Abstract
The implementation of a variety of immunosuppressive therapies has made drug-associated progressive multifocal leukoencephalopathy (PML) an increasingly prevalent clinical entity. The purpose of this study was to investigate its diagnostic characteristics and to determine whether differences herein exist between the multiple sclerosis (MS), neoplasm, post-transplantation, and autoimmune disease subgroups. Reports of possible, probable, and definite PML according to the current diagnostic criteria were obtained by a systematic search of PubMed and the Dutch pharmacovigilance database. Demographic, epidemiologic, clinical, radiological, cerebrospinal fluid (CSF), and histopathological features were extracted from each report and differences were compared between the disease categories. In the 326 identified reports, PML onset occurred on average 29.5 months after drug introduction, varying from 14.2 to 37.8 months in the neoplasm and MS subgroups, respectively. The most common overall symptoms were motor weakness (48.6 %), cognitive deficits (43.2 %), dysarthria (26.3 %), and ataxia (24.1 %). The former two also constituted the most prevalent manifestations in each subgroup. Lesions were more often localized supratentorially (87.7 %) than infratentorially (27.4 %), especially in the frontal (64.1 %) and parietal lobes (46.6 %), and revealed enhancement in 27.6 % of cases, particularly in the MS (42.9 %) subgroup. Positive JC virus results in the first CSF sample were obtained in 63.5 %, while conversion after one or more negative outcomes occurred in 13.7 % of cases. 52.2 % of patients died, ranging from 12.0 to 83.3 % in the MS and neoplasm subgroups, respectively. In conclusion, despite the heterogeneous nature of the underlying diseases, motor weakness and cognitive changes were the two most common manifestations of drug-associated PML in all subgroups. The frontal and parietal lobes invariably constituted the predilection sites of drug-related PML lesions.
Electronic supplementary material
The online version of this article (doi:10.1007/s00415-016-8217-x) contains supplementary material, which is available to authorized users.
Keywords: Progressive multifocal leukoencephalopathy, Medication, Drugs, Side effect, Adverse event
Introduction
Progressive multifocal leukoencephalopathy (PML) is a JC virus (JCV) related demyelinating disorder of the central nervous system that occurs almost exclusively in immunocompromised patients [1]. It is characterized histopathologically by a lytic infection of oligodendrocytes, astrocytes, and/or neurons in the white matter, cortical gray matter, and/or gray-white matter junction giving rise to a plethora of clinical phenotypes [2]. Quintessential radiological lesions are hyperintense on T2-weighted and FLAIR images and hypointense on T1-weighted images. In 2013, diagnostic criteria have been established which include clinical, imaging, laboratory, and histopathological features. Prerequisite for a definite diagnosis of PML is either the presence of characteristic pathoanatomic findings in a biopsy specimen or a combination of the appropriate clinical symptoms, radiological features, and the detection of JCV DNA in cerebrospinal fluid (CSF) [3]. Cases that do not fulfill these criteria might be classified as possible or probable PML and may be missed in official statistics.
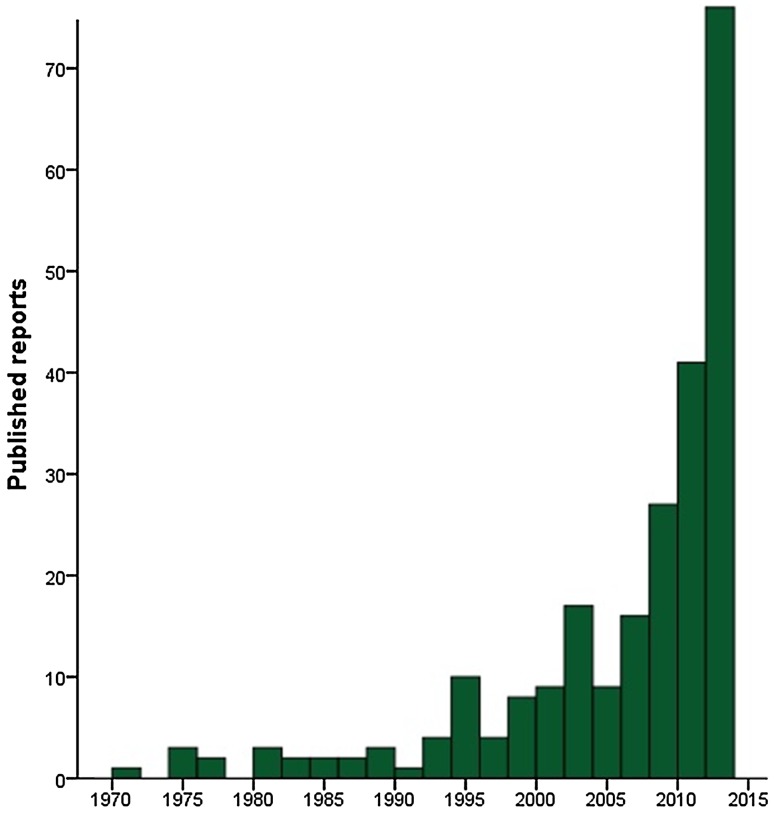
PML has traditionally been associated with an intrinsically compromised immune system. In the past few years, a considerable number of immunosuppressive drugs has been approved by the authorities and implemented into clinical practice to broaden the therapeutic spectrum of a variety of medical conditions [4–6]. Consequently, medication-associated PML has become an increasingly prevalent clinical entity, as reflected by an exponential increase in the number of published cases (Fig. 1). Because of the frequently poor prognosis of PML, it is of paramount importance to establish this diagnosis at an early stage. However, a comprehensive, quantitative analysis of the clinical, radiological, and CSF features of drug-associated PML and its subgroups, based on the underlying disease categories, has never been conducted thus far. Nevertheless, for an early diagnosis it is crucial for clinicians across various specialties to be aware of the preferential clinical and radiological presentation and the potential differences herein between the subgroups. In this study, we aimed to address this urgent medical need for advances in knowledge on drug-associated PML by outlining its specific clinical, radiological, and CSF characteristics. A quantitative assessment of the degree of association between any particular drug and PML has been reported previously, and therefore fell beyond the scope of this paper [7].
