Abstract
Increased longevity, expressed as number of individuals surviving to older adulthood, represents one of the ways the human life history pattern differs from other primates. We believe it is a critical demographic factor in the development of human culture. Here, we examine when changes in longevity occurred by assessing the ratio of older to younger adults in four hominid dental samples from successive time periods, and by determining the significance of differences in these ratios. Younger and older adult status is assessed by wear seriation of each sample. Whereas there is significant increased longevity between all groups, indicating a trend of increased adult survivorship over the course of human evolution, there is a dramatic increase in longevity in the modern humans of the Early Upper Paleolithic. We believe that this great increase contributed to population expansions and cultural innovations associated with modernity.
The human life history pattern differs from that observed in the great apes in its delayed maturation, slower growth, higher fertility, and increased longevity, which is associated with menopause in women (refs. 1–4, and see figure 1 in ref. 2 for mortality differences between recent hunter-gatherers and chimpanzees). These are evolutionary changes that have implications for the development of human culture. Longevity, in particular, may be necessary for the transgenerational accumulation and transfer of information that allows for complex kinship systems and other social networks that are uniquely human. It is also a focal point of the grandmother hypothesis, which posits that increased longevity is important in enhancing the inclusive fitness of grandmothers who, perhaps as early as the first Homo populations, invested in their reproductive-age daughters and their offspring (4–6).
Therefore, the details of how longevity increases over the course of human evolution are of great interest (7). Other aspects of the hominid life history pattern, in particular, rates of maturation and growth and development, have been the focus of intensive study in fossil hominids (see, for example, refs. 8–11 and references therein), but changes in longevity itself have not been empirically assessed. Longevity is usually discussed through its correlation with other variables, such as body size, encephalization, and growth and development patterns (12, 13). On the basis of these correlations, some have argued that increased longevity appears quite early in the evolution of Homo (5, 6). Observation of larger brain and body size implied prolonged maturation in Homo ergaster (11, 14, 15), leading some workers to suggest longer lifespan in this taxon. On the other hand, it has also been suggested that early hominids may not have lived long enough to become grandmothers, based on published ages from a few key sites (16). However, a large sample, amenable to meaningful statistical treatment of this issue, has not been analyzed. The actual pattern of change in adult survivorship critical to testing the reality of correlations between brain size and longevity in the human lineage, the predictions of the grandmother hypothesis, and any of the other questions surrounding the evolution of human longevity, has yet to be empirically established. Here, we address the basic issue of whether different hominid groups from successive time periods have different patterns of longevity, regardless of differences in brain size and maturation rates, and whether the fossil record supports the prediction of increased longevity in early Homo.
By longevity, we are not concerned with the maximum life span attainable by members of a species, but with the number of adults who live to be old. In our view, it is the number of individuals living to older adulthood that provides evidence of selection favoring the survivorship of older adults and is important for many of the evolutionary issues surrounding changes in longevity. To avoid the taphonomic and demographic problems involved with the inclusion of juveniles, we limited the present study to adults, examining changes in the ratio of older to younger adults (OY ratio) in the death distribution over time. Although this is not the OY ratio that would be expected in the living populations (17), it does reflect it, and its changes provide insight into the evolution of age structure in the human fossil record.
We tested the null hypothesis of no difference in the composition of older and younger adults among four hominid groups represented by 768 dental individuals, estimated conservatively so that no specimen could be counted more than once: later australopithecines (including specimens attributed to Australopithecus and Paranthropus), Early and Middle Pleistocene Homo, Neandertals from Europe and Western Asia, and post-Neandertal Early Upper Paleolithic Europeans (Table 1). The habilines were excluded from both the Homo and australopithecine samples because their taxonomic placement is uncertain. They may be more closely associated with australopithecines than with early Homo (18, 19), with whom they are penecontemporary, and the sample is too small to stand alone as a separate group. As discussed below, their inclusion in either category would not change results significantly. The four groups were chosen because they provide large samples amenable to dental wear seriation and categorical adult age assessment. Each broad group represents several different sites with different taphonomic histories, reducing the chance that the circumstances of one particular site will significantly bias the results. We chose specimens for whom we had reasonable confidence in both their categorical age assessment and the likelihood that they represented a unique individual. Significance of the differences in OY ratios between the groups was tested by using distributions generated by random resampling with replacement.
