Abstract
BACKGROUND/OBJECTIVES
Evidence indicates that berry anthocyanins are anti-atherogenic, antioxidant, and anti-inflammatory. However, berries differ vastly in their anthocyanin composition and thus potentially in their biological and metabolic effects. The present study compared hypolipidemic, antioxidant, and anti-inflammatory properties of blueberry (BB), blackberry (BK), and blackcurrant (BC) in a diet-induced obesity (DIO) mouse model.
MATERIALS/METHODS
Male C57BL/6J mice were fed a high fat (HF; 35% fat, w/w) control diet or a HF diet supplemented with freeze-dried 5% BB, 6.3% BK or 5.7% BC for 12 weeks (10 mice/group) to achieve the same total anthocyanin content in each diet. Plasma lipids, antioxidant status and pro-inflammatory cytokines were measured. The expression of genes involved in antioxidant defense, inflammation, and lipid metabolism was determined in the liver, epididymal adipose tissue, proximal intestine, and skeletal muscle. Histological analysis was performed to identify crown-like structure (CLS) in epididymal fat pads to determine macrophage infiltration.
RESULTS
No differences were noted between the control and any berry-fed groups in plasma levels of liver enzymes, insulin, glucose, ferric reducing antioxidant power, superoxide dismutase, and tumor necrosis factor α. However, BK significantly lowered plasma triglyceride compared with the HF control and other berries, whereas BC significantly reduced F4/80 mRNA and the number of CLS in the epididymal fat pad, indicative of less macrophage infiltration.
CONCLUSIONS
The present study provides evidence that BB, BK and BC with varying anthocyanin composition differentially affect plasma lipids and adipose macrophage infiltration in DIO mice, but with no differences in their antioxidant capacity and anti-inflammatory potential.
Keywords: Berry anthocyanins, antioxidant capacity, hypolipidemic effects, inflammation, macrophage
INTRODUCTION
Oxidative stress and chronic inflammation in obesity are linked to the pathogenesis of chronic diseases, including cardiovascular disease (CVD), type 2 diabetes, and fatty liver disease [1,2,3,4]. Hyperlipidemia, a contributor to oxidative stress and inflammation, is a major risk factor for CVD and is positively associated with obesity [5].
Evidence from epidemiological and clinical studies indicates that a high daily intake of fruits and vegetables reduces the risk of chronic diseases [6,7] and prevents inflammation and oxidative stress in humans [8,9]. Among other components of fruits and vegetables, phenolic compounds, such as anthocyanins, possess antioxidant and anti-inflammatory properties. In particular, berries rich in anthocyanins have been shown to mitigate oxidative stress and inflammation, thus reducing disease risk and promoting health [10,11,12,13,14,15].
Anthocyanins are the primary polyphenols in berries. There are approximately 200 anthocyanin derivatives differing in their aglycone (anthocyanidin) and glycone (sugar) moieties. Six most prevalent aglycones in berries, i.e., cyanidin (cya), delphinidin (del), malvidin (mal), peonidin (peo), pelargonidin (pel) and petunidin (pet), attach to sugar residues, such as glucose, xylose, galactose, arabinose, and rutinose [16]. Anthocyanins are shown to exert different degrees of antioxidant and anti-inflammatory activities in vitro, depending on their chemical structures [17,18]. However, little is known about their differential antioxidant and anti-inflammatory effects in vivo. The hypothesis of this study was that anthocyanin composition is important to exert hypolipidemic, antioxidant, and anti-inflammatory properties of berries. The present study was conducted to compare the antioxidant, anti-inflammatory and hypolipidemic effects of blueberry (BB), blackberry (BK) and blackcurrant (BC), which are different in their anthocyanin compositions, in diet-induced obesity (DIO) mouse model.
