Abstract
There is rising incidence and prevalence of inflammatory bowel disease (IBD) in India topping the Southeast Asian (SEA) countries. The common genes implicated in disease pathogenesis in the West are not causal in Indian patients and the role of “hygiene hypothesis” is unclear. There appears to be a North-South divide with more ulcerative colitis (UC) in north and Crohn’s disease (CD) in south India. IBD in second generation Indian migrants to the West takes the early onset and more severe form of the West whereas it retains the nature of its country of origin in migrants to SEA countries. The clinical presentation is much like other SEA countries (similar age and sex profile, low positive family history and effect of smoking, roughly similar disease location, use of aminosalicylates for CD, low use of biologics and similar surgical rates) with some differences (higher incidence of inflammatory CD, lower perianal disease, higher use of aminosalicylates and azathioprine and lower current use of corticosteroids). UC presents more with extensive disease not paralleled in severity clinically or histologically, follows benign course with easy medical control and low incidence of fulminant disease, cancer, complications, and surgery. UC related colorectal cancer develop in an unpredictable manner with respect to disease duration and site questioning the validity of strict screening protocol. About a third of CD patients get antituberculosis drugs and a significant number presents with small intestinal bleed which is predominantly afflicted by aggressive inflammation. Biomarkers have inadequate diagnostic sensitivity and specificity for both. Pediatric IBD tends to be more severe than adult. Population based studies are needed to address the lacunae in epidemiology and definition of etiological factors. Newer biomarkers and advanced diagnostic techniques (in the field of gastrointestinal endoscopy, molecular pathology and genetics) needs to be developed for proper disease definition and treatment.
Keywords: Inflammatory bowel disease, Ulcerative colitis, Crohn’s disease, India, Review
Core tip: There is growing interest in inflammatory bowel disease (IBD) in India due to its rising incidence. This review addresses the current state of knowledge on different aspects of Indian IBD patients like epidemiology, genetics, mechanisms, clinical presentations and treatment (compared to other south Asian countries) in the context of which future areas of research is highlighted. The disease is milder in India. Well-designed population based studies are needed. To address the obscure pathogenesis and uncertain disease course (behaviour, activity, treatment response, development of cancer and prognosis) studies on mechanisms, biomarkers, advanced endoscopic techniques need to be done to decrease the morbidity burden.
INTRODUCTION
Inflammatory bowel disease (IBD) encompasses ulcerative colitis (UC) and Crohn’s disease (CD), two chronic inflammatory disease of uncertain etiology affecting the gut. IBD started gaining special attention in India only after mid 1980s with wider availability of colonoscope. In the era before 1985, UC was often difficult to distinguish from more prevalent infectious colitis and CD was only reported on surgical specimens[1] and even its existence was questioned[2]. Clearly the studies had limitations as due to lack of equipments the extent and severity of bowel involvement, biopsy taking from proximal gut areas and diagnosis of superadded infections was difficult and treatment options were limited. The period 1986-2000 saw a resurgence of interest in IBD (especially UC) not only due to availability of better resources but also increased reporting of IBD in the 1st and 2nd generation Indian diasporas settled abroad. This period saw well described clinical studies on UC. The period 2000 - 2010 saw further spread of the speciality of gastroenterology all over India with availability of even better infrastructure especially technology to visualise the small gut (capsule and balloon endoscopes). This spurred an increase in number of well documented publications on CD (and intestinal tuberculosis which made comparison easy between the two). With increase in patient awareness, spending potential and availability of health insurance, access to specialised health care became a reality at least for a section of common people who otherwise could not have afforded it. This increased the number of patients available for detailed study. After 2010 with availability of better opportunities for research in basic science focus has been on genetic aspects, deeper insight into the disease mechanisms and development of new drugs.
EPIDEMIOLGY
In North America and northern Europe (areas with highest IBD occurrence), the incidence of UC and CD (6-12/105 and 5-7/105 population respectively) are much higher than southern Europe (2-8/105 and 0.1 - 4/105 population respectively). IBD was traditionally thought to be of low occurrence in eastern Europe, Asia and Africa till recently. The temporal trend is variable in different geographic areas. In North America and northern Europe, UC initially increased from 1935 to 1964, then decreased till 1979 and thereafter have been stable. The incidence is increasing in southern Europe with a tendency to catch up with the north. CD started increasing from 1960 with plateauing after 1975 but recently have been rising all over the world[3-5].
Recent reports show IBD to be rising in Asia albeit much less than Europe[6]. In India the first case of CD was reported about 23 years after UC though in surgical specimen[1]. Since then 2 epidemiologic studies have been conducted in north India on UC only[7,8]. A house to house survey of 4796 houses including 21921 persons (> 14 years age) in Haryana state revealed 10 cases (5 each in both sexes) which gave a prevalence of 45.5/105 population (42.8/105 for males and 48.6/105 for females)[7]. In a later study from the neighbouring state of Punjab[8] where cluster sampling method was employed the crude incidence and prevalence of UC was found to be 6.02/105 and 44.8/105 population which was the highest in Asia but still less than that of North America and Europe. This was similar to the prevalence reported in the study 17 years earlier indicating stability over time. There have been no epidemiologic study from any other parts of India though the recent IBD task force data[9] fulfils some of the gaps especially for CD. The IBD Task Force was set up in 2003 to collect data prospectively by questionnaire method from all over India. Participants were all qualified gastroenterologists from all corners of India. Of 1159 questionnaires analysed, UC: CD was 750:409 and region wise distribution were North 220 (148:72), East 159 (90:69), Central 255 (227:28), South 466 (235:231), West 59 (50:9). Thus CD was much higher in the south followed by east compared to other regions. Most of this data was however hospital based.
Other indirect evidence of prevalence comes also from hospital based series on diarrhoea, GI bleed and malabsorption which are the principle symptoms of IBD. In an early study in 1990 on patients attending mission hospitals in India, Nepal, Pakistan and Bangladesh, IBD was diagnosed in only 74 cases (UC 56, CD18) out of 12272 cases of bloody diarrhoea[10]. Later three colonoscopic studies from north India found IBD in 19.3%[11] and 5.5%[12] cases of lower GI bleed and in 25% cases of chronic large bowel diarrhoea[13].
With the advent of better radiologic and endoscopic modalities for visualising the small gut after 2000, CD is being diagnosed with increasing frequency. Two series on the etiology of malabsorption in 275 and 94 cases from north India, found the diagnosis in 2% cases each[14,15] whereas in a study of 124 cases from south India, 15.3% had CD[16]. By small gut investigative modalities like capsule and balloon enteroscopy for symptoms of pain abdomen, malabsorption and obscure GI bleed (OGIB), CD was diagnosed in 12%-20% cases from south[17-19], 11%-20% cases from east[20-22]. Thus in India the general belief is that IBD is rising and a north-south divide is apparent between UC and CD. This divide may be the result of differences in genetics, hygiene and diet (see below).
RACE
The nature of IBD in Indians as a race is well highlighted by early epidemiologic studies in the diasporas and subsequently supported by Indian studies. In the early 90s, studies on South Asians in Leicestershire[23,24] showed increased incidence of CD in South Asians (2.4/105 in Hindus, 3.4/105 in Sikhs and 5.4/105 in Muslims) and of UC in Sikhs (16.5/105) only but not in Europeans and other South Asians. The subjects had less number of complications and operations. In this context, it is interesting to note that the study from Punjab, a state with a Sikh majority in India, show relatively high incidence and prevalence of UC[8]. Similarly, young Asian Indians born in Britain were more likely to have IBD by 26 years age compared to indigenous Europeans (OR = 6.1, CI: 2.14-17.33)[25] and South Asians (Indians, Bangladeshis, Pakistanis) to have more pancolitis (63% vs 42.5%) and colonic CD (46.8%) but with less penetrating disease and need for surgery[26].
Other studies on Indians in Canada[27] and South Africa[28] showed that the majority had pancolitis but clinically mild disease and Canadian born patients were younger than migrants.
In a very recent analysis of 30812 IBD patients from United States diagnosed between 2008 - 2013 (20308 with UC, 7706 with CD, and 2798 with indeterminate colitis), UC was more commonly associated with Indian and Jewish ethnicity and less with East Asian and Hispanic ethnicity. Similar patterns also applied to CD and to all types of IBD analyzed jointly[29]. Studies in multiracial Asian countries also project similar findings. Indians in Fiji, Singapore and Malaysia constitute the majority of cases on UC compared to the local Chinese and other races, have more extensive disease yet least number of surgery[30-33].
Thus there is a rapid rise in IBD among Indians settled abroad with younger age of onset and higher incidence of pancolitis (simulating the population of their country of settlement) but yet has less clinical severity, complications and surgery indicating a benign course. This is peculiar to Asian Indians as a race compared to Europeans and other Asians. Most Indian studies (to be discussed below) also show similar features. A particular disease trait in a specific race might indicate a genetic link. The other point of note is that IBD in 2nd generation Indian diasporas settled in the West (Europe and North America) resemble their country of settlement, but in those settled in other Asian countries, it retains features of their country of origin. This might indicate some common environmental factor in Asian countries (diet, lifestyle) which has less effect on alteration of disease expression after migration.
GENETICS
Involvement of genes in the pathogenesis of a disease can be deciphered from the disease occurrence in family members, siblings and twins. The Indian IBD task force data showed positive family history of IBD in 2.9% cases only (UC 2.3% and CD 4.6%). In one study there was no association of HLA DR and DQ with CD[34]. Studies on causative role of individual genes have only started recently in India especially with the discovery of single nucleotide polymorphisms (SNP) of 163 genes associated with IBD (at various points of its immune mechanism) in Caucasian populations (of whom NOD2 gene shows the most definite association with disease phenotype). The association of different genes with IBD in India studied till now are shown in Figure 1[35-55]. Multiple studies have negated the association of NOD2 gene with CD in India[52-54] but SNP 268Pro/Ser increased relative risk of UC (OR = 1.72, 1.14-2.52)[51].
