Abstract
Stochastic niche theory resolves many of the differences between neutral theory and classical tradeoff-based niche theories of resource competition and community structure. In stochastic niche theory, invading species become established only if propagules can survive stochastic mortality while growing to maturity on the resources left unconsumed by established species. The theory makes three predictions about community structure. First, stochastic niche assembly creates communities in which species dominate approximately equally wide “slices” of the habitat's spatial heterogeneity. These niche widths generate realistic distributions of species relative abundances for which, contrary to neutral theory but consistent with numerous observations, there are strong correlations among species traits, species abundances, and environmental conditions. Second, slight decreases in resource levels are predicted to cause large decreases in the probability that a propagule would survive to be an adult. These decreases cause local diversity to be limited by the inhibitory effects of resource use by established species on the establishment (recruitment) of potential invaders. If resource pulses or disturbance allowed invaders to overcome this recruitment limitation, many more species could indefinitely coexist. Third, the low invasibility of high diversity communities is predicted to result not from diversity per se, but from the uniformly low levels of resources that occur in high-diversity communities created by stochastic competitive assembly. This prediction provides a potential solution to the invasion paradox, which is the tendency for highly diverse regions to be more heavily invaded.
Both tradeoff-based theories of interspecific competition (1–10) and neutral theories (11–13) have been suggested as potential explanations for the assembly, dynamics, and structure of ecological communities. Both approaches have provided insights, and both have shortcomings (11, 14–20). For instance, tradeoff-based theories of resource competition predict patterns of species traits and species separation on nutrient gradients similar to those observed (4, 5, 8–10, 21–24). These theories also provide a potential explanation for the high diversity of nature, predicting that habitat heterogeneity can allow a potentially unlimited number of species to coexist if species that are better at dealing with one environmental constraint are necessarily worse at dealing with another (4, 8, 9, 25). Classical tradeoff-based theories, however, do not predict a limit to diversity, because each point on a tradeoff surface represents traits of a species that could potentially invade into and coexist with any other species from that tradeoff surface (4, 5, 8–10, 26). This feature also means that classical tradeoff theory does not provide a general explanation for species relative abundances (11).
Neutral theory assumes that species are ecologically equivalent in their responses to all constraints and thus have no interspecific tradeoffs (11–13); it predicts that high species diversity could result from a balance between speciation and stochastic extinction caused by random drifts in population size (11). The stochastic drift of neutral theory is the basis for an elegantly simple analytical explanation for species relative abundance patterns (11). However, neutral theory predicts, contrary to numerous observations (e.g., refs. 4, 5, 14–21, and 22–24), that there should be no relation among species traits and their abundances or among community composition and environmental conditions. According to neutral theory, species are rare or abundant not because of their traits and the traits of their competitors but rather solely because of stochastic drift in densities of competitively identical species (11).
In this article, I develop stochastic niche theory, which modifies competitive tradeoff theory by including stochastic processes, such as those that underlie neutral theory. The historical roots of this theory lie in the work of MacArthur, May, Turelli, and others (27–31) on competition and limiting similarity. Stochastic niche theory offers an explanation for diversity, species composition, relative species abundance patterns, and invasion dynamics in ecological communities that resolves many of the shortcomings of both classical tradeoff theory and neutral theory.
Three observations form the heart of stochastic niche theory. First, community assembly results from the success or failure of propagules of invaders that, because of their rarity, are potentially subject to loss by means of demographic stochasticity. Second, successful propagules must be able to grow immensely in biomass and survive long enough to become reproductively successful adults while using the resources left unconsumed by established species. Third, the probability of successful establishment should depend on the resource requirements of an invader relative to those of established species, i.e., on how different an invader is from established species. Note that I call any species that enters a habitat in which it does not currently exist an “invader” or “invading species” whether or not it is a member of the regional flora or fauna or from a different biogeographic realm.
Resources and Invader Establishment
Community assembly is an iterative process. After arriving at a site, propagules either fail or they grow and spread through the community, impacting its resource levels and species abundances. Because propagules are rare and subject to the vagaries of stochastic but resource-dependent death, the establishment of novel species is a potentially important, although stochastic, sieve determining community composition and diversity.
The arrival of a novel propagule is the start of a potential invasion event. For terrestrial vascular plants, a seedling must both survive and increase in mass by a factor of 103 to 107 or more to become a reproductive adult. Juveniles have much higher mortality rates than adults, and survival can be growth rate- and resource-dependent. The analyses presented here incorporate resource and growth rate-dependent survival by assuming that ms, the probability of mortality for a seedling or juvenile per unit time, is constant, but that the amount of time spent exposed to this mortality as a juvenile, and thus the chance of survival to adult, depends on resources and resultant growth rates.
Let p be the probability that a germinated propagule would survive to become an adult and let y be the time required for it to grow in mass from that of a seed (Bs) to that of an adult (Ba). Then
 |
[1] |
Time to maturity, y, depends on the amount that a juvenile must grow to become an adult and on its resource-dependent vegetative growth rate, U. The probability of survival to adult is a strongly decreasing function of y. For instance, if ms = .75 yr–1, 1 in 103 propagules would become adult if this required 5 yr of growth (y = 5 yr), but only 1 in 106 would do so if lower resource caused y = 10 yr. Time to maturity, y, is determined by the amount of resource, R, that is left unconsumed by established species and by invader resource requirements. Let an invader have a vegetative growth rate that is a Monod function of R, of the single limiting resource. Then
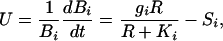 |
[2] |
where the subscript i refers to an invading individual; gi is its maximal growth rate; Bi is its biomass; Ki is the value of R at which it achieves half of its maximal growth rate; and Si is its rate of biomass loss to senescence, herbivory, etc. Note that this individual will have a vegetative growth rate greater than 0 only if R > KiSi/(gi – Si).