MATERIALS AND METHODS
Measurement of total anthocyanins using a pH differential method
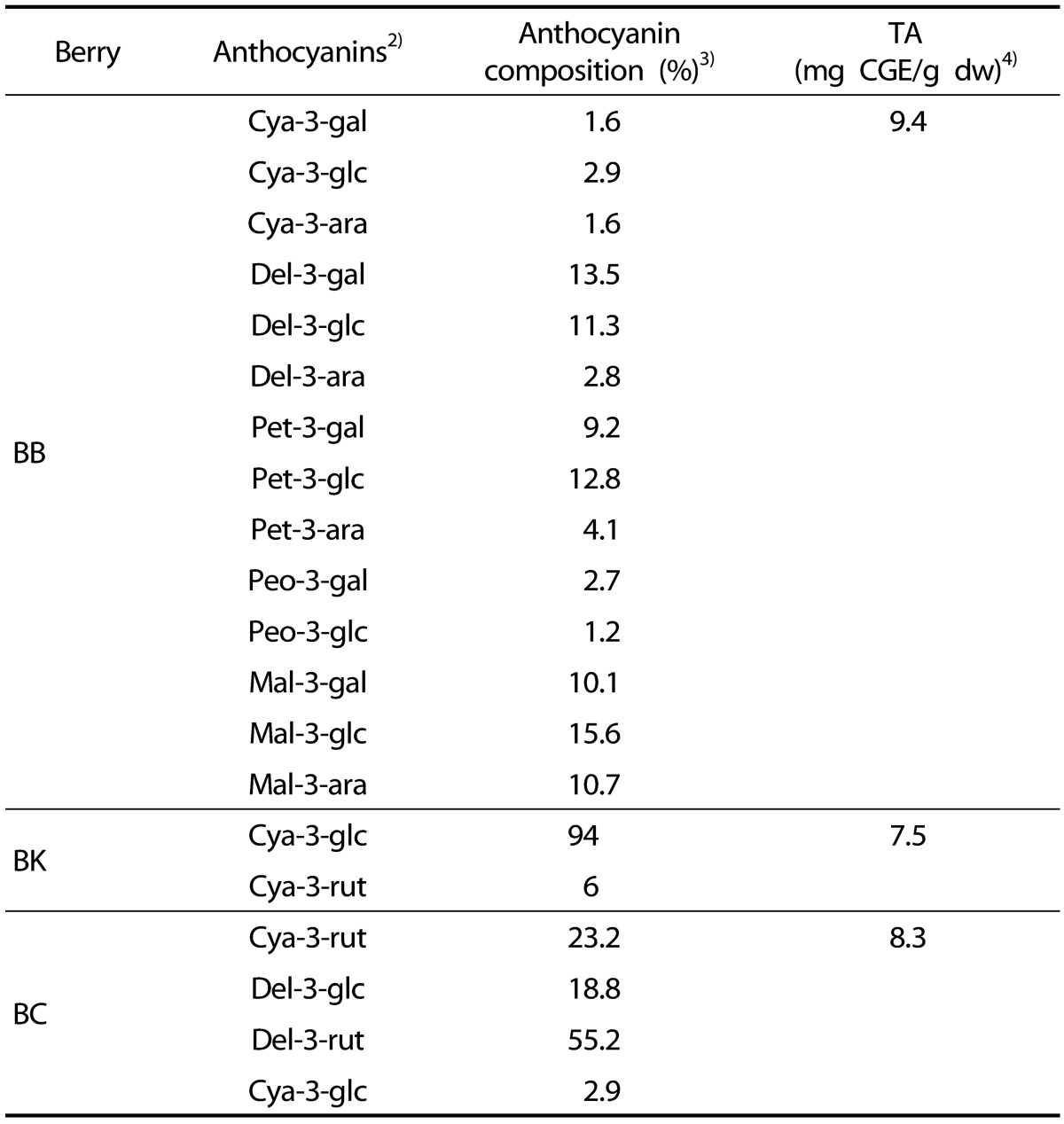
Three hundred grams of dried berry powders were extracted into 9:1 mixture of 100 mL of aqueous formic acid (8.5%, v/v) and acetonitrile/methanol mixture (85/15, v/v) for 3 min by vortexing followed by centrifugation at 720 x g for 5 min. The supernatants were subjected to anthocyanin analysis and total anthocyanin (TA) contents were measured using a pH differential method [19]. TA contents were expressed as mg cyanidin-3-glucoside equivalent (CGE)/g dry weight (dw) (Table 1).
Table 1. Analysis of anthocyanin compositions and TA of BB, BK and BC1).
1) TA, BB, BK and BC stand for total anthocyanin content, blueberry, blackberry, and blackcurrant, respectively.
2) Gal, glc, rut, and ara stand for galactoside, glucoside, rutinoside, and arabinoside, respectively. Cya, del, mal, pet, and peo stand for cyanidin, delphinidin, malvidin, petunidin, and peonidin, respectively.
3) Anthocyanin compositions were analyzed using HPLC method with CGE.
4) TA contents of three berries were determined by pH differential method. CGE stands for cyanin-3-glucoside equivalent.
Determination of the major anthocyanins of three berries using high performance liquid chromatography (HPLC)
Anthocyanin compositions of freeze-dried powder of BB, BK and BC (kindly provided by VDF FutureCeuticals, Momence, IL) were analyzed by reverse phase HPLC using an Agilent HPLC system (Agilent Model 1,200, Palo Alto, CA, USA) equipped with a photodiode array (PDA) detector and a C18 symmetry analytical column (5 µm 250 mm×4.6 mm, Waters, Milford, MA, USA). Wu's method [20] was slightly modified for the gradient of mobile phases (solvent A, 5% H3PO4 in HPLC grade water; solvent B, HPLC grade methanol). The anthocyanins were detected at 520 nm. Fourteen, 2 and 4 anthocyanins of BB, BK, and BC, respectively, were identified by comparison with reference chromatograms [20]. Anthocyanin compositions of berries were expressed as % of TA contents in Table 1.
Animals and diets
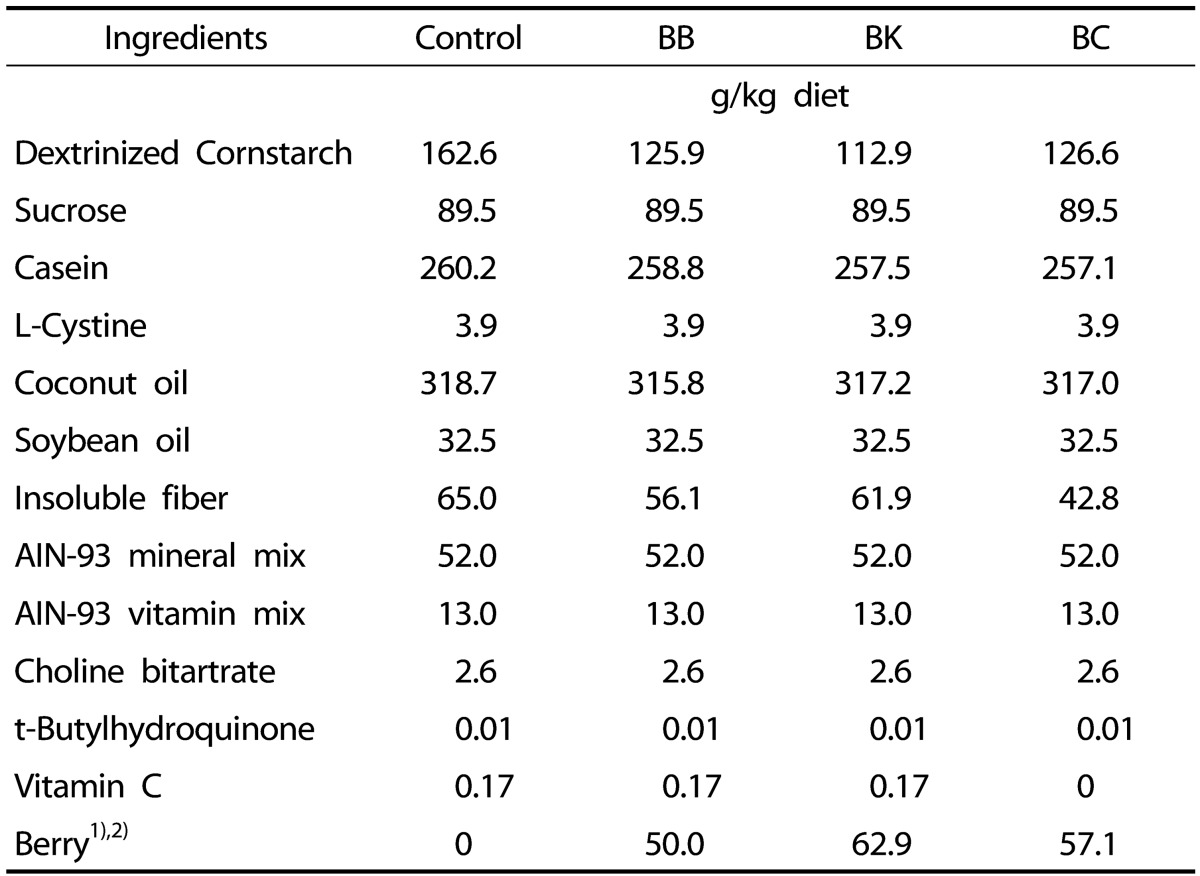
Forty male DIO C57BL/6J mice at age of 12 weeks were purchased from Jackson Laboratory (Bar Harbor, ME) and randomly assigned to the following groups (n = 10/group): 1) a control group fed a high fat (HF) (35% fat w/w); 2) the HF diet supplemented with BB (5%, w/w); 3) the HF diet with BK (6.3%, w/w); and 4) the HF diet with BC (5.7%, w/w). Berry contents in the experimental diets were adjusted to achieve the same TA contents. In order to adjust TA contents in our diet, 50 g of BB power (5%), 62.9 g of BK powder (6.3%), and 57.1 g of BC powder (5.7%) were added in each diet so that all of the diet contain 470 mg CGE/kg diets. Total phenolic contents of each berry powder measured by Folin-Ciocalteu's reagent methods were 2,706.7 ± 96.0 mg gallic acid equivalent (GAE)/kg diet for BB, 2,835.9 ± 63.8 mg GAE/kg diet for BK, and 2,382.4 ± 60.8 mg GAE/kg diet for BC. Also, to avoid a confounding effect of varying levels of berry vitamin C, its levels were adjusted to the vitamin C level of BC. By HPLC, BC was shown to contain 290 mg of vitamin C per 100 g dry powder, whereas BB and BK did not contain detectable amounts of the vitamin. The compositions of 4 different diets are shown in Table 2.
