Abstract
Hepatitis C is a global health issue and constitutes a major cause of chronic liver disease worldwide. In this article, a comprehensive literature search was conducted for the prevalence of hepatitis C virus (HCV) infection in Greece, since data on the HCV prevalence, viremia and genotypes are important for developing strategies to manage or eliminate HCV infection. In addition, the pattern of HCV infection was analyzed according to the geographic region and the risk factors. These differences reflect not only distinct epidemiological characteristics among populations, but also differences on the strategy of data acquisition and quantification. Although there are not enough data, the estimation of the current prevalence of Hepatitis C in Greece ranges from 0.5% to 2%. The most important risk factors for HCV infection include blood product transfusion, intravenous drug use, chronic hemodialysis, organ transplantation, occupational exposure, sexual transmission, and vertical transmission. Because of lack of vaccine or effective post-exposure prophylaxis for HCV, the main focus of prevention is to recognize and control these risk factors. HCV infection in Greece is closely associated with the development of chronic liver disease, cirrhosis, and primary hepatocellular carcinoma. As far as the genotype distribution is concerned genotype 1 estimated to be 45%-47% and it constitutes the prevalent genotype in Greece, followed by genotype 3.
Keywords: Prevalence, Hepatitis C virus infection, Hepatitis C, Greece, Epidemiology
Core tip: Hepatitis C virus (HCV) is a major health issue, it constitutes one of the most often causes of liver disease not only in Greece but also worldwide and is a potential cause of morbidity and mortality in the future. Epidemiological studies in Greece suggest that prevalence of HCV infection varies from 0.5% to 2%. The annual average incidence of HCV infection is estimated at 0.62 in 100000 people without significant difference between the two genders. These data are particularly important because they contribute to a better apprehension of the HCV infection in Greece since health authorities are able to create a more efficient health policy in a country which is hard affected from the economic crisis.
INTRODUCTION
Hepatitis C is a contagious liver disease and constitutes a major global health issue. Hepatitis C virus (HCV) is a hepatotropic RNA virus which was discovered in 1989. It is a small, enveloped, single-stranded, positive-sense RNA virus and the structural domain contains two regions, the core and the envelope (E1 and E2). Approximately 60%-80% of infected people may progress to chronic liver disease and 20% of them will develop cirrhosis[1]. Eventually, 5%-7% of these patients die from HCV-related complications[2].
Parenteral exposure to HCV is the main cause of HCV transmission and it occurs via contaminated blood exposure, sexual transmission or vertical transmission from mother to child during perinatal period[3]. Before 1990 the group with the highest risk of HCV transmission was the transfused patients. However, the post-transfusion HCV related hepatitis decreased dramatically, when the virus was isolated and the screening of blood and blood products carried out. Nowadays, in developed countries the most common route of HCV infection is through the use of drugs[4]. Other groups of great risk are patients with history of surgical procedures (including abortion or uterine curettage), dental surgical procedures, chronic hemodialysis and tattooing[5]. Sexual transmission and vertical transmission are less common ways of HCV infection[3].
According to World Health Organization and recent studies, about 3% of the global population has been infected with HCV[1,6,7]. It is estimated that chronic carriers of HCV are more than 170 million and they constitute a high risk group for developing cirrhosis and Hepatocellular carcinoma (HCC)[7]. In addition, at Eastern Mediterranean Region, 17 million people suffer already from chronic hepatitis C and 800000 individuals are infected each year with HCV[8]. However, the epidemiological patterns among different countries of Eastern Mediterranean Region are not similar. Prevalence of HCV infection in countries such as Libya and Lebanon is reported to be 1.5%-3%[8], whereas in other countries, such as Egypt, prevalence is much higher (14%-20%)[9]. Epidemiological studies in Greece suggest that prevalence of HCV infection varies from 0.5% to 2%[10-13]. Consequently, epidemiological data depend not only on the geographical region and the population group but also on the strategy of data acquisition (national registries, phone surveys, etc.) and quantification. This procedure points out the need for more robust epidemiology studies which will be able to quantify HCV in the general population taking into account the urban, rural and marginalized populations [Intravenous drug users (IVDUs), people in institutions, etc.].
We have conducted a literature review from January 1992 to January 2016 using Medline, Scopus, and Google Scholar databases and an amount of information was collected from the official site of Hellenic Center for Disease Control and Prevention (HCDCP).
PREVALENCE OF HEPATITIS C IN GENERAL POPULATION
The estimation of current prevalence of Hepatitis C in Greece ranges from 0.5% to 2%. HCDCP reports that from 1998 to 2011, 200000 individuals have been infected with HCV[3] and 958 individuals have been infected with acute hepatitis C. The annual average incidence of HCV infection is estimated at 0.62 in 100000 people[3] without significant difference between the two genders[5]. A new epidemiological study which cites data from a recent phone survey[14], reports an age-adjusted anti-HCV prevalence of 1.79% (1.87% high-risk individuals-adjusted) for the population of the study (ages 18-70), and a total estimated prevalence of 1.47%[15]. According to the above studies, 58% of diagnosed chronic HCV patients have never been treated. This corresponds to approximately 15700 treated patients in 2011.
Furthermore, 16% of the total liver transplantations performed in Greece in the recent years (2011-2013) were attributable to HCV[15]. Another study which included multinational study groups, reported an estimated 133000 total number of viremic infections in 2013 and it forecasted a decline to 106700 viremic infections in 2030[16].