Fig. 1.
Number of published reports on drug-associated PML throughout the years, as included in this paper
Methods
Inclusion of published reports
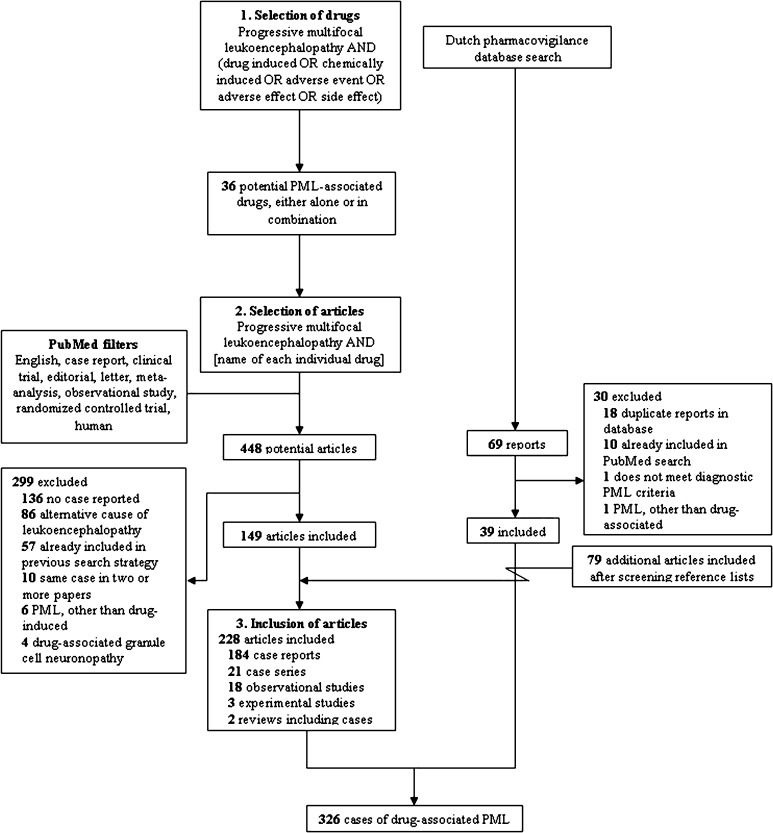
References were identified by means of a three-step PubMed search conducted on August 30, 2015 (Fig. 2). To obtain a first inventory of the drugs that might trigger PML occurrence, the search terms ‘progressive multifocal leukoencephalopathy’, ‘drug induced’, ‘chemically induced’, ‘medication induced’, ‘adverse event’, ‘adverse effect’, and ‘side effect’ were used in the ‘all fields’ menu without limits of time. Second, combinations of ‘progressive multifocal leukoencephalopathy’ and the names of each of the individual drugs that had been identified in the first step were applied to collect all reports of potentially drug-associated PML. Finally, reference lists of all included articles were screened for any additional relevant reports.
Fig. 2.
Flowchart of the search strategy, selection, and inclusion of articles and reports from the Dutch pharmacovigilance database
We included cases of possible, probable, and definite PML according to the current American Academy of Neurology criteria [3] that were published in English language journals in case reports, case series, clinical trials, editorials, letters, meta-analyses, and observational studies. In addition, articles were included when they described a simultaneous immune reconstitution inflammatory syndrome (IRIS), either occurring already during treatment with or only after withdrawal of the causative drug. With respect to the latter, IRIS was defined as deterioration of neurologic deficits following drug removal, corroborated by inflammatory changes on neuroimaging [8]. Articles were excluded if an identical case had already been described in a previous report, when PML was not related to a drug, or if an alternative diagnosis was more likely, e.g., posterior reversible encephalopathy syndrome (PRES) associated with tacrolimus, cyclosporine, cisplatin, and methotrexate. Finally, cases of medication-related granule cell neuronopathy were excluded [9, 10]. When imaging features played a pivotal role in the distinction between ‘possible PML’ and ‘not PML’, the decision of inclusion or exclusion was made after analysis by an experienced neuroradiologist with special expertise in the field of inflammatory diseases of the central nervous system (MPW).
Dutch pharmacovigilance database
In addition to the cases published in the literature, reports on medication-associated PML were obtained by querying the database of the Netherlands Pharmacovigilance Centre Lareb. This database contains detailed accurately verified information of adverse drug reactions reported by Dutch healthcare professionals and manufacturers. Care was taken to exclude duplicates that had already been included in the literature search.