Table 2. Composition of experimental diets.
1) The amounts of berry powders were adjusted to equilibrate total anthocyanin contents in the berry diets as 470 mg/ kg diets.
2) Total phenolic contents of each diet supplemented by berry powders are 1,353.4 mg gallic acid equivalent (GAE)/kg diet (BB), 1,783.8 mg GAE/kg diet (BK), and 1,360.4 mg GAE/kg diet (BC).
Mice were housed in a polycarbonate cage under a 12 h light/dark cycle and had free access to water and food throughout. Body weights and food intake were recorded weekly. At the end of 12 weeks, mice were starved for 4 h and anesthetized with ketamine HCl/xylazine (120/6 mg per kg body weight) (Henry Schein, Melville, NY). Blood was collected by cardiac puncture and placed into tubes containing 3.5 mg EDTA (BD Vacutainer). Plasma was separated by centrifugation and stored at -80℃. Liver, epididymal and retroperitoneal adipose fat pads, soleus/gastrocnemius muscle, and intestine were snap frozen in liquid nitrogen and stored at -80℃ until use. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Connecticut (A13-026).
Plasma analysis
Plasma total cholesterol (TC) and triglyceride (TG) were determined enzymatically by using reagents from Pointe Scientific (Canton, MI) and a TG kit from Wako Chemical USA (Richmond, VA) as previously described [21,22]. High-density lipoprotein cholesterol (HDLC) was measured by after precipitating apolipoprotein B-containing lipoprotein by Mg2+/dextran sulfate (Pointe Scientific), and non-HDLC was measured by subtracting HDLC from TC. Plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined using Cholestech LDX System (Cholestech, Waltham, MA) [22]. Plasma tumor necrosis factor α (TNFα) was measured using an ELISA kit from eBioscience (San Diego, CA). Plasma insulin and glucose levels were determined using an ELISA kit from Alpco (Salem, NH) and a kit from Abcam (Cambridge, MA), respectively.
Ferric reducing antioxidant power (FRAP) assay was used to measure plasma antioxidant capacity [23]. Briefly, 20 µl of plasma was diluted in 60 µl of deionized H2O and mixed with 600 µl of pre-warmed FRAP reagent consisting of 300 mM acetate buffer, 10 mM of 2, 4, 6-tripyridyl-s-triazine in 40 mM HCl, and 20 mM of ferric chloride hexahydrate (10:1:1) at 37℃ for 15 min. The absorbance of the mixture was measured at 593 nm. The antioxidant capacity was expressed as µM trolox equivalent (TE)/ml. Plasma superoxide dismutase (SOD) was measured using a commercial kit from Cayman (Ann Arbor, MI).
Liver lipids
Liver lipids were extracted using the Folch method. TC and TG were measured enzymatically as previously described [24].
Adipocyte size and number of crown-like structure in epididymal fat pad
Formalin-fixed epididymal fat pads were embedded in paraffin and cut into 5 µm sections. Subsequently, the sections were stained with hematoxylin and eosin at the Connecticut Veterinary Medical Diagnostic Laboratory (Storrs, CT) and viewed at 10× magnification using an Axiovert Observer A1 microscope (Carl Zeiss Microscopy, Peabody, MA). The number of crown-like structure (CLS) in one field of a slide per mouse at 10× magnification was manually counted.
Quantitative realtime PCR (qRT-PCR)
Expression of genes in the liver, adipose tissue, proximal intestine, and skeletal muscle was measured using SYBR Green procedure and CFX96 real-time PCR detection system (Bio-Rad) as previously described [25,26]. Primer sequences were designed using Beacon Designer software (Premier Biosoft) according to GenBank database and will be available upon request.