Prevalence of HCV infection depends on the geographical regions of Greece. For example, prevalence in Crete is higher than the average prevalence reported in mainland Greece[5,17,18]. More specifically the highest prevalence of anti-HCV antibodies is reported in Rethymnon (0.52%), whereas the lowest percentage of seropositivity is reported in Chania[19] (0.23%). Furthermore, Thessaly, in central Greece, is a region with low prevalence of Hepatitis C (0.34%). However, there are three municipalities in Thessaly (Nea Ionia in Magnesia and Palamas and Sofades in Karditsa) where the percentage of anti-HCV antibodies is high[20]. Moreover, some areas in North-Western Greece, such as Achaia (in Peloponnese) and Corfu are mentioned as regions with low prevalence of HCV infection (0.5%)[12,21-23]. Another study which conducted in a Greek island called Zakinthos with a well-defined mixed (urban and rural) population, identified that the overall anti-HCV prevalence was 1.25%, while there was a significant higher prevalence (6.8%) in a well-defined rural area[24]. A more recent study about the endemicity of viral hepatitis C in Cretan-population indicates that there is a higher prevalence of anti-HCV among younger individuals[17]. It is worth mentioning that in the same area the hepatitis C markers are absent in the children. This fact may raise some questions about the possible sources of transmission of hepatitis C during the preceding years such as drug or alcohol abuse, tattoos or piercing[25].
PREVALENCE OF HEPATITIS C IN HIGH RISK GROUPS
Transfused patients
In the 1970s and 1980s blood transfusion was the most common way of HCV transmission(known as Non–A, Non-B at the specific timeline)[26]. Lauer et al[27] in 2001 noted that the probability of post-transfusion HCV transmission amounts to 1 case per 103000 blood units. However, the most recent screening methods for HCV in blood banks appreciate that the risk of transmission in the modern era is significantly decreased and it considered to be about 1 chance per 2 million units transfused [European Centre for Disease Prevention and Control (ECDC)]. No survey has been conducted about the risk of post–transfusion HCV transmission in the Greek population. Furthermore, among blood donors, the regular systematic donors have lower anti-HCV prevalence than the military and replacement (family) donors (percentage of 0.23%, 0.26% and 0.42% respectively)[28].
One study which conducted in Greece revealed that from 434 patients infected with HCV, 167 (38.5%) had a history of blood transfusion[4]. Nonetheless, study groups of the above trial included patients with post transfusion hepatitis (PTH) acquired prior to the 1990s (and thus before the era of systematic blood screening), a time point characterized by the expansion of IVDUs in Greece[4]. In this study, it has been also reported that the genotype 1 was the most prevalent genotype in PTH patients with a prevalence of 57% prior to 1981, followed by a decrease to 48% post 1981 and a subsequent increase of genotype 3 from 13% to 30%[4]. Another study, which also conceals the rapid epidemiologic expansion of genotype 3 in this time-frame in the general population, reports that the reduction of the prevalent genotype is due to the fact that individuals infected by transfusion are less likely to practice high-risk behaviors[29].
There are other groups of patients, such as patients with β-thalassemia, who constitute an especially high risk group. In transfusion-dependent thalassemia, prevalence of HCV infection is very high. Various studies suggest that the percentage ranges from 23% tο 74% depending on the number of patients at the studies[30-32]. Nevertheless, chronic hepatitis C does not impair the overall survival of patients with transfusion-dependent thalassemia[30].
Intravenous drug users
Considering that the blood screening has significantly reduced the possibility of disease transmission via transfusion, intravenous drug use is an important risk factor of HCV transmission[9,33,34]. Many epidemiology studies in Greece suggest that 20%-40% of individuals with chronic hepatitis C have history of intravenous drug use[4,9-11]. In addition, studies report that approximately 73% of IVDUs suffer from HCV infection as well as that 11% of the total HCV infections come from active IVDUs[35]. The present socioeconomic status in Greece seems to affect negatively the prevalence of HCV among IVDUs. Data before and during the economic recession in Greece (2008-today)[16] report that the decline in Gross Domestic Product was inversely correlated with the annual prevalence rates of human immunodeficiency virus (HIV) and HCV among IVDUs[36]. The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) and the ECDC confirm this evidence[37].
The exact rate of HCV-HBV co-infection in the IVDU group is not well known. A study reports that the prevalence of HbsAg positive patients constitutes the 5% of the whole IVDUs, while at the same time the prevalence of anti-HCV positive patients is about 73% of the mentioned group. The expected prevalence of co-infection in this group does not agree with the rather high rates reported in the international literature[38].
HCV infected IVDUs are more frequently male, from urban areas as opposed to rural or semi-rural areas, with higher education, younger at study entry and less frequently immigrants[11]. Particularly after 1992, nine out of ten HCV infections belong to the IVDUs group and one out of two IVDUs were younger than 20 years when they acquired the infection. Some proof of the aforementioned speculations can be sought in the multinational/multicenter study conducted by Hatzakis et al[16]. Experienced stuff determined the outcomes in each country. Data from Greece forecast an increase in the number of HCC cases to 35% by 2030. The number of liver related deaths will likewise be increased to 35%, while decompensated cirrhosis and compensated cirrhosis infections will be increased 30% and 15% respectively[16]. The results from this study concerns the general population but to some extent they reflect the younger age of infection’s acquisition and thus the prolonged course of disease and the increase in the disease burden. In the present era international guidelines encourage physicians to treat HCV infected IVDUs since this strategy reduce the disease burden.
The prevalent genotype seems to be genotype 3 and especially subtype 3a, followed by genotypes 1a and 4a[4,29,33,35]. Savvas et al[4] suggest that after 1981 prevalence of genotype 1 was reduced and prevalence of genotype 3 was increased. This change has been observed both for IVDUs and transfused patients.