Table 1. Sample summary: Sample sizes and OY ratio for each group.
| Old | Young | Total | OY | |
|---|---|---|---|---|
| Australopithecines | 37 | 316 | 353 | 0.12 |
| Early Homo | 42 | 166 | 208 | 0.25 |
| Neandertals | 37 | 96 | 113 | 0.39 |
| Early Upper Paleolithic | 50 | 24 | 74 | 2.08 |
| All groups combined | 166 | 602 | 768 | 0.28 |
Our sample derives from our own age estimates and age estimates provided by M. H. Wolpoff and D. W. Frayer.
Dental age estimates, based on wear seriation, were used to place specimens into categories of older or younger adults. These estimates should not be thought of as absolute numbers, but as ranges within which the actual age probably lies, with resolution depending on condition and age of the specimen, knowledge of the population from which it comes, a number of assumptions about eruption schedule, and, to some extent, the constancy of wear rates between populations and tooth classes (20–24). Wear-based seriation was pioneered by Miles (25) and described and successfully used by many workers (26–30). By using this method, rates of wear are estimated by observing the degree of molar wear at the time of occlusal eruption of subsequent molars on immature specimens and these rates are then extrapolated to older individuals (Fig. 1). Because our sample is categorical (older or younger adult) and not composed of the age estimates themselves, we do not require that the ages have a high level of resolution. Although determination of the categories is not always unambiguous, the procedures we used minimize error.
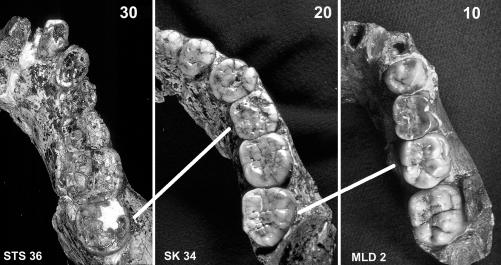
Fig. 1.
Three australopithecine dentitions of different ages shown to the same approximate size: MLD 2 (Right), with a deciduous molar, permanent M1, and unerupted M2; SK 34 (Center), a younger adult; and STS 36 (Left), an older adult. We use these dentitions to illustrate how specimens were placed into younger and older adult categories. M3 eruption was considered to indicate the age of reproductive maturation, and older adults were defined as twice the age of reproductive maturation, the age at which one could theoretically first become a grandparent. As shown, the wear on the M3 of SK 34 is comparable with that on the M1 of MLD 2, indicating ≈5 years of wear, by using the human model discussed in the text. This finding would indicate an age of ≈20 years (15 plus 5), within our younger adult category. Also as indicated, the M3 of STS 36 exhibits more wear than the M1 of SK 34 (i.e., >14 years), indicating a probable age of >30 years, within our older-adult category. The use of different eruption schedules produces the same categorical assessment. If the australopithecine molars in this illustration erupted at 3, 7, and 11 years of age for M1, -2, and -3, respectively, to use one chimpanzee model, the wear would represent less time (≈3 years of wear on the M1 of MLD2), but adulthood and older adulthood would begin at ages 11 and 22, respectively. SK 34 would remain in the young adult category (11 plus 3), and STS 36 would remain in the older adult category (>11 years of wear on the M3). See text for further details.
Individuals with M3s in occlusal eruption were considered adult, and individuals double that age were considered older adult. As discussed below, although we estimated ages based on a single eruption schedule, these ages were used to allocate specimens to life history categories that are themselves independent of actual ages. Regardless of chronological age, M3 eruption marks the end of the juvenile period and is correlated with physiological maturation, including reproductive maturation, in primates (31, 32). However, it was convenient for purposes of comparison and necessary for objective and repeatable assessment of when individuals were double the age of M3 eruption, to estimate an age at death. In making these assessments, we accept the rationale for M3 eruption at age 15 (in contrast to a later age in recent humans), based on observations of minimal M2 wear at the time of M3 occlusal eruption in Neandertal and older fossil populations (19, 30, 33). Although 15 years is younger than the mean age at first birth reported for women in many modern populations, including the Aché and the!Kung, it is close to that reported for Inuit and some Australian populations and is within the range of human population means (3). Therefore, it is not unreasonable to think of 15 years of age as representative of dental and reproductive maturation in fossil and some recent humans. Older adults were defined as twice the age of reproductive maturation, the age at which one could theoretically first become a grandmother; i.e., 30 years if women had their first child at the age of 15.