Statistical analysis
One-way analysis of variance (ANOVA) and Tukey's Post Hoc test or unpaired t-test when appropriate were used to detect differences between groups by GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA). Pearson correlation was used when appropriate. P-values less than 0.05 were considered significant and values were expressed as mean ± SEM.
RESULTS
Identification and quantification of the major anthocyanins of to BB, BK and BC
The anthocyanin composition of three berries differed substantially as shown in Table 1. Fourteen anthocyanins were identified in BB including del-3-galactoside (del-3-gal, 13.5%), del-3-glucoside (del-3-glc, 11.3%), pet-3-glucoside (pet-3-glc, 12.8%) and mal-3-glucosie (mal-3-glc, 15.6%) as major anthocyanins. cya-3-glucoside accounted for 94% of anthocyanins in BK. BC contained del-3-rutinoside (del-3-rut, 55.2%), cya-3-rutinoside (cya-3-rut, 23.2%) and del-3-glucoside (del-3-glc, 18.8%) as major anthocyanins
General observations
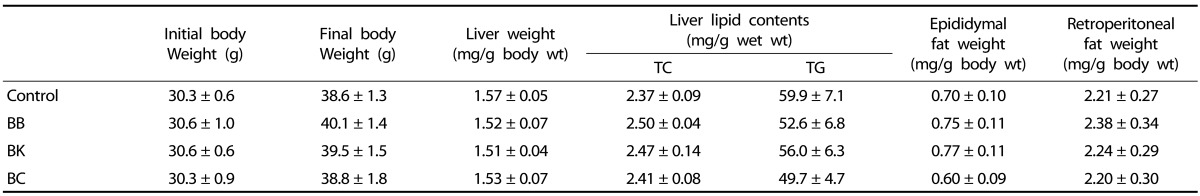
No significant differences were noted among groups in food intake (data not shown), body and liver weights, liver TG and TC, and epididymal and retroperitoneal fat weights (Table 3).
Table 3. Body and tissue weights and liver lipids in male C57BL/6J mice fed a HF control diet or a HF diet supplemented with a berry for 12 weeks1),2).
1) Data represent mean ± SEM; n = 10.
2) One-way analysis of variance with Tukey's Post Hoc Test was used to evaluate a statistical difference.
Plasma lipids and other markers
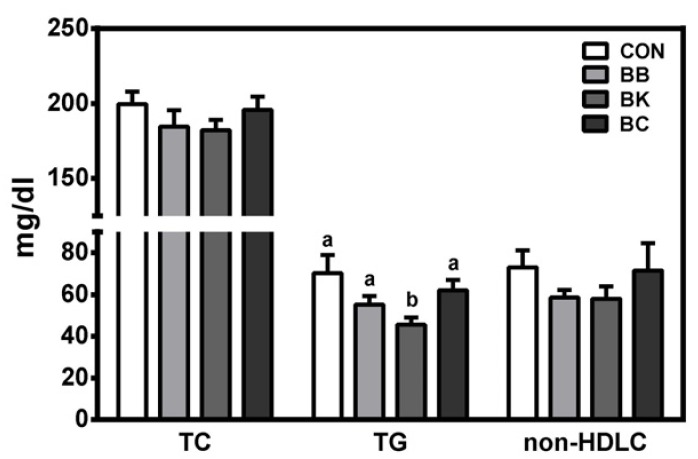
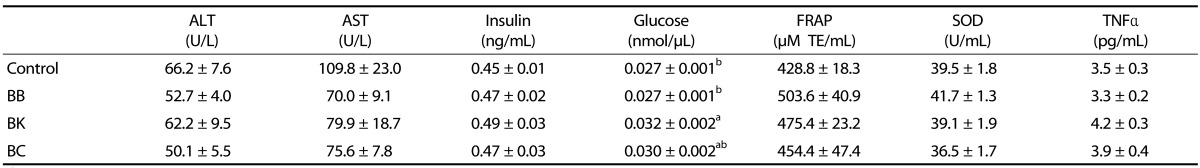
Plasma TG was significantly lower in mice fed BK than controls and other berry-fed groups. No significant differences were noted among groups in plasma TC and non-HDLC (Fig. 1), hepatic damage markers (ALT and AST), oxidative stress markers (FRAP and SOD), and TNFα (Table 4). Also, plasma insulin and glucose were not significantly altered by berry diets. Interestingly, however, plasma glucose was significantly elevated in mice fed BK, compared with controls and mice fed BB.
Fig. 1. Plasma lipid levels of male C57BL/6J mice fed a HF control diet or a HF diet supplemented with a berry for 12 weeks.
TC, total cholesterol; TG, triglycerides; non-HDLC, non-high-density lipoprotein cholesterol; CON, control; BB, blueberry; BK, blackberry; BC, blackcurrant. Mean ± SEM, n = 10. Bars with a different letter are significantly different (P < 0.05). One-way analysis of variance with Tukey's Post Hoc Test was used to evaluate a statistical difference.
Table 4. Plasma enzymes, insulin, glucose, antioxidant status and inflammatory cytokine level in male C57BL/6J mice fed a HF control diet or a HF diet supplemented with a berry for 12 weeks1),2),3).
1) Data represent mean ± SEM; n = 10.
2) One-way analysis of variance with Tukey's Post Hoc Test was used to evaluate a statistical difference.
3) Values with a different superscript in a column are statistically significant (P < 0.05).