The age of drug users and the duration of drug usage increase the possibility of HCV infection in IVDUs[5]. However, the most HCV infections come from needle sharing among the drug users[39]. A recent study suggests that during the period 2005-2006 the number of IVDUs did not change but needle sharing became more common[11]. This phenomenon commonly occurs among prisoners who are IVDUs and for this reason they develop a higher rate of HCV infections in relation with the general population[39,40]. As far as the prevention measures are concerned, it is evident that opioid substitution therapy and needle/syringe programs are among the most effective measures for preventing infectious diseases among IVDUs. Data from European monitoring services (EMCDDA and ECDC) in 2011 underline the fact that Greece is among the countries which use less the above prevention strategies[37]. Specifically, Greece is placed 13th on the list of 18 European Union/European Economic Area (EU/EEA) countries on the topic of opioid substitution treatment, and 9th on the list of 12 EU/EEA countries.
Finally, high prevalence of anti-HCV antibodies (9%) is reported among patients in a Psychiatric hospital, and it is about ten times higher relative to general population. This is observed due to psychiatric morbidity since they have more possibilities to use intravenous drugs which are associated with HCV infection. In addition, the treatment for HCV is related to psychiatric adverse events[41].
Patients in chronic hemodialysis
HCV infection is common among Greek patients with end-stage renal disease. Prevalence of anti-HCV ranges from 10% to 65% depending on the geographical region[42]. Boletis et al[43] suggest that anti-HCV prevalence among hemodialysis patients of Laiko General Hospital of Athens is 24.3%. In a study of 366 hemodialysis patients, HCV infection was detected in 36%[44]. HCV infection in hemodialysis patients occurs due to prolonged vascular access and exposure to infected patients and contaminated equipment. After the implementation of universal precautions, the incidence of seroconversion was reduced to 0.56% and then to 0%[42]. It is reported that prevalence of HCV transmission is related to hemodialysis duration and number of transfusions[42,43,45]. In renal transplant patients, the prevalence of HCV infection is similar to hemodialysis patients[43]. The most prevalent genotype concerning hemodialysis patients differs in various studies. According to Rigopoulou et al[44] and Diamantis et al[46] genotype 3a is the most common prevalent genotype. However, Griveas et al[42] suggest that the majority of HCV infections is genotype 1b. Katsoulidou et al[47] in an earlier study observed that genotype 4 is the most common genotype of HCV infections.
Health care personnel/Warship personnel
Health care workers and other groups such as policemen, prison staff and social workers constitute a high risk group for HCV exposure[48]. The participation of the stuff in invasive procedures such as surgeries, hemodialysis and venipunctures is a great importance risk factor[26]. Each patient has 0.024% possibilities to infect another individual, whereas an HCV-infected patient has 1.8% possibilities to transmit the HCV virus[48]. According to a Greek study, exposure to HCV can occur by percutaneous injury (82%), mucosal exposure (14%), excoriations and abraded skin exposure (3%), and by human bites (1%)[48]. Prevalence of HCV infection among health care workers is estimated to be 1%[26] and it seems to be lower in relation to the prevalence in general population[48]. It is worth mentioning that many cases of occupational exposure are not reported. In addition, Falagas et al[49] demonstrate that the risk of a percutaneous exposure is bigger in health workers and nurses than doctors.
Mazokopakis et al[50] examined if there are a link between the seroprevalence of hepatitis A, B and C among the warship personnel in Greece. As far as the HCV prevalence is concerned, seems that there is no association between this group of individuals and HCV, since the anti-HCV prevalence in this group is similar to those reported in a Greek study among blood donors (0.5%-3%).
Diabetic patients
Allison et al[51] studied the association between diabetes mellitus (DM) and HCV and they noted that 50% of HCV positive patients developed DM, in contrast to 9% of HCV negative patients. Thus, they proposed that may exist a direct effect of HCV upon pancreatic islet cells or an autoimmune procedure. In a same study in Greece the results were opposite since they noted low anti-HCV prevalence in diabetic patients (1.65%). According to this study the diabetic population cannot establish as a group at high risk for HCV since the prevalence of the HCV in the general population in Greece was higher (> 2%)[52].
Renal transplant patients
A study which conducted in Greece reports that the prevalence of HCV infection in renal transplant recipients is high. Furthermore, the prevalence seems to be proportional to the haemodialysis time before transplant and inversely proportional to the time after transplant[53].
RISK FACTORS
Medical procedures
The risk of HCV transmission via medical procedures is very low, especially nowadays. The hospital stuff follows the safety measures and the common medical procedures are associated with low prevalence of HCV transmission[26]. As far as the dental procedures and the surgeries, the rate of HCV infections is small (10% of reported HCV infections)[11]. In the past, infection rates were higher due to the use of non-sterilized, multiple-use glass syringes for medical purposes and warm glass bottles for folk remedies (for congesting blood in the muscles)[20]. In the majority of cases the poor sanitation and sterilization methods were the cause for the HCV transmission.
Perinatal transmission
HCV transmission from HCV-infected pregnant women to their children may occur during pregnancy, labor or postnatal period[54]. Two Greek studies have demonstrated that 0.80%-1.95% of the pregnant women tested positive for anti-HCV antibodies, with the incidence presenting a linear increase over the passing years[55,56]. This elevated rate of HCV infection in this subpopulation is due to the extremely high rates of seropositivity among pregnant refugees[55,56]. Raptopoulou-Gigi et al[56] suggest that mother-to-child transmission rate is 6% when mother is viraemic, whereas no transmission is reported when the mother is non-viraemic[54]. Except from viral load, HCV/HIV co-infection and intravenous drug use history are also important risk factors for vertical transmission[54,56]. As reported by Syriopoulou et al[54] viral transmission from mother to child was not influenced by mother’s age, mode of delivery, genotype or type of feeding.