These age estimates use one of the fastest eruption schedules observed in human populations (34), transformed from gingival to occlusal eruption after Wolpoff (30). Our rationale for this estimate derives from evidence that eruption schedules are, in part, influenced by the rate of exfoliation of deciduous teeth (34), which in turn is related to deciduous tooth wear. Therefore, this indigenous Australian schedule may be appropriate for hunting/gathering groups with heavy masticatory stresses, as one would expect in Paleolithic groups (20, 35, 36). However, we slightly modified the schedule reported by Brown et al. (34) with respect to M3 eruption, as discussed above (19, 30).
Although, in constituting our categories, we used the same eruption schedule for all fossil groups, we recognize that the maturation rate for australopithecines and early Homo is controversial (8–11, 26, 37). However, our use of OY ratios circumvents this debate because the categories are independent of actual ages as long as hominid dental development is tied to physiological development, as it is in other primates (31). M3 eruption indicates adulthood, and the relative wear indicators of older adulthood apply regardless of actual age (Fig. 1). Therefore, for consistency and comparability we determined age estimates according to the eruption schedule discussed above, but we emphasize that the categories reflect relative wear determined through seriation and not the numerical ages attributed to them. They represent physiological age groups, and are therefore minimally affected by variation in maturation rates. This reason is why we chose a categorical approach.
Wear-based seriation was performed independently for different fossil groups, so the assumption of reasonably constant wear rates across populations was made only for samples with no subadults to seriate. Although there is evidence that molar wear rates across fossil populations are reasonably comparable (22, 30), this conclusion has not been established across earlier fossil species, especially those with specialized diets like the robust australopithecines. However, the dietary specializations and associated craniofacial architecture among australopithecines that could contribute to greater tooth wear are also associated with larger teeth and thicker enamel that reduce rates of wear, and there is no direct evidence that wear rates are faster in the more specialized australopithecines. In addition, although different molars (tooth classes) may have slightly different wear rates, Miles (21) suggests that this variation ultimately makes little difference to estimates of age.
The categorical treatment of ages circumvents much of the inaccuracy associated with dental age assessment. Our categories are unaffected by the most common inaccuracies associated with wear-based aging, methods that tend to overage modern humans over the age of 35 and underage very old individuals over the age of 50 (21). Our “older” category, age 30 and above, is an age at which age estimates are still quite accurate. Treating the data categorically also allowed us to maximize our data set by including categorical assessments (for example, as in the case of very old individuals, whose teeth are too worn for age assessment) as well as numerical ages as described above, depending on the condition of specimens and the source of the assessment.
We tested the null hypothesis of no difference in longevity between the fossil groups by comparing the number of older adults relative to that of younger adults in the samples of hominid dental remains. These OY ratios and sample sizes for each of the four groups are given in Table 1. OY ratios increase over time. The significance of the differences among OY ratios was assessed by establishing the probability of finding the observed OY ratio of one fossil group within distributions of OY ratios sampled from the earlier group. These distributions were generated through random resampling with replacement. The approach of data resampling provides a way to solve problems that lie outside the analytical boundaries of classical statistics (38), for example, by using ratios as the statistic of interest as in this study. Resampling addresses the problem of interdependence of data within the death distribution and the potential further dependence of the successive samples on earlier ones because of evolution.
Our null hypothesis, that there is no difference in OY ratios among the different hominid groups, can be stated as a question of probability: how likely is it to observe an OY ratio of a particular hominid group in an earlier group? We rejected the null hypothesis if a ratio the same or greater than the observed OY ratio was found in <5% of the distribution generated from the earlier group. Thus, for example, we tested the prediction that there is a significant increase in longevity with the emergence of Homo by assessing the probability of observing the Homo OY ratio in an australopithecine sample of the same size. We generated a distribution of ratios from the australopithecines, each ratio in the distribution representing a dental sample of the same size as the observed early Homo group. We randomly drew 208 individuals (the number of early Homo in our data) with replacement from the combined australopithecine young and old adults, generating an OY ratio based on that run. This procedure was repeated 10,000 times, producing a distribution of ratios. The probability of drawing 0.25 (the observed Homo OY ratio) or greater from this distribution was then assessed. This same method was used to assess the significance of the difference in OY ratios between Neandertal and Early/Middle Pleistocene Homo samples and post-Neandertal Early Upper Paleolithic and Neandertal samples.