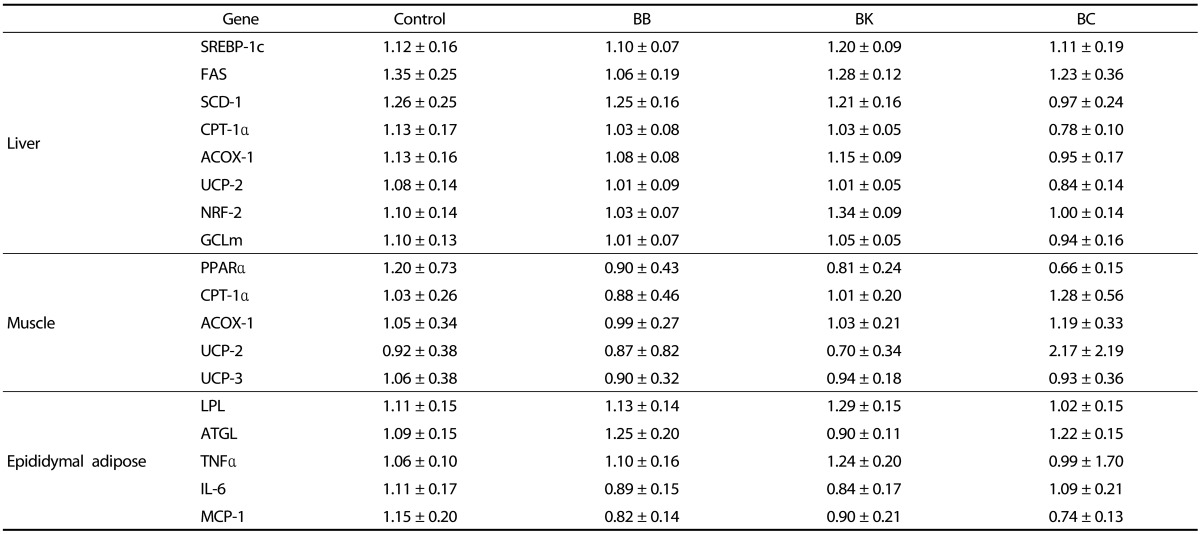
Expression of genes involved in lipogenesis, fatty acid oxidation, and antioxidant defense in the liver, muscle, adipose tissue and intestine
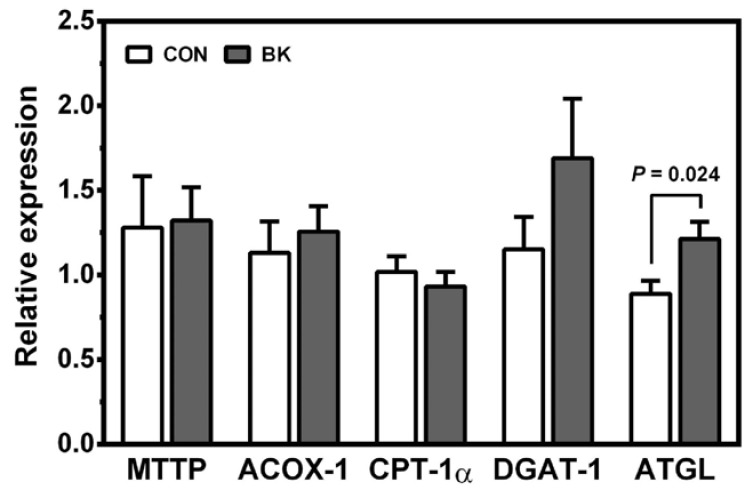
To gain insight into the TG-lowering effect of BK, genes involved in lipogenesis and fatty acid β-oxidation in the liver, skeletal muscle and proximal intestine were examined. Berries had no significant effects on the expression of liver lipogenic genes, including sterol regulatory element binding protein 1c (SREBP-1c), fatty acid synthase (FAS) and stearoyl CoA desaturase 1 (SCD-1) (Table 5). Likewise, no differences were observed in the mRNA levels of peroxisome proliferator activated receptor α (PPARα), carnitine palmitoyltransferase 1α (CPT-1α), and acyl-CoA oxidase 1 (ACOX-1) in the liver, muscle and intestine (Table 5 and Fig. 2). The genes involved in energy uncoupling as well as blocking proton leaking in the mitochondria [27], e.g., uncoupling protein 2 (UCP-2) and UCP-3, were also not significantly altered by berry supplementations. Although there were no significant differences in the expression of microsomal triglyceride transfer protein (MTTP) and diglyceride acyltransferase (DGAT1), adipose triglyceride lipase (ATGL) mRNA levels were significantly increased in mice fed BK compared to controls. In the epididymal adipose tissue, none of the berry supplementations significantly altered lipoprotein lipase (LPL) and ATGL mRNA levels.
Table 5. Gene expressions in the liver, muscle, and epididymal adipose tissue of male C57BL/6J mice fed a HF control diet or a HF diet supplemented with a berry for 12 weeks1),2).
1) Data are shown in relative to control and represent mean ± SEM; n = 10.
2) One-way analysis of variance with Tukey's Post Hoc Test was used to evaluate a statistical difference.
Fig. 2. Expression of genes in the proximal intestine of male C57BL/6J mice fed a HF control diet or a HF diet supplemented with BK for 12 weeks.
Values are relative expression to control. CON, control; BB, blueberry; BK, blackberry; BC, blackcurrant. Mean ± SEM, n = 10. Unpaired t-test was used to evaluate a statistical difference between two groups.
Berry supplementations also did not affect the expression of nuclear factor E2 related factor 2 (NRF-2) and its target gene, glutamate-cysteine ligase modifier subunit (GCLm) in the liver.
