Abstract
Purpose
To analyze clinical signs and symptoms of ocular surface disease in patients with diabetes mellitus (DM), based on severity of diabetic peripheral neuropathy (DPN).
Methods
This cross-sectional study included participants who were carefully phenotyped by a multidisciplinary team and categorized into groups based on severity of DPN. All study participants underwent ophthalmic evaluation and completed the Ocular Surface Disease Index (OSDI) and Visual Function Questionnaire (VFQ-25).
Results
The 34 study participants were: healthy controls (n=9), patients with DM and mild or no DPN (n=16), and patients with DM and severe DPN (n=9). Tear osmolarity was increased, and corneal nerve fiber length was decreased, with increasing severity of DPN. In addition, patients with DM were found to have decreased Schirmer’s test values when compared to healthy controls. No statistically significant differences were found between groups in OSDI, tear break-up time, or corneal sensitivity. No statistically significant correlations were noted between the OSDI or VFQ-25 scores and clinical signs of dry eyes.
Conclusions
This study demonstrates increased clinical signs of ocular surface disease, but not an increase in subjective symptoms of dry eyes, with increasing severity of DPN. Furthermore, no significant correlation was found between OSDI scores and clinical signs of dry eye. A periodic evaluation of the ocular surface is important for patients with DM, in addition to retinopathy screening, as they may be asymptomatic but have severe dry eye disease, which can lead to further ocular surface complications such as corneal ulceration.
Keywords: ocular surface disease, dry eye, diabetes, diabetes mellitus, diabetic peripheral neuropathy
Introduction
According to the CDC, the prevalence of diabetes mellitus (DM) in the United States in 2010 was 25.8 million, or 8.3% of the population.1 It is well known that DM is a common cause, or risk factor, for ocular disease including retinopathy, cataract, and glaucoma.2 There is also evidence of increased ocular surface disease in DM that includes: dry eye disease (DED), recurrent corneal erosions, neurotrophic corneal ulceration, and delayed epithelial healing.3,4,5
In addition to ocular manifestations of DM there are also a plethora of well-known systemic abnormalities associated with DM, including vasculopathy and diabetic peripheral neuropathy (DPN). DPN is the most common complication of DM and is estimated to affect up to 50% of patients with DM.6,7 The risk of DPN has been found to increase with age and duration of diabetes.8
Previous studies have established a correlation between DED and diabetic retinopathy.9 However, a recent study did not find a significant correlation between DED and DPN, using the Michigan neuropathy screening instrument (MNSI) to detect DPN.10 However, the MNSI, although a sensitive clinical instrument for detecting the presence of DPN, was not designed to evaluate DPN severity.
The goal of this study was to analyze clinical signs and symptoms of DED in patients with DM and different DPN severities, as defined using comprehensive, gold-standard evaluations11 performed by a multidisciplinary team.
Methods
This cross-sectional study included participants who were either healthy controls or had diabetes. This study was approved by the Institutional Review Board of the University of Michigan and is in compliance with the Declaration of Helsinki. All participants signed a written consent.
Main inclusion criteria were: age ≥ 18 years of age and presence of diabetes as defined by the American Diabetes Association12 for the diabetic group. Exclusion criteria included the following: 1) any systemic neuropathy other than DPN; 2) history of any ocular surgery or corneal disease; 3) history of cancer; 4) history of stroke; 5) history of previous back surgery or spinal stenosis; 6) any neurodegenerative condition like multiple sclerosis.13
All participants were carefully phenotyped using nerve conduction and quantitative sensory testing by a multidisciplinary team to determine the presence, and severity of DPN. Participants were classified as having mild DPN if they had the following: 1) Small fiber dysfunction, as defined by abnormal quantitative sensory testing (QST) and an abnormal neurologic examination performed by a board certified neurologist; 2) the presence of ≥ 1 abnormal attribute (of amplitude, latency, F-wave, or nerve conduction velocity) in ≥ 2 separate nerves among the median, peroneal, and sural nerves in nerve conduction studies (NCS).13
Participants were classified as having severe DPN if they had the following: a combination of neuropathic symptoms and signs of distal sensorimotor polyneuropathy with ≥ 2 of the following: decreased/absent distal sensation (as diagnosed by a neurological examination from a board certified neurologist and abnormal QST), unequivocally decreased or absent ankle reflexes, abnormalities in NCS as described above, and the absence of a recordable sural nerve amplitude.13
Participants were: healthy controls (group 1), patients with DM and mild or no DPN (group 2), and patients with DM and severe DPN (group 3). Gender, age, race, body mass index (BMI), hemoglobin A1c, type and duration of DM were recorded for all participants. All study participants completed the Ocular Surface Disease Index (OSDI; Allergan, Inc, Irvine, CA, USA),14 a subjective 12-question survey for dry eye patients that gives a score denoting severity of dry eye symptoms, and the National Eye Institute Visual Function Questionnaire (VFQ-25),15 which is designed to measure vision-targeted health status. The VFQ-25 measures the influence of visual disability and visual symptoms on generic health domains such as emotional well being and social functioning, in addition to task-oriented domains related to daily visual functioning.