The biomass dynamics of an established species are also governed by Eq. 2, except that an additional loss term must be added to the equation: loss to plant mortality, mp. This addition is necessary because Eq. 2 applies to the vegetative growth of a single living individual, not to a whole population. At equilibrium, an established species (“Species e”) would reduce the limiting resource to R*e, where
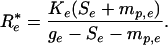 |
[3] |
The R* of a species determines its competitive ability; the species with the lowest R* displaces all other species at equilibrium when species compete for a single resource (4). In combination, Eqs. 1–3 show that p, the probability of the propagule of an invading species surviving to adulthood, is
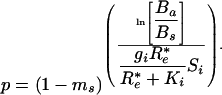 |
[4] |
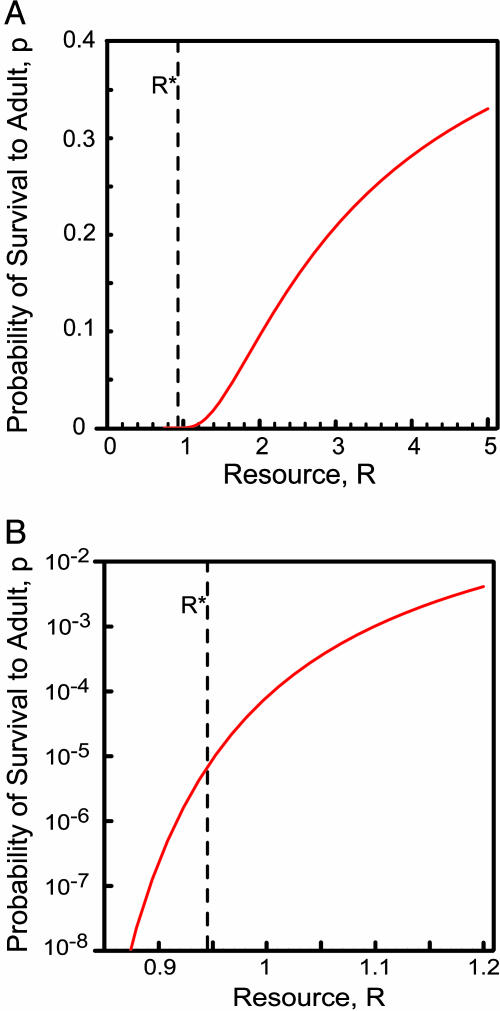
This equation shows that p very strongly depends on R*e (Fig. 1). It is extremely unlikely that a rare invader would successfully establish if the invader had an R* similar to or greater than that of an established species (Fig. 1B). For this example, the chance of invader survival to adult is <10–5 when an invader enters a habitat in which the established plant had reduced the resource down to the R* of the invader. The invader's probability of survival to adult increases by three orders of magnitude, to 10–2, if the invader enters a habitat with just 20% more resource than its R*. This low predicted survival rate for a species invading a habitat with the resource at its own R* is consistent with the observation that many plant species produce 104 to 106 seeds per year, live for many years, and yet, on average, produce only one reproductively successful offspring per adult.
Fig. 1.
Resource-dependent survival. (A) Effect of ambient resource levels on the probability (p; red curve) that a propagule would survive to become a reproductive adult (i.e., probability of invasion) based on Eq. 4. R* is the level to which an equilibrial population of the invading species would reduce the resource. (B) The same curve is shown, except that p is on a log scale, showing the large impacts on p of slight differences in R. Parameters were set as follows: mp = 0.2 yr–1, ms = 0.4 yr–1, Bs = 0.1, Ba = 10, Ki = 3, Si = 1.0 yr–1, and ri = 5.0 yr–1.
This article addresses stochastic competitive community assembly for a simple case for which classical analytical tradeoff theory predicts unlimited diversity (25, 26). This case assumes that (i) species compete for a single resource, (ii) maximal growth rates are a Gaussian function of temperature, (iii) species are identical except in optimal temperatures, and (iv) the habitat has spatial heterogeneity in temperature. The heterogeneous habitat can be visualized as a continuum of site temperatures that might occur, for instance, from low to high elevations or from the north-facing to the south-facing slopes. The theory directly applies to factors other than temperature, such as substrate redox potential or soil pH. In addition, this simple model provides a caricature of other interspecific tradeoffs for which each point on a tradeoff surface represents a viable species, such as competitive tradeoffs for two or more limiting resources.
For habitats with temperature heterogeneity, the vegetative growth rate of an invading individual in a particular habitat is still determined by Eq. 2, except that gi in Eq. 2 (and in all other equations) would be a Gaussian function of temperature:
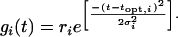 |
[5] |
Here, t is temperature; topt,i is the temperature at which species i grows optimally; ri is the maximal rate of biomass growth (units of dB/Bdt); and σi is the standard deviation of this Gaussian function (Fig. 2A).