Inflammation in the epididymal adipose tissue
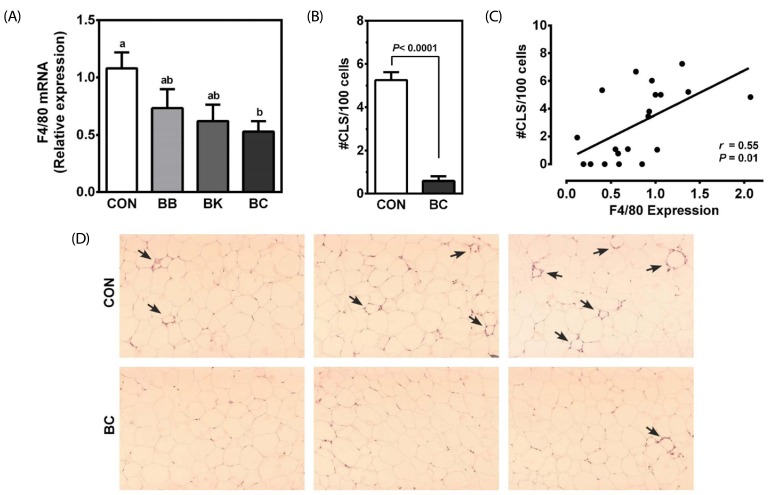
No differences were noted among groups in the expression of pro-inflammatory genes, including TNFα, interleukin 6 (IL-6), and monocyte chemoattractant protein 1 (MCP-1) in the epididymal adipose tissue (Table 5). However, the mRNA abundance of F4/80, a macrophage marker, was significantly lower in the mice fed BC than controls (Fig. 3A). Consistently, the number of CLS, representing a dying adipocyte surrounded by macrophages, was also significantly lower in the BC-fed mice than controls (Fig. 3B and 3D). A significant correlation was observed between F4/80 mRNA and the number of CLS (r = 0.55, P = 0.01) (Fig. 3C).
Fig. 3. Inflammatory markers in the epididymal adipose tissue of male C57BL/6J mice fed a HF control diet or a HF diet supplemented with a berry for 12 weeks.
(A) qRT-PCR was conducted to measure F4/80 mRNA levels. Mean ± SEM, n = 10. Bars with a different letter are significantly different (P < 0.05). (B) CLS number was counted in a field of H&E stained adipose tissue samples and expressed as CLS number per 100 adipocytes. Mean ± SEM, n = 10. (C) Pearson correlation between F4/80 mRNA and the number of CLS. (D) Representative H&E stained adipose tissue sections from control (CON) and blackcurrant (BC)-fed mice. Arrows indicate CLS. One-way analysis of variance with Tukey's Post Hoc Test or unpaired t-test was used to evaluate a statistical difference. CLS: Crown-like structure.
DISCUSSION
We previously reported that BB contains more than 10 different anthocyanins including pet-3-glc, del-3-gal, mal-3-glc, mal-3-galactoside and mal-3-arabinoside, whereas BK contains cya-3-glc as the major anthocyanin in BK, accounting for ~94% of its TA content and BC contains four major anthocyanidins; del-3-rut, cya-3-rut, del-3-glc and cya-3-glc [28]. The present study examined if the compositionally distinct berries differentially display their antioxidant, anti-inflammatory, and lipidlowering effects in DIO mice. Our data here presents new evidence that among the 3 berries, BK uniquely lowered plasma TG. BC inhibited the expression of F4/80 with a concomitant reduction in CLS number, an indicator of macrophage infiltration, in the epididymal adipose tissue compared with other berries. The BK's TG-lowering and BC's inhibitory effects on the macrophage infiltration were independent of their antioxidant capacity.
In our recent study, we found that the anthocyanin fractions of the same berries repressed the expression of pro-inflammatory genes to a similar degree in LPS-treated RAW macrophages as well as bone marrow-derived macrophages in vitro [28]. In the present study, however, we did not observe systemic anti-inflammatory and antioxidant effects of any berries in vivo, as assessed by plasma TNFα, FRAP and SOD. Given that the low bioavailability of anthocyanins [29], it is possible that the levels of berry anthocyanins may not be sufficiently high to elicit a systemic anti-inflammatory effect.
We found that BK, but not BB and BC, exerted a TG-lowering effect compared to control. This is consistent with the earlier observation that BK nectar reduced serum TG in hypercholesterolemic hamsters [30]. None of the berries significantly affect the expression of genes involved in lipogenesis and in fatty acid β-oxidation. Also, the mRNA levels of UCP-2 and UCP-3, which potentially affect plasma TG by uncoupling ATP synthesis, were not significantly affected. Interestingly, the intestinal expression of ATGL critical to TG hydrolysis, was significantly greater in BK-fed mice than controls. As the intestine is the site for chylomicron formation [31,32], BK may enhance the intracellular hydrolysis of TG absorbed and inhibit TG synthesis needed chylomicron formation, thus lowering fat absorption and ultimately plasma TG. Studies have also shown that cya-3-glc, the primary anthocyanin in BK, lowers plasma TG by increasing PPARα expression/activity [33,34] and by increasing LPL activity in the skeletal muscle [35] in obese mice. In addition, it has been shown that plasma protocatechuic acid, a major metabolite of cya-3-glc, accounts for 44% of ingested cya-3-glc [36] and that protocatechuic acid can lower plasma TG in diabetic mice [37]. Further studies are warranted to define the mechanisms underlying the TG-lowering effect of BK.
Chronic low-grade inflammation in the adipose tissue leads to systemic inflammation and metabolic disturbances, which contribute to the development of insulin resistance, type 2 diabetes, and CVD [38,39,40]. In the lipid-laden adipose tissue in obesity, the adipocyte produces MCP-1 that facilitates the recruitment of circulating monocytes and infiltration of macrophages. In the present study, in the adipose tissue of mice fed BC, the expression of F4/80, a macrophage marker [41], was significantly lower than mice fed control diet. Furthermore, the number of CLS, a marker of dying adipocytes, was significantly reduced in the mice fed BC compared with controls, with a significant positive correlation between F4/80 expression and CLS number in the epididymal tissue. Although macrophages present in the adipose tissue are thought to originate from circulating monocytes recruited in response to MCP-1 [42], a recent evidence shows that macrophages locally proliferate in the adipose tissue and substantially contribute to the number of macrophages [43]. Therefore, the lower numbers of macrophages and CLS in the epididymal fat of BC-fed mice suggest that BC may inhibit the proliferation of macrophages in the adipose tissue without directly affecting the expression of pro-inflammatory genes.
