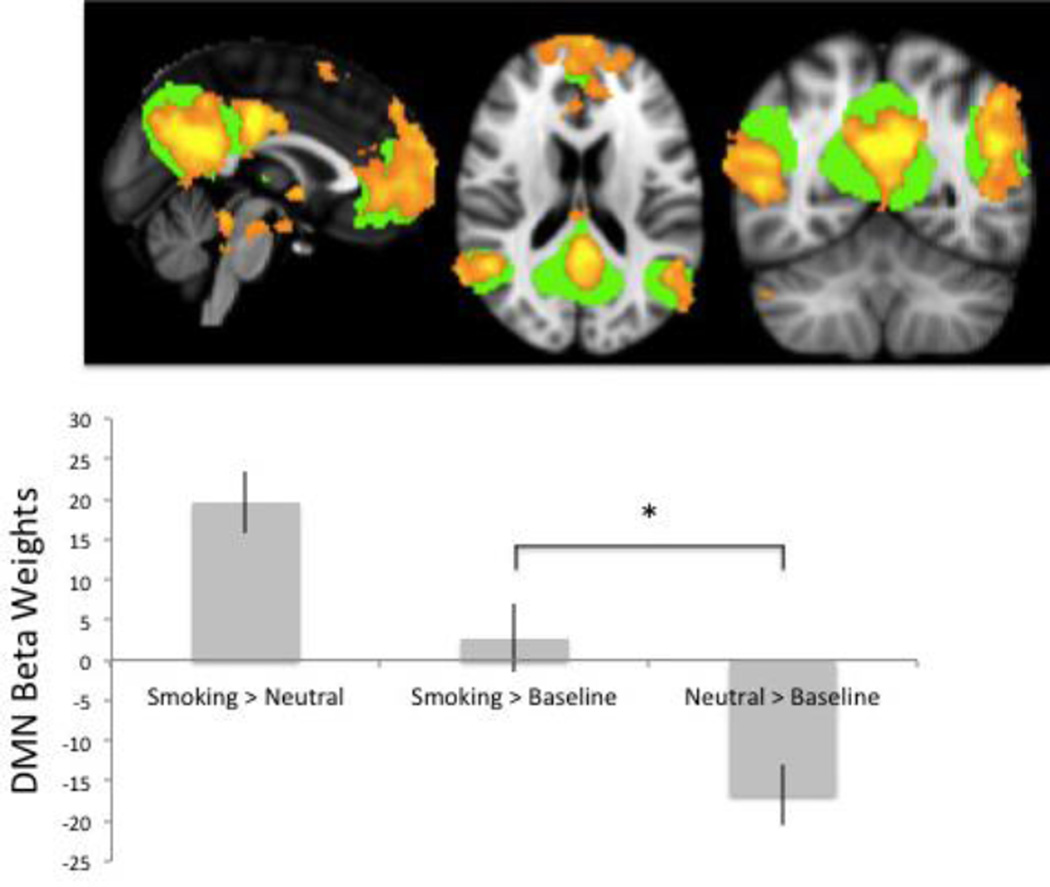
Figure 1.
Default mode network activation is significantly less suppressed when smokers view smoking cues. The green overlay on the brain images shows the DMN ROI taken from Smith et al 2009 used in all relevant analyses. The orange overlay shows the whole brain analysis results of the smoking vs. neutral contrast for the primary cohort, which significantly overlaps with the DMN ROI. The bar graph shows data from the primary cohort indicating that there is elevated DMN activity for the smoking > neutral contrast, which is driven by a lack of DMN suppression when smokers view smoking images. The asterisk indicates a significant difference between DMN reactivity to smoking cues and neutral cues (p < 0.001). This finding was confirmed by the replication cohort.