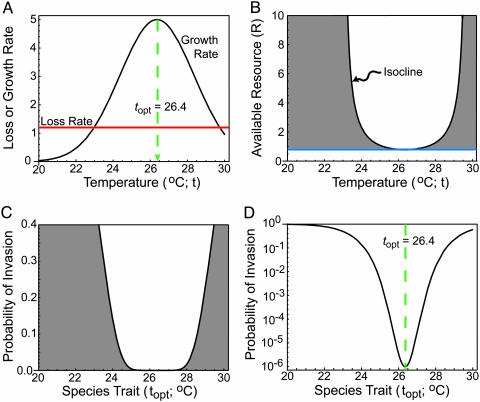
Fig. 2.
Gaussian growth, resource use, and invasion success. (A) Temperature-dependence of growth rate, g, based on Eq. 5. (B) The temperature- and resource-dependent zero net-growth isocline (black curve) of the species of A. The isocline shows the level to which R is reduced by an equilibrial population of this species. The shaded region shows resource levels available to potential invaders. (C) Dependence of probability of invasion (probability of propagule survival to adult) on the topt of an invader for the case illustrated in B. This function assumes that a propagule has landed in a site with its optimum temperature and that the established species is at equilibrium. (D) The same curve as in C but shown on a log scale. For these cases and those of Fig. 3, mp = 0.2 yr–1, ms = 0.33 yr–1, Bs = 0.05, Ba = 50, Ki = 2.5, Si = 1.0 yr–1, ri = 5.0 yr–1, σi = 2°C, and f = 105. Species differ only in topt values.
Once established, a species would reduce the resource to its temperature-dependent R* as specified by Eqs. 3 and 5. This isocline (Fig. 2B) shows resource levels that an invader would experience. Note that this species (and all other species) can only survive with resource levels on or above the horizontal line, which is the interspecific tradeoff curve. The shaded region above this line shows the resources available to invaders. These levels determine (by means of Eq. 4 with gi as defined by Eq. 5) the survival probabilities of invaders. For instance, the Gaussian growth curve of Fig. 2 A leads to the temperature-dependent resource isocline of Fig. 2B, which leads to probabilities of invasion shown in Fig. 2 C and D. These invasion curves show the probability that a species with a given topt would invade a site with a temperature equal to its topt in the presence of the established species. This model shows a “best case scenario,” because most invading propagules would colonize sites with a temperature other than their topt (which is the case modeled below). In contrast, established species reach greatest abundance and thus deposit most propagules in sites at or near their topt (also as modeled below).
Established species strongly inhibit invasion of species similar to them. Each established species decreases the likelihood of invader establishment over a rather broad range of invader topt values, creating a stochastic “limit” to similarity. This limit occurs because the Gaussian growth curve (Fig. 2 A) is flattened when inverted to give the resource-dependent zero net-growth isocline (Fig. 2B), which is even more flattened to give the invasion probability curve (Fig. 2C). The magnitude of this effect is illustrated by the log of the probability of invader establishment (Fig. 2D).
Stochastic Individual-Based Community Assembly
To determine how resource competition, spatial heterogeneity, and stochastic resource-dependent establishment interact to determine community properties, I performed simulations of community assembly. Species abundances and resource levels were determined by Eqs. 1–5. Simulations incorporated several stochastic elements. First, the topt of each potential invader was chosen by random draw from a normal or uniform distribution. Second, the temperature of the habitat invaded by a propagule was randomly drawn from the probability distribution (normal or uniform) of all possible habitat types. Third, the resource- and temperature-dependent survival probability and reproduction of each invader were used, by means of stochastic demographic simulation, to determine whether it either went extinct or survived and whether its offspring survived, etc.
Simulations of community assembly, performed in mathematica, proceeded as follows. A propagule and the habitat in which it landed were independently and randomly chosen. The survival probability, p, of the propagule was determined by using Eqs. 4 and 5, with Re* being the resource level imposed by the then-established species that was the best competitor at the temperature of the habitat into which the invading individual had fallen. This survival probability was compared to Q, a uniform random number on the {0, 1} interval. If p < Q, the invading individual survived to be reproductive; otherwise, the invading individual died and another propagule and habitat were chosen. If a propagule survived to become a reproductive adult, birth–death processes were followed for this adult and for all its progeny for 40 iterations to determine whether it went extinct or whether a viable population was established. Each adult had a fecundity of f seeds per year (f = 105 for simulations in this study). Each of its seed had a probability of p, as specified in Eqs. 4 and 5, of surviving to become an adult. If a viable population was established, the species was allowed to spread across the heterogeneous habitat and go to competitive equilibrium with all previously established species. Any established species whose equilibrium population density fell to less than one individual (out of a community normalized to contain 10,000 individuals for the total habitat) was considered extinct, and new equilibrial densities were determined. The resource levels imposed along the gradient by this new equilibrial community were the resource levels experienced by subsequent invading individuals until a new invader again survived to establish a viable population. This entire process was iterated for a fixed number of propagule invasions, usually 106 to 108.