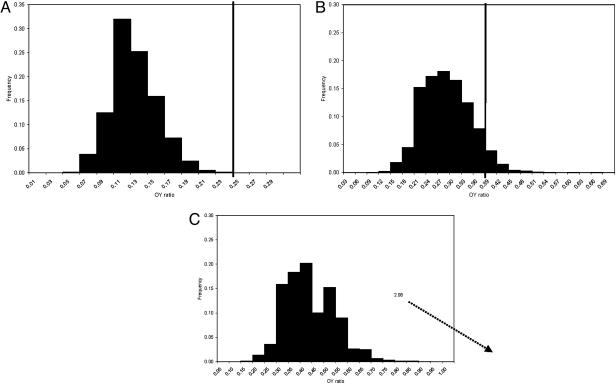
The differences among the ratios of all groups are significant at the P = 0.05 level, refuting the null hypothesis of no difference for each interval (Fig. 2). Fig. 2A shows the significance of the increase in longevity in early Homo compared with australopithecines. Distribution of OY ratios generated from 10,000 sampling runs, each with sample size of 208, ranged from 0.05 to 0.24, and the observed early Homo ratio (0.25) was never once reproduced. The inclusion of the habilines would not significantly change these results. Of 11 adult habilines for which we have wear data, all are in our younger adult category. Their inclusion in early Homo would change that OY ratio from 0.25 to 0.24; their inclusion in the australopithecines would change the australopithecine OY ratio from 0.12 to 0.11.
Fig. 2.
Probability of finding observed OY ratios for three hominid groups in distributions of OY ratios generated from hominids from an earlier time period by resampling. Each OY ratio is created by randomly drawing an adult sample of the same size as the test (later) group from the earlier one and calculating the OY ratio in it (sample sizes are shown in Table 1). This procedure was performed 10,000 times in each case, and the generated samples describe the probability of observing the OY ratio from the test (later) sample in the earlier one. For example, each one of the 10,000 OY ratios in A is a random sample of 208 australopithecine adults, because the actual Homo sample size is 208. (A) The observed early Homo OY ratio (vertical bar at 0.25) compared with ratios generated from the australopithecine sample. The observed Homo ratio was never reproduced in 10,000 OY ratios in draws of 208 generated from the australopithecine sample. (B) Observed Neandertal OY ratio (0.39) compared with ratios generated from the Early and Middle Pleistocene Homo sample in 10,000 draws of 133. OY ratios of 0.39 and larger occurred in 2.26% of the generated distribution. (C) The observed Early Upper Paleolithic OY ratio (2.08) compared with ratios generated from the Neandertal sample in 10,000 draws of 74. The observed ratio is far outside this distribution, representing a major increase in the number of adults over the age of 30 in the death distribution
The probability of drawing the Neandertal ratio (0.39) out of the Early/Middle Pleistocene Homo distribution is quite low (Fig. 2B). The 10,000 generated OY ratios range from 0.07 to 0.64. The observed Neandertal OY ratio of 0.39 or greater was found in 2.26% of the generated ratio distribution, rejecting the null hypothesis of no difference.
The large increase in ratios associated with the Upper Paleolithic is most significant (Fig. 2C). The observed Upper Paleolithic ratio (2.08) lies well outside maximum possible OY ratio generated from the Neandertal sample. Because of the unexpected magnitude of this increase, we questioned whether the formulation of our age categories could have introduced bias that influenced the results. For this reason, we used an adulthood criterion of 15 years for the Early Upper Paleolithic as we did for the earlier samples. It is probable that Early Upper Paleolithic M3 eruption was closer to 16–18 years, as it is for some living hunter–gatherers, but the average age at first birth in these populations may have been earlier, as reported for Inuit and some Australian groups (3). Hence, although we may have erred by including some subadults in the Early Upper Paleolithic young adult sample, this approach is the most conservative one because it increases the size of the younger adult portion of the sample. Because the significant change is in the increased OY ratio, this approach would underestimate, rather than overestimate, this increase.