Figure 1.
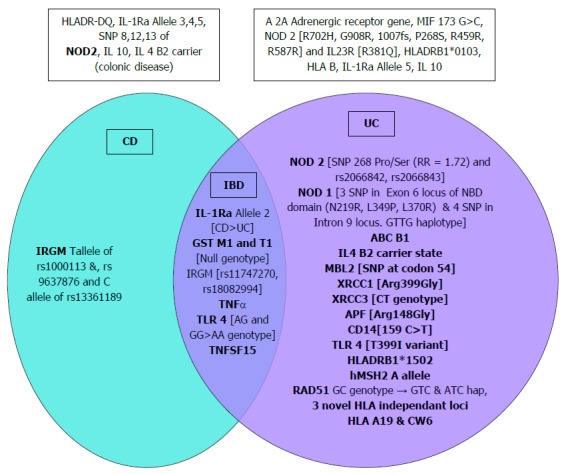
Association of different genes with inflammatory bowel disease, ulcerative colitis and Crohn’s disease. Genes shown outside the Venn diagram were not associated. CD: Crohn’s disease; IBD: Inflammatory bowel disease; UC: Ulcerative colitis.
Few studies have demonstrated relation to UC phenotype but majority show increased risk or negative association only. Specific haplotypes of MDR1 (ABC B1) gene were well associated with early age (< 29 years) of disease onset, left sided disease and steroid response in UC[48]. TNF alpha 863 AA genotype increased risk of both UC and CD, more for UC especially pancolitis. IL4 B2 carrier state was less in left sided colitis than proctosigmoiditis and absent in colonic CD[42].
Of the SNPs associated with IBD in Caucasians detected by metanalysis of GWAS studies, only 5 of 59 index ones studied in North India were found significant showing limited replication in Indians[44] and there was varying contribution to risk by the HLA region with 3 novel HLA independent risk loci for UC[38]. HLA DRB1*0103 was absent while HLA DRB1*1502 was increased in Indian UC patients compared to controls and Europeans. There was no significant difference between the ethnic groups in NOD 2 and IL23 gene[56]. Thus the common causative genes in Caucasian population are non-causal in India which could be due to differences in microbiome (the first line interact of NOD 2 gene) or secondary factors like diet, pollution, use of antibiotics and proton pump inhibitors which differentially affects the phenotypic outcome either by themselves or by altering the microbiome.
A traditional thinking in India has been that of a genetic difference between the North and South Indians which is supported by 2 studies, one undertaken by the Indian Genome Variation Consortium[57] and another from Singapore which compared the genes of two distinct migrant groups from India - the Indo-European language speaking Gujrati expatriates in United States and the Dravidian language speaking Tamil expatriates in Singapore[58]. A genetic analysis of 132 individuals of 25 diverse groups in India predicted the existence of two ancestral groups in the pre-historic India: an “ancestral North Indian (ANI)”, which shared genetic affinity with the populations of the Middle East, Central Asia and Europe (30% to 70%), and an “ancestral South Indian (ASI)”, which has no relation with any population outside India i.e., they were the indigenous population of India[59]. The epidemiologic features of UC in North Indians (who are supposed to have originated from the above regions as Indo - Aryans) resembling the Caucasians may reflect this fact. The caste system in India maintained this genetic distinctness due to marriages within one’s own caste. But the present-day Indian populations are an admixture of both ANI and ASI as shown in a most recent study[60]. This is an obvious outcome of rapid migration of population from one region to another in India due to socioeconomic and political upheavals brought by rapid industrialisation with increase in intercaste marriages. This might obfuscate any further chance to study the pristine genetics and their bearing on disease phenotype in these populations in future.
MECHANISM
IBD results from a strong genetic predisposition on which environmental factors act to produce dysregulated gut mucosal immune response to luminal antigens (the intestinal microbiota, normal or abnormal). The genetic findings discussed above may predispose, yet the increasing number of IBD cases reported within limited period of time in India cannot be explained totally by genomic changes but provide evidence for the importance of exposure to environmental factors in disease pathogenesis. Childhood infections, lack of breast feeding, exposure to helminths, smoking, repeated use of drugs like antibiotics and proton pump inhibitors, dietary and psychosocial factors have all been implicated in IBD pathogenesis and may act by altering this balance between the gut and microbiota either by themselves or by altering the latter[61]. Childhood infections by producing tolerance of immunoregulatory T cells to external antigens early in life (immune conditioning) protects against IBD in adult life. This is exemplified by the (1) increasing incidence and earlier onset of IBD in children of Indian diasporas settled in cleaner and more hygienic environment of Europe and North America compared to their parents and counterparts settled in India as well as children of the local Caucasian population; and (2) increasing incidence of allergic and autoimmune diseases with decreasing childhood infection in Western epidemiologic studies[62]. The increasing incidence of CD (over UC) was first observed in Western countries as the sanitation - hygiene started improving with disappearance of helminthic (which promote Th2 response protective against CD) and other infections which is now happening in developing countries. Other pointers to this association are the increasing CD incidence in urban vs rural setting, higher vs lower socioeconomic strata and in whites vs natives. In India the highest reporting of CD comes from Kerala, a south Indian state with the highest literacy rate and better hygiene[63].
In a study from south India, T cell activation [increase in CD3(+) CD69(+) population] by hookworm antigen and interferon-γ ELISPOT responses to hookworm antigens were significantly higher in controls compared to CD patients[64]. Other studies on environmental factors are summarised in Table 1[65-67]. In the survey by the IBD Task Force, more number of CD cases had appendicectomy though rates of smoking and oral contraceptives intake were same as UC[9]. While some of these findings are similar (less effect of smoking, presence of hot water tap and flush toilet in childhood protective for UC) to other south east (SE) Asian countries but many are not (owning pet increase risk of UC, daily tea/coffee consumption has no effect on both UC and CD) and the effect of some have not been studied (breast fed > 12 mo, antibiotic use, daily physical activity, western diet)[68]. Type of diet consumed varies widely in different regions of India. In general rice based diets are common in south and east (areas with more CD) whereas wheat is consumed more in north India (area with more UC). Studies are very few (listed in Table 1) and the findings need confirmation by more number of detailed community based cohort studies.
Table 1.
Environmental factors involved in inflammatory bowel disease causation in India
| Study type (region) | Sample size |
Factors significant on univariate analysis |
Factors significant on multivariate analysis, OR (CI) | Factors not significant | |||
|
Ulcerative Colitis |
Crohn’s disease |
||||||
| Favours | Protects | Favours | Protects | ||||
| Case control[65] (south)1 | 200/200 | Urban residence (birth/current) | Cattle in house compound (current) | Cattle in house compound (current) 0.57 (0.35-0.92) | Age, closed toilet in house, tooth cleanser use, pets in house (childhood/current), Cattle in house compound (childhood), appendicectomy, smoking, regular meat consumption | ||
| Treated drinking water (childhood/current) | Regular fish consumption (> 1/wk) | Regular fish consumption (> 1/wk) 0.52 (0.33-0.80) | |||||
| Piped water supply in house (childhood/current) | Treated drinking water (childhood/current) 1.59 (1.02-2.47) | ||||||
| Lactovegetarian | |||||||
| High socioeconomic score | |||||||
| Case control[66] (north)1 | 513/188 | Higher education (graduation and beyond) | Using private bed | Using private bed 0.25 (0.16-0.39) | Age, smoking, water source (municipal vs tubewell), illness in family, number of sibs | ||
| Hand washing | Owning a pet 2.02 (1.14-3.59) | ||||||
| Having personal towel | RCA/flush type latrine | RCA latrine 0.29 (0.14-0.60) | |||||
| Owning a pet | Flush type latrine 0.43 (0.23-0.82) | ||||||
| Death in family | Death in family 2.19 (1.58-4.07) | ||||||
| Case control[67] (east) | 50/50 | Higher intake of refined sugarLow intake of fruits | Higher intake of refined sugar 1.78 (1.03-6.9) | Age, Sex, socioeconomic status, regular smoking and alcohol intake, rice, wheat, meat, fish, fried food, tea/coffee, green leafy vegetables2 | |||
| Low intake of fruits 0.28 (0.15-0.65) | |||||||
More females had disease than controls;
Higher intake of green leafy vegetables (P = 0.05) tended towards protective association.
Microorganisms in pathogenesis
A number of studies have found altered number, type and location of colonic bacterial flora in IBD. Butyrate producing bacteria (Clostridium coccides and leptum) were significantly decreased in UC and differentially positive according to severity which increased with disease remission[69,70]. A ten times increase in total bacterial count with increase in unusual anaerobes and facultative aerobes (Proteobacteria) and significant decrease in bacterial diversity from phylum to species was noted in colonic flora of severe UC compared to mild and moderate disease[71]. Other studies found clear delineation in bacterial concentration in mucosal layer between predominant and subdominant genera between UC and CD indicating involvement of different subsets of bacteria in pathogenesis[72] and more mucosal adherence of E. coli in the colon of CD patients[73]. Other organisms that have been associated with exacerbations of UC (detected in stool in 17%-30% cases of moderate to severe disease[74-76]) are CMV in 2.3%-15.8% cases[74,77]. C. difficile (toxin detected in 3.4%-32%[74,78-80]) and rarely Giardia, Strongyloides, hookworm, herpes simplex, E.hystolytica, cryptosporidium and salmonella[74,75]. The exact significance of these in disease pathogenesis is unclear. In CD pathogenesis the contribution of Mycobacterium paratuberculosis remains contentious and that of Mycobacterium tuberculosis is discussed below.