Results
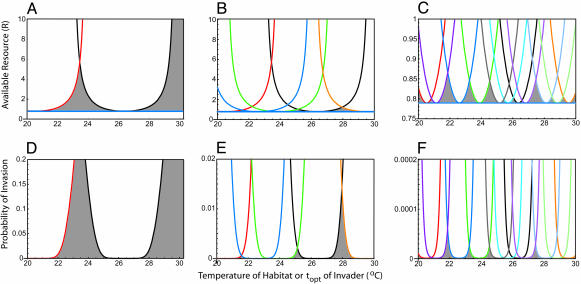
A Typical Case. A typical pattern of community assembly for a case in which there were uniform distributions of site temperatures and of propagule topt values is shown in Figs. 2 and 3. Because the habitat initially was replete with the limiting resource, species of any temperature optimum were equally likely to be the first successful colonist. For the simulation shown, the first successful invader had a temperature optimum of 26.4°C, giving the resource isocline (Fig. 2B) and resulting invasion probabilities (Fig. 2C) already discussed. After 51 invasion events, a second species (topt = 20.5°C) became established. These two species reduced resource levels (Fig. 3A), impacting invasion probabilities (Fig. 3D). After 7,600 invasion events, an additional three species had established (Fig. 3B). Their temperature optima (22.6, 23.9, and 29.6°C) fell in the two regions of high invisibility created by the first two species. The levels and pattern of resources left unconsumed by these five species (Fig. 3B) determined the probabilities of subsequent invasion (Fig. 3E). After 580,000 invasion events, six more species had become established. These 11 species created a landscape with sufficiently low and uniform resources (Fig. 3C) and invasion probabilities (Fig. 3F) that none of the subsequent 420,000 invaders became established. The greatest probability of further invasion is about 0.00005; this probability applies only once a species with a temperature optimum of about 21.9°C colonizes a site with a temperature close to its optimum.
Fig. 3.
Stochastic competitive assembly and resource use. An example of stochastic competitive assembly, based on simulation of Eqs. 1–5 for a case in which the temperature of a habitat and the topt of a propagule are both randomly drawn from uniform distributions on the temperature interval from 20°Cto30°C. Levels of available resources for the community after the second species becomes established (A, red isocline), after five species become established (B), and after 11 species become established (C; note the 10-fold change in scale for the y axis) in the community. Probabilities of subsequent invasion for the cases of A, B, and C, assuming that each species colonizes a site with a temperature equal to its topt. The two regions of high invasion probability for the two-species community (D) were the regions invaded by the next three species (E). The resources left unconsumed by the five-species community created zones with higher invasion probability (E; note 10-fold change in scale of y axis), which were the areas subsequently invaded (C and F; note 1,000-fold change in y axis for F).
Throughout the assembly process, each successful invader reduces resources in sites with conditions near its optimum, reducing the chance of invasion by species with similar traits (Fig. 2D). Each successful invader thus preempts habitats close to its optimum. Although this is not a fixed “limit to similarity,” the consequences are similar.
General Features of Stochastic Competitive Assembly. As shown by hundreds of additional simulations, communities created by stochastic competitive assembly have several general features. First, species number increases rapidly initially and then levels off (Fig. 4A). In particular, species number increases as the log of invasion events (Fig. 4A Inset) despite there being an unlimited pool of potential colonizing species. A habitat that contained 20 species after 10 years and 26 species after 100 years would, for instance, have 32 species after 1,000 years and only 44 species after 100,000 years.
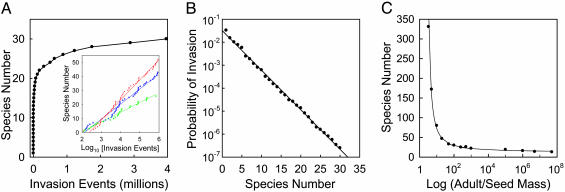
Fig. 4.
Diversity and invasion. (A) Dynamics of species accumulation during stochastic competitive assembly shown for a simulation with Gaussian distributions of both habitat temperatures (mean, 25°C; σ = 2°C) and propagule topt values (mean, 25°C; σ = 2°C). Parameters were otherwise identical to those of Figs. 2 and 3, except that σi = 0.4°C. (Insert) Shown are three additional simulations differing only in the extent of habitat heterogeneity (σ), each illustrating that species number increases as the log of invasion events. (B) Log of the probability of future invasion (proportion of propagules that become established) declines linearly with species number. Results are for the same simulation shown in A.(C) Species number after 106 invasion events for cases like those of A but with a range of values for Bs and Ba.
This dramatic slowing of invader success is caused by the lower and more uniform resource levels created as assembly proceeds. This pattern of resource reduction causes the probability of invader establishment to decline exponentially as diversity increases (Fig. 4B). For the case of Fig. 4A, for instance, the probability of establishment by an invading propagule would be about 10–7 once the assembled community contained 32 species and 10–8 once it contained 38 species. Thus, as diversity increases, the chance of successful recruitment (establishment of a new species) declines dramatically. This recruitment limitation occurs although all potential invaders have positive biomass growth rates and thus would “invade” if the classical, nonstochastic definition were used.
Another factor that might impact species number is the amount by which a seed must increase in mass to become a reproductive adult, i.e., the value of Ba relative to that of Bs (Eq. 4). The dependence of species number on Ba/Bs is surprisingly flat once Ba/Bs exceeds about 103 (Fig. 4C). At that point a 105-fold increase in the ratio led to only a 1.4-fold decrease in species number. Interestingly, once Ba/Bs is in the range observed for vascular plants, diversity is essentially independent of seed and adult sizes.
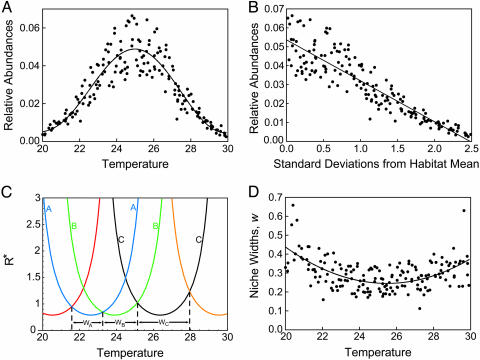
A second major feature of stochastic competitive community assembly is that contrary to neutral theory the relative abundances of species depend on their traits and on environmental conditions. The most abundant species, on average, are the best competitors for the most common conditions. For instance, Fig. 5A shows relative abundances of species for five replicate cases of community assembly with a Gaussian distribution of site temperatures (mean = 25°C, s = 3). The most abundant species were, on average, those with topt values of about 25°C. The relative abundances of species declined based on the number of standard deviations (σ for the Gaussian distribution of habitat temperatures) by which their topt differed from the mean temperature of the habitat (Fig. 5B).