3. RESULTS
3.1 Demographics
All demographics are presented in table 1. The primary and replication cohorts differed in age (t = 2.3, p < 0.04), expired CO at intake (t = 2.2, p < 0.04), and nicotine dependence level as measured by the FTND (t = 2.3 p < 0.02), where the primary cohort was older, had lower expired CO, and was less nicotine dependent. However, the FTND scores indicate that both cohorts are moderately nicotine dependent. Subjective evaluation of all images shown during the in-scanner tasks indicated that participants reported higher craving to smoking than neutral cues in the primary (t = 4.2, p < 0.001) and replication cohorts (t = 5.6, p< 0.001). Following scanning, there was a significant decrease in expired carbon monoxide within the primary (t = 5.2, p < 0.01) and replication cohorts (t = 5.9, p < 0.001).
3.2 Functional magnetic resonance imaging
Within the primary cohort, the smoking vs. neutral contrast has been presented in our prior work (Janes et al., 2015a). There was substantial overlap between this map generated by the whole brain smoking vs. neutral contrast and the DMN ROI (r = 0.46, Fig. 1). When evaluating brain reactivity to smoking cues using an ROI approach, the DMN was significantly less suppressed when individuals were exposed to smoking cues in comparison to neutral cues (t = 5.2 p < 0.001; Fig. 1). This finding was confirmed in our replication cohort (t = 3.9 p < 0.002)
3.3 Magnetic resonance spectroscopy
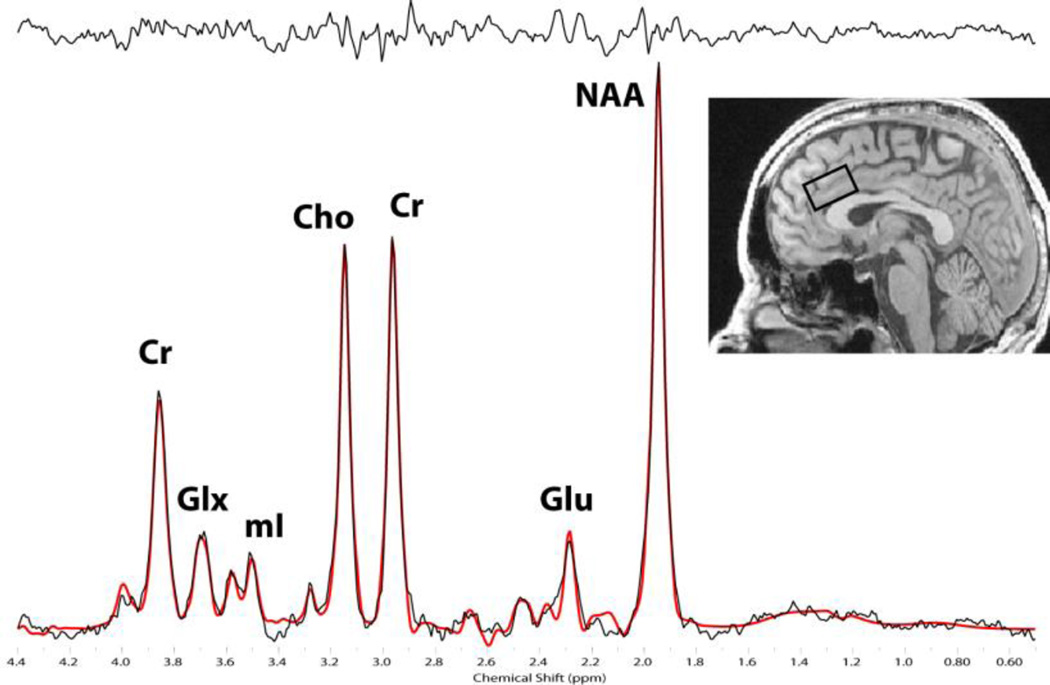
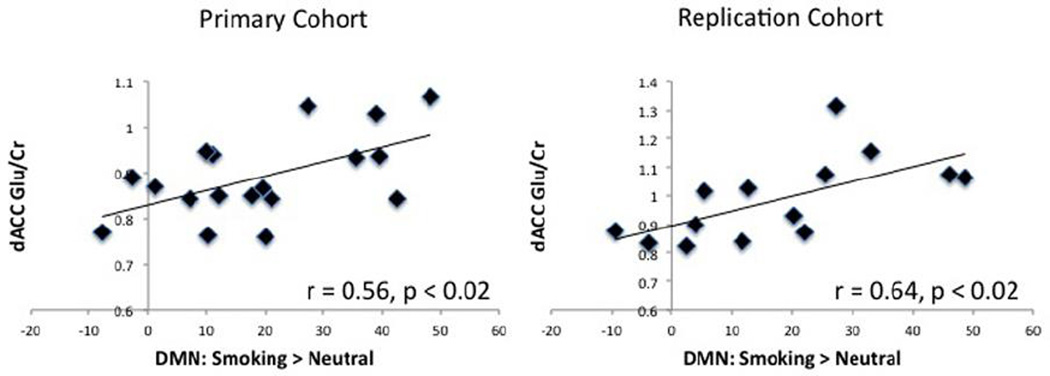
Participants in the primary cohort had an average Cramer Rao lower bound of 5.4% ± 0.3 for Glu and 1.8% ± 0.12 for Cr (representative spectra shown in Fig. 2). The values for the replication cohort were comparable with 5.2% ± 0.16 for Glu and 1.7% ± 0.16 for Cr. Dorsal ACC Glu/Cr was positively correlated with DMN reactivity for the smoking > neutral contrast within the primary cohort (r = 0.56, p < 0.02, Fig. 3). This finding