Data acquisition
The following data, when available, were extracted from each report: study type, number of cases, age, sex, underlying disease, immunosuppressive agent(s), time elapsed between drug introduction and either first symptoms or asymptomatic radiological findings that could be attributed to PML in retrospect, mortality rate (and time from first symptoms to death), and clinical, radiological, CSF, and histopathological features. When a combination of therapies rather than a single pharmaceutical was presumed to be responsible for the phenotype, the most likely contributing drugs—based on mechanism of action, dose, and duration of use—were noted up to a maximum of three. The localization of PML lesions was determined by the description provided by the authors of each report and the MR and/or CT images shown, if any. In addition, the presence of contrast enhancement, its distribution pattern (i.e., patchy, punctate, homogeneous, or unknown), and the dissemination pattern of lesions were listed. With respect to the latter, we distinguished between unilobar, multilobar (i.e., two or more contiguous lobes affected), and widespread (i.e., two or more noncontiguous lobes affected and/or the presence of lesions in both hemispheres). For the sake of clinical relevance and vigilance, symptoms and radiological lesions that appeared after establishing the diagnosis of PML, attributable to either progression of the disease or a consecutive emerging IRIS, were excluded.
Statistical analysis
Differences between the four subgroups, based on the underlying disease categories, were analyzed using Chi-square tests and one-way ANOVA. Post-hoc Bonferroni corrections have been applied and significance levels were set at 0.0083 (i.e., 0.05/6). Due to the conservative nature of the Bonferroni method [11], it seems likely that potential differences between the subgroups represent genuine dissimilarities rather than mere coincidence.
Results
Demographic and epidemiologic features
The literature search yielded 287 cases of drug-related PML in 184 case reports, 21 case series, 18 observational studies, three experimental studies, and two review articles [12–239]. Furthermore, an additional 39 reports were identified in the Dutch pharmacovigilance database, resulting in a total number of 326 documented cases. In eight cases, consensus was reached after initial doubt on the diagnosis of PML; five of them have been included, while the remaining three were finally labeled as ‘not PML’. Natalizumab, predniso(lo)ne, (dimethyl) fumarate, fludarabine, rituximab, and brentuximab vedotin were the most common single agents that have been demonstrated to trigger PML (Online Resource 1). Additionally, glucocorticoids with either azathioprine or cyclophosphamide and a regime containing four or more pharmaceuticals [usually courses of chemotherapy, frequently including rituximab (R-CHOP)] comprised the most prevalent composite culprits. The multifarious underlying diseases could be subdivided into four main categories, i.e. multiple sclerosis (MS), (other) immune-mediated disorders, neoplasms (93.8 % lymphoproliferative, 3.1 % myeloproliferative, 2.1 % solid neoplasm, 1.0 % unknown), and the post-transplantation setting (Fig. 3). Because of the relatively large number of natalizumab-associated PML cases, the former was not included in the group of immune-mediated diseases, but rather considered a distinct entity. As immunosuppressive drugs are also frequently used in the prevention of relapses in neuromyelitis optica spectrum disorder after a first episode, one might expect drug-associated PML to have been described in this patient population. However, no such reports have been identified in our search. The most common drugs associated with PML per subgroup are summarized in Online Resource 2. In general, there was considerably few drug overlap between the various disease categories, an exception being the combination of azathioprine and glucocorticoids which was used in both autoimmune disorders and the post-transplantation setting.
Fig. 3.
Distribution of cases of drug-associated progressive multifocal leukoencephalopathy among the major disease categories (a) and subdivisions of the post-transplantation setting (b), the autoimmune diseases (c), and the neoplasms (d). Because of the large number of natalizumab-associated PML cases, multiple sclerosis was considered a distinct entity and was not included in the group of autoimmune disorders. CML chronic myeloid leukemia, CLL chronic lymphocytic leukemia, GPA granulomatosis with polyangiitis (formerly Wegener’s disease), MDS myelodysplastic syndrome, NHL non-Hodgkin lymphoma, NSCLC non-small-cell lung cancer, RA rheumatoid arthritis, SLE systematic lupus erythematosus, WM Waldenström’s macroglobulinemia
The age and sex distribution of drug-related PML among the subgroups were in accordance with the epidemiologic features of the particular diseases, with a higher percentage of younger women and older men in the MS and neoplasm subgroups, respectively (Table 1). The mean time elapsed from introduction of the drug(s) to first symptoms was 28.9 (95 % CI 25.5–32.2) months, while the time span to the occurrence of asymptomatic lesions (only in natalizumab-induced PML) was found to be 36.4 (95 % CI 27.0–45.9) months. Significantly shorter periods were observed in the neoplasm group [mean 14.2 months (95 % CI 9.3–19.2)] compared to the three other disease categories (p < 0.001). A delay of several weeks to even months between the occurrence of first symptoms and the final diagnosis of PML was noticed in a considerable number of cases [23, 28, 50, 67, 78, 101, 103, 117, 122, 149, 158, 185, 201, 213, 223, 224, 226, 233, 235, 236]. This diagnosis was established on combined clinical, radiological, and CSF grounds in 69.4 % of cases. In the remaining 30.6 %, histopathological analysis was necessary to provide a greater degree of certainty, either because PCR for JCV in the CSF was not available or (repeatedly) negative. Brain biopsies were conducted most commonly in patients with underlying autoimmune diseases (43.8 %) and neoplasms (47.8 %), but in only 8.3 % of natalizumab-induced PML cases. The overall mortality rate was 52.2 % (95 % CI 46.5–58.0 %), with values of 12.0 % (95 % CI 5.2–18.7 %), 56.2 % (95 % CI 44.5–67.8 %), 83.3 % (95 % CI 75.5–91.2 %), and 68.4 % (95 % CI 52.9–83.9 %) in the MS, immune-mediated, neoplasm and post-transplantation subgroups, respectively.