Fig. 5.
Results of stochastic competitive assembly. (A) Results of stochastic competitive assembly for five replicate simulations in which there were Gaussian distributions of both habitat temperatures and propagule topt values. Parameters equal those reported for Fig. 4 A and B. The distribution of relative abundances of habitat temperatures (solid curve) closely mimics the observed relative species abundances (points). (B) Relative abundance data of A graphed against the number of standard deviations of habitat heterogeneity (σ = 2°C) by which optimum temperatures of species differ from habitat mean temperature. The most abundant species are those with topt values corresponding to the most common habitat types. (C) Niche width is defined as the range of temperatures over which a species is the competitive dominant one. (D) Observed niche widths for the five simulations of A and B.
This seemingly intuitive result holds a deeper insight. The correspondence between species traits and abundances occurs because species capture roughly equally wide “slices” of environmental conditions, as illustrated by their “niche widths.” The best competitors for the most frequent conditions are highly abundant precisely because they are able to prevent establishment by species similar to themselves. Niche width is defined here as the range of temperatures through which a given species is competitively dominant. A species is competitively dominant for those temperatures for which it has the lowest R*. For example, species A, B, and C have niche widths of wA, wB, and wC (Fig. 5C).
Stochastic competitive assembly causes species to have niche widths that are roughly equal. In contrast, niche widths would be more variable if resources were partitioned randomly among species. We can compare niche widths for stochastic competitive communities (Fig. 5D) with niche widths for communities composed of n species with topt values randomly located along a temperature gradient of length L. Clearly, the average niche width for each species would be L/n for both randomly chosen communities and stochastic competitive communities. The critical issue is the variation in niche widths. For a randomly chosen community, the variance in niche widths, σ2, is
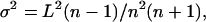 |
[6] |
which is derived in Lehman and Tilman (26) for the broken stick model and modified here to have a length of L rather than 1 and to have n represent species number rather than the number of “breaks.” I performed a paired t test comparing the standard deviation of niche widths between each stochastic competitive community and a randomly chosen community with identical species number. These standard deviations were highly significantly different (paired t test; n = 5, t = 23.8, P < 0.0001), with the niche widths of randomly chosen communities being 3.6× more variable, as measured by standard deviations, than of stochastic competitively assembled communities. Similar analyses, performed on the results of stochastic competitive assembly for 43 other cases spanning a large variety of conditions yielded similar results (paired t test; n = 43, t = 5.36, P < 0.001). Thus, stochastic competitive assembly creates communities with relatively uniformly spaced species. Expressed another way, stochastic competitive assembly imposes a limit, albeit a stochastic limit, to similarity (31). Niche widths are, of course, somewhat greater for the successful invaders of rarer habitats (Fig. 5C), but this trend in niche widths is small relative to the trend in habitat frequencies (Fig. 5A).
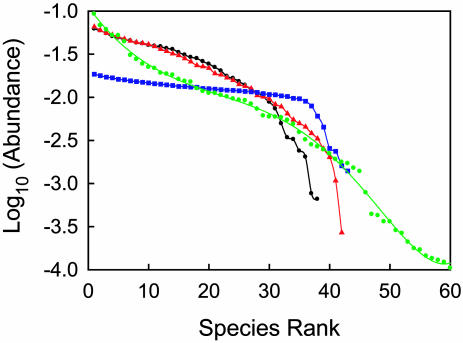
Stochastic competitive community assembly determines the distributions of species relative abundances (Fig. 6). The shapes of these curves are predicted to result mainly from the interplay of the approximately equally wide slices of habitat held by each species and the shape of the probability distribution of habitat types but also to depend on the distribution of traits of potential colonists. The curve predicted for habitats with a uniform distribution of both habitat types and species traits (Fig. 6, blue curve) is much flatter (many equally abundant species) than observed in nature, whereas cases with Gaussian habitat heterogeneity and/or Gaussian distributions of traits give curves that better mimic observed patterns.
Fig. 6.
Stochastic competitive assembly and species abundances. Relative species abundance curves (average of five simulations, 107 invasion events each) for four cases: Uniform distributions of both habitat temperature and topt values (blue; parameters equal those reported for Figs. 2 and 3, except that σi = 0.4°C); Gaussian habitat and uniform topt values (red; parameters equal those of the blue curve, but with habitat mean = 25°C and σ = 2°C); Gaussian habitat and Gaussian topt values [black (case shown in Figs. 4 and 5) and green (parameters equal those reported for Figs. 4 and 5, except that σi = 0.09°C with a habitat mean = 28.5°C and its σ = 2°C and a propagule topt mean = 21.5°C and its σ = 2.0°C)].