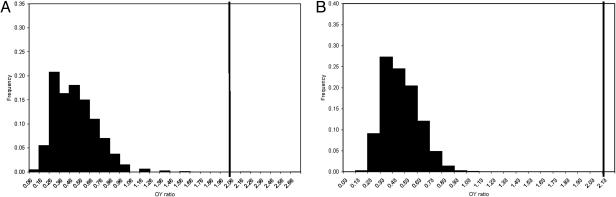
Our data do not directly address the causes of life history differences, the high representation of young adults in earlier samples, or the increased representation of older adults in the Upper Paleolithic. We expect that the data reflect variation in the causes of young adult mortality, among other factors; the systematic nature of the OY ratio changes and the many different site histories sampled make a purely taphonomic explanation unlikely. To further examine the possibility of taphonomic bias, we questioned whether mortuary practices, specifically an increased number of burials, are responsible for the magnitude of the relative increase of the number of older adults in the Upper Paleolithic. Table 2 shows the OY ratios based on burials and nonburials within the Upper Paleolithic sample. The Upper Paleolithic OY ratio based on nonburials (2.06) is slightly lower than the Upper Paleolithic OY ratio based on burials (2.13), but the difference is not significant. OY ratio distributions were generated from the Neandertal sample with sample size of 49 for the nonburials and 25 for the burials, and the probability of observing ratios of 2.06 and 2.13 (respectively) was assessed. Both cases rejected the null hypothesis (Fig. 3). Burials cannot account for the large increase in older individuals.
Table 2. OY ratios for burials and nonburials in the Early Upper Paleolithic.
| Old | Young | Total | OY | |
|---|---|---|---|---|
| Nonburials | 33 | 16 | 49 | 2.06 |
| Burials | 17 | 8 | 25 | 2.13 |
Fig. 3.
The effect of mortuary practices on the increase in Early Upper Paleolithic longevity that is shown in Fig. 2C. Although the OY ratio of Early Upper Paleolithic burials is slightly higher than that of nonburials, both are significantly larger than the largest OY ratios generated from the Neandertal sample. (A) The observed OY ratio of Early Upper Paleolithic nonburials (2.06), compared with the ratios generated from the Neandertal sample in 10,000 draws of 49. (B) The observed OY ratio of Early Upper Paleolithic burials (2.13), compared with the ratios generated from the Neandertal sample in 10,000 draws of 25.
Two important conclusions emerge from this study: first, there is significant increased longevity between all groups, indicating a trend of increased survivorship of older adults through human evolution. Second, the increase is by far the greatest in the early modern humans of the Upper Paleolithic, when for the first time there are a larger number of older adults than younger adults in the death distribution. Whereas high levels of young adult mortality have been noted for Neandertals (39), the magnitude of the increase in OY ratios in the Upper Paleolithic is nevertheless surprising.
The larger OY ratio in Homo relative to australopithecines weakly supports the predictions of the grandmother hypothesis as applied to Homo erectus (6). The OY ratio doubles in the Early/Middle Pleistocene Homo sample, but is still very low. In contrast, the increased longevity in the Upper Paleolithic is dramatically larger, with OY ratios five times greater than that seen in Middle Paleolithic hominids. Because the OY ratio increases so significantly at this time, we suggest that theories involving the evolutionary value of senescence may be most applicable to the Middle/Upper Paleolithic transition.
Although longevity may be under direct selection as suggested in some kin selection models (40), or may be linked to other traits under direct selection, it is not clear that the increase in adult survivorship reported here necessarily has a genetic basis. Long individual lifespans occur in nonhuman primates (41), not just in humans, and therefore increase in the number of individuals living to older age may not reflect a major genetic change affecting maximum lifespan. However, whether the result of cultural factors, other forms of relaxed selection affecting the mortality of young adults, and/or biological change, the increase in adult survivorship would have considerable evolutionary impact. If related to senescence, it reflects an adaptation that must help compensate for the disabilities and diseases of older age, when gene expressions uncommon in younger adults became more frequent. We believe that this adaptation involves the increased importance of transgenerational relationships that may be critical to the development and survival of social groups with large amounts of complex information to transmit. Increased adult survivorship strengthens those relationships and information transmission by extending the time over which people can learn from older individuals and by the increase in the number of older people, which promotes the acquisition and transmission of specialized knowledge such as that reflected in the Upper Paleolithic.