CLINICAL FEATURES
The chief clinical features of IBD in India (as per the ISG Task Force data representative of the whole country) is summarised in Table 2 along with those of other countries of SE Asia and Australia (which approximates that of the West in incidence, female dominance and higher positive family history) for a comparative view[9,81,82] The chief similarities with SE Asia are UC more than CD, age profile (with unimodal peak for UC), male dominance, low percentage of positive family history, less effect of smoking on disease, similar disease location (except for lower proctitis and higher pancolitis in case of UC like Australia), low use of biologics, high use of 5ASA for CD and similar surgical rates (including low colectomy rates for UC). The issue of biologics vs surgery in refractory cases is a matter of debate and mostly depends on cost of hospital stay and medicines, availability of expertise and infrastructure, ability to deal with stoma care and proper follow up.
Table 2.
Comparative clinical features of inflammatory bowel disease in different countries
| Country | India[9] | South East Asia[81,82] | Australia[81,82] | |||
| Factors | UC | CD | UC | CD | UC | CD |
| Median Age (yr) | 38.5 | 35.9 | 42 | 34 | 42 | 34 |
| Sex (M: F) | 1.4:1 | 1.3:1 | 1.4:1 | 1.6:1 | 1:1.2 | 1:1.1 |
| Smokers | 21.3% | 24.2% | 6% | 11% | 8% | 10% |
| Disease location | ||||||
| L1 | 28.9% | 31% | 31% | |||
| L2 | 31.4% | 24% | 24% | |||
| L3 | 39.6% | 45% | 45% | |||
| L4 | 5.8% | 5% | 5% | |||
| Disease behaviour | ||||||
| B1 | 76.8% | 66% | 88% | |||
| B2 | 18.8% | 17% | 10% | |||
| B3 | 4.4% | 19% | 2% | |||
| Perianal | 6.9% | 18% | 12% | |||
| Disease extent | ||||||
| Proctitis | 18.3% | 37% | 32% | |||
| Distal colitis | 38.8% | 32% | 27% | |||
| Extensive colitis | 42.8% | 31% | 41% | |||
| Treatment | ||||||
| 5ASA | 89.7% | 58.4% | 68% | 49% | 86% | 71% |
| Corticosteroids | 29.1% | 26.9% | 21% | 42% | 50% | 67% |
| Immunosuppressive | 29.8% | 62.9% | 8% | 35% | 0 | 14% |
| Biologics | 0 | 2.2% | 1% | 5% | 0 | 0 |
| Antibiotic | 0 | 0 | 25% | 19% | 0 | 21% |
| Probiotics | 6.1% | 7.6% | ||||
| Surgery | 4.0% | 15.2% | 1.6% | 11.6% | 5.9% | 14.3% |
| Positive family history (%) | 2.3% | 4.6% | 31% | 17%1 | ||
| EIM | ||||||
| Joints | 33.3% | 26.3% | 13%1 | 3.6%1 | ||
| Backache | 31.5% | 36.4% | 3.3[AS]1 | |||
| Skin | 2.4% | 3.4% | 4.8%1 | 3.6%1 | ||
| Incidence per 105 population | 6.07%[8] | 0.76% | 0.54% | 7.33% | 14% | |
Present in overall inflammatory bowel disease population. EIM: Extraintestinal manifestations; AS: Ankylosing spondylitis; UC: Ulcerative colitis; CD: Crohn’s disease.
The chief differences are the higher incidence of extraintestinal manifestations (EIM), disease behaviour in CD (higher inflammatory and lower penetrating disease similar to Australia), much lower perianal disease, higher use of 5ASA (similar to Australia especially for UC) and azathioprine (AZP) (compared to both SE Asia and Australia), lower current use of corticosteroids (especially for CD), no use of antibiotics for CD and a possibility of medicine discontinuation (17.5% of UC and 6.9% of CD cases). The reason is that in Indian patients steroids are used intermittently (either at induction or for flares, so 2/3rd have history of past use[9]), is tapered quickly to be replaced by AZP along with 5ASA as most cases are mild to moderate in severity with easy control and only few are steroid resistant or dependant. A latest study on disease behaviour over long follow up upto 15 years showed that most patients had aggressive inflammatory disease at presentation (as is the point incidence in Task Force data) which decreased over time with simultaneous increase in penetrating disease whereas stricturing disease remained stable. This was independant of age or disease location[83]. Another large series spanning 13 years showed that small intestinal CD more often followed aggressive course and needed surgical intervention[84]. Regarding higher incidence of EIM it must be mentioned that studies are very heterogenous regarding the methods (and hence the extent and minuteness) by which they are searched for and often terms like arthralgia and backache have been synonymously with EIM without proper documentation of lesions.
It was believed in the 80s that UC is a mild disease in India, the outcome variables being shorter length of colonic involvement, low rate of complications, surgery and mortality. Though in recent times there is increasing presentation with extensive disease in majority, the disease still runs a relatively benign course as reflected by easy medical control, low incidence of fulminant disease, cancer and requirement for surgery even in the longrun. This general disease pattern in Indians is reflected even in recent series published from multiracial Asian countries (see above).
The other difference in CD cases with the West is the higher percentage of OGIB at presentation representing small intestinal bleed. This is likely due to increasing presentation at younger age with aggressive inflammatory type small intestinal disease. The earliest Indian series on OGIB where small gut was visualised only by barium studies and intraoperative endoscopy, CD was confirmed in 20%[22]. Subsequent series using capsule endoscopy, single and double balloon enteroscopy for symptoms of pain abdomen, malabsorption and OGIB have respectively yielded CD in 0-38%, 0-100%, 0-19%, cases, overall being 11.3%-29.2%[17-21].
BIOMARKERS IN IBD
(1) ANCA positivity has been reported in 3%-32% of UC[35,85] and 3.8%-10% of CD[86,87]; (2) fecal lactoferrin is also a good marker of UC activity (sensitivity 94%, specificity 100%) being higher in cases and more severe disease and decreased in parallel with improvement of Mayo severity score with treatment[88]. It has also been suggested to reflect disease exacerbation caused by C. difficile[80]; (3) fecal calprotectin level was found to be higher in UC than ileocolonic CD and correlate well with clinical (by Truelove-Witts score) and also endoscopic severity (by Mayo score) but not disease extent. A level of 800 mcg/gm stool best differentiated active from inactive disease[89]; (4) positivity of ASCA in CD has been reported overall in 30%-62%, IgA in 34%-38% and IgG in 38%-50% all higher than in controls. The corresponding figures for UC were 26%-40%, 28% and 24% respectively, but only IgG ASCA was significantly lower than in CD. ASCA also does not differentiate CD from ITB[86,87]. Thus ASCA does not appear to have diagnostic value for CD strong enough to differentiate with certainty from UC and ITB as in the West. However more studies are needed to elucidate the exact role of these biomarkers in disease definition.
COLORECTAL CANCER IN UC
Only 5 series[1,90-93] present data on UC related colorectal cancer (CRC) from India listed in Table 3. In all the disease duration was long and there was predominance of pancolitis yet low rates of CRC. How can these facts be reconciled? The answer may lie in the fact that most of the pancolitis cases are not clinically or histologically severe and can be well controlled with medicines. The control of inflammation stops the impetus for carcinogenetic changes. The other factor may be the background low sporadic CRC rates in Asia and India possibly related to yet unidentified protective genes. (These UC related CRC rates though much lower than in the West are still higher than the prevailing low sporadic CRC rates in India). To address these issues properly, medically compliant high risk UC cases needs to be followed up for development of polyps, low grade dysplasia (LGD), high grade dysplasia (HGD), dysplasia associated lesion or mass and CRC in the sequence followed by sporadic CRC. Unfortunately the duration of disease after which screening should start and the intervals at which colonoscopy is to be done are unsettled and has to be tailored according to local conditions (15%-20% of CRC cases develop at less than10 years of disease duration). Recent studies have tried to address these issues. In the retrospective study, 5 (0.94%) developed CRC and 1 (0.19%) HGD over 25 years. Surveillance was done for 6 years. Of the 5 CRC, one each had LGD and HGD detected 5 and 3 years earlier respectively and another had LGD in rectum 1 year earlier which persisted while CRC developed in descending colon. First CRC developed after 10 years of disease. Six patients had LGD at different areas (single or multifocal) which either disappeared or did not progress in next1 to 2.5 years follow up[90]. It thus appears that development of CRC is unpredictable and may occur at site different from dysplastic areas even in synchronous manner[94]. This suggests molecular and pathological heterogeneity during multiclonal origin of UC related CRC, where UC randomly damages multiple carcinogenetic genes in epithelial cells. This also put question mark on the usefulness of strict screening protocol with respect to time interval and number of segmental biopsies. In another prospective study, only 29/41 (70.7%) eligible UC cases (55% pancolitis > 7 years and rest limited segment colitis > 10 years duration) could be enrolled for colonoscopic surveillance over 42 mo (done by magnification chromoendoscopy). Initial screening showed LGD in 5 (17.2%) and HGD in 3 (10.3%). Only 12 follow up colonoscopies could be done in 9 cases at intervals of 6-24 mo which detected 3 new LGD cases and adenocarcinoma in one HGD case[92]. This study showed surveillance to be useful but acceptance and proper follow up to be suboptimal in India. Albeit these uncertainties of disease progression, cost effectiveness and acceptability of screening protocol, it seems reasonable for surveillance to start after 8-10 years in cases of pancolitis (10-12 years for limited colitis) and subsequent intervals determined by the findings at baseline of LGD (3-5 years), HGD (1-2 years) and also by frequency of relapses, quality of medical control and the expected disease duration at initial diagnosis.