Discussion
Stochastic niche theory has its conceptual roots in the classic theory of niche overlap (27–30), but it incorporates three important mechanisms that are absent in classic niche overlap theory. First, it incorporates the impacts of demographic stochasticity on rare invaders (31). Second, stochastic niche theory explicitly considers the mechanisms of competition. Third, it addresses the constraint that a potential invader must be able to survive, grow, and reproduce by using the resources left unconsumed by established species. As the analyses presented here show, it is precisely because new species enter a community as rare propagules that must survive and grow on resources left unconsumed by existing species that demographic stochasticity interacts with resource competition to have profound effects on community structure. Stochastic competitive assembly causes local diversity to be limited not by competitive interactions among established species but by inhibitory effects of established species on recruitment. If this recruitment limitation were overcome, such as by addition of large numbers of propagules of novel species, many more species are predicted to indefinitely coexist locally than ever would occur with natural assembly.
To determine how this prediction of stochastic niche theory and the others presented in this paper might depend on the methods used to simulate stochasticity, a limited number of simulations were also performed by using a spatially explicit stochastic model in which the dispersal, growth, resource use, reproduction, and mortality of each individual was followed separately. These simulations provided qualitative support for the results presented in Figs. 3, 4, 5, 6, but additional work will be needed to elucidate other effects that might arise in fully stochastic multispecies dynamics.
Several lines of field evidence support the assumptions and predictions of stochastic niche theory. First, it has frequently been observed that there is a strong positive correlation between the number of propagules released into an environment and establishment success. This correlation occurs, for example, for both introduced passerine birds and introduced biological control agents (32, 33). Second, if sufficient amounts of seed from species enter a site to allow them to overcome recruitment limitation, many native or naturalized plant species can become established and persist in sites from which they were absent. This finding suggests that the occurrence of these plant species is recruitment-limited and that their traits fall along a tradeoff surface similar to that of the established species. For instance, a one-time addition of seed from many plant species led to a long-term near-doubling of local diversity in native, high-diversity grassland, with no evidence of competitive displacement (34). A review of ≈50-seed addition experiments showed that about a fourth of the species added as seed to communities became established adults (35). Also, introductions of exotic plant species to oceanic islands have caused few plant extinctions. Rather, exotic and native plants have persisted, causing total plant diversity on many oceanic islands to double (36). Similarly, exotic plant species introduced to California have increased its flora by ≈20% (36).
Stochastic competitive community assembly illustrates that “recruitment limitation” and “biotic limitation of diversity” are interacting and interdependent processes. The stochasticity of the colonization process causes different localities to differ in their species compositions. As local diversity increases during community assembly, the probability of local recruitment by additional species decreases. This inverse relationship results in many regional species that are capable of persisting in a locality being absent because of their low probability of establishment from propagules once species similar to themselves are established in a site. Furthermore, stochastic niche theory predicts that periodic disturbance, resource pulses, and other processes that allow species to overcome resource-dependent recruitment limitation would increase local species diversity (37–39).
Unlike neutral models, for which random walks to extinction are ultimately unavoidable because there are no stabilizing forces (11), stochastic but niche-tradeoff-based interactions have several features that lead to long-term persistence of species once established. First, the additional simulations done with the spatially explicit and fully stochastic version of this model show that species that have sufficient resources to become established also often attain sufficiently high equilibrial densities once established that they rarely suffer extinction from drift. Moreover, interspecific tradeoffs mean that all species have stable equilibria. The more that demographic stochasticity might reduce a population below its equilibrial density, the greater would be its per capita population growth rate and thus its rate of recovery. This density-dependent stabilization in combination with relatively high adult densities, relatively low adult mortality, and high seed production makes resource-dependent stochasticity during invasion the major step limiting local diversity.
Stochastic niche theory predicts, as do many other models (e.g., refs. 40–42), that, all else being equal, greater local diversity should lead to lower invasion rates. This prediction is supported by some (43–45) but not all (46) field studies, perhaps because in nature all else is rarely equal. Because the species that theory predicts should recruit are those that exploit patterns of underutilized resources, a more unique prediction of stochastic niche theory is that community invasibility depends on the degree to which established species exploit habitat heterogeneity in limiting resources.
This interpretation offers a potential resolution to the invasion paradox (47), i.e., to the negative correlation between diversity and invasion in both experiments and local comparisons but the positive correlation between diversity and invasion in regional comparisons (48, but see 49). Small nearby plots are likely to have similar within-plot spatial heterogeneity. Differences in their diversity would thus correspond to differences in the degree to which the existing species exploited that heterogeneity. On much larger scales, the regional species pool should be sufficient to fully exploit the heterogeneity of areas with low spatial heterogeneity, but it would be insufficient to do so in areas with the greatest spatial heterogeneity. This insufficiency would cause the most diverse regions to be the most readily invaded because their greater heterogeneity would be the least fully exploited. This possibility, which was supported by simulations of invasions into communities of differing spatial heterogeneities created by stochastic competitive assembly but with a restricted regional species pool, is thus consistent with the suggestion that the same processes that allow high diversity may also allow high rates of invasion (46).
A test of stochastic niche theory is provided by a study in which the seed of 27 grassland species was added to each of 147 plots in a biodiversity experiment. The total biomass attained after 3 yr by the added species declined >10-fold, and the number of added species that became established declined >2-fold along the experimental diversity gradient (50). The lower biomass and species number of invaders at higher diversity corresponded with the lower levels of soil nitrate at higher diversity. Perhaps the most interesting result was the pattern of inhibition of invaders by established species. All resident and invading species were classified as being in one of four functional groups. For all four possible comparisons, the strongest inhibitory effect of each established functional group was on invaders in that same functional group (50). This result supports the prediction of stochastic niche theory that established species should more strongly inhibit invaders that are more similar to themselves.