Table 1.
Demographic and epidemiologic features of drug-associated progressive multifocal leukoencephalopathy
| Multiple sclerosisa | Autoimmune diseases | Neoplasms | Post-transplantation | Total | |
|---|---|---|---|---|---|
| Number of cases | 113 | 78 | 97 | 38 | 326 |
| Literature | 89 (31.0) | 75 (26.1) | 86 (30.0) | 37 (12.9) | 287 |
| Lareb | 24 (61.5) | 3 (7.7) | 11 (28.2) | 1 (2.6) | 39 |
| Age (years) | 45.0 ± 10.4*# | 54.5 ± 14.2* | 58.9 ± 12.4#¶ | 49.7 ± 14.3¶ | 52.2 ± 13.7 |
| Sex (% male) | 41/111 (36.9)¶† | 27/76 (35.5)*# | 57/92 (62.0)*¶ | 25/38 (65.8)#† | 150/317 (47.3) |
| Time to PML onset (months) | 37.8 ± 17.0# | 30.6 ± 20.5* | 14.2 ± 21.4*#¶ | 34.9 ± 37.6¶ | 29.5 ± 24.4 |
| To first symptoms | 38.0 ± 17.5# | 30.6 ± 20.5* | 14.2 ± 21.4*#¶ | 34.9 ± 37.6¶ | 28.8 ± 25.3 |
| To “silent” MRI lesionsb | 36.4 ± 12.2 | N/A | N/A | N/A | 36.4 ± 12.2 |
| Diagnosis (%) | |||||
| Clinical–MRI–CSF | 97/98 (99.0)*#¶ | 42/73 (57.5)* | 52/93 (55.9)# | 18/37 (48.6)¶ | 209/301 (69.4) |
| Histopathology | 1/98 (1.0)*#¶ | 31/73 (42.5)* | 41/93 (44.1)# | 19/37 (51.4)¶ | 92/301 (30.6) |
| PML classification (%) | |||||
| Possible | 11/99 (11.1) | 4/76 (5.3) | 9/94 (9.6) | 3/38 (7.9) | 27/307 (8.8) |
| Probable | 12/99 (12.1)*#¶ | 0/76 (0.0)* | 1/94 (1.1)# | 0/38 (0.0)¶ | 13/307 (4.2) |
| Definite | 75/99 (75.8) | 63/76 (82.9) | 79/94 (84.0) | 28/38 (73.7) | 245/307 (79.8) |
| Unknown | 1/99 (1.0)* | 9/76 (11.8) | 5/94 (5.3) | 7/38 (18.4)* | 22/307 (7.2) |
| Mortality rate (%) | 11/92 (12.0)*#¶ | 41/73 (56.2)*† | 75/90 (83.3)#† | 26/38 (68.4)¶ | 153/293 (52.2) |
| Time to death (months) | 5.2 ± 3.2 | 7.0 ± 10.4 | 3.9 ± 4.5 | 3.4 ± 3.6 | 4.8 ± 6.7 |
Categorical data have been displayed as frequency (percentage), continuous data as mean ± standard deviation
#, *, ¶, † significant differences between subgroups (p < 0.05/6 = 0.0083)
aBecause of the large number of natalizumab-associated PML cases, multiple sclerosis was considered a distinct entity and was not included in the group of autoimmune disorders
bAsymptomatic PML cases were only observed in natalizumab-treated patients
Clinical characteristics
In descending order of magnitude, the most common overall symptoms and signs of drug-related PML were motor weakness (48.6 %), cognitive deficits (43.2 %), dysarthria (26.3 %), ataxia (24.1 %), aphasia (22.7 %), and behavioral changes (21.9 %) (Table 2). Although some deviations were observed between the subgroups, motor weakness and cognitive changes constituted the two most frequently encountered symptoms in all disease categories. Behavioral changes were significantly more common in the post-transplantation group than in any of the other disease categories (MS p < 0.001, immune-mediated disorders p = 0.001, neoplasms p = 0.002). The highest prevalences of cognitive deficits and seizures were also found in this disease category (60.0 and 22.9 %, respectively). Symptoms and signs of cerebellar and brainstem involvement, i.e., ataxia, vertigo, eye movement disorders, and dysphagia tended to be more prevalent in the group of autoimmune disorders. Furthermore, dysarthria, gait abnormalities and sensory deficits (37.3, 26.9, and 20.9 %, respectively) occurred most commonly in the group of immune-mediated diseases. A notable feature of drug-related PML among the neoplasms was the high prevalence of visual deficits (25.8 %), especially when compared to the autoimmune disorders (p = 0.001). Asymptomatic PML cases were only observed in natalizumab-treated patients (10.3 %).
Table 2.