Participants also underwent clinical testing for: tear osmolarity (Tearlab, San Diego, CA), tear break up time (TBUT), corneal sensitivity (Cochet-Bonnet esthesiometry), Schirmer’s test (with anesthesia), and ocular surface staining with fluorescein and lissamine green. Clinical evaluations were done on both eyes and the mean of the two eyes was used for analysis.
Corneal confocal microscopy (CCM) was performed to determine corneal nerve fiber length (CNFL). CCM was performed only on the right eye of study participants. All subjects underwent CCM imaging of the central corneal sub-basal nerve plexus using the Heidelberg Retina Tomograph-2 (HRT-2) Rostock cornea module (Heidelberg Engineering, Heidelberg, Germany). After instilling one drop of topical proparacaine hydrochloride 0.5% into the right eye, the patient placed his/her head into the headrest of the confocal microscope. A small amount of Genteal eye gel (Novartis Pharmaceuticals, East Hanover, NJ) was applied to the lens of the microscope, and a sterile plastic cap (Tomocap, Heidelberg Engineering, Heidelberg, Germany) was placed over the lens. The patient was instructed to look straight ahead as the plastic-covered lens contacted the patient’s cornea. “Sequence” mode was used to record consecutive 400 × 400 µm images of the sub-basal nerve layer at 8 frames per second.13
Analysis of the corneal nerve images began with selection of five representative, focused, non-overlapping images of the sub-basal nerve plexus from the central cornea from each participant. A masked grader used NeuronJ software (publicly available at http://www.imagescience.org/meijering/software/neuronj/) to calculate the sum length of all nerve fibers within each image. From this number, the CNFL was derived by dividing the sum length of the nerve fibers in each image by the area of the image (1600 µm2). The five CNFL measurements from each individual were averaged to determine the final CNFL per patient.13
Statistical analysis was performed using STATA Data Analysis and Statistical Software (Release11.StataCorpLP, College Station, TX). Analysis of variance (ANOVA) was used to analyze differences between groups depending on severity of peripheral neuropathy and correlation testing was performed between areas of interest, using the Bonferroni correction for multiple comparisons. The chi square test was used to analyze differences amongst the groups with regards to fluorescein and lissamine green staining, race, sex, and type of DM. The T-test was utilized to compare continuous variables including duration of diabetes between the two diabetic groups.
Results
The 34 study participants were: age-matched healthy controls (group 1, n=9), patients with DM and mild or no DPN (group 2, n=16), and patients with DM and severe DPN (group 3, n=9). Their characteristics (age, gender, race, BMI, hemoglobin A1c, type and duration of DM) are shown in Table 1. As perhaps expected, patients with DM and more severe DPN were older and had a longer duration of DM.
Table 1.
Patient Characteristics
| Normal Controls (N=9) |
DM With No/Mild DPN (N=16) |
DM with Severe DPN (N=9) |
P- Value |
||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| Age [years] | 43.93 (10.24) | 52.01 (12.78) | 55.43 (9.21) | 0.098a | |
| Duration of DM [years] |
NA | 7.57 (3.78) | 11.94 (5.32) | 0.087b | |
| Body Mass Index | 22.66 (3.29) | 36.84 (6.21) | 31.93 (4.93) | 0.000a | |
| Hemoglobin A1c [mmol/mol] |
5.41 (0.16) | 7.86 (1.07) | 8.09 (1.47) | 0.001a | |
| N (%) | N (%) | N (%) | |||
| Gender | 0.053c | ||||
| Female | 6 (66.67) | 6 (37.5) | 1 (11.11) | ||
| Male | 3 (33.33) | 10 (62.5) | 8 (88.89) | ||
| Race | 0.813c | ||||
| Caucasian | 7 (77.78) | 12 (75.0) | 7 (77.78) | ||
| African-American | 2 (22.22) | 2 (12.5) | 1 (11.11) | ||
| Other | 0 (0) | 2 (12.5) | 1 (11.11) | ||
| Type of DM | 0.158c | ||||
| Type 1 | 0 (0) | 6 (37.5) | 1 (11.11) | ||
| Type 2 | 0 (0) | 10 (62.5) | 8 (88.89) | ||
Abbreviations: DPN, Diabetic Peripheral Neuropathy; DM, Diabetes Mellitus; NA, Not Available.