Sexual transmission
Sexual transmission of HCV in general population remains controversial. Percent of 15 of seropositive individuals report at least one high risk sexual contact in the last six months[26]. In addition, prevalence of anti-HCV antibodies among prostitutes is higher than prevalence in general population[26,57]. On the other hand, studies from other countries assert that sexual transmission of HCV among monogamous heterosexual couples is unlikely to happen[58,59] and the maximum incidence rate is 0.07%[58]. However, there is no similar Greek study to evaluate the risk of sexual transmission of HCV among couples.
HCV ROLE IN CHRONIC LIVER DISEASE AND HCC
Chronic liver disease is closely associated with HCV infection in Greece comparable to that reported in most European countries. The majority of patients with parenterally transmitted acute NANB hepatitis and almost 2/3 of those with sporadic NANB CLD were found to be associated with chronic HCV infection. Generally, chronic HCV infection was the cause of the single aetiological factor or co-factor, for about 1/4 (23.7%) of Greek patients presenting with CLD of any aetiology. Therefore, chronic HCV infection ranked second after chronic HBV infection in the aetiology of CLD in Greece[60].
The association of anti-HCV with HCC has been examined in several studies. HBV and HCV have an interacting role in the origin of hepatocellular carcinoma and in hepatocarcinogenesis which, in some patients, appears to be related to underlying replication of both viruses[60].
HIV/HCV CO-INFECTION
In Greece, the rates of HIV/HCV co-infection have ranged from 8.2% to 13.8%[61-63] and 8.1% of patients with HIV/HCV co-infection are homosexual[61]. In IVDUs population, HIV/HCV co-infection rates were increased over the last years[36]. In the majority of patients with co-infection, HCV (85.7%) viral load is very high[62]. However, in cases of HIV/HCV co-infection anti-HCV antibodies titer may be reduced or undetectable, because of the immunodeficiency[26].
REFUGEES
Immigrants who live in Greece develop higher rates of infectious diseases. Seropositivity rate for HCV is 2.3%[11,64] and it concerns mainly the refugees from the ex-Union of Soviet Socialist Republics and Africa (i.e., Egypt) and less the refugees from Albania[9,25,65-67].
GENOTYPE DISTRIBUTION
Genotypes 1a, 1b, 3a and 4a appeared in the Greek population around 1965, 1958, 1975 and 1967 respectively[29]. In addition, it is mentioned that during 1978-1990 genotype 3 increased rapidly, whereas the other subtypes increased slowly during 1960-1990[29,68]. Recent studies demonstrate genotype distribution in Greece. According to many studies, genotype 1 is estimated to be 45%-47% and it constitutes the prevalent genotype in Greece, followed by genotype 3[4,11,68]. The incidence of the remainder genotypes is genotype 4 (13%), genotype 2 (9%), genotype 5 (1.2%) and genotype 6 (0%)[4,11,69] (Figure 1). As far as subtypes are concerned, the most prevalent is 3a (32.9%), followed by 1b (26.8%) and 2a (6.1%).
Figure 1.
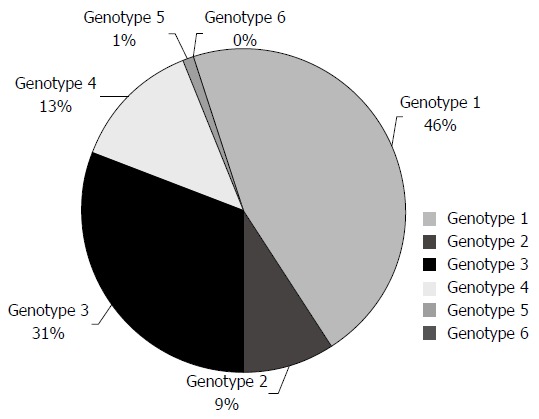
Genotype distribution in the Greek population.
Genotype distribution depends on various factors. Prevalence of genotypes differs between the genders. Genotype 1 is more prevalent in women, whereas genotype 3 is more common in men[11,68]. In addition, genotype 1 is related to contaminated blood transfusion and genotype 3 is related to intravenous drug use[4,11,29]. A recent study demonstrates that genotype 4 is more common among immigrants (18.8%) compared to Greeks (13.2%). These refugees are more likely to come from Egypt[11]. Finally, Karatapanis et al[70] report a significantly high prevalence of genotype 5 in Rhodes, an island in South-East Greece compared to the rest regions of the country.
CONCLUSION
Hepatitis C constitutes a global health problem because of its adverse impact on quality of life and survival of patients. The definition of epidemiological patterns of infection will help to control the HCV transmission in Greece. Thus, more multicenter Greek studies are needed for the precise evaluation of prevalence of HCV infection in Greece.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Triantos Christos has received fees for serving as a speaker for Gilead Sciences and Bristol-Myers Squibb, and as an advisory board member for Gilead Sciences and for MSD.
Peer-review started: April 9, 2016
First decision: June 20, 2016
Article in press: August 10, 2016
P- Reviewer: Julie NL, Mendez-Sanchez N S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
References
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 2.Mohamed AA, Elbedewy TA, El-Serafy M, El-Toukhy N, Ahmed W, Ali El Din Z. Hepatitis C virus: A global view. World J Hepatol. 2015;7:2676–2680. doi: 10.4254/wjh.v7.i26.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikolopoulou G, Zisouli A. Viral hepatitis. Available from: http://www.keelpno.gr/en-us/home.aspx.