Table 3.
Comparative data on ulcerative colitis related colorectal cancer in different Indian series
| Study type | Incidence of CRC | Time duration of FU in years | Presented with CRC | CRC developed at FU | Risk of CRC at time in years | Risk Factors | LGD | HGD | Detected on surveillance/symptoms |
| Retrospective[91] | 8/436 (1.8%) | 12.1 (7-25) | 4 | 4 (2/4 after 7, 8 yr of FU) | Pancolitis 6/8 (66.7%) | NM | NM | Symptoms | |
| Retrospective[90] | 5/532 (0.94%) | 25 | 0 | 5 (in FU of 6 yr) | 0 at 10, 2.3% (4.4% for pancolitis) at 20, 5.8% (10.2% for pancolitis) at > 20 | Pancolitis, disease duration > 10 yr | 2 progressed to CRC (1 after 5 yr, 1 at a different site after 1 yr). 6 did not progress in 1- 2 1/2 yr | 2 (1 CRC after 3 yr, another operated immediately) | 2 CRC on routine surveillance |
| Partly Retrospective, partly prospective[1] | 5/50 (10%), [0/21 (prospective), 5/19 (retrospective)] | 9.35 (1-30), prospective 4.5 (1-12), retrospective 15 (8-30) | 0 | 5 (1 after 10 yr, 4 after 20 yr) | 0 at 10, 1% at 20, 7% at > 20 | Disease duration > 10 yr | No surveillance | No surveillance | 5 at symptoms |
| Prospective[93] | 12/430 (2.8%) | Median 6 (1-39) | 0 | After median 18 yr from onset (6-27), 3 at 6, 6, 7 yr FU | Incidence density per 103 PYD was 2.3 at 10, 3.3 at 20 , 7 at > 20 | Pancolitis, longer disease duration | NM | NM | 1 on surveillance after 11 yr/11 at symptoms, (unifocal in 10 and multifocal in 2) |
| Prospective surveillance study[92] | 1/29 (3.4%) | 10 (7.5-14.5) | 0 | 1 (after 1 yr) | Pancolitis in 55% | 5 at baseline, 3 new cases on surveillance | 3 (1 CRC after 1 yr) | 1 over 42 mo surveillance |
CRC: Colorectal cancer; FU: Follow up; LGD: Low grade dysplasia; HGD: High grade dysplasia; PYD: Person years of disease duration; NM: Not mentioned.
In a study of culprit CRC genes, 21.4% high risk UC cases developed neoplasia, none of whom had microsatellite instability (MSI) which was found in 16.7% sporadic CRC cases. p53 mutation increased from 3.3% in low risk UC cases to 27.3% in high risk UC cases without CRC, to 50% in high risk UC cases with CRC and 33.3% in sporadic CRC. KRAS mutation was present only in presence of neoplasia but BRAF mutation was absent in all cases. Therefore the mutations are similar to the West except MSI[95]. The same group also reports increasing number of copy number variations spanning multiple chromosomes to be associated with the progression of UC to CRC from low risk through high risk cases, dysplasia cases to CRC[96] some of which are common and unique to both UC associated and sporadic CRC. These are present in 10 overlapping regions of a number of chromosomes (highest in 12p and 8q chromosome) and have overall accuracy of 29% and 54% for detecting these cancers respectively[97].
DIFFERENTIATION OF CD FROM ITB
Indian CD patients are diagnosed after a average gap of 1 and 1/2 years from symptom onset mainly because of diagnostic confusion with ITB. The clinical, serologic, radiologic, endoscopic, microbiogical and histologic features which distinguishes ITB from CD are discussed at length in recent review[98] and series[99-101] and the reader is referred to these articles for detailed discussion. However in spite of such in depth theoretical differentiation, 36.7% receive antituberculosis drugs at some point in their disease course[9]. This underscores the practical difficulty of differentiating the two in community setting where experts and advanced diagnostic facilities are not widely available. A recent attempt at ultrastructural and molecular differentiation can be seen e.g., (1) claudin 2 expressed in the upper part of intercellular junction with maintained tight junction in ITB compared to CD where it is increased along the whole length of intercellular junction[102]; (2) mesenchymal cell marker CD73 being expressed only in ITB granuloma but not CD granuloma[103]; and (3) increased expression of growth related oncogene alpha mRNA in biopsy specimen and of IL17 in peripheral blood mononuclear cells of ITB cases compared to CD whereas increased IL 1, IL 6 and IL 8 in peripheral blood mononuclear cells and increased gamma interferon, TLR 5 and 9 and decreased RANTES in biopsy specimen of CD cases compared to ITB[104].
The important questions are whether the two diseases can coexist throughout their course, or whether Mycobacterium tuberculosis initiate CD by producing altered gut mucosal immunity like other gut microbiota and more recently whether it can cause disease exacerbation in patients on immunosuppressives. It is now clear that a large portion of IBD risk loci are shared with other immune-mediated diseases, primary immunodeficiencies and mycobacterial disease, pointing towards common pathogenic mechanisms between different diseases. CD is associated with genes which code for proteins involved in autophagy and innate immunity highlighting the importance of defective processing of intracellular bacteria in its pathogenesis. It is intriguing to note that seven susceptibility loci for infection with Mycobacterium leprae, including NOD2, IL23R, RIPK2 and TNFSF15, have also been associated with CD though their final effects are in different directions. It remains to be investigated whether this genetic overlap signifies a true causative role for mycobacteria in CD, or rather represent the result of convergent evolutionary adaptations to different pathogens[61]. A very recent report of intestinal tuberculosis diagnosed in a long standing case of CD on immunosuppressive and it to be causally associated with Takayasu disease is interesting enough to raise question[105].
PEDIATRIC IBD
There are very few published series on the topic. Only very recently a multicentre questionnaire survey involving large number of pediatric cases both from north and south India have been published[106] which endorses the previous two small series only on CD from south India[107,108]. Very interestingly the north south divide between UC and CD, clinical features, EIM and treatment results are all similar to their adult counterparts. Whereas the UC:CD ratio is 2:1 among north Indian patients, it is just the reverse among cases from south India. Pancolitis was the commonest mode of presentation in UC (70.9%) though clinically it was moderate in 84%. Eighty-eight percent required steroids of whom 43% needed AZP subsequently for maintainance in addition to 5ASA. Biologicals were used in 6 (0.8%) but 4 needed surgery. For CD, ileocolonic involvement was commonest (72.9% of whom 76.2% required AZP for maintenance) while upper GI and perianal modifiers were present in 18% each. Eighty-four percent needed steroids of whom 76.2% were maintained on 5-ASA + AZP and 12.2% were given infliximab. Twenty point four percent developed fistulae, stricture, and perforation of whom 8.2% needed surgery. The differences were (1) CD commoner than UC; (2) growth failure (76.2%, 40%) and anemia (64.7%, 43%) are common in CD, UC; (3) no gender difference in both UC and CD; (4) CD was more severe, 76.2% had moderately severe disease and 19.6 % had fulminant disease; and (5) more than 80% of both UC and CD needed steroids. Similar facts have been noticed recently in pediatric South Asian population in America also[109,110].
CONCLUSION
IBD occurs much more commonly in India than previously thought with incidence and prevalence topping Asian countries and approaching the lower limit of that of North Europe and America which might have involvement of both genetic (inheritance from Caucasian origin) and environmental (causal for the recent quick rise in incidence) factors. The common genes implicated in pathogenesis in the West are not causal in Indian patients and the causal environmental factors needs larger community based cohort studies to be pinpointed. There appears to be a North-South divide with UC being higher in north than south India while the situation is reverse for CD though population based studies are lacking from south India. IBD in 2nd generation Indian diasporas settled in the West resemble their country of settlement but in those settled in other SE Asian countries retains features of their country of origin. The clinical presentation is much like other SE Asian countries (similar age and sex profile, low positive family history and effect of smoking, roughly similar disease location, high use of 5ASA for CD, low use of biologics and similar surgical rates) with some differences (higher incidence of inflammatory CD with much lower perianal disease, higher use of 5ASA and AZP and lower current use of corticosteroids). UC presents more with extensive disease not paralleled in severity clinically or histologically, follows benign course with easy medical control and low incidence of fulminant disease, cancer, complications, and surgery. This specific disease behaviour in Indians as a race might also be genetically determined with environmental modifiers. UC related CRC develop in an unpredictable manner with respect to disease duration, site and dysplastic areas questioning the validity of strict screening protocol. The diagnostic confusion of CD with ITB is still prevalent and in spite of multiple investigations for differentiation, about a third of CD patients still get antituberculosis drugs. A significant number of CD cases presents with small intestinal bleed where the disease predominantly affects with aggressive inflammation. A significant number require surgery in the longrun. Biomarkers have inadequate diagnostic sensitivity and specificity for both. Pediatric IBD tends to be more severe than adult.
FUTURE
Presently there is scarcity of well-designed population based studies from India to authenticate the apparent temporal rise and the regional differences in incidence and prevalence of IBD. These have to be undertaken from different parts of the country, especially from south where CD seems to be highly prevalent compared to north. Ideal epidemiologic study should ensure universal accessibility to health care, a population based sample, standardised case definition, relevant database/registries with appropriate validation and prospective data collection[6]. Most published studies are hospital based which are likely to underestimate incidence as more severe disease is only seen. But there might be true increase in incidence due to rapid industrialisation, urbanisation and change in diet and lifestyle. With rapid mixing of population all over India forced by political and socioeconomic factors, the genetic distinctness and hence disease phenotype is also likely to change and may further obfuscate the unclear present scenario.