was confirmed in the replication cohort (r = 0.64, p < 0.02). A correlation between the smoking > neutral contrast and Cr was conducted and showed that the association between Glu/Cr and cue reactivity was not driven by Cr in either the primary (r = 0.04, p = 0.87) or replication cohort (r = −2.7, p = 0.53). Follow-up analyses suggest that this relationship between DMN smoking > neutral cue-reactivity and dACC Glu/Cr is driven by reactivity to smoking images. Specifically, there was a trend association between DMN reactivity to smoking cues (relative to baseline when a fixation is presented) and dACC Glu/Cr in our primary (r = 0.41, p < 0.09) and replication cohorts (r = 0.46, p < 0.09). The relationship between dACC Glu/Cr and DMN reactivity to smoking cues becomes significant when both cohorts are combined into a single group (r = 0.39, p < 0.03). Such an association was not found between dACC Glu/Cr and DMN reactivity to neutral cues (relative to baseline) in either the primary (r = 0.1, p = 0.75) or replication cohort (r = 0.1, p = 0.70).
Figure 2.
Representative J-resolved spectra showing the Glu and Cr peaks used in the analysis. Brain image shows the sagittal view of a representative T1-weighted image illustrating the 2 × 2 × 3cm voxel placement in the dACC.
Figure 3.
There is a significant association between dACC Glu/Cr (y-axis) and the amount of DMN activation from the smoking > neutral contrast (x-axis) for both the primary and replication cohort.
3.4 Voxel Tissue
On average, for both groups, the dACC voxel was comprised of 56.86 ± 0.91 percent gray matter, 27.6 ± 1.1 percent white matter, and 15.9 ± 1.2 percent CSF. No correlation was found between dACC Glu/Cr and the volume of grey matter (primary cohort: r = −0.18, replication cohort: r = 0.09), white matter (primary cohort: r = − 0.03, replication cohort, r = − 0.25), or CSF (primary cohort: r = 0.19, replication cohort r = 0.15) within the dACC voxel used for all MRS measurements.