- 4.Savvas SP, Koskinas J, Sinani C, Hadziyannis A, Spanou F, Hadziyannis SJ. Changes in epidemiological patterns of HCV infection and their impact on liver disease over the last 20 years in Greece. J Viral Hepat. 2005;12:551–557. doi: 10.1111/j.1365-2893.2005.00614.x. [DOI] [PubMed] [Google Scholar]
- 5.Lionis C, Vlachonikolis IG, Skliros S, Symeonidis A, Merkouris BP, Kouroumalis E. Do undefined sources of hepatitis C transmission exist? The Greek study in General Practice. J Viral Hepat. 2000;7:218–224. doi: 10.1046/j.1365-2893.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- 6.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29 Suppl 1:74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee MH, Yang HI, Yuan Y, L’Italien G, Chen CJ. Epidemiology and natural history of hepatitis C virus infection. World J Gastroenterol. 2014;20:9270–9280. doi: 10.3748/wjg.v20.i28.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatzakis A, Van Damme P, Alcorn K, Gore C, Benazzouz M, Berkane S, Buti M, Carballo M, Cortes Martins H, Deuffic-Burban S, et al. The state of hepatitis B and C in the Mediterranean and Balkan countries: report from a summit conference. J Viral Hepat. 2013;20 Suppl 2:1–20. doi: 10.1111/jvh.12120. [DOI] [PubMed] [Google Scholar]
- 9.Giannousis IP, Papatheodoridis GV, Deutsch MJ, Manolakopoulos SG, Manesis EK, Koskinas JS, Archimandritis AJ. The burden and recent epidemiological changes of the main chronic liver diseases in a Greek referral tertiary centre. Eur J Gastroenterol Hepatol. 2010;22:172–179. doi: 10.1097/MEG.0b013e328331115b. [DOI] [PubMed] [Google Scholar]
- 10.Sypsa V, Touloumi G, Tassopoulos NC, Ketikoglou I, Vafiadis I, Hatzis G, Tsantoulas D, Akriviadis E, Delladetsima J, Demonakou M, et al. Reconstructing and predicting the hepatitis C virus epidemic in Greece: increasing trends of cirrhosis and hepatocellular carcinoma despite the decline in incidence of HCV infection. J Viral Hepat. 2004;11:366–374. doi: 10.1111/j.1365-2893.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 11.Raptopoulou M, Touloumi G, Tzourmakliotis D, Nikolopoulou G, Dimopoulou M, Giannoulis G, Vasiliadis T, Skoutelis A, Anagnostou O, Hatzis G, et al. Significant epidemiological changes in chronic hepatitis C infection: results of the nationwide HEPNET-GREECE cohort study. Hippokratia. 2011;15:26–31. [PMC free article] [PubMed] [Google Scholar]
- 12.Gogos CA, Fouka KP, Nikiforidis G, Avgeridis K, Sakellaropoulos G, Bassaris H, Maniatis A, Skoutelis A. Prevalence of hepatitis B and C virus infection in the general population and selected groups in South-Western Greece. Eur J Epidemiol. 2003;18:551–557. doi: 10.1023/a:1024698715741. [DOI] [PubMed] [Google Scholar]
- 13.Koulentaki M, Ergazaki M, Moschandrea J, Spanoudakis S, Tzagarakis N, Drandakis PE, Spandidos DA, Kouroumalis EA. Prevalence of hepatitis B and C markers in high-risk hospitalised patients in Crete: a five-year observational study. BMC Public Health. 2001;1:17. doi: 10.1186/1471-2458-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papatheodoridis G, Sypsa V, Kantzanou M, Nikolakopoulos I, Hatzakis A. Estimating the treatment cascade of chronic hepatitis B and C in Greece using a telephone survey. J Viral Hepat. 2015;22:409–415. doi: 10.1111/jvh.12314. [DOI] [PubMed] [Google Scholar]
- 15.Saraswat V, Norris S, de Knegt RJ, Sanchez Avila JF, Sonderup M, Zuckerman E, Arkkila P, Stedman C, Acharya S, Aho I, et al. Historical epidemiology of hepatitis C virus (HCV) in select countries - volume 2. J Viral Hepat. 2015;22 Suppl 1:6–25. doi: 10.1111/jvh.12350. [DOI] [PubMed] [Google Scholar]
- 16.Hatzakis A, Chulanov V, Gadano AC, Bergin C, Ben-Ari Z, Mossong J, Schréter I, Baatarkhuu O, Acharya S, Aho I, et al. The present and future disease burden of hepatitis C virus (HCV) infections with today’s treatment paradigm - volume 2. J Viral Hepat. 2015;22 Suppl 1:26–45. doi: 10.1111/jvh.12351. [DOI] [PubMed] [Google Scholar]
- 17.Drositis I, Bertsias A, Lionis C, Kouroumalis E. Epidemiology and molecular analysis of hepatitis A, B and C in a semi-urban and rural area of Crete. Eur J Intern Med. 2013;24:839–845. doi: 10.1016/j.ejim.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Lionis C, Koulentaki M, Biziagos E, Kouroumalis E. Current prevalence of hepatitis A, B and C in a well-defined area in rural Crete, Greece. J Viral Hepat. 1997;4:55–61. doi: 10.1046/j.1365-2893.1997.00125.x. [DOI] [PubMed] [Google Scholar]