ANOVA,
T-Test,
Chi Square.
Clinical evaluation of ocular surface parameters revealed statistically significant differences between groups with regards to tear osmolarity, Schirmer’s test, corneal nerve fiber length (Figure 1), and VFQ-25 score. Tear osmolarity was greater in patients with DM than controls, and increased with severity of DPN. Schirmer’s test values were higher in controls than in diabetic patients, but did not decrease with increasing severity of DPN. Corneal nerve fiber lengths were higher in controls than in patients with DM, and declined with increasing DPN severity (Figure 2). The VFQ-25 score was greatest in controls, but did not decrease with increasing DPN severity.
Figure 1.
Differences in dry eye signs and symptoms between the following groups: healthy control (N=9), DM with mild/no DPN (N=16) and DM with severe DPN (N=9) in A: Mean tear osmolarity (p=0.007); B: Mean corneal nerve fiber length (p=0.004); C: Mean Schirmer's test values (p=0.019); D: Mean total OSDI score (p=0.260). Abbreviations: DPN, Diabetic Peripheral Neuropathy; DM, Diabetes Mellitus; OSDI, Ocular Surface Disease Index.
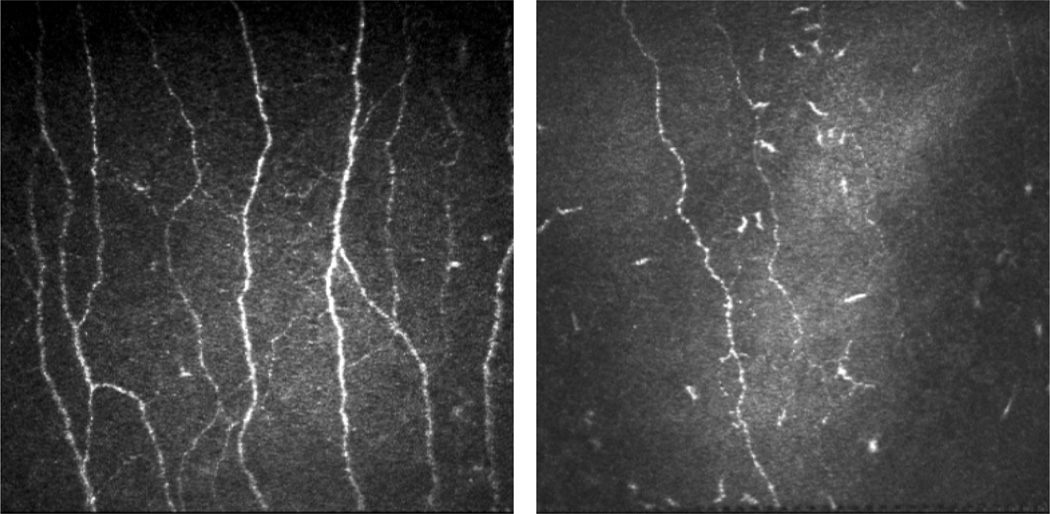
Figure 2.
Representative images of confocal microscopy of the sub-basal nerve plexus of a healthy control (left) compared with a patient with diabetes and peripheral neuropathy (right).
No statistically significant differences were found between groups with the OSDI (Figure 1), TBUT, or corneal sensitivity. OSDI scores tended to be lower in controls than in the groups with DM, but decreased with severity of DPN. There was a correlation between decreased corneal sensitivity and increasing DPN severity. In addition, no significant differences were noted between the 3 groups when comparing fluorescein (positive staining noted in 22% of group 1, 31% of group 2, and 33% of group 3 patients, p = 0.85) and lissamine green staining (positive staining noted in 11% of group 1, 40% of group 2, and 33% of group 3 patients, p = 0.32). No statistically significant correlations were noted between the OSDI or VFQ-25 score and the following clinical signs of dry eyes: tear osmolarity, TBUT, and Schirmer’s test. Likewise, no significant correlations were present between CNFL and the clinical signs of DED or corneal sensation.