4. DISCUSSION
Numerous studies indicate that smokers have significant reactivity to smoking cues in brain regions overlapping with the DMN (Brody et al., 2007; Claus et al., 2013; Engelmann et al., 2012; Janes et al., 2015a, 2015b). Further, DMN regions such as the precuneus and posterior cingulate cortex (PCC) are linked with craving and nicotine dependence (Brewer et al. 2013; Courtney et al. 2013). By studying the DMN directly, the results of the present study confirm that the DMN is less suppressed when smokers view smoking cues relative to neutral cues. This point was further validated by showing a strong overlap between the DMN ROI as defined by Smith et al (2009) and the whole brain reactivity to smoking cues presented in the current work. Further, the strength of DMN reactivity to smoking > neutral cues is positively associated with dACC Glu/Cr, a finding that was confirmed in an independent sample. Follow-up analyses suggest that this relationship between DMN smoking > neutral cue-reactivity and dACC Glu/Cr is driven by reactivity to smoking images.
Prior work has shown that individuals with greater dACC Glu had enhanced activity in regions of the DMN during a low cognitive demand task (Falkenberg et al., 2012). Together with the present findings, these data indicate a relationship exists between of higher dACC Glu and DMN engagement when this network is typically active, namely during periods of low cognitive demand and during exposure to personally relevant stimuli. The link between dACC Glu and DMN reactivity to smoking cues also is supported by the finding that varenicline induced the suppression of both dACC Glu and DMN activity during a cognitive task in participants (Wheelock et al. 2014) suggesting a relationship between these neurobiological factors. Further, linking the interaction between these regions is that others have shown that the structural integrity of the SN, which is comprised of the dACC and insula, influences DMN activity (Bonnelle et al 2012), making it tempting to speculate that neurochemical variations within nodes of the SN, such as the dACC, may also impact DMN activity.
While evidence suggests that the SN modulates DMN activity (Seeley et al. 2007), we are unable to confirm that it is variation in dACC Glu that is driving the association with DMN activity to smoking vs. neutral cues. Despite this limitation, there is a growing body of literature implicating the importance of interactions between the SN and DMN (or nodes of these networks) in nicotine dependence.
For instance, stronger dACC-precuneus structural and functional interactions are associated with greater nicotine physical dependence (Huang et al., 2013, 2014) and such functional dACC-precuneus interactions increase during withdrawal (Huang et al., 2014). As the dACC and precuneus are primary nodes of the SN and DMN respectively, the work of Haung et al is consistent with the finding that SN-DMN interactions also increase during withdrawal (Lerman et al., 2014). It has been suggested that these enhanced interactions may bias individuals to engage the DMN and attend to internal states contributing to craving (Sutherland et al., 2012). Our work extends these findings by showing that even during satiety, individuals with higher dACC Glu are more prone to engage the DMN when exposed to self-relevant smoking cues. The possibility that dACC Glu may modulate such aspects of brain function is consistent with prior work. Specifically, heightened Glu in more rostral regions of the ACC, influenced an electroencephalogram (EEG) measure of resting brain function, as well as the perceived self-relatedness of stimuli (Bai et al., 2015).
Our prior work suggests that a relationship exists between dACC Glu and treatment outcome as individuals who are more likely to relapse have reduced dACC Glu (Mashhoon et al., 2011). These findings are somewhat in contrast to our current work, which suggests higher dACC Glu enhances self-relevant processing of smoking cues, which could be considered a risk factor for relapse. However, there are several key differences between these two studies. For instance, there is a significant age difference between the population of smokers in each study where the prior work focused on individuals in their mid to late 40s, suggesting that age-related interactions between dACC Glu and smoking should be considered. Further, while both studies focused on the dACC, voxel placement was slightly more rostral in the prior work. Finally, the work of Mashhoon et al was highly preliminary and involved an extremely small sample size that only included women. While these methodological differences may account for the apparent incongruity, it is also possible that heightened dACC Glu does not directly contribute to relapse vulnerability. Instead, dACC Glu may contribute to one of a constellation of risk factors contributing to relapse, specifically being more prone to self-referential processing during conditions such as exposure to smoking cues. Additionally, it is possible that existing treatments may aid cessation by targeting these neurobiological systems. For example, a 12-week course of varenicline reduced dACC Glu as well as DMN activation during the Stroop color-naming task, which requires external focus (Wheelock et al., 2014). It is unclear whether varenicline disrupts the association between dACC Glu and DMN reactivity to smoking relative to neutral cues.