- 19.Koulentaki M, Spanoudakis S, Kantidaki E, Drandakis P, Tzagarakis N, Biziagos E, Moschandrea J, Kouroumalis EA. Prevalence of hepatitis B and C markers in volunteer blood donors in Crete. A 5-year study. J Viral Hepat. 1999;6:243–248. doi: 10.1046/j.1365-2893.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 20.Gatselis NK, Rigopoulou E, Stefos A, Kardasi M, Dalekos GN. Risk factors associated with HCV infection in semi-rural areas of central Greece. Eur J Intern Med. 2007;18:48–55. doi: 10.1016/j.ejim.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Zervou EK, Boumba DS, Liaskos C, Georgiadou S, Tsianos EV, Dalekos GN. Low prevalence of HCV, HIV, and HTLV-I/II infection markers in northwestern Greece: results of a 3-year prospective donor study (1995-1997) Eur J Intern Med. 2003;14:39–44. doi: 10.1016/s0953-6205(02)00185-1. [DOI] [PubMed] [Google Scholar]
- 22.Zervou E, Dalekos G, Gerolymatou A, Boumba D, Tsianos E. Prevalence of hepatitis B and C markers in blood donors in Epirus. Acta Microbiologica Hellenica. 1998;43:482–489. [Google Scholar]
- 23.Andriotis V, Tzilianos M, Nasoula E, Staveri X, Sagias G. Epidemiological report of infection by HCV, HIV-1, HTLV1/2 in blood donor population. MedChron Northwestern Greece. 2011:7. [Google Scholar]
- 24.Goritsas C, Plerou I, Agaliotis S, Spinthaki R, Mimidis K, Velissaris D, Lazarou N, Labropoulou-Karatza C. HCV infection in the general population of a Greek island: prevalence and risk factors. Hepatogastroenterology. 2000;47:782–785. [PubMed] [Google Scholar]
- 25.Lionis C, Frangoulis E, Koulentakis M, Biziagos E, Kouroumalis E. Prevalence of hepatitis A, B, and C markers in school children of a rural area of Crete, Greece. Eur J Epidemiol. 1997;13:417–420. doi: 10.1023/a:1007378608773. [DOI] [PubMed] [Google Scholar]
- 26.Triantos C. Epidemiology of viral hepatitis. Hospital Chron. 2006;68:206–213. [Google Scholar]
- 27.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 28.Foudoulaki-Paparizos L, Kyriakis KP, Kourendi K, Sofroniadou K. HCV seroprevalence among military, sporadic and regular blood donors in Greece. A ten year seroepidemiologic study (1992-2001) HAEMA. 7:72–78. [Google Scholar]
- 29.Magiorkinis G, Sypsa V, Magiorkinis E, Paraskevis D, Katsoulidou A, Belshaw R, Fraser C, Pybus OG, Hatzakis A. Integrating phylodynamics and epidemiology to estimate transmission diversity in viral epidemics. PLoS Comput Biol. 2013;9:e1002876. doi: 10.1371/journal.pcbi.1002876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Triantos C, Kourakli A, Kalafateli M, Giannakopoulou D, Koukias N, Thomopoulos K, Lampropoulou P, Bartzavali C, Fragopanagou H, Kagadis GC, et al. Hepatitis C in patients with β-thalassemia major. A single-centre experience. Ann Hematol. 2013;92:739–746. doi: 10.1007/s00277-013-1692-6. [DOI] [PubMed] [Google Scholar]
- 31.Kountouras D, Tsagarakis NJ, Fatourou E, Dalagiorgos E, Chrysanthos N, Berdoussi H, Vgontza N, Karagiorga M, Lagiandreou A, Kaligeros K, et al. Liver disease in adult transfusion-dependent beta-thalassaemic patients: investigating the role of iron overload and chronic HCV infection. Liver Int. 2013;33:420–427. doi: 10.1111/liv.12095. [DOI] [PubMed] [Google Scholar]
- 32.Katsanos K, Chaidos A, Christodoulou D, Tzambouras N, Zervou E, Bourandas K, Tsianos E. Epidemiological and clinical characteristics of HCV infection in transfusion-dependent thalassemia. Ann Gastroenterol. 2005;18:56. [Google Scholar]
- 33.Hadziyannis SJ, Koskinas JS. Differences in epidemiology, liver disease and treatment response among HCV genotypes. Hepatol Res. 2004;29:129–135. doi: 10.1016/j.hepres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Dalekos GN, Zervou E, Merkouropoulos MH, Tsianos EV. Prevalence of hepatitis B and C viruses infection in chronic alcoholics with or without liver disease in Ioannina, Greece: low incidence of HCV infection. Eur J Epidemiol. 1996;12:21–25. doi: 10.1007/BF00144423. [DOI] [PubMed] [Google Scholar]
- 35.Gigi E, Sinakos E, Sykja A, Androulakis G, Tanis C, Stayridou V, Tsirogianni E, Zouridakis K, Bellou AL, Orfanou E, et al. Epidemiology, clinical data, and treatment of viral hepatitis in a large cohort of intravenous drug users. J Addict Med. 2013;7:52–57. doi: 10.1097/ADM.0b013e318279756f. [DOI] [PubMed] [Google Scholar]
- 36.Paraskevis D, Nikolopoulos G, Fotiou A, Tsiara C, Paraskeva D, Sypsa V, Lazanas M, Gargalianos P, Psichogiou M, Skoutelis A, et al. Economic recession and emergence of an HIV-1 outbreak among drug injectors in Athens metropolitan area: a longitudinal study. PLoS One. 2013;8:e78941. doi: 10.1371/journal.pone.0078941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiessing L, Likatavicius G, Hedrich D, Guarita B, van de Laar MJ, Vicente J. Trends in HIV and hepatitis C virus infections among injecting drug users in Europe, 2005 to 2010. Euro Surveill. 2011:16. [PubMed] [Google Scholar]