Clinical characteristics of drug-associated progressive multifocal leukoencephalopathy
| Multiple sclerosis (n = 87) | Autoimmune diseases (n = 67) | Neoplasms (n = 89) | Post-transplantation (n = 35) | Total (n = 278) | |
|---|---|---|---|---|---|
| Motor weaknessa | 34 (39.1) | 34 (50.7) | 49 (55.1) | 18 (51.4) | 135 (48.6) |
| Cognitive deficitsb | 30 (34.5) | 25 (37.3) | 44 (49.4) | 21 (60.0) | 120 (43.2) |
| Dysarthria | 13 (14.9)* | 25 (37.3)* | 27 (30.3) | 8 (22.9) | 73 (26.3) |
| Ataxia | 14 (16.1) | 21 (31.3) | 23 (25.8) | 9 (25.7) | 67 (24.1) |
| Aphasiac | 20 (23.0) | 19 (28.4) | 20 (22.5) | 4 (11.4) | 63 (22.7) |
| Behavioral changed | 14 (16.1)# | 12 (17.9)* | 18 (20.2)¶ | 17 (48.6)#*¶ | 61 (21.9) |
| Gait abnormalities | 14 (16.1) | 18 (26.9) | 13 (14.6) | 5 (14.3) | 50 (18.0) |
| Visual deficitse | 10 (11.5) | 4 (6.0)* | 23 (25.8)* | 6 (17.1) | 43 (15.5) |
| Sensory deficits | 7 (8.0) | 14 (20.9)# | 5 (5.6)# | 6 (17.1) | 32 (11.5) |
| Seizure | 9 (10.3) | 5 (7.5) | 6 (6.7) | 8 (22.9) | 28 (10.1) |
| Facial palsy | 5 (5.7) | 10 (14.9) | 6 (6.7) | 6 (17.1) | 27 (9.7) |
| Dysphagia | 2 (2.3) | 8 (11.9) | 6 (6.7) | 4 (11.4) | 20 (7.2) |
| Apraxia | 3 (3.4) | 3 (4.5) | 11 (12.4) | 2 (5.7) | 19 (6.8) |
| Vertigo | 4 (4.6) | 6 (9.0) | 3 (3.4) | 3 (8.6) | 16 (5.8) |
| Eye movement disordersf | 2 (2.3) | 6 (9.0) | 8 (9.0) | 0 (0.0) | 16 (5.8) |
| Headache | 2 (2.3) | 3 (4.5) | 3 (3.4) | 3 (8.6) | 11 (4.0) |
| Parkinsonismg | 1 (1.1) | 6 (9.0) | 2 (2.2) | 2 (5.7) | 11 (4.0) |
| Depression | 4 (4.6) | 2 (3.0) | 1 (1.1) | 1 (2.9) | 8 (2.9) |
| Asymptomatic | 9 (10.3)*# | 0 (0.0)# | 0 (0.0)* | 0 (0.0) | 9 (3.2) |
Data have been displayed as frequency (percentage)
Because of the large number of natalizumab-associated PML cases, multiple sclerosis was considered a distinct entity and was not included in the group of autoimmune disorders
aMonoparesis, hemiparesis, tetraparesis, hemiplegia, and tetraplegia
bConfusion and memory deficits
cTrue aphasia and word finding difficulties
dPersonality changes, apathy, lethargy, and agitation
eVisual field defects and reduced visual acuity
fOphtalmoparesis, strabismus, and diplopia
gTremor, bradykinesia, and ‘parkinsonism’
#, *, ¶ significant differences between subgroups (p < 0.05/6 = 0.0083)
Radiological findings
Whereas infratentorial lesions were present in 27.4 % of cases, drug-associated PML was considerably more prevalent in the supratentorial brain (87.7 %) (Table 3). In all disease categories, lesions were most often situated in the frontal and parietal lobes (64.1 and 46.6 %, respectively). The temporal (21.4 %) and occipital (22.7 %) lobes were less commonly affected. Involvement of the latter, however, was observed relatively frequently in the neoplasm subgroup (36.2 %), in particular when compared to the autoimmune and MS groups (p = 0.003 and p = 0.01, respectively). Infratentorial lesion localization was especially prevalent in drug-related PML associated with immune-mediated disorders, with cerebellar involvement being significantly more common than in the MS and neoplasm categories (p = 0.001 and p = 0.007, respectively). Furthermore, there was a trend towards a higher level of brainstem involvement in the group of autoimmune diseases (MS p = 0.013; neoplasm p = 0.077). In the natalizumab-induced PML subgroup, lesions were most commonly distributed in one lobe (42.9 % vs. 21.4 % multilobar and 27.1 % widespread). In contrast, widespread dissemination was by far the most frequently encountered pattern in the remaining three disease categories (autoimmune disorders 52.5 %, neoplasms 52.1 %, post-transplantation 68.0 %). Contrast enhancement was present in 27.6 % of cases, particularly in the natalizumab-induced (42.9 %) and post-transplantation (31.6 %) subgroups. In the former, punctate and patchy enhancement patterns were observed to an equal extent (19.6 %). In the other subgroups, only patchy contrast enhancement was found.
Table 3.