Hubbell (11) expressed concern about the classic broken stick model of niche preemption because it made the unjustified assumption that each successive species in a competitive hierarchy would obtain the same constant fraction of the remaining resources. This assumption, he said, “remains unexplained by any biological mechanism, and borders on the mystical.” Unlike the broken stick model, stochastic niche theory makes no a priori assumption about the fraction of resources that are captured by a species. Rather, this fraction is the result of two factors: the relatively constant niche widths generated by stochastic competitive assembly and the frequency distribution of habitat conditions (i.e., Fig. 5A, solid curve). For stochastic niche theory, the abundance of a species is, in essence, proportional to the niche width of the species times the height of its slice. This height is the proportion of the habitat that has the environmental conditions that the species requires. Because niche widths, although variable, are approximately equal, the more abundant species of a habitat are, on average, those that are better competitors for the more frequent conditions of the habitat. Rarer species are better competitors for less frequent environmental conditions (Fig. 5 A and B). This competitive process generates patterns of relative species abundances that can be qualitatively similar to those of the broken stick model. However, stochastic niche theory generates these without any a priori assumption of sequential resource partitioning.
Neutral theory predicts patterns of relative species abundances that closely match those often observed in nature (11, 17–19), but neutral theory also predicts that there should be no relationship between species traits and their abundances. Rather, abundances are predicted to result solely from structured random walks (11), leaving unexplained the correlations between species traits and their abundances within habitats and along environmental gradients (e.g., see refs. 4, 5, and 21). However, observations and experiments in a wide variety of communities have shown that species abundances change in a continuous manner along gradients in environmental factors, such as soil nutrients and moisture (4, 5, 22–24); that abundances are related to species traits (4, 5, 22–25); and that widely separated communities that experience similar environmental conditions converge on similar compositions (15). The importance of species traits is shown also by the global convergent evolution of plant form and functioning in each biome type. The prediction of stochastic niche theory is thus consistent with the frequent observation that the dominant species of a habitat have morphological, physiological, and/or behavioral traits that make them the superior competitors for the most common conditions in a habitat (5, 21) and that species abundances change along environmental gradients in ways that are consistent with their traits. Neither pattern is predicted by neutral theory.
It has been suggested that resource competition theory might be inherently incapable of providing a general theory of relative species abundance patterns (11). This is a valid concern for classic resource competition theory because it provides no simple limit to diversity; relative species abundances do not converge but rather merely reflect the traits of the species living in or invading into a habitat. In contrast, the limit to similarity that arises in stochastic niche theory causes convergence on a particular pattern of relative species abundances that is mainly determined by environmental heterogeneity and the niche widths of species. Because neutral theory and stochastic niche theory can predict essentially identical relative species abundance curves, each having a different underlying cause, the shapes of such curves may not be particularly insightful (51). The difference between the two theories is that stochastic niche theory also predicts that species traits and abundances should be correlated with environmental conditions, whereas neutral theory predicts that they should not be correlated in this manner. Given these predictions, it would seem more fruitful to test for relationships between species traits and abundances both within habitats and along gradients rather than to test for the goodness of fit of relative abundance patterns.
In total, although interspecific competition for resources has long been recognized as an important mechanism structuring communities, stochastic niche theory suggests that many additional insights arise once demographic stochasticity and its impacts on invasion and establishment are considered, which suggests that stochastic niche theory might be fruitfully broadened to consider other tradeoff-based mechanisms of competition, mutualism, disease, and multitrophic-level interactions.
Acknowledgments
I thank Janneke Hille Ris Lambers, Joseph Fargione, Chris Clark, Ray Dybzinski, Ross Noreen, Daniel Klein, Helene Muller-Laundau, Simon Levin, Jay Stachowicz, and Jonathan Levine for comments. This study was supported in part by National Science Foundation Grant DEB 0080382.
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 30, 2002.
See accompanying Biography on page 10851.
References
- 1.Hastings, A. (1980) Theor. Popul. Biol. 18, 363–373. [DOI] [PubMed] [Google Scholar]
- 2.Levins, R. (1979) Am. Nat. 114, 765–783. [Google Scholar]
- 3.Tilman, D. (1976) Science 192, 463–465. [DOI] [PubMed] [Google Scholar]
- 4.Tilman, D. (1982) Resource Competition and Community Structure, Monographs in Population Biology (Princeton Univ. Press, Princeton). [PubMed]
- 5.Tilman, D. (1988) Plant Strategies and the Dynamics and Structure of Plant Communities (Princeton Univ. Press, Princeton).
- 6.Tilman, D. (1994) Ecology 75, 2–16. [Google Scholar]
- 7.Chesson, P. L. (1986) in Community Ecology, eds. Diamond, J. & Case, T. J. (Harper & Row, New York) pp. 240–256.
- 8.Chesson, P. (2000) Annu. Rev. Ecol. Syst. 31, 343–366. [Google Scholar]
- 9.Huisman, J. & Weissing, F. J. (1999) Nature 402, 407–410. [Google Scholar]
- 10.Rees, M., Condit, R., Crawley, M., Pacala, S. & Tilman, D. (2001) Science 293, 650–655. [DOI] [PubMed] [Google Scholar]
- 11.Hubbell, S. P. (2001) The Unified Neutral Theory of Biodiversity and Biogeography, Monographs in Population Biology (Princeton Univ. Press, Princeton).