Lastly, it should be noted that delphinidin, the major anthocyanidin in BC, has been shown to have higher antiinflammatory properties than cyanidin and malvidin, the major anthocyanidin of BK and BB, respectively, due to a presence of an ortho-dihydroxyphenyl group in the B ring of delphinidin [18]. The strong anti-inflammatory of BC compared to the BK and BB may be also attributable to a synergistic effect of delphinidin and cyanidin that is also present in BC [44]. An earlier study documented that a mixture of del-3-glc and cya-3-glc showed a synergistic repression of the production of C-reactive protein in HepG2 cells in vitro [44]. Different types of glycones in anthocyanins may also contribute to a different degree of anti-inflammatory properties of anthocyanins. Evidence shows that anthocyanins containing rutinoside, a main glycone of BC anthocyanins, are more stable than those containing a monosaccaride, possibly adding to its potent anti-inflammatory effect [45].
In conclusion, although confounding effects of other phenolic compounds in each berry would be a limitation of current study, our study here showed that BK, BC and BB, with distinctly different in their anthocyanin composition, exert different metabolic and inflammatory effects in DIO mice. BK lowered plasma TG, whereas BC markedly inhibited macrophage infiltration and decreased the number of CLS in the adipose tissue. Such effects of BK and BC were independent of their TA contents. Further studies are needed to investigate mechanisms underlying the unique effects of BK and BC.
Footnotes
CONFLICT OF INTEREST: The authors declare no potential conflicts of interests.
References
- 1.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm. 2010;2010:535918. doi: 10.1155/2010/535918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26:968–976. doi: 10.1161/01.ATV.0000216787.85457.f3. [DOI] [PubMed] [Google Scholar]
- 4.Mathieu P, Poirier P, Pibarot P, Lemieux I, Després JP. Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension. 2009;53:577–584. doi: 10.1161/HYPERTENSIONAHA.108.110320. [DOI] [PubMed] [Google Scholar]
- 5.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 6.Boeing H, Bechthold A, Bub A, Ellinger S, Haller D, Kroke A, Leschik-Bonnet E, Müller MJ, Oberritter H, Schulze M, Stehle P, Watzl B. Critical review: vegetables and fruit in the prevention of chronic diseases. Eur J Nutr. 2012;51:637–663. doi: 10.1007/s00394-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Colditz G, Ascherio A, Rosner B, Spiegelman D, Willett WC. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001;134:1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 8.Holt EM, Steffen LM, Moran A, Basu S, Steinberger J, Ross JA, Hong CP, Sinaiko AR. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J Am Diet Assoc. 2009;109:414–421. doi: 10.1016/j.jada.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazzano LA, Serdula MK, Liu S. Dietary intake of fruits and vegetables and risk of cardiovascular disease. Curr Atheroscler Rep. 2003;5:492–499. doi: 10.1007/s11883-003-0040-z. [DOI] [PubMed] [Google Scholar]
- 10.Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J Agric Food Chem. 2006;54:4069–4075. doi: 10.1021/jf060300l. [DOI] [PubMed] [Google Scholar]
- 11.Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res. 2007;51:675–683. doi: 10.1002/mnfr.200700002. [DOI] [PubMed] [Google Scholar]
- 12.Prior RL, Wilkes SE, Rogers TR, Khanal RC, Wu X, Howard LR. Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J Agric Food Chem. 2010;58:3970–3976. doi: 10.1021/jf902852d. [DOI] [PubMed] [Google Scholar]
- 13.Riso P, Klimis-Zacas D, Del Bo' C, Martini D, Campolo J, Vendrame S, Møller P, Loft S, De Maria R, Porrini M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur J Nutr. 2013;52:949–961. doi: 10.1007/s00394-012-0402-9. [DOI] [PubMed] [Google Scholar]
- 14.Serraino I, Dugo L, Dugo P, Mondello L, Mazzon E, Dugo G, Caputi AP, Cuzzocrea S. Protective effects of cyanidin-3-O-glucoside from blackberry extract against peroxynitrite-induced endothelial dysfunction and vascular failure. Life Sci. 2003;73:1097–1114. doi: 10.1016/s0024-3205(03)00356-4. [DOI] [PubMed] [Google Scholar]
- 15.Gopalan A, Reuben SC, Ahmed S, Darvesh AS, Hohmann J, Bishayee A. The health benefits of blackcurrants. Food Funct. 2012;3:795–809. doi: 10.1039/c2fo30058c. [DOI] [PubMed] [Google Scholar]
- 16.He J, Giusti MM. Anthocyanins: natural colorants with health-promoting properties. Annu Rev Food Sci Technol. 2010;1:163–187. doi: 10.1146/annurev.food.080708.100754. [DOI] [PubMed] [Google Scholar]
- 17.Chang HJ, Choi EH, Chun HS. Quantitative structure-activity relationship (QSAR) of antioxidative anthocyanidins and their glycosides. Food Sci Biotechnol. 2008;17:501–507. [Google Scholar]
- 18.Hou DX, Yanagita T, Uto T, Masuzaki S, Fujii M. Anthocyanidins inhibit cyclooxygenase-2 expression in LPS-evoked macrophages: structure-activity relationship and molecular mechanisms involved. Biochem Pharmacol. 2005;70:417–425. doi: 10.1016/j.bcp.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Giusti MM, Wrolstad RE. Current Protocols in Food Analytical Chemistry. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Hoboken (NJ): John Wiley & Sons, Inc.; 2001. pp. F1.2.1–F1.2.13. [Google Scholar]
- 20.Wu X, Prior RL. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: fruits and berries. J Agric Food Chem. 2005;53:2589–2599. doi: 10.1021/jf048068b. [DOI] [PubMed] [Google Scholar]