Radiological and cerebrospinal fluid features of drug-associated progressive multifocal leukoencephalopathy
| Multiple sclerosisa | Autoimmune diseases | Neoplasms | Post-transplantation | Total | |
|---|---|---|---|---|---|
| Radiological features | |||||
| Supratentorial | 66/72 (91.7) | 46/61 (75.4)* | 71/76 (93.4)* | 24/27 (88.9) | 207/236 (87.7) |
| Frontal lobe | 49/69 (71.0) | 32/57 (56.1) | 43/69 (62.3) | 17/25 (68.0) | 141/220 (64.1) |
| Parietal lobe | 22/69 (31.9) | 31/58 (53.4) | 36/69 (52.2) | 14/25 (56.0) | 103/221 (46.6) |
| Temporal lobe | 9/69 (13.0) | 12/57 (21.1) | 21/69 (30.4) | 5/25 (20.0) | 47/220 (21.4) |
| Occipital lobe | 10/69 (14.5)# | 9/57 (15.8) | 25/69 (36.2)# | 6/25 (24.0) | 50/220 (22.7) |
| Basal ganglia | 1/69 (1.4) | 3/57 (5.3) | 3/69 (4.3) | 3/25 (12.0) | 10/220 (4.5) |
| Thalamus | 3/69 (4.3) | 6/58 (10.3) | 8/69 (11.6) | 3/25 (12.0) | 20/221 (9.0) |
| Capsula interna | 3/69 (4.3) | 4/59 (6.8) | 2/69 (2.9) | 3/25 (12.0) | 12/222 (5.4) |
| Corpus callosum | 2/69 (2.9) | 3/59 (5.1) | 6/69 (8.7) | 2/25 (8.0) | 13/222 (5.9) |
| Infratentorial | 13/69 (18.8)* | 25/60 (41.7)* | 15/69 (21.7) | 8/25 (32.0) | 61/223 (27.4) |
| Cerebellum | 8/69 (11.6)* | 21/60 (35.0)*# | 10/69 (14.5)# | 6/25 (24.0) | 45/223 (20.2) |
| Brain stem | 7/69 (10.1) | 16/59 (27.1) | 10/69 (14.5) | 5/25 (20.0) | 38/222 (17.1) |
| Lesion distribution | |||||
| Unilobar | 30/70 (42.9)*# | 13/59 (22.0) | 13/71 (18.3)* | 1/25 (4.0)# | 57/225 (25.3) |
| Multilobar | 15/70 (21.4) | 13/59 (22.0) | 17/71 (23.9) | 6/25 (24.0) | 51/225 (22.7) |
| Widespread | 19/70 (27.1)*#¶ | 31/59 (52.5)* | 37/71 (52.1)# | 17/25 (68.0)¶ | 104/225 (46.2) |
| Unknown | 6/70 (8.6) | 2/59 (3.4) | 4/71 (5.6) | 1/25 (4.0) | 13/225 (5.8) |
| Contrast enhancement | 24/56 (42.9)* | 5/43 (11.6)* | 10/45 (22.2) | 6/19 (31.6) | 45/163 (27.6) |
| Punctate | 11/56 (19.6)*# | 0/43 (0.0)* | 0/45 (0.0)# | 0/19 (0.0) | 11/163 (6.7) |
| Patchy | 11/56 (19.6) | 3/43 (7.0) | 8/45 (17.8) | 4/19 (21.1) | 26/163 (16.0) |
| Homogeneous | 1/56 (1.8) | 0/43 (0.0) | 0/45 (0.0) | 0/19 (0.0) | 1/163 (0.6) |
| Unknown | 1/56 (1.8) | 2/43 (4.7) | 2/45 (4.4) | 2/19 (10.5) | 7/163 (4.3) |
| CSF features | |||||
| PCR JC virus | |||||
| Negative | 11/95 (11.6)# | 15/53 (28.3) | 21/64 (32.8)# | 6/21 (28.6) | 53/233 (22.7) |
| Directly positive | 64/95 (67.4) | 31/53 (58.5) | 39/64 (60.9) | 14/21 (66.7) | 148/233 (63.5) |
| Positive after ≥2 LPs | 20/95 (21.1)* | 7/53 (13.2) | 4/64 (6.3)* | 1/21 (4.8) | 32/233 (13.7) |
| Leukocytes/ul | |||||
| 0–4 | 17/19 (89.5) | 38/41 (92.7) | 34/41 (82.9) | 17/18 (94.4) | 106/119 (89.1) |
| ≥5 | 2/19 (10.5) | 3/41 (7.3) | 7/41 (17.1) | 1/18 (5.6) | 13/119 (10.9) |
Data have been displayed as frequency (percentage)
LP lumbar puncture
*, #, ¶ significant differences between subgroups (p < 0.05/6 = 0.0083)
aBecause of the large number of natalizumab-associated PML cases, multiple sclerosis was considered a distinct entity and was not included in the group of autoimmune disorders
CSF results
JCV DNA remained (repeatedly) undetectable in 22.7 % (95 % CI 17.3–28.1 %) of drug-associated PML cases. Furthermore, nearly one out of each seven patients (13.7 %; 95 % CI 9.3–18.1 %) initially displayed one or more negative outcomes before PCR finally converted to positive, ranging from 4.8 % in the post-transplantation (95 % CI 0.0–14.7 %) subgroup to 21.1 % (95 % CI 12.7–29.4 %) in the MS subgroup. Positive results in the first CSF sample were obtained in 63.5 %. Pleocytosis was present in 10.9 % of patients, especially in the neoplasm group (17.1 %).
Discussion
Despite the exponential increase in the number of drug-associated PML cases throughout the years, a comprehensive, quantitative analysis of its diagnostic characteristics that also takes into account the differences between the various underlying disease categories has not been performed thus far. Our study demonstrates that motor weakness and cognitive deficits are the most common presenting symptoms in all subgroups. Several dissimilarities were observed, however, between the disease categories in the remaining clinical manifestations. While behavioral and cognitive changes were most prevalent among the post-transplantation group, cerebellar and brainstem symptoms occurred most commonly in the autoimmune category. Visual disturbances appeared most frequently in drug-related PML associated with neoplasms. However, a sound explanation for these differences seems to be missing.