The potential demographic consequences of increased adult survivorship are significant. Our results are compatible with archeological and genetic evidence suggesting population expansions in the Upper Paleolithic that may be a consequence of population growth (42, 43). There is reason to think that increased longevity would have a direct affect on population growth. Not only does increased survivorship imply greater lifetime fertility for individuals, the investment of older individuals in their children's families may provide a selective advantage promoting further population increase. The large OY ratio we observe may therefore be a significant factor in the evolution of modernity not only through its importance for transgenerational information transfer but also because of its demographic impact. Recent models (43) suggest demographic factors are responsible for the cultural innovations associated with modernity. Population expansion may have provided social pressures that led to extensive trade networks, increased mobility, and more complex systems of cooperation and competition between groups, resulting in increased personal ornamentation and other material expressions of individual and group identity.
In summary, while the fossil record by its very nature reflects sampling biases and there is a great need to develop and refine techniques for aging older adults to address the evolution of senescence and to understand the changes presented here in more detail, these results suggest a major increase in adult survivorship in the Upper Paleolithic. We suggest that this increase in longevity addresses the meaning of modernity itself. Modernity is a complex concept, incorporating both biological and cultural variables, that has proven difficult, if not impossible, to define (44). However, if there is a single fundamental factor related to biology that underlies the behavioral innovations of modernity, this increase in adult survivorship may be it. We therefore think significant longevity came late in human evolution and was a fundamental demographic component tied to the population expansions and related behavioral innovations associated with modern humans.
Acknowledgments
We thank David W. Frayer (University of Kansas, Lawrence) and Milford H. Wolpoff (University of Michigan, Ann Arbor) for additional age estimates; Mark Flinn, Thomas Roèek, and three anonymous reviewers for advice and comments that significantly improved this manuscript; and the Anthropos Institute, Morvaské Muzeum (Brno, Czech Republic), Antropologické Oddĕlení, Národní Museum (Prague), Hrvatski Prirodoslovni Muzej (Zagreb, Croatia), Institute for Paleontology and Quaternary Geology, Croatian Academy of Sciences and Arts (Zagreb), Natural History Museum (London), Paleoanthropology Laboratory, Université de Bordeaux (France), Institute for Vertebrate Paleontology and Paleoanthropology (Beijing), Transvaal Museum (Pretoria, South Africa), and the Department of Anatomy, University of the Witwatersrand (Johannesburg) for permission to study the fossils in their care.
Abbreviation: OY ratio, ratio of older to younger adults.
See Commentary on page 10847.
References
- 1.Hill, K. & Kaplan, H. (1999) Annu. Rev. Anthropol. 28, 397–430. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan, H., Hill, K., Lancaster, J. & Hurtado, A. M. (2000) Evol. Anthropol. 9, 156–185. [Google Scholar]
- 3.Hill, K. & Hurtado, A. M. (1996) Ache Life History: The Ecology and Demography of a Foraging People (de Gruyter, New York).
- 4.Hawkes, K., O'Connell, J. F., Blurton Jones, N. G., Charnov, E. L. & Alvarez, H. P. (1998) Proc. Natl. Acad. Sci. USA 95, 1336–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawkes, K. (2003) Am. J. Hum. Biol. 15, 380–400. [DOI] [PubMed] [Google Scholar]
- 6.O'Connell, J. F., Hawkes, K. & Blurton Jones, N. G. (1999) J. Hum. Evol. 36, 461–485. [DOI] [PubMed] [Google Scholar]
- 7.Rogers, A. R. (2003) Proc. Natl. Acad. Sci. USA 100, 9114–9115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mann, A. E., Lampl, M. & Monge, J. (1990) Yearbook Phys. Anthropol. 33, 111–150. [Google Scholar]
- 9.Smith, B. H. & Tompkins, R. L. (1995) Annu. Rev. Anthropol 24, 257–279. [Google Scholar]
- 10.Dean, C. (2000) J. Anat. 197, 77–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean, C., Leakey, M. G., Reid, D., Schrenk, F., Schwartz, G. T., Stringer, C. & Walker, A. (2001) Nature 414, 628–631. [DOI] [PubMed] [Google Scholar]
- 12.Sacher, G. A. (1975) in Primate Functional Morphology and Evolution, ed. Tuttle, R. (Mouton, The Hague), pp. 417–441.
- 13.Hammer, M. and Foley, R. A. (1996) Hum. Evol. 11, 61–66. [Google Scholar]
- 14.Smith, B. H. (1993) in The Nariokotome Homo erectus Skeleton, eds. Walker, A. & Leakey, R. (Harvard Univ. Press, Cambridge, MA), pp. 195–220.