- 21.Kim B, Ku CS, Pham TX, Park Y, Martin DA, Xie L, Taheri R, Lee J, Bolling BW. Aronia melanocarpa (chokeberry) polyphenol-rich extract improves antioxidant function and reduces total plasma cholesterol in apolipoprotein E knockout mice. Nutr Res. 2013;33:406–413. doi: 10.1016/j.nutres.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y, Seo JM, Nguyen A, Pham TX, Park HJ, Park Y, Kim B, Bruno RS, Lee J. Astaxanthin-rich extract from the green alga Haematococcus pluvialis lowers plasma lipid concentrations and enhances antioxidant defense in apolipoprotein E knockout mice. J Nutr. 2011;141:1611–1617. doi: 10.3945/jn.111.142109. [DOI] [PubMed] [Google Scholar]
- 23.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 24.Carr TP, Andresen CJ, Rudel LL. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin Biochem. 1993;26:39–42. doi: 10.1016/0009-9120(93)90015-x. [DOI] [PubMed] [Google Scholar]
- 25.Kim B, Park Y, Wegner CJ, Bolling BW, Lee J. Polyphenol-rich black chokeberry (Aronia melanocarpa) extract regulates the expression of genes critical for intestinal cholesterol flux in Caco-2 cells. J Nutr Biochem. 2013;24:1564–1570. doi: 10.1016/j.jnutbio.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Ku CS, Pham TX, Park Y, Kim B, Shin MS, Kang I, Lee J. Edible blue-green algae reduce the production of pro-inflammatory cytokines by inhibiting NF-kappaB pathway in macrophages and splenocytes. Biochim Biophys Acta. 2013;1830:2981–2988. doi: 10.1016/j.bbagen.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mailloux RJ, Harper ME. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med. 2011;51:1106–1115. doi: 10.1016/j.freeradbiomed.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 28.Lee SG, Kim B, Yang Y, Pham TX, Park YK, Manatou J, Koo SI, Chun OK, Lee JY. Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-κB independent of NRF2-mediated mechanism. J Nutr Biochem. 2014;25:404–411. doi: 10.1016/j.jnutbio.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Crozier A, Del Rio D, Clifford MN. Bioavailability of dietary flavonoids and phenolic compounds. Mol Aspects Med. 2010;31:446–467. doi: 10.1016/j.mam.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira de Araujo PR, da Silva Santos V, Rodrigues Machado A, Gevehr Fernandes C, Silva JA, da Silva Rodrigues R. Benefits of blackberry nectar (Rubus spp.) relative to hypercholesterolemia and lipid peroxidation. Nutr Hosp. 2011;26:984–990. doi: 10.1590/S0212-16112011000500010. [DOI] [PubMed] [Google Scholar]
- 31.Prior RL, Wu X, Gu L, Hager T, Hager A, Wilkes S, Howard L. Purified berry anthocyanins but not whole berries normalize lipid parameters in mice fed an obesogenic high fat diet. Mol Nutr Food Res. 2009;53:1406–1418. doi: 10.1002/mnfr.200900026. [DOI] [PubMed] [Google Scholar]
- 32.Perseghin G. Muscle lipid metabolism in the metabolic syndrome. Curr Opin Lipidol. 2005;16:416–420. doi: 10.1097/01.mol.0000174401.07056.56. [DOI] [PubMed] [Google Scholar]
- 33.Jia Y, Kim JY, Jun HJ, Kim SJ, Lee JH, Hoang MH, Kim HS, Chang HI, Hwang KY, Um SJ, Lee SJ. Cyanidin is an agonistic ligand for peroxisome proliferator-activated receptor-alpha reducing hepatic lipid. Biochim Biophys Acta. 2012;1831:698–708. doi: 10.1016/j.bbalip.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Um MY, Ahn J, Ha TY. Hypolipidaemic effects of cyanidin 3-glucoside rich extract from black rice through regulating hepatic lipogenic enzyme activities. J Sci Food Agric. 2013;93:3126–3128. doi: 10.1002/jsfa.6070. [DOI] [PubMed] [Google Scholar]
- 35.Wei X, Wang D, Yang Y, Xia M, Li D, Li G, Zhu Y, Xiao Y, Ling W. Cyanidin-3-O-beta-glucoside improves obesity and triglyceride metabolism in KK-Ay mice by regulating lipoprotein lipase activity. J Sci Food Agric. 2011;91:1006–1013. doi: 10.1002/jsfa.4275. [DOI] [PubMed] [Google Scholar]
- 36.Vitaglione P, Donnarumma G, Napolitano A, Galvano F, Gallo A, Scalfi L, Fogliano V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J Nutr. 2007;137:2043–2048. doi: 10.1093/jn/137.9.2043. [DOI] [PubMed] [Google Scholar]
- 37.Lin CY, Huang CS, Huang CY, Yin MC. Anticoagulatory, antiinflammatory, and antioxidative effects of protocatechuic acid in diabetic mice. J Agric Food Chem. 2009;57:6661–6667. doi: 10.1021/jf9015202. [DOI] [PubMed] [Google Scholar]
- 38.Ohman MK, Shen Y, Obimba CI, Wright AP, Warnock M, Lawrence DA, Eitzman DT. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2008;117:798–805. doi: 10.1161/CIRCULATIONAHA.107.717595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010;17:332–341. doi: 10.5551/jat.3939. [DOI] [PubMed] [Google Scholar]
- 40.Wajchenberg BL, Nery M, Cunha MR, Silva ME. Adipose tissue at the crossroads in the development of the metabolic syndrome, inflammation and atherosclerosis. Arq Bras Endocrinol Metabol. 2009;53:145–150. doi: 10.1590/s0004-27302009000200005. [DOI] [PubMed] [Google Scholar]
- 41.Coenen KR, Gruen ML, Chait A, Hasty AH. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes. 2007;56:564–573. doi: 10.2337/db06-1375. [DOI] [PubMed] [Google Scholar]
- 42.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19:162–171. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Y, Ling W, Guo H, Song F, Ye Q, Zou T, Li D, Zhang Y, Li G, Xiao Y, Liu F, Li Z, Shi Z, Yang Y. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: a randomized controlled trial. Nutr Metab Cardiovasc Dis. 2013;23:843–849. doi: 10.1016/j.numecd.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Prior RL, Wu X. Anthocyanins: structural characteristics that result in unique metabolic patterns and biological activities. Free Radic Res. 2006;40:1014–1028. doi: 10.1080/10715760600758522. [DOI] [PubMed] [Google Scholar]