The relationship between dACC Glu/Cr and DMN reactivity to smoking cues was confirmed in a smaller replication sample where participants were shown cues in the context of a working memory task. Given the difference in task (cue reactivity vs. memory task) it is plausible that individuals were responding to smoking cues differently. However, the fact that there was still a strong association between dACC Glu/Cr and DMN activity to smoking cues in both samples supports the robustness of these findings. There also were significant differences between the groups in age, expired CO on entry into the study, and FTND score. These group differences further suggest the robustness of the primary findings, as variation in these domains did not diminish the association between dACC Glu and DMN activity. It should be noted that while the groups did differ in age, both groups were comprised of relatively young adults (primary cohort range: 22–41 years, replication cohort: 18–33 years). Thus, we are unable to determine whether the reported association will be found other age groups such as adolescents or older adults. Additionally, despite group differences in FTND scores, both groups were identified as moderately nicotine dependent and we are unable to say whether individuals with mild or severe nicotine dependence would show the same relationship between dACC Glu and DMN activity. Additionally, we did not find any association between FTND and DMN smoking vs. neutral reactivity or dACC Glu/Cr in the primary (DMN: r = − 0.21, p > 0.4, dACC Glu/Cr: 0.04, p > 0.87) or replication cohorts (DMN: r = 0.9, p > 0.01, dACC Glu/Cr: −0.23, p > 0.4). This lack of an association is consistent with the work of others where dACC Glu showed no relationship with smoking variables such as age of first cigarette, daily cigarette consumption, or lifetime cigarette exposure (Gallinat and Schubert, 2007). Such findings suggest that while dACC Glu does not have an overt relationship with traditional smoking measures, dACC Glu is associated with brain function that may prime individuals to engage in self-referential processing under certain conditions such as during exposure to smoking cues.
There are several study limitations of this study that warrant discussion. First, MRS is unable to determine whether the measured Glu is related to neurotransmission, metabolism, or both. As such we cannot determine the specific mechanism through which heightened Glu may interact with DMN function and dACC connectivity. The approach that we took in the present study does not permit us to identify the causal link between these measures. However, the converging evidence across disciplines linking midline cortical Glu and DMN activity indicates an important relationship (Hu et al., 2013; Falkenberg et al., 2012; Wheelock et al., 2014). Most telling is that varenicline-induced suppression of both dACC Glu and DMN reactivity, which supports the concept that these neurobiological factors are associated (Wheelock et al., 2014). Whether smoking cessation treatments disrupt the association between dACC Glu and DMN cue reactivity should be studied further. Additionally, future research is needed to clarify the exact mechanism for the relationship between dACC Glu and DMN cue reactivity. Such work would be supported by the investigation of the DMN during tasks unrelated to smoking involving healthy control participants to determine whether the relationship between dACC Glu/Cr extends to DMN engagement beyond smoking cue-reactivity. Healthy controls were not included in the current work as enhanced responding to smoking cues should only be noted in drug users, a concept supported by the fact that no reliable cuereactivity has been reported in non-smokers (Engleman et al., 2011). Despite these limitations, the current findings indicate that smokers with relatively greater levels of dACC Glu/Cr are more likely to engage the DMN when exposed to smoking cues suggesting that they are engaging in self-referential processing and may have difficulty disengaging from these internal smoking-related thoughts.
Highlights.
The default mode network (DMN) is involved in internally focused cognition.
We show less DMN suppression when nicotine-dependent smokers view smoking cues.
The dorsal anterior cingulate cortex (dACC) may play a role in modulating the DMN.