- 15.Clegg, M. & Aiello, L. (1999) Am. J. Phys. Anthropol. 110, 81–93. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy, G. E. (2003) J. R. Anthropol. Inst. 9, 549–572. [Google Scholar]
- 17.Deevey, E. S., Jr. (1947) Q. Rev. Biol. 22, 283–314. [DOI] [PubMed] [Google Scholar]
- 18.Wood, B. A. & Collard, M. (1999) Science 284, 65–71. [DOI] [PubMed] [Google Scholar]
- 19.Wolpoff, M. H. (1999) Paleoanthropology (McGraw–Hill, New York), 2nd Ed.
- 20.Molnar, S. (1971) Am. J. Phys. Anthropol. 34, 175–190. [DOI] [PubMed] [Google Scholar]
- 21.Miles, A. E. W. (2001) J. Archaeol. Sci. 28, 973–982. [Google Scholar]
- 22.Skinner, M. (1997) J. Archaeol. Sci. 24, 677–700. [Google Scholar]
- 23.Lucy, D., Pollard, A. M. & Roberts, C. A. (1995) J. Archaeol. Sci. 22, 417–428. [Google Scholar]
- 24.Mays, S. (2002) J. Archaeol. Sci. 29, 861–871. [Google Scholar]
- 25.Miles, A. E. W. (1963) in Dental Anthropology, ed. Brothwell, D. (Pergamon, Oxford), pp. 191–209.
- 26.Mann, A. E. (1975) Some Paleodemographic Aspects of the South African Australopithecines (Univ. of Pennsylvania Publications in Anthropology, Number 1, Philadelphia).
- 27.Lovejoy, C. O. (1985) Am. J. Phys. Anthropol. 68, 47–56. [DOI] [PubMed] [Google Scholar]
- 28.Lovejoy, C. O., Meindl, R. S., Mensforth, R. P. & Barton, T. J. (1985) Am. J. Phys. Anthropol. 68, 1–14. [DOI] [PubMed] [Google Scholar]
- 29.Nowell, G. W. (1978) Am. J. Phys. Anthropol. 49, 271–276. [DOI] [PubMed] [Google Scholar]
- 30.Wolpoff, M. H. (1979) Am. J. Phys. Anthropol. 50, 67–114. [DOI] [PubMed] [Google Scholar]
- 31.Kelley, J. & Smith, T. M. (2003) J. Hum. Evol. 44, 307–329. [DOI] [PubMed] [Google Scholar]
- 32.Schultz, A. H. (1960) in Human Growth, ed. Tanner, J. M. (Pergamon, Oxford), pp. 1–30.
- 33.Ramirez Rozzi, F. V. & Bermudez De Castro, J.-M. (2004) Nature 428, 936–939. [DOI] [PubMed] [Google Scholar]
- 34.Brown, T., Jenner, J. D., Barrett, M. J. & Lees, G. H. (1978) Occ. Pap. Hum. Biol. 1, 47–70. [Google Scholar]
- 35.Molnar, S. (1972) Curr. Anthropol. 13, 511–525. [Google Scholar]
- 36.Smith, B. H. (1984) Am. J. Phys. Anthropol. 63, 39–56. [DOI] [PubMed] [Google Scholar]
- 37.Lampl, M. A., Mann, A. E. & Monge, J. (2000) Anthropologie 38, 51–62. [Google Scholar]
- 38.Efron, B. & Tibshirani, R. J. (1993) An Introduction to the Bootstrap (Chapman & Hall, New York).
- 39.Trinkaus, E. (1995) J. Archaeol. Sci. 22, 121–142. [Google Scholar]
- 40.Carey, J. R. (2003) Longevity: The Biology and Demography of Life Span (Princeton Univ. Press, Princeton).
- 41.Hill, K., Boesch, C., Goodall, J., Pusey, A., Williams, J. & Wrangham, R. (2001) J. Hum. Evol. 40, 437–450. [DOI] [PubMed] [Google Scholar]
- 42.Templeton, A. R. (2002) Nature 416, 45–51. [DOI] [PubMed] [Google Scholar]
- 43.Shennan, S. (2001) Cambr. Archaeol. J. 11, 5–16. [Google Scholar]
- 44.Wolpoff, M. H. & Caspari, R. (1997) in Conceptual Issues in Modern Human Origins Research, eds. Clark, G. A & Willermet, C. M. (de Gruyter, New York), pp. 28–44.