- 38.Reimer J, Lorenzen J, Baetz B, Fischer B, Rehm J, Haasen C, Backmund M. Multiple viral hepatitis in injection drug users and associated risk factors. J Gastroenterol Hepatol. 2007;22:80–85. doi: 10.1111/j.1440-1746.2006.04358.x. [DOI] [PubMed] [Google Scholar]
- 39.Malliori M, Sypsa V, Psichogiou M, Touloumi G, Skoutelis A, Tassopoulos N, Hatzakis A, Stefanis C. A survey of bloodborne viruses and associated risk behaviours in Greek prisons. Addiction. 1998;93:243–251. doi: 10.1046/j.1360-0443.1998.9322438.x. [DOI] [PubMed] [Google Scholar]
- 40.Chatziarsenis M, Miyakis S, Faresjo T, Trell E, Vlachonikolis J, Lionis C. Is there room for general practice in penitentiary institutions: screening and vaccinating high-risk groups against hepatitis. Fam Pract. 1999;16:366–368. doi: 10.1093/fampra/16.4.366. [DOI] [PubMed] [Google Scholar]
- 41.Kakisi OK, Grammatikos AA, Karageorgopoulos DE, Athanasoulia AP, Papadopoulou AV, Falagas ME. Prevalence of hepatitis B, hepatitis C, and HIV infections among patients in a psychiatric hospital in Greece. Psychiatr Serv. 2009;60:1269–1272. doi: 10.1176/ps.2009.60.9.1269. [DOI] [PubMed] [Google Scholar]
- 42.Griveas I, Germanidis G, Visvardis G, Morice Y, Perelson AS, Pawlotsky JM, Papadopoulou D. Acute hepatitis C in patients receiving hemodialysis. Ren Fail. 2007;29:731–736. doi: 10.1080/08860220701460160. [DOI] [PubMed] [Google Scholar]
- 43.Boletis JN. Epidemiology and mode of transmission of hepatitis C virus infection after renal transplantation. Nephrol Dial Transplant. 2000;15 Suppl 8:52–54. doi: 10.1093/ndt/15.suppl_8.52. [DOI] [PubMed] [Google Scholar]
- 44.Rigopoulou EI, Stefanidis I, Liaskos C, Zervou EK, Rizos C, Mina P, Zachou K, Syrganis C, Patsidis E, Kyriakopoulos G, et al. HCV-RNA qualitative assay based on transcription mediated amplification improves the detection of hepatitis C virus infection in patients on hemodialysis: results from five hemodialysis units in central Greece. J Clin Virol. 2005;34:81–85. doi: 10.1016/j.jcv.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Boletis J, Stathakis C, Papastathi H, Vafiadi I, Goumenos D, Miriagou B, Hatzakis A, Kostakis A, Vosnides G. Antibodies against hepatitis C virus among renal transplant patients in Greece. Transpl Int. 1992;5 Suppl 1:S51–S53. doi: 10.1007/978-3-642-77423-2_16. [DOI] [PubMed] [Google Scholar]
- 46.Diamantis I, Vafiadis I, Voskaridou E, Dellatetsima J, Jäggi N, Gyr K, Battegay M. Genotype distribution of hepatitis C virus infection in Greece: correlation with different risk factors and response to interferon therapy. Eur J Gastroenterol Hepatol. 1998;10:75–79. doi: 10.1097/00042737-199801000-00014. [DOI] [PubMed] [Google Scholar]
- 47.Katsoulidou A, Paraskevis D, Kalapothaki V, Arvanitis D, Karayiannis P, Hadjiconstantiou V, Hatzakis A. Molecular epidemiology of a hepatitis C virus outbreak in a haemodialysis unit. Multicentre Haemodialysis Cohort Study on Viral Hepatitis. Nephrol Dial Transplant. 1999;14:1188–1194. doi: 10.1093/ndt/14.5.1188. [DOI] [PubMed] [Google Scholar]
- 48.Drakopoulos V. Hepatitis viruses B, C and health care personnel. Medical BHMA 2007: 22-34 [Google Scholar]
- 49.Falagas ME, Karydis I, Kostogiannou I. Percutaneous exposure incidents of the health care personnel in a newly founded tertiary hospital: a prospective study. PLoS One. 2007;2:e194. doi: 10.1371/journal.pone.0000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazokopakis E, Vlachonikolis J, Philalithis A, Lionis C. Seroprevalence of hepatitis A, B and C markers in Greek warship personnel. Eur J Epidemiol. 2000;16:1069–1072. doi: 10.1023/a:1010857128629. [DOI] [PubMed] [Google Scholar]
- 51.Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21:1135–1139. doi: 10.1016/s0168-8278(05)80631-2. [DOI] [PubMed] [Google Scholar]
- 52.Skliros EA, Sotiropoulos A, Lionis C, Tassopoulos NC. Hepatitis B and C virus infection in patients with high serum transaminases. Postgrad Med J. 1998;74:511. doi: 10.1136/pgmj.74.874.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vafiadis I, Boletis J, Papastathi H, Delladetsima J, Stathakis C, Hatzakis A, Kostakis A, Vosnides G. Hepatitis C virus infection among Greek renal transplant patients. Gut. 1993;34:S57–S58. doi: 10.1136/gut.34.2_suppl.s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Syriopoulou V, Nikolopoulou G, Daikos GL, Theodoridou M, Pavlopoulou I, Nicolaidou P, Manolaki N. Mother to child transmission of hepatitis C virus: rate of infection and risk factors. Scand J Infect Dis. 2005;37:350–353. doi: 10.1080/00365540510032105. [DOI] [PubMed] [Google Scholar]
- 55.Panagopoulos P, Economou A, Kasimi A, Spyropoulou P, Kanellopoulos N, Dadiotis L, Salamalekis E. Prevalence of hepatitis B and C in the maternity department of a Greek district hospital. J Matern Fetal Neonatal Med. 2004;16:106–110. doi: 10.1080/14767050400003751. [DOI] [PubMed] [Google Scholar]