Because of the frequently grim prognosis and lethal outcomes, it is of paramount importance to consider the diagnosis of medication-associated PML at an early stage during treatment with immunosuppressive drugs. The clinical phenotype in the articles reviewed, however, was not seldom initially misinterpreted as an exacerbation or the cerebral manifestations of the underlying disease, i.e., an MS relapse, neuropsychiatric lupus, or central nervous system vasculitis [23, 28, 50, 67, 78, 101, 103, 117, 122, 149, 158, 185, 201, 213, 223, 224, 226, 233, 235, 236]. It was only after further clinical and radiological worsening upon high doses of immunosuppressive therapies that the possibility of PML was considered in these cases. Apart from the intrinsically dismal prognosis of PML, this delay of several weeks to even months and additional assault on the immune system certainly may have contributed to the high mortality rate. Sometimes, acute symptoms and signs were attributed to cerebral infarctions [21, 38, 109]. Finally, ocular and vestibular symptoms have occasionally been misjudged as cataract and Meniere’s disease, respectively [45, 120, 155]. It is possible that PML symptoms have been misinterpreted in these cases because of the relatively long interval between drug introduction and first central nervous system deficits, on average almost 2.5 years.
The differential diagnosis of medication-associated PML depends on the context in which the specific immunosuppressive drug is applied. In the post-transplantation setting, it is important to distinguish between drug-associated PML and PRES, which usually affects the parieto-occipital lobes and frequently resolves spontaneously after withdrawal of the culprit(s). In MS patients, on the other hand, the distinction between a relapse and natalizumab-induced PML can be particularly troublesome. A correct and early diagnosis, however, has important therapeutic and prognostic implications. In this regard, several clinical and MRI features may direct the physician to the right diagnosis. Acute spinal cord or brainstem presentations and focal, sharp-edged, periventricular locations are generally more frequently encountered in an MS exacerbation. In contrast, PML is characterized by a subacute onset, progressive nature of (sub)cortical symptoms, and large, ill-defined, confluent T2-weighted lesions, deep gray matter involvement, and/or crescent cerebellar lesions [240, 241]. This study confirms these findings, the most common natalizumab-associated PML symptoms being motor weakness, cognitive deficits, and aphasia, and lesions mainly localized in the frontal and parietal lobes. Isolated brainstem involvement, however, does not exclude PML at all [201, 233]. New imaging procedures such as the application of susceptibility weighted imaging and ultra high-field 7 Tesla MRI have shown promising preliminary data in the distinction between PML and MS lesions and might finally play a pivotal role when regular techniques fail to attain full assurance [242]. However, further work is required before they can be implemented in everyday clinical practice.
Due to the raised level of radiological vigilance in natalizumab-treated patients including high-frequency MRI monitoring according to recent expert opinion guidelines [243, 244], cases of PML diagnosed at an asymptomatic stage are identified with increasing frequency [19, 27, 123, 137, 153, 162, 221]. These cases are only classified as probable PML in the presence of JCV DNA in the CSF detected by PCR, indicating the conservative nature of the current diagnostic criteria [3]. The predicament of negative or inconclusive results across different laboratories in view of asymptomatic patients displaying MRI lesions has been touched upon previously [221] and stresses the need of a revision of the diagnostic PML criteria.
Definitive establishment of the PML diagnosis was hampered by initially negative PCR outcomes in CSF samples in nearly one out of each seven patients before it finally converted to positive. These results are strongly dependent on the disease stage and the characteristics of the assay, especially its lower detection limit, the sensitivity of the older assays being approximately 75 % [3]. Samples were frequently analyzed not only in the institute’s own laboratory, but also in external facilities, with the National Institutes of Health laboratory being the one with the most ultrasensitive assay (i.e., a detection limit of 10 JCV DNA copies per milliliter). Thus, physicians need to be aware that the lack of detection of JCV DNA in CSF does not preclude the diagnosis of PML. Repeated lumbar punctures, follow-up MRI, and possibly additional techniques currently under evaluation such as the assessment of anti-JCV antibodies in CSF [245], and in a proportion of cases, biopsy may be required to confirm the diagnosis of PML.
A clear radiological anterior-posterior gradient was observed, with the frontal and parietal lobes being more often involved than their temporal and occipital counterparts. A widespread lesion distribution pattern was found to be most common in drug-related PML associated with autoimmune disorders, neoplasms, and the post-transplantation setting, while (asymptomatic) unilobar involvement was most often observed in the natalizumab-induced subgroup. Contrast enhancement was present in about one fourth of cases of drug-associated PML, especially in the MS and post-transplantation subgroups. Although the underlying mechanisms and evolution of this ‘inflammatory PML’ variant remain to be elucidated, experimental evidence in natalizumab-induced PML suggests that lymphocyte trafficking continues to occur via alternative pathways due to upregulation of different adhesion molecules, thereby inducing a state of incomplete immune surveillance [246].
In contrast to what its name may suggest, the thalamus and basal ganglia—obviously deep gray matter structures—were involved in the PML disease process in 9.0 and 4.5 % of cases, respectively. Berger et al. previously demonstrated lesions in these areas in 14 and 12 % in PML associated with human immunodeficiency virus [247]. Furthermore, seizures which are generally assumed to be generated by synchronous cortical activity were present in 10.1 % of patients. In previous studies, even higher prevalences of 18 and 34.7 % were found, with causative PML lesions being situated adjacent to the T1-hyperintense cerebral cortical gray matter [248, 249]. It can be inferred from both observations that the ‘leukoencephalopathy’ phrase in the term PML, suggesting a white matter disease, is actually kind of a misnomer and rather confusing for clinicians who are not familiar with this disease.