Fortunately IBD has not yet attained the dimension of a pressing public health problem and hence it is difficult to visualise active participation of government in addressing its problems in near future. Yet as people live long with the disease, the morbidity burden is going to rise and patients will mandate special support in the longrun from all specialities involved in the management of this complex disease (gastroenterologist, surgeon, radiologist, nutritionist, stoma care specialist, pathologist) which will skyrocket cost of treatment. The government, national and state societies (ISG, SGEI) and other related foundations (CCFI) can cooperate to establish proper registries, conduct well designed epidemiologic studies, set up patient care centres (IBD centres) in different states where patients needing lifelong special care can get support at affordable cost. Case definition and treatment protocol needs to be standardised, widely circulated and adherence ensured. It will also be the responsibility of physicians to refer appropriate cases early to IBD centres. Newer biomarkers and diagnostic modalities needs to be made generally available for proper diagnosis in all aspects of the disease. Treatment options (both existing and newer) needs to be tested rigorously in randomised controlled trials (RCT) especially costly drugs like biologics, ciclosporin and others to define appropriate use of each (especially in view of the milder disease in India) to avoid unnecessary spending by patients. In a low prevalence country like India, the usefulness and feasibility of screening for CRC surveillance has to be carefully addressed. The disease duration after which the surveillance should start, the frequency of follow up, type of endoscopy to be used (magnification, chromo, confocal endomicroscopy) and the number and colonic areas of biopsy has to be fixed. Considering the low patient number, options will be difficult to test in RCTs.
Lastly some of the problems facing IBD management all over the world, not the least in India, has to be addressed also. These are (1) an obscure pathogenesis which prohibit formulation of uniform diagnostic and therapeutic strategies; (2) uncertain disease course including behaviour, activity, treatment response, development of cancer and prognosis; and (3) differential diagnosis of unclassified IBD colitis.
IBD results from an interplay of host genetic factors and environmental factors with the multiple microbial ecosystems present in different areas of the human bowel (both luminal and mucosal). Mucosal surface of the intestinal tract (which is continuously exposed to a large number of microorganisms) produce a diverse array of antimicrobial proteins, some constitutively and some in response to invasion of pathogens or enteric microbiota into the mucosal barrier. An interplay of diverse pathogenetic pathways (both stimulatory and regulatory) first produce an array of mucosal inflammatory mediators (inflammasome) for control of offending bacteria which are downregulated after elimination of the offence so that host tissues are not damaged. IBD occurs when these regulatory pathways go awry predisposed by genetic abnormalities. Basic research regarding intestinal inflammation may reveal new insights into the role of the inflammasome, and the macromolecular complex of metabolites formed by intestinal bacteria (metagenomics) which will lead to the development of biomarkers that target specific pathogenic mechanisms of IBD. The advanced knowledge of antimicrobial protein expression in IBD can lead to its potential use as biomarkers for disease activity and also help in analysing the pathophysiology of IBD from their mechanism of protection against pathogens.
Biomarker also have great potential role in all phases of care of IBD patients. For those with suspected IBD, biomarkers can select patients unlikely to have IBD for whom further testing may be avoided. In diagnosed cases, biomarkers can be used to (1) determine the type of IBD and to predict disease course; (2) identify patients most likely to respond to therapies and those that may require more aggressive therapies, thus determining the prognosis; and (3) differentiate patients with active inflammation from those likely to have symptoms from other causes in patients with recurrent symptoms.
As treatment strategies (medical and surgical) and prognostic outcomes become more and more disease-specific, refinement in disease classification becomes mandatory. The present approach based on clinical features, existing biomarkers, radiology, endoscopy and histopathology falls well short of the desired target. On the contrary, emerging diagnostic techniques in the field of gastrointestinal endoscopy, molecular pathology, genetics, epigenetics, metabolomics and proteomics is showing promising results. Novel advanced endoscopic imaging techniques (like magnification endoscopy, chromoendoscopy, narrow band imaging, confocal microendoscopy, etc.,) and biomarkers can shed new light for the differential diagnosis of IBD, better reflecting diverse disease behaviours based on specific pathogenic pathways.
Hence progress in basic research is most important in India and should focus on identifying the genetic links and pathogenetic mechanisms of Indian IBD patients. Already studies have shown that the common genetic mutations associated with CD in the west are absent in Indian patients, novel mutations are being discovered and it is likely that pathogenetic mechanisms and hence treatment approach will not be the same as in the west. This will have to be aided by progress in the fields of “OMICS” (genomics, metagenomics, cellomics, proteomics, metabolomics), endoscopy and molecular pathology. Also detailed community based cohort studies are needed to define the causal environmental factors and these should include the effects of (1) highly prevalent diabetes mellitus; (2) increasing obesity; and (3) repeated use of antibiotics and prolonged use proton pump inhibitors, all of which are known to alter the gut microbiota and can be involved in disease pathogenesis.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest relevant to this article were reported.
Peer-review started: June 30, 2016
First decision: July 29, 2016
Article in press: August 23, 2016
P- Reviewer: Lakatos PL, Sparrow MP S- Editor: Qi Y L- Editor: A E- Editor: Ma S
References
- 1.Ray G. Inflammatory bowel disease in India--changing paradigms. Int J Colorectal Dis. 2011;26:635–644. doi: 10.1007/s00384-010-1084-5. [DOI] [PubMed] [Google Scholar]
- 2.Antia FP. Crohn’s conundrum in Indians. Indian J Gastroenterol. 1986;5:79–80. [PubMed] [Google Scholar]
- 3.Andres PG, Friedman LS. Epidemiology and the natural course of inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:255–281, vii. doi: 10.1016/s0889-8553(05)70056-x. [DOI] [PubMed] [Google Scholar]
- 4.Lucendo AJ, Hervías D, Roncero Ó, Lorente R, Bouhmidi A, Angueira T, Verdejo C, Salueña I, González-Castillo S, Arias Á. Epidemiology and temporal trends (2000-2012) of inflammatory bowel disease in adult patients in a central region of Spain. Eur J Gastroenterol Hepatol. 2014;26:1399–1407. doi: 10.1097/MEG.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 5.Lovasz BD, Golovics PA, Vegh Z, Lakatos PL. New trends in inflammatory bowel disease epidemiology and disease course in Eastern Europe. Dig Liver Dis. 2013;45:269–276. doi: 10.1016/j.dld.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia-Pacific area: a comparison with developed countries and regional differences. J Dig Dis. 2010;11:134–147. doi: 10.1111/j.1751-2980.2010.00429.x. [DOI] [PubMed] [Google Scholar]
- 7.Khosla SN, Girdhar NK, Lal S, Mishra DS. Epidemiology of ulcerative colitis in hospital and select general population of northern India. J Assoc Physicians India. 1986;34:405–407. [PubMed] [Google Scholar]
- 8.Sood A, Midha V, Sood N, Bhatia AS, Avasthi G. Incidence and prevalence of ulcerative colitis in Punjab, North India. Gut. 2003;52:1587–1590. doi: 10.1136/gut.52.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makharia GK, Ramakrishna BS, Abraham P, Choudhuri G, Misra SP, Ahuja V, Bhatia SJ, Bhasin DK, Dadhich S, Dhali GK, et al. Survey of inflammatory bowel diseases in India. Indian J Gastroenterol. 2012;31:299–306. doi: 10.1007/s12664-012-0258-1. [DOI] [PubMed] [Google Scholar]
- 10.Probert CS, Mayberry JF, Mann R. Inflammatory bowel disease in the rural Indian subcontinent: a survey of patients attending mission hospitals. Digestion. 1990;47:42–46. doi: 10.1159/000200475. [DOI] [PubMed] [Google Scholar]
- 11.Goenka MK, Kochhar R, Mehta SK. Spectrum of lower gastrointestinal hemorrhage: an endoscopic study of 166 patients. Indian J Gastroenterol. 1993;12:129–131. [PubMed] [Google Scholar]