Discussion
The goal of this study was to analyze clinical signs and symptoms of ocular surface disease in patients with DM, based on severity of DPN. Our results demonstrated that patients with DM have decreased Schirmer’s test results and increased tear osmolarity when compared to healthy controls, and that tear osmolarity increases with severity of DPN. In addition, corneal nerve fiber length was found to decrease with increasing severity of DPN.
Even though there were increased clinical findings of DED with increasing severity of DPN, dry eye symptoms, as measured by OSDI, were not significantly different based on severity of DPN. OSDI scores were higher in patients with DM vs. healthy controls, but were actually found to decrease with increasing DPN severity. This suggests that patients with increasing severity of DPN may have some pathology, like hypoesthesia of the cornea, which prevents them from being severely symptomatic even in the presence of advanced clinical findings. Our study demonstrated a non-statistically significant correlation between decreasing corneal sensitivity, as measured by Cochet-Bonnet, and increasing severity of DPN (r=−.022, p=.23). This may help explain the lack of subjective dry eye symptoms in patients with severe DPN. Previous studies16 have found statistical significance when comparing corneal sensitivities of diabetic and non-diabetic patients. Our small sample size likely precluded us from obtaining a similar statistical significance, but a correlation was noted. The OSDI has been shown to be a good measure of subjective symptoms in patients with dry eyes in the general population, but this may not hold true specifically for patients with DM and DPN. On a similar note, previous studies have also noted that patients with diabetic retinopathy seldom complain of dry eye symptoms despite clinical manifestations.17,18
The VFQ-25, in contrast to the OSDI, assesses a more comprehensive visual quality of life. Previous studies have shown a decrease in the VFQ-25 score in patients with dry eyes,19 and our study adds to that by demonstrating a significant decrease in VFQ-25 scores with increasing severity of DPN. Given the lack of correlation between dry eye symptoms and clinical signs in patients with DM, we suspect that VFQ-25 scores may be decreased in patients with severe DPN because of the association of DPN with other systemic and ocular complications of DM that decrease vision related quality of life.
It has previously been established that patients with diabetes have an increased prevalence of ocular surface disease and DED.19 Previous studies have shown that DM has been associated with decreased TBUT, Schirmer’s test values, subbasal nerve densities, and corneal sensitivity and increased tear osmolarity, and fluorescein and lissamine green staining.3–5, 18–21 A recent study also found that corneal nerve fiber lengths differed significantly between healthy controls and patients with DM, and progressively worsened with increasing neuropathy severity.22 Our study confirms these associations. Several other studies have also analyzed structural and metabolic corneal abnormalities that contribute to ocular surface disease in patients with DM.10,16 It is notable that the Schirmer’s test is comparably decreased in patients with DM regardless of level of DPN. There is known decrease in exocrine function in DM, and it is possible that DM affects tear function early in the disease but is not necessarily progressive.23
This study has several strengths. First, DPN was evaluated and quantified with a method that utilized nerve conduction velocity as part of a multidisciplinary evaluation. Unlike our study that finds increased clinical signs of dry eyes with increasing severity of DPN, a recent study in 2013 did not find a significant correlation between DED and DPN.10 However, this study used the Michigan neuropathy screening instrument (MNSI) for diagnosis of DPN, which the authors pointed out is not as accurate as methods such as nerve conduction velocities. Second, measurements of CNFL in our study were done in a masked fashion by the same person in all of the study participants, which minimizes potential bias in image assessment. Our study had several limitations, including the relatively small number of subjects which likely limited our ability to find greater statistical significance. We were also unable to avoid some potential confounding factors, including differences in age, gender, body mass index, and duration of DM.
In conclusion, this study demonstrates increased clinical signs of ocular surface disease, but not an increase in subjective symptoms of dry eyes, with increasing severity of DPN. Furthermore, no significant correlation was found between OSDI scores and clinical signs of dry eye. It is well known that routine screening for diabetic retinopathy is beneficial for patients with DM. The results of this study suggest that a periodic evaluation of the ocular surface is also important for DM patients, as they may be asymptomatic but have severe DED, which can lead to further ocular surface complications such as corneal ulceration.
Acknowledgments
This work was supported by: Michigan Diabetes Research Center funded by NIH P60DK020572 from the National Institute of Diabetes and Digestive and Kidney Diseases (RMS) and 1R01 HL102334 (RPB).The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
No conflicting relationship exists for any author regarding any material discussed in this manuscript.
Portions of this analysis were presented as a poster at the Association for Research in Vision and Ophthalmology Annual Meeting in Orlando, FL in 2014.