We show an association between dACC glutamate and DMN reactivity to smoking cues.
Acknowledgments
Funding Sources This work was supported by the National Institute on Drug Abuse Grant K01DA029645 (AJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors:
ACJ conceptualized the experiment, analyzed data, wrote the manuscript, and integrated comments from all other authors. JB collected data, organized study days, and provided manuscript feedback. JEJ set up, and analyzed all spectroscopy data and provided manuscript feedback. SES offered guidance during the study and provided manuscript feedback. All authors have approved the final article.
The other authors have no financial disclosures to report
Conflicts of interest: none for all authors.
REFERENCES
- Bai Y, Nakao T, Xu J, Qin P, Chaves P, Heinzel A, Duncan N, Lane T, Yen N-S, Tsai S-Y, Northoff G. Resting state glutamate predicts elevated pre-stimulus alpha during self-relatedness: a combined EEG-MRS study on “rest-self overlap.”. Soc. Neurosci. 2015;11:249–263. doi: 10.1080/17470919.2015.1072582. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Garrison KA, Whitfield-Gabrieli S. What about the “self” is processed in the posterior cingulate cortex? Front. Hum. Neurosci. 2013;7:647. doi: 10.3389/fnhum.2013.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural substrates of resisting craving during cigarette cue exposure. Biol. Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Blaine SK, Filbey FM, Mayer AR, Hutchison KE. Association between nicotine dependence severity, BOLD response to smoking cues, and functional connectivity. Neuropsychopharmacol. 2013;38:2363–2372. doi: 10.1038/npp.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, London ED, Ray LA. The association between cue-reactivity in the precuneus and level of dependence on nicotine and alcohol. Drug Alcohol Depend. 2014;141:21–26. doi: 10.1016/j.drugalcdep.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU – brief) in laboratory and clinical settings. Nicotine Tob. Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Duncan NW, Wiebking C, Tiret B, Marjańska M. Glutamate concentration in the medial prefrontal cortex predicts resting-state cortical-subcortical functional connectivity in humans. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. NeuroImage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict. Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Falkenberg LE, Westerhausen R, Specht K, Hugdahl K. Resting-state glutamate level in the anterior cingulate predicts blood-oxygen level-dependent response to cognitive control. Proc. Natl. Acad. Sci. U S A. 2012;109:5069–5073. doi: 10.1073/pnas.1115628109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FMRIB Software Library; Analsysis Group. Oxford, UK: FMRIB; http://www.fmrib.ox.ac.uk/fsl. [Google Scholar]
- Friedman SD, Baker LD, Borson S, Jensen JE, Barsness SM, Craft S, Merriam GR, Otto RK, Novotny EJ, Vitiello MV. Growth hormone–releasing hormone increases brain gaba levels in mild cognitive impairment and healthy aging. JAMA Neurol. 2013;70:883–890. doi: 10.1001/jamaneurol.2013.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Schubert F. Regional cerebral glutamate concentrations and chronic tobacco consumption. Pharmacopsychiatry. 2007;40:64–67. doi: 10.1055/s-2007-970144. [DOI] [PubMed] [Google Scholar]
- Henry ME, Lauriat TL, Shanahan M, Renshaw PF, Jensen JE. Accuracy and stability of measuring GABA, glutamate, and glutamine by proton magnetic resonance spectroscopy: a phantom study at 4 tesla. J. Magn. Reson. 2011;208:210–218. doi: 10.1016/j.jmr.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Chen X, Gu H, Yang Y. Resting-state glutamate and gaba concentrations predict task-induced deactivation in the default mode network. J. Neurosci. 2013;33:18566–18573. doi: 10.1523/JNEUROSCI.1973-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, DiFranza JR, Kennedy DN, Zhang N. Progressive levels of physical dependence to tobacco coincide with changes in the anterior cingulum bundle microstructure. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, King JA, Ursprung WWS, Zheng S, Zhang N, Kennedy DN, Ziedonis D, DiFranza JR. The development and expression of physical nicotine dependence corresponds to structural and functional alterations in the anterior cingulate-precuneus pathway. Brain Behav. 