- 12.Anand AC, Patnaik PK, Bhalla VP, Chaudhary R, Saha A, Rana VS. Massive lower intestinal bleeding--a decade of experience. Trop Gastroenterol. 2001;22:131–134. [PubMed] [Google Scholar]
- 13.Garg PK, Singh J, Dhali GK, Mathur M, Sharma MP. Microscopic colitis is a cause of large bowel diarrhea in Northern India. J Clin Gastroenterol. 1996;22:11–15. doi: 10.1097/00004836-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Ghoshal UC, Mehrotra M, Kumar S, Ghoshal U, Krishnani N, Misra A, Aggarwal R, Choudhuri G. Spectrum of malabsorption syndrome among adults & amp; factors differentiating celiac disease & amp; tropical malabsorption. Indian J Med Res. 2012;136:451–459. [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav P, Das P, Mirdha BR, Gupta SD, Bhatnagar S, Pandey RM, Makharia GK. Current spectrum of malabsorption syndrome in adults in India. Indian J Gastroenterol. 2011;30:22–28. doi: 10.1007/s12664-011-0081-0. [DOI] [PubMed] [Google Scholar]
- 16.Dutta AK, Balekuduru A, Chacko A. Spectrum of malabsorption in India--tropical sprue is still the leader. J Assoc Physicians India. 2011;59:420–422. [PubMed] [Google Scholar]
- 17.Ramchandani M, Reddy DN, Gupta R, Lakhtakia S, Tandan M, Rao GV, Darisetty S. Diagnostic yield and therapeutic impact of single-balloon enteroscopy: series of 106 cases. J Gastroenterol Hepatol. 2009;24:1631–1638. doi: 10.1111/j.1440-1746.2009.05936.x. [DOI] [PubMed] [Google Scholar]
- 18.Dutta AK, Sajith KG, Joseph AJ, Simon EG, Chacko A. Learning curve, diagnostic yield and safety of single balloon enteroscopy. Trop Gastroenterol. 2012;33:179–184. doi: 10.7869/tg.2012.45. [DOI] [PubMed] [Google Scholar]
- 19.Sriram PV, Rao GV, Reddy DN. Wireless capsule endoscopy: experience in a tropical country. J Gastroenterol Hepatol. 2004;19:63–67. doi: 10.1111/j.1440-1746.2004.03220.x. [DOI] [PubMed] [Google Scholar]
- 20.Goenka MK, Majumder S, Goenka U. Capsule endoscopy: Present status and future expectation. World J Gastroenterol. 2014;20:10024–10037. doi: 10.3748/wjg.v20.i29.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das K, Sarkar R, Dasgupta J, Ray S, Ghatak S, Das K, Mridha AR, Dhali GK, Chowdhury A. Obscure GI bleeding in the tropics: impact of introduction of double-balloon and capsule endoscopies on outcome. Gastrointest Endosc. 2010;72:292–300. doi: 10.1016/j.gie.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 22.Ray G, Banerjee PK, Ghoshal UC, Dhar K, Pal BB, Biswas AD, Das U, Saha ML, Acharya AN, Majumdar S. Etiology and management of obscure gastrointestinal bleed--an appraisal from eastern India. Indian J Gastroenterol. 2001;20:90–93. [PubMed] [Google Scholar]
- 23.Probert CS, Jayanthi V, Pinder D, Wicks AC, Mayberry JF. Epidemiological study of ulcerative proctocolitis in Indian migrants and the indigenous population of Leicestershire. Gut. 1992;33:687–693. doi: 10.1136/gut.33.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayanthi V, Probert CS, Pinder D, Wicks AC, Mayberry JF. Epidemiology of Crohn’s disease in Indian migrants and the indigenous population in Leicestershire. Q J Med. 1992;82:125–138. [PubMed] [Google Scholar]
- 25.Montgomery SM, Morris DL, Pounder RE, Wakefield AJ. Asian ethnic origin and the risk of inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1999;11:543–546. doi: 10.1097/00042737-199905000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Walker DG, Williams HR, Kane SP, Mawdsley JE, Arnold J, McNeil I, Thomas HJ, Teare JP, Hart AL, Pitcher MC, et al. Differences in inflammatory bowel disease phenotype between South Asians and Northern Europeans living in North West London, UK. Am J Gastroenterol. 2011;106:1281–1289. doi: 10.1038/ajg.2011.85. [DOI] [PubMed] [Google Scholar]
- 27.Freeman HJ. Inflammatory bowel diseases in Indo-Canadians with and without antineutrophil cytoplasmic autoantibodies. Can J Gastroenterol. 2000;14:21–26. doi: 10.1155/2000/349082. [DOI] [PubMed] [Google Scholar]
- 28.Rajput HI, Seebaran AR, Desai Y. Ulcerative colitis in the Indian population of Durban. S Afr Med J. 1992;81:245–248. [PubMed] [Google Scholar]
- 29.Malhotra R, Turner K, Sonnenberg A, Genta RM. High prevalence of inflammatory bowel disease in United States residents of Indian ancestry. Clin Gastroenterol Hepatol. 2015;13:683–689. doi: 10.1016/j.cgh.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 30.Probert CS, Jayanthi V, Mayberry JF. Inflammatory bowel disease in Indian migrants in Fiji. Digestion. 1991;50:82–84. doi: 10.1159/000200743. [DOI] [PubMed] [Google Scholar]
- 31.Ling KL, Ooi CJ, Luman W, Cheong WK, Choen FS, Ng HS. Clinical characteristics of ulcerative colitis in Singapore, a multiracial city-state. J Clin Gastroenterol. 2002;35:144–148. doi: 10.1097/00004836-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Lee YM, Fock K, See SJ, Ng TM, Khor C, Teo EK. Racial differences in the prevalence of ulcerative colitis and Crohn’s disease in Singapore. J Gastroenterol Hepatol. 2000;15:622–625. doi: 10.1046/j.1440-1746.2000.02212.x. [DOI] [PubMed] [Google Scholar]
- 33.Hilmi I, Singh R, Ganesananthan S, Yatim I, Radzi M, Chua AB, Tan HJ, Huang S, Chin KS, Menon J, et al. Demography and clinical course of ulcerative colitis in a multiracial Asian population: a nationwide study from Malaysia. J Dig Dis. 2009;10:15–20. doi: 10.1111/j.1751-2980.2008.00357.x. [DOI] [PubMed] [Google Scholar]
- 34.Thakur S, Ranjan P, Ghoshal UC, Muller-Myhsok B, Khan F, Talwar S, Agarwal S. Association of human leucocyte DR and DQ antigens in Crohn’s disease in Asian Indians: a family study. Trop Gastroenterol. 2003;24:185–188. [PubMed] [Google Scholar]
- 35.Habeeb MA, Rajalingam R, Dhar A, Kumar A, Sharma MP, Mehra NK. HLA association and occurrence of autoantibodies in Asian-Indian patients with ulcerative colitis. Am J Gastroenterol. 1997;92:772–776. [PubMed] [Google Scholar]
- 36.Bardia A, Tiwari SK, Vishwakarma SK, Habeeb MA, Nallari P, Sultana SA, Pasha SA, Reddy YP, Khan AA. Haplotype analyses of DNA repair gene polymorphisms and their role in ulcerative colitis. PLoS One. 2014;9:e108562. doi: 10.1371/journal.pone.0108562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baskaran K, Pugazhendhi S, Ramakrishna BS. Association of IRGM gene mutations with inflammatory bowel disease in the Indian population. PLoS One. 2014;9:e106863. doi: 10.1371/journal.pone.0106863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juyal G, Negi S, Sood A, Gupta A, Prasad P, Senapati S, Zaneveld J, Singh S, Midha V, van Sommeren S, et al. Genome-wide association scan in north Indians reveals three novel HLA-independent risk loci for ulcerative colitis. Gut. 2015;64:571–579. doi: 10.1136/gutjnl-2013-306625. [DOI] [PubMed] [Google Scholar]
- 39.Meena NK, Verma R, Verma N, Ahuja V, Paul J. TLR4 D299G polymorphism modulates cytokine expression in ulcerative colitis. J Clin Gastroenterol. 2013;47:773–780. doi: 10.1097/MCG.0b013e31828a6e93. [DOI] [PubMed] [Google Scholar]
- 40.Verma R, Ahuja V, Paul J. Detection of single-nucleotide polymorphisms in the intron 9 region of the nucleotide oligomerization domain-1 gene in ulcerative colitis patients of North India. J Gastroenterol Hepatol. 2012;27:96–103. doi: 10.1111/j.1440-1746.2011.06832.x. [DOI] [PubMed] [Google Scholar]
- 41.Sivaram G, Tiwari SK, Bardia A, Anjum F, Vishnupriya S, Habeeb A, Khan AA. Macrophage migration inhibitory factor, Toll-like receptor 4, and CD14 polymorphisms with altered expression levels in patients with ulcerative colitis. Hum Immunol. 2012;73:201–205. doi: 10.1016/j.humimm.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Ahirwar DK, Kesarwani P, Singh R, Ghoshal UC, Mittal RD. Role of tumor necrosis factor-alpha (C-863A) polymorphism in pathogenesis of inflammatory bowel disease in Northern India. J Gastrointest Cancer. 2012;43:196–204. doi: 10.1007/s12029-010-9238-9. [DOI] [PubMed] [Google Scholar]
- 43.Bardia A, Tiwari SK, Gunisetty S, Anjum F, Nallari P, Habeeb MA, Khan AA. Functional polymorphisms in XRCC-1 and APE-1 contribute to increased apoptosis and risk of ulcerative colitis. Inflamm Res. 2012;61:359–365. doi: 10.1007/s00011-011-0418-2. [DOI] [PubMed] [Google Scholar]
- 44.Juyal G, Prasad P, Senapati S, Midha V, Sood A, Amre D, Juyal RC, BK T. An investigation of genome-wide studies reported susceptibility loci for ulcerative colitis shows limited replication in north Indians. PLoS One. 2011;6:e16565. doi: 10.1371/journal.pone.0016565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sivaram G, Tiwari SK, Bardia A, Manoj G, Santhosh B, Saikant R, Aejaz H, Vishnupriya S, Khan AA, Habibullah C. Association of genetic variants of mannan-binding (MBL) lectin-2 gene, MBL levels and function in ulcerative colitis and Crohn’s disease. Innate Immun. 2011;17:526–531. doi: 10.1177/1753425910384531. [DOI] [PubMed] [Google Scholar]