- 56.Raptopoulou-Gigi M, Orphanou E, Lalla TH, Lita A, Garifallos A. Prevalence of hepatitis C virus infection in a cohort of pregnant women in northern Greece and transmission of HCV from mother to child. Eur J Epidemiol. 2001;17:263–266. doi: 10.1023/a:1017951605272. [DOI] [PubMed] [Google Scholar]
- 57.Tsakris A, Kyriakis KP, Chryssou S, Papoutsakis G. Infection by hepatitis B and C virus in female and transsexual Greek prostitutes with serological evidence of active syphilis. Int J STD AIDS. 1997;8:697–699. doi: 10.1258/0956462971919075. [DOI] [PubMed] [Google Scholar]
- 58.Terrault NA, Dodge JL, Murphy EL, Tavis JE, Kiss A, Levin TR, Gish RG, Busch MP, Reingold AL, Alter MJ. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology. 2013;57:881–889. doi: 10.1002/hep.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vandelli C, Renzo F, Romanò L, Tisminetzky S, De Palma M, Stroffolini T, Ventura E, Zanetti A. Lack of evidence of sexual transmission of hepatitis C among monogamous couples: results of a 10-year prospective follow-up study. Am J Gastroenterol. 2004;99:855–859. doi: 10.1111/j.1572-0241.2004.04150.x. [DOI] [PubMed] [Google Scholar]
- 60.Hadziyannis SJ, Giannoulis G, Hadziyannis E, Kaklamani E, Alexopoulou A, Dourakis S, Trichopoulos D. Hepatitis C virus infection in Greece and its role in chronic liver disease and hepatocellular carcinoma. J Hepatol. 1993;17 Suppl 3:S72–S77. doi: 10.1016/s0168-8278(05)80428-3. [DOI] [PubMed] [Google Scholar]
- 61.Dimitrakopoulos A, Takou A, Haida A, Molangeli S, Gialeraki A, Kordossis T. The prevalence of hepatitis B and C in HIV-positive Greek patients: relationship to survival of deceased AIDS patients. J Infect. 2000;40:127–131. doi: 10.1053/jinf.1998.0636. [DOI] [PubMed] [Google Scholar]
- 62.Elefsiniotis IS, Glynou I, Brokalaki H, Magaziotou I, Pantazis KD, Fotiou A, Liosis G, Kada H, Saroglou G. Serological and virological profile of chronic HBV infected women at reproductive age in Greece. A two-year single center study. Eur J Obstet Gynecol Reprod Biol. 2007;132:200–203. doi: 10.1016/j.ejogrb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 63.Wiessing L, Ferri M, Grady B, Kantzanou M, Sperle I, Cullen KJ, Hatzakis A, Prins M, Vickerman P, Lazarus JV, et al. Hepatitis C virus infection epidemiology among people who inject drugs in Europe: a systematic review of data for scaling up treatment and prevention. PLoS One. 2014;9:e103345. doi: 10.1371/journal.pone.0103345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roussos A, Goritsas C, Pappas T, Spanaki M, Papadaki P, Ferti A. Prevalence of hepatitis B and C markers among refugees in Athens. World J Gastroenterol. 2003;9:993–995. doi: 10.3748/wjg.v9.i5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malamitsi-Puchner A, Papacharitonos S, Sotos D, Tzala L, Psichogiou M, Hatzakis A, Evangelopoulou A, Michalas S. Prevalence study of different hepatitis markers among pregnant Albanian refugees in Greece. Eur J Epidemiol. 1996;12:297–301. doi: 10.1007/BF00145420. [DOI] [PubMed] [Google Scholar]
- 66.Papadakis G, Okoba NA, Nicolaou C, Boufidou F, Ioannidis A, Bersimis S, Chatzipanagiotou S. Serologic markers for HBV, HCV and HIV in immigrants visiting the Athens’ polyclinic of ‘Doctors of the World - Greece’. Public Health. 2013;127:1045–1047. doi: 10.1016/j.puhe.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 67.Gregoriadou A, Delidou-Tsogia K, Dardavessis T, Pigadas A, Tirodimos I, Katsougiannopoulos V. Prevalence of hepatitis C markers among Greek origin refugees from the former Soviet Union. Acta Microbiologica Hellenica. 1999;44:46–50. [Google Scholar]
- 68.Katsoulidou A, Sypsa V, Tassopoulos NC, Boletis J, Karafoulidou A, Ketikoglou I, Tsantoulas D, Vafiadi I, Hatzis G, Skoutelis A, et al. Molecular epidemiology of hepatitis C virus (HCV) in Greece: temporal trends in HCV genotype-specific incidence and molecular characterization of genotype 4 isolates. J Viral Hepat. 2006;13:19–27. doi: 10.1111/j.1365-2893.2005.00649.x. [DOI] [PubMed] [Google Scholar]
- 69.Stamouli M, Panagiotou I, Kairis D, Michopoulou A, Skliris A, Totos G. Genotype distribution in chronic hepatitis C patients in Greece. Clin Lab. 2012;58:173–176. [PubMed] [Google Scholar]
- 70.Karatapanis S, Tsoplou P, Papastergiou V, Vasiageorgi A, Stampori M, Saitis I, Tsitsopoulos E, Lisgos P, Skorda L, Ketikoglou I, et al. Hepatitis C virus genotyping in Greece: unexpected high prevalence of genotype 5a in a Greek island. J Med Virol. 2012;84:223–228. doi: 10.1002/jmv.22249. [DOI] [PubMed] [Google Scholar]