2014;4:408–417. doi: 10.1002/brb3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Farmer S, Frederick BD, Nickerson LD, Lukas SE. An increase in tobacco craving is associated with enhanced medial prefrontal cortex network coupling. PLoS One. 2014;9:e88228. doi: 10.1371/journal.pone.0088228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Farmer S, Peechatka AL, Frederick BB, Lukas SE. Insula-dorsal anterior cingulate cortex coupling is associated with enhanced brain reactivity to smoking cues. Neuropsychopharmacology. 2015a;40:1561–1568. doi: 10.1038/npp.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Jensen JE, Farmer SL, Frederick BD, Pizzagalli DA, Lukas SE. GABA Levels in the dorsal anterior cingulate cortex associated with difficulty ignoring smoking-related cues in tobacco-dependent volunteers. Neuropsychopharmacology. 2013;38:1113–1120. doi: 10.1038/npp.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Nickerson LD, Frederick B, de B, Kaufman MJ. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depend. 2012;125:252–259. doi: 10.1016/j.drugalcdep.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, Frederick BB, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol. Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Ross RS, Farmer S, Frederick BB, Nickerson LD, Lukas SE, Stern CE. Memory retrieval of smoking-related images induce greater insula activation as revealed by an fMRI-based delayed matching to sample task. Addict. Biol. 2015b;20:349–356. doi: 10.1111/adb.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JE, Licata SC, Ongur D, Friedman SD, Prescot AP, Henry ME, Renshaw PF. Quantification of j-resolved proton spectra in two-dimensions with lcmodel using GAMMA-simulated basis sets at 4 tesla. NMR Biomed. 2009;22:762–769. doi: 10.1002/nbm.1390. [DOI] [PubMed] [Google Scholar]
- Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry. 2014;71:523–530. doi: 10.1001/jamapsychiatry.2013.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPresti M, Schon K, Tricarico MD, Swisher JD, Celone KA, Stern CE. Working memory for social cues recruits orbitofrontal cortex and amygdala: a functional magnetic resonance imaging study of delayed matching to sample for emotional expressions. J. Neurosci. 2008;28:3718–3728. doi: 10.1523/JNEUROSCI.0464-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon Y, Janes AC, Jensen JE, Prescot AP, Pachas G, Renshaw PF, Fava M, Eden Evins A, Kaufman MJ. Anterior cingulate proton spectroscopy glutamate levels differ as a function of smoking cessation outcome. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:1709–1713. doi: 10.1016/j.pnpbp.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J. Cogn. Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, London ED, Northoff G. Neuroimaging markers of glutamatergic and GABAergic systems in drug addiction: relationships to resting-state functional connectivity. Neurosci. Biobehav. Rev. 2016;61:35–52. doi: 10.1016/j.neubiorev.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc. Natl. Acad. Sci. U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1099. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat. Rev. Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schon K, Hasselmo ME, LoPresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: a functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. J. Neurosci. 2004;24:11088–11097. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim ASN. The common basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 2009;32:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: lessons learned and a road ahead. NeuroImage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MD, Reid MA, To H, White DM, Cropsey KL, Lahti AC. Open label smoking cessation with varenicline is associated with decreased glutamate levels and functional changes in anterior cingulate cortex: preliminary findings. Front. Pharmacol. 2014;5:158. doi: 10.3389/fphar.2014.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileyto P, Patterson F, Niaura R, Epstein L, Brown R, Audrain-McGovern J, Hawk L, Lerman C, Patterson F. Do small lapses predict relapse to smoking behavior under bupropion treatment? Nicotine Tob Res. 2004;6:357–366. doi: 10.1080/1462220042000202463. [DOI] [PubMed] [Google Scholar]