- 46.Sikander A, Rana SV, Sharma SK, Sinha SK, Arora SK, Prasad KK, Singh K. Association of alpha 2A adrenergic receptor gene (ADRAlpha2A) polymorphism with irritable bowel syndrome, microscopic and ulcerative colitis. Clin Chim Acta. 2010;411:59–63. doi: 10.1016/j.cca.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Verma R, Ahuja V, Paul J. Frequency of single nucleotide polymorphisms in NOD1 gene of ulcerative colitis patients: a case-control study in the Indian population. BMC Med Genet. 2009;10:82. doi: 10.1186/1471-2350-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juyal G, Midha V, Amre D, Sood A, Seidman E, Thelma BK. Associations between common variants in the MDR1 (ABCB1) gene and ulcerative colitis among North Indians. Pharmacogenet Genomics. 2009;19:77–85. doi: 10.1097/FPC.0b013e32831a9abe. [DOI] [PubMed] [Google Scholar]
- 49.Mittal RD, Manchanda PK, Bid HK, Ghoshal UC. Analysis of polymorphisms of tumor necrosis factor-alpha and polymorphic xenobiotic metabolizing enzymes in inflammatory bowel disease: study from northern India. J Gastroenterol Hepatol. 2007;22:920–924. doi: 10.1111/j.1440-1746.2006.04538.x. [DOI] [PubMed] [Google Scholar]
- 50.Mittal RD, Bid HK, Ghoshal UC. IL-1 receptor antagonist (IL-1Ra) gene polymorphism in patients with inflammatory bowel disease in India. Scand J Gastroenterol. 2005;40:827–831. doi: 10.1080/00365520510015629. [DOI] [PubMed] [Google Scholar]
- 51.Juyal G, Amre D, Midha V, Sood A, Seidman E, Thelma BK. Evidence of allelic heterogeneity for associations between the NOD2/CARD15 gene and ulcerative colitis among North Indians. Aliment Pharmacol Ther. 2007;26:1325–1332. doi: 10.1111/j.1365-2036.2007.03524.x. [DOI] [PubMed] [Google Scholar]
- 52.Pugazhendhi S, Santhanam S, Venkataraman J, Creveaux I, Ramakrishna BS. NOD2 gene mutations associate weakly with ulcerative colitis but not with Crohn’s disease in Indian patients with inflammatory bowel disease. Gene. 2013;512:309–313. doi: 10.1016/j.gene.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 53.Mahurkar S, Banerjee R, Rani VS, Thakur N, Rao GV, Reddy DN, Chandak GR. Common variants in NOD2 and IL23R are not associated with inflammatory bowel disease in Indians. J Gastroenterol Hepatol. 2011;26:694–699. doi: 10.1111/j.1440-1746.2010.06533.x. [DOI] [PubMed] [Google Scholar]
- 54.Pugazhendhi S, Amte A, Balamurugan R, Subramanian V, Ramakrishna BS. Common NOD2 mutations are absent in patients with Crohn’s disease in India. Indian J Gastroenterol. 2008;27:201–203. [PubMed] [Google Scholar]
- 55.Baskaran K, Pugazhendhi S, Ramakrishna BS. Protective association of tumor necrosis factor superfamily 15 (TNFSF15) polymorphic haplotype with Ulcerative Colitis and Crohn’s disease in an Indian population. PLoS One. 2014;9:e114665. doi: 10.1371/journal.pone.0114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker DG, Williams HR, Bancil AS, Rai P, Pantelidis P, Chambers J, Kooner JS, Sato H, Orchard TR. Ethnicity differences in genetic susceptibility to ulcerative colitis: a comparison of Indian asians and white northern Europeans. Inflamm Bowel Dis. 2013;19:2888–2894. doi: 10.1097/01.MIB.0000437567.12067.e7. [DOI] [PubMed] [Google Scholar]
- 57.Indian Genome Variation Consortium. Genetic landscape of the people of India: a canvas for disease gene exploration. J Genet. 2008;87:3–20. doi: 10.1007/s12041-008-0002-x. [DOI] [PubMed] [Google Scholar]
- 58.Ali M, Liu X, Pillai EN, Chen P, Khor CC, Ong RT, Teo YY. Characterizing the genetic differences between two distinct migrant groups from Indo-European and Dravidian speaking populations in India. BMC Genet. 2014;15:86. doi: 10.1186/1471-2156-15-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tamang R, Singh L, Thangaraj K. Complex genetic origin of Indian populations and its implications. J Biosci. 2012;37:911–919. doi: 10.1007/s12038-012-9256-9. [DOI] [PubMed] [Google Scholar]
- 60.Juyal G, Mondal M, Luisi P, Laayouni H, Sood A, Midha V, Heutink P, Bertranpetit J, Thelma BK, Casals F. Population and genomic lessons from genetic analysis of two Indian populations. Hum Genet. 2014;133:1273–1287. doi: 10.1007/s00439-014-1462-0. [DOI] [PubMed] [Google Scholar]
- 61.Ek WE, D’Amato M, Halfvarson J. The history of genetics in inflammatory bowel disease. Ann Gastroenterol. 2014;27:294–303. [PMC free article] [PubMed] [Google Scholar]
- 62.Rajan TV. Inflammatory bowel disease: maladaptation of the vigilant genotype in a hyper-clean world? Perspect Biol Med. 2006;49:171–177. doi: 10.1353/pbm.2006.0029. [DOI] [PubMed] [Google Scholar]
- 63.Desai HG, Gupte PA. Increasing incidence of Crohn’s disease in India: is it related to improved sanitation? Indian J Gastroenterol. 2005;24:23–24. [PubMed] [Google Scholar]
- 64.Kabeerdoss J, Pugazhendhi S, Subramanian V, Binder HJ, Ramakrishna BS. Exposure to hookworms in patients with Crohn’s disease: a case-control study. Aliment Pharmacol Ther. 2011;34:923–930. doi: 10.1111/j.1365-2036.2011.04824.x. [DOI] [PubMed] [Google Scholar]
- 65.Pugazhendhi S, Sahu MK, Subramanian V, Pulimood A, Ramakrishna BS. Environmental factors associated with Crohn’s disease in India. Indian J Gastroenterol. 2011;30:264–269. doi: 10.1007/s12664-011-0145-1. [DOI] [PubMed] [Google Scholar]
- 66.Sood A, Amre D, Midha V, Sharma S, Sood N, Thara A, Bansal M, Juyal G, Thelma BK, Seidman E. Low hygiene and exposure to infections may be associated with increased risk for ulcerative colitis in a North Indian population. Ann Gastroenterol. 2014;27:219–223. [PMC free article] [PubMed] [Google Scholar]
- 67.Ray G. Association of dietary factors with ulcerative colitis in India. J Gastroenterol Hepatol Res. 2015;4:1649–1652. [Google Scholar]
- 68.Ng SC, Tang W, Leong RW, Chen M, Ko Y, Studd C, Niewiadomski O, Bell S, Kamm MA, de Silva HJ, et al. Environmental risk factors in inflammatory bowel disease: a population-based case-control study in Asia-Pacific. Gut. 2015;64:1063–1071. doi: 10.1136/gutjnl-2014-307410. [DOI] [PubMed] [Google Scholar]
- 69.Kumari R, Ahuja V, Paul J. Fluctuations in butyrate-producing bacteria in ulcerative colitis patients of North India. World J Gastroenterol. 2013;19:3404–3414. doi: 10.3748/wjg.v19.i22.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kabeerdoss J, Sankaran V, Pugazhendhi S, Ramakrishna BS. Clostridium leptum group bacteria abundance and diversity in the fecal microbiota of patients with inflammatory bowel disease: a case-control study in India. BMC Gastroenterol. 2013;13:20. doi: 10.1186/1471-230X-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walujkar SA, Dhotre DP, Marathe NP, Lawate PS, Bharadwaj RS, Shouche YS. Characterization of bacterial community shift in human Ulcerative Colitis patients revealed by Illumina based 16S rRNA gene amplicon sequencing. Gut Pathog. 2014;6:22. doi: 10.1186/1757-4749-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Verma R, Verma AK, Ahuja V, Paul J. Real-time analysis of mucosal flora in patients with inflammatory bowel disease in India. J Clin Microbiol. 2010;48:4279–4282. doi: 10.1128/JCM.01360-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patra S, Samal SC, Kang G, Pulimood A, Mathan M, Ramakrishna BS. Adherent Escherichia coli in colorectal mucosal biopsies: a histological and ultrastructural evaluation. Indian J Pathol Microbiol. 2012;55:485–489. doi: 10.4103/0377-4929.107786. [DOI] [PubMed] [Google Scholar]
- 74.Iyer VH, Augustine J, Pulimood AB, Ajjampur SS, Ramakrishna BS. Correlation between coinfection with parasites, cytomegalovirus, and Clostridium difficile and disease severity in patients with ulcerative colitis. Indian J Gastroenterol. 2013;32:115–118. doi: 10.1007/s12664-012-0302-1. [DOI] [PubMed] [Google Scholar]
- 75.Banerjee D, Deb R, Dar L, Mirdha BR, Pati SK, Thareja S, Falodia S, Ahuja V. High frequency of parasitic and viral stool pathogens in patients with active ulcerative colitis: report from a tropical country. Scand J Gastroenterol. 2009;44:325–331. doi: 10.1080/00365520802556809. [DOI] [PubMed] [Google Scholar]
- 76.Ghoshal UC, Alexender G, Ghoshal U, Tripathi S, Krishnani N. Strongyloides stercoralis infestation in a patient with severe ulcerative colitis. Indian J Med Sci. 2006;60:106–110. [PubMed] [Google Scholar]
- 77.Kishore J, Ghoshal U, Ghoshal UC, Krishnani N, Kumar S, Singh M, Ayyagari A. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance and outcome. J Med Microbiol. 2004;53:1155–1160. doi: 10.1099/jmm.0.45629-0. [DOI] [PubMed] [Google Scholar]
- 78.Kochhar R, Ayyagari A, Goenka MK, Dhali GK, Aggarwal R, Mehta SK. Role of infectious agents in exacerbations of ulcerative colitis in India. A study of Clostridium difficile. J Clin Gastroenterol. 1993;16:26–30. doi: 10.1097/00004836-199301000-00008. [DOI] [PubMed] [Google Scholar]