- 12.Bell, G. (2000) Am. Nat. 155, 606–617. [DOI] [PubMed] [Google Scholar]
- 13.Bell, G. (2001) Science 293, 2413–2418. [DOI] [PubMed] [Google Scholar]
- 14.Brokaw, N. & Busing, R. T. (2000) Trends Ecol. Evol. 15, 183–188. [DOI] [PubMed] [Google Scholar]
- 15.Clark, J. S. & McLachlan, J. S. (2003) Nature 423, 635–638. [DOI] [PubMed] [Google Scholar]
- 16.Harte, J. (2003) Nature 424, 1006–1007. [DOI] [PubMed] [Google Scholar]
- 17.Magurran, A. E. & Henderson, P. A. (2003) Nature 422, 714–718. [DOI] [PubMed] [Google Scholar]
- 18.McGill, B. J. (2003) Nature 422, 881–885. [DOI] [PubMed] [Google Scholar]
- 19.Volkov, I., Banavar, J. R., Hubbell, S. P. & Maritan, A. (2003) Nature 424, 1035–1037. [DOI] [PubMed] [Google Scholar]
- 20.Whitfield, J. (2002) Nature 417, 480–481. [DOI] [PubMed] [Google Scholar]
- 21.Chabot, B. F. & Mooney, H. A., eds. (1985) Physiological Ecology of North American Plant Communities (Chapman & Hall, London).
- 22.Beadle, N.C.W. (1966) Ecology 47, 992–1007. [Google Scholar]
- 23.Beard, J. S. (1955) Ecology 36, 89–100. [Google Scholar]
- 24.Beard, J. S. (1967) J. Ecol. 55, 271–290. [Google Scholar]
- 25.Tilman, D. (1999) Ecology 80, 1455–1474. [Google Scholar]
- 26.Lehman, C. L. & Tilman, D. (2000) Am. Nat. 156, 534–552. [DOI] [PubMed] [Google Scholar]
- 27.MacArthur, R. H. (1970) Theor. Popul. Biol. 1, 1–11. [DOI] [PubMed] [Google Scholar]
- 28.MacArthur, R. H. (1972) Geographical Ecology: Patterns in the Distribution of Species (Harper & Row, New York).
- 29.May, R. M. & MacArthur, R. H. (1972) Proc. Natl. Acad. Sci. USA 69, 1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turelli, M. (1981) Theor. Popul. Biol. 20, 1–56. [Google Scholar]
- 31.Hurtt, G. C. & Pacala, S. W. (1995) J. Theor. Biol. 176, 1–12. [Google Scholar]
- 32.Hopper, K. R. & Roush, R. T. (1993) Ecol. Entomol. 18, 321–331. [Google Scholar]
- 33.Duncan, R. P. (1997) Am. Nat. 149, 903–915. [DOI] [PubMed] [Google Scholar]
- 34.Tilman, D. (1997) Ecology 78, 81–92. [Google Scholar]
- 35.Turnbull, L. A., Crawley, M. J. & Rees, M. (2000) Oikos 88, 225–238. [Google Scholar]
- 36.Sax, D. F., Gaines, S. D. & Brown, J. H. (2002) Am. Nat. 160, 766–783. [DOI] [PubMed] [Google Scholar]
- 37.Huenneke, L. F., Hamburg, S. P., Koide, R., Mooney, H. A. & Vitousek, P. M. (1990) Ecology 71, 478–491. [Google Scholar]
- 38.Burke, M. J. W. & Grime, J. P. (1996) Ecology 77, 776–790. [Google Scholar]
- 39.Davis, M. A., Thompson, K. & Grime, J. P. (2001) Diversity and Distributions 7, 97–102. [Google Scholar]
- 40.Post, W. M. & Pimm, S. L. (1983) Math. Biosci. 64, 169–192. [Google Scholar]
- 41.Case, T. J. (1990) Proc. Natl. Acad. Sci. USA 87, 9610–9614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Law, R. & Morton, R. D. (1996) Ecology 77, 762–775. [Google Scholar]
- 43.Naeem, S., Knops, J. M. H., Tilman, D., Howe, K. M., Kennedy, T. & Gale, S. (2000) Oikos 91, 97–108. [Google Scholar]
- 44.Knops, J. M. H., Tilman, D., Haddad, N. M., Naeem, S., Mitchell, C. E., Haarstad, J., Ritchie, M. E., Howe, K. M., Reich, P. B., Siemann, E. & Groth, J. (1999) Ecol. Lett. 2, 286–293. [DOI] [PubMed] [Google Scholar]
- 45.Kennedy, T. A., Naeem, S., Howe, K. M., Knops, J. M. H., Tilman, D. & Reich, P. (2002) Nature 417, 636–638. [DOI] [PubMed] [Google Scholar]
- 46.Levine, J. M. & D'Antonio, C. M. (1999) Oikos 87, 15–26. [Google Scholar]
- 47.Renne, I. J. & Tracy, B. F. (2003) Front. Ecol. Environ. 1, 122. [Google Scholar]
- 48.Stohlgren, T. J., Barnett, D. T. & Kartesz, J. T. (2003) Front. Ecol. Environ. 1, 11–14. [Google Scholar]
- 49.Rejmanek, M. (2003) Front. Ecol. Environ. 1, 122–123. [Google Scholar]
- 50.Fargione, J., Brown, C. S. & Tilman, D. (2003) Proc. Natl. Acad. Sci. USA 100, 8916–8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chave, J., Muller-Landau, H. C. & Levin, S. A. (2002) Am. Nat. 159, 1–23. [DOI] [PubMed] [Google Scholar]