References
- 1.Centers for Disease Control and Prevention. Diabetes Public Health Resource. [Accessed 28 June, 2014]; Available at: http://www.cdc.gov/diabetes/pubs/estimates11.htm.
- 2.Rosenblatt BJ, Benson WE. Diabetic retinopathy. In: Yanoff M, Duker JS, Augsburger JJ, editors. Ophthalmology. 3rd. chap 6.19. Philadelphia, Pa: Elsevier Mosby; 2008. [Google Scholar]
- 3.Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology. 2001;18:586–592. doi: 10.1016/s0161-6420(00)00599-6. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez-Thorin JC. The cornea in diabetes mellitus. Int Ophthalmol Clin. 1998;38:19–36. [PubMed] [Google Scholar]
- 5.Fuerst N, Langelier N, Massaro-Giordano M, et al. Tear osmolarity and dry eye symptoms in diabetics. Clin Ophthalmol. 2014;8:507–515. doi: 10.2147/OPTH.S51514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasnoor M, Dimachkie MM, Kluding P, et al. Diabetic neuropathy part 1: overview and symmetric phenotypes. Neurol Clin. 2013;31:425–445. doi: 10.1016/j.ncl.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ang L, Jaiswal M, Martin C, Pop-Busui R. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14:528. doi: 10.1007/s11892-014-0528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Said G. Diabetic neuropathy. Handb Clin Neurol. 2013;115:579–589. doi: 10.1016/B978-0-444-52902-2.00033-3. [DOI] [PubMed] [Google Scholar]
- 9.Manaviat MR, Rashidi M, Afkhami-Ardekani M, et al. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol. 2008;8:10. doi: 10.1186/1471-2415-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Najafi L, Malek M, Valojerdi AE, et al. Dry eye and its correlation to diabetes microvascular complications in people with type 2 diabetes mellitus. J Diabetes Complications. 2013;27:459–462. doi: 10.1016/j.jdiacomp.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Dyck PJ, Albers JW, Andersen H, et al. Diabetic Polyneuropathies: Update on Research Definition, Diagnostic Criteria and Estimation of Severity. Diabetes Metab Res Rev. 2011;27:620–628. doi: 10.1002/dmrr.1226. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association. Executive summary: Standards of medical care in diabetes-2014. Diabetes Care. 2014;37(Suppl):S5–S13. doi: 10.2337/dc14-S005. [DOI] [PubMed] [Google Scholar]
- 13.Stem MS, Hussain M, Lentz SI, et al. Differential reduction in corneal nerve fiber length in patients with type 1 or type 2 diabetes mellitus. J Diabetes Complications. 2014;28:658–661. doi: 10.1016/j.jdiacomp.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 15.The National Eye Institute 25-Item Visual Function Questionnaire (VFQ25) [Accessed 16 June 2014];Version 2000. Available at: http://www.nei.nih.gov/resources/visionfunction/manual_cm2000.pdf.
- 16.Cousen P, Cackett P, Bennett H, et al. Tear production and corneal sensitivity in diabetes. J Diabetes Complications. 2007;21:371–373. doi: 10.1016/j.jdiacomp.2006.05.008. 22. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen NV, Lund FS. Diabetic polyneuropathy, corneal sensitivity, vibratory perception and Achilles tendon reflex in diabetes. Acta Neurol Scand. 1979;59:15–22. doi: 10.1111/j.1600-0404.1979.tb02906.x. [DOI] [PubMed] [Google Scholar]
- 18.Nepp J, Abela C, Polzer I, et al. Is there a correlation between the severity of diabetic retinopathy and keratoconjunctivitis sicca? Cornea. 2000;19:487–491. doi: 10.1097/00003226-200007000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Le Q, Zhou X, Ge L, et al. Impact of dry eye syndrome on vision-related quality of life in a non-clinic-based general population. BMC Ophthalmol. 2012;12:22. doi: 10.1186/1471-2415-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain S. Dry eyes in diabetes. Diabetes Care. 1998;21:1375. doi: 10.2337/diacare.21.8.1375. [DOI] [PubMed] [Google Scholar]
- 21.Hom M, De Land P. Self-reported dry eyes and diabetic history. Optometry. 2006;77:554–558. doi: 10.1016/j.optm.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Petropoulos IN, Alam U, Fadavi H, et al. Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes Care. 2013;36:3646–3651. doi: 10.2337/dc13-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dogru M. Tear secretion and tear film function in insulin dependent diabetics. Br J Ophthalmol. 2000;84:1210. doi: 10.1136/bjo.84.10.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]