- 79.Balamurugan R, Balaji V, Ramakrishna BS. Estimation of faecal carriage of Clostridium difficile in patients with ulcerative colitis using real time polymerase chain reaction. Indian J Med Res. 2008;127:472–477. [PubMed] [Google Scholar]
- 80.Vaishnavi C, Kochhar R, Bhasin D, Thennarasu K, Singh K. Simultaneous assays for Clostridium difficile and faecal lactoferrin in ulcerative colitis. Trop Gastroenterol. 2003;24:13–16. [PubMed] [Google Scholar]
- 81.Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145:158–165.e2. doi: 10.1053/j.gastro.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 82.Ng SC, Zeng Z, Niewiadomski O, Tang W, Bell S, Kamm MA, Hu P, de Silva HJ, Niriella MA, Udara WS, et al. Early Course of Inflammatory Bowel Disease in a Population-Based Inception Cohort Study From 8 Countries in Asia and Australia. Gastroenterology. 2016;150:86–95.e3; quiz e13-4. doi: 10.1053/j.gastro.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 83.Kalaria R, Desai D, Abraham P, Joshi A, Gupta T, Shah S. Temporal Change in Phenotypic Behaviour in Patients with Crohn’s Disease: Do Indian Patients Behave Differently from Western and Other Asian Patients? J Crohns Colitis. 2016;10:255–261. doi: 10.1093/ecco-jcc/jjv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goel A, Dutta AK, Pulimood AB, Eapen A, Chacko A. Clinical profile and predictors of disease behavior and surgery in Indian patients with Crohn’s disease. Indian J Gastroenterol. 2013;32:184–189. doi: 10.1007/s12664-012-0293-y. [DOI] [PubMed] [Google Scholar]
- 85.Makharia GK, Sachdev V, Gupta R, Lal S, Pandey RM. Anti-Saccharomyces cerevisiae antibody does not differentiate between Crohn’s disease and intestinal tuberculosis. Dig Dis Sci. 2007;52:33–39. doi: 10.1007/s10620-006-9527-0. [DOI] [PubMed] [Google Scholar]
- 86.Amarapurkar DN, Patel ND, Rane PS. Diagnosis of Crohn’s disease in India where tuberculosis is widely prevalent. World J Gastroenterol. 2008;14:741–746. doi: 10.3748/wjg.14.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ghoshal UC, Ghoshal U, Singh H, Tiwari S. Anti-Saccharomyces cerevisiae antibody is not useful to differentiate between Crohn’s disease and intestinal tuberculosis in India. J Postgrad Med. 2007;53:166–170. doi: 10.4103/0022-3859.33857. [DOI] [PubMed] [Google Scholar]
- 88.Masoodi I, Kochhar R, Dutta U, Vaishnavi C, Prasad KK, Vaiphei K, Kaur S, Singh K. Fecal lactoferrin, myeloperoxidase and serum C-reactive are effective biomarkers in the assessment of disease activity and severity in patients with idiopathic ulcerative colitis. J Gastroenterol Hepatol. 2009;24:1768–1774. doi: 10.1111/j.1440-1746.2009.06048.x. [DOI] [PubMed] [Google Scholar]
- 89.Samant H, Desai D, Abraham P, Joshi A, Gupta T, Dherai A, Ashavaid T. Fecal calprotectin and its correlation with inflammatory markers and endoscopy in patients from India with inflammatory bowel disease. Indian J Gastroenterol. 2015;34:431–435. doi: 10.1007/s12664-015-0608-x. [DOI] [PubMed] [Google Scholar]
- 90.Venkataraman S, Mohan V, Ramakrishna BS, Peter S, Chacko A, Chandy G, Kurian G, Kurian S, Mathan M, Mathan VI, et al. Risk of colorectal cancer in ulcerative colitis in India. J Gastroenterol Hepatol. 2005;20:705–709. doi: 10.1111/j.1440-1746.2005.03810.x. [DOI] [PubMed] [Google Scholar]
- 91.Kochhar R, Goenka MK, Kaushik SP, Gupta NM, Nagi B, Mehta SK. Colorectal carcinoma in Indian patients with idiopathic ulcerative colitis. Eur J Cancer Prev. 1992;1:293–296. doi: 10.1097/00008469-199206000-00003. [DOI] [PubMed] [Google Scholar]
- 92.Shivakumar BM, Lakshmankumar B, Rao L, Bhat G, Suvarna D, Pai CG. Colorectal neoplasia in long-standing ulcerative colitis - a prospective study from a low-prevalence area. Colorectal Dis. 2013;15:e462–e468. doi: 10.1111/codi.12276. [DOI] [PubMed] [Google Scholar]
- 93.Desai D, Shah S, Deshmukh A, Abraham P, Joshi A, Gupta T, Deshpande R, Khandagale V, George S. Colorectal cancers in ulcerative colitis from a low-prevalence area for colon cancer. World J Gastroenterol. 2015;21:3644–3649. doi: 10.3748/wjg.v21.i12.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shivakumar BM, Rao L, Satyamoorthy K, Pai GC. Synchronous colorectal neoplasms in ulcerative pancolitis of 6 years duration: pathological and molecular heterogeneity. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-200172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shivakumar BM, Kumar BL, Bhat G, Suvarna D, Rao L, Pai CG, Satyamoorthy K. Molecular alterations in colitis-associated colorectal neoplasia: study from a low prevalence area using magnifying chromo colonoscopy. J Crohns Colitis. 2012;6:647–654. doi: 10.1016/j.crohns.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 96.Shivakumar BM, Rotti H, Vasudevan TG, Balakrishnan A, Chakrabarty S, Bhat G, Rao L, Pai CG, Satyamoorthy K. Copy number variations are progressively associated with the pathogenesis of colorectal cancer in ulcerative colitis. World J Gastroenterol. 2015;21:616–622. doi: 10.3748/wjg.v21.i2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shivakumar BM, Chakrabarty S, Rotti H, Seenappa V, Rao L, Geetha V, Tantry BV, Kini H, Dharamsi R, Pai CG, et al. Comparative analysis of copy number variations in ulcerative colitis associated and sporadic colorectal neoplasia. BMC Cancer. 2016;16:271. doi: 10.1186/s12885-016-2303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pulimood AB, Amarapurkar DN, Ghoshal U, Phillip M, Pai CG, Reddy DN, Nagi B, Ramakrishna BS. Differentiation of Crohn’s disease from intestinal tuberculosis in India in 2010. World J Gastroenterol. 2011;17:433–443. doi: 10.3748/wjg.v17.i4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dutta AK, Sahu MK, Gangadharan SK, Chacko A. Distinguishing Crohn’s disease from intestinal tuberculosis--a prospective study. Trop Gastroenterol. 2011;32:204–209. [PubMed] [Google Scholar]
- 100.Larsson G, Shenoy T, Ramasubramanian R, Balakumaran LK, Småstuen MC, Bjune GA, Moum BA. Routine diagnosis of intestinal tuberculosis and Crohn’s disease in Southern India. World J Gastroenterol. 2014;20:5017–5024. doi: 10.3748/wjg.v20.i17.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sood A, Midha V, Singh A. Differential diagnosis of Crohn’s disease versus ileal tuberculosis. Curr Gastroenterol Rep. 2014;16:418. doi: 10.1007/s11894-014-0418-9. [DOI] [PubMed] [Google Scholar]
- 102.Das P, Goswami P, Das TK, Nag T, Sreenivas V, Ahuja V, Panda SK, Gupta SD, Makharia GK. Comparative tight junction protein expressions in colonic Crohn’s disease, ulcerative colitis, and tuberculosis: a new perspective. Virchows Arch. 2012;460:261–270. doi: 10.1007/s00428-012-1195-1. [DOI] [PubMed] [Google Scholar]
- 103.Banerjee R, Balaji M, Sasikala M, Anuradha S, Rao GV, Nageshwar Reddy D. Granulomas of intestinal tuberculosis and Crohn’s disease can be differentiated by CD73 cell surface marker expression: a pilot study. Dig Dis Sci. 2013;58:2301–2307. doi: 10.1007/s10620-013-2667-0. [DOI] [PubMed] [Google Scholar]
- 104.Pugazhendhi S, Jayakanthan K, Pulimood AB, Ramakrishna BS. Cytokine gene expression in intestinal tuberculosis and Crohn’s disease. Int J Tuberc Lung Dis. 2013;17:662–668. doi: 10.5588/ijtld.12.0600. [DOI] [PubMed] [Google Scholar]
- 105.Baijal R, Chogle A, Kumar P, Shah N, Kulkarni S, Doshi S, Gupta D, Amarapurkar D. A Case of Tuberculous Colitis with Associated Takayasu’s Arteritis. J Assoc Physicians India. 2015;63:62–65. [PubMed] [Google Scholar]
- 106.Sathiyasekaran M, Bavanandam S, Sankaranarayanan S, Mohan N, Geetha M, Wadhwa N, Kehar M, Biradar V. A questionnaire survey of pediatric inflammatory bowel disease in India. Indian J Gastroenterol. 2014;33:543–549. doi: 10.1007/s12664-014-0507-6. [DOI] [PubMed] [Google Scholar]
- 107.Avinash B, Dutta AK, Chacko A. Pediatric inflammatory bowel disease in South India. Indian Pediatr. 2009;46:639–640. [PubMed] [Google Scholar]
- 108.Sathiyasekaran M, Raju BB, Shivbalan S, Rajarajan K. Pediatric Crohn’s disease in South India. Indian Pediatr. 2005;42:459–463. [PubMed] [Google Scholar]
- 109.Li BH, Guan X, Vittinghoff E, Gupta N. Comparison of the presentation and course of pediatric inflammatory bowel disease in South Asians with Whites: a single center study in the United States. J Pediatr. 2013;163:1211–1213. doi: 10.1016/j.jpeds.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 110.Pinsk V, Lemberg DA, Grewal K, Barker CC, Schreiber RA, Jacobson K. Inflammatory bowel disease in the South Asian pediatric population of British Columbia. Am J Gastroenterol. 2007;102:1077–1083. doi: 10.1111/j.1572-0241.2007.01124.x. [DOI] [PubMed] [Google Scholar]