The discrepancies in survival between the different categories of PML—not only the drug-associated cases, but also the ones related to an intrinsically compromised immune system—can probably be explained by their various degrees of ‘generalized’ immunosuppression. The more specific the immune system is targeted, the lower the mortality rate in general. We demonstrated a significantly lower mortality rate in the natalizumab-induced PML subgroup compared to the other disease categories and found the highest value in the neoplasm subgroup. As natalizumab impedes the interaction between alpha-4 integrin on lymphocytes and vascular cell adhesion molecule 1 on the endothelium of the vessel wall, it specifically prevents the diapedesis of these cells through the blood brain barrier without reducing the total number of leukocytes in the blood or their function. Therefore, in contrast to the various antineoplastic agents and immunosuppressive drugs used in cancer and the post-transplantation setting, the immune system may be more specifically suppressed. Depression of the immune system may not only result from the drugs administered, but is also an intrinsic consequence of the disease process in hematological malignancies which accounts for the highest mortality rate in this subgroup. Furthermore, the high level of radiological vigilance and occurrence of asymptomatic PML cases among MS patients treated with natalizumab frequently led to a quick drug suspension which probably contributed to a better prognosis.
Several intrinsic limitations of this study need to be addressed. Although we identified a large number of reports, not all cases of medication-related PML are published in the literature, especially when the association between the drug and the side effect has been described before. Therefore, our study design does not allow calculating the treatment-related risk of developing PML for each specific drug. Second, the contrast between underreporting in the oncology field on the one hand and the high clinical and radiological vigilance in MS and autoimmune disorders on the other has possibly led to a skewed distribution of published cases. Furthermore, the causal relationship between the mentioned drugs and PML may have been blurred by a variety of reasons. First, a state of immunosuppression could have already been present as a result of the underlying disease itself, e.g., due to lymphopenia in SLE, sarcoidosis, or leukemia. Indeed, PML has been described in these disorders in the absence of immunosuppressive medication [250, 251]. Therefore, it seems plausible to assume that PML in the group of neoplasms and in some but not all autoimmune disorders results from the interaction and synergistic immunosuppressive effects of the given drug(s) and the underlying disease. In the MS and post-transplantation setting, on the other hand, PML seems to be entirely attributable to iatrogenic reasons. Second, it is not inconceivable that several reports only mentioned the drug(s) that were taken at the time of PML onset, without giving notice to previous use of immunosuppressive therapies or long-standing leukopenia. As a result, the drugs mentioned may just have given the final push to the diagnosis of PML rather than being fully responsible. In most cases of this study (277/326, 85 %), the risk of medication-associated PML seemed related to a maximum of three drugs. However, in this respect the heterogeneity of the data set needs to be appreciated and one should take into account several other factors like duration of treatment, concomitant medication use, and comorbidity.
In conclusion, drug-associated PML represents an emerging yet underrecognized clinical entity. We demonstrated that the various subgroups share several clinical and radiological characteristics despite the highly heterogeneous nature of the underlying diseases. In all subgroups, motor weakness and cognitive changes comprised the two most common clinical manifestations, while the frontal and parietal lobes invariably appeared to be the predilection sites of PML lesions. Nonetheless, it is important to be aware of subtle—and sometimes more obvious—differences in preferential presentations, e.g., discrepancies in the patterns of lesion dissemination and contrast enhancement. In the end, meticulous clinical and radiological vigilance in recognizing the phenotype at an early stage coupled with prompt withdrawal and clearance of the culprit, appropriate supportive therapies, and accurate monitoring and treatment of a consecutive IRIS remain crucial to obtain better outcomes in the battle against this frequently relentless disease.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Ethical standards
The manuscript does not contain clinical studies or patient data.
Conflicts of interest
Roderick Maas reports no disclosures. Annemarie Muller-Hansma reports no disclosures. Rianne Esselink reports no disclosures. Jean-Luc Murk received personal fees from Biogen. Clemens Warnke received compensation for consulting and/or research support from Novartis, Biogen, Bayer, and TEVA. Joep Killestein accepted speaker and consulting fees from Merck-Serono, Biogen Idec, TEVA, Genzyme, and Novartis. Mike Wattjes serves as a consultant for Roche, Novartis, and Biogen. MS Center Amsterdam of the VU University Medical Center received financial support for research activities from Bayer Schering Pharma, Biogen Idec, GlaxoSmithKline, Merck-Serono, Novartis, and TEVA.
Funding
Not applicable.
References
- 1.Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9:425–437. doi: 10.1016/S1474-4422(10)70040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gheuens S, Wuthrich C, Koralnik IJ. Progressive multifocal leukoencephalopathy: why gray and white matter. Annu Rev Pathol. 2013;8:189–215. doi: 10.1146/annurev-pathol-020712-164018. [DOI] [PubMed] [Google Scholar]
- 3.Berger JR, Aksamit AJ, Clifford DB, Davis L, Koralnik IJ, Sejvar JJ, Bartt R, Major EO, Nath A. PML diagnostic criteria: consensus statement from the AAN neuroinfectious disease section. Neurology. 2013;80:1430–1438. doi: 10.1212/WNL.0b013e31828c2fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaheer F, Berger JR. Treatment-related progressive multifocal leukoencephalopathy: current understanding and future steps. Ther Adv Drug Saf. 2012;3:227–239. doi: 10.1177/2042098612453849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy associated with immunosuppressive therapy in rheumatic diseases: evolving role of biologic therapies. Arthritis Rheum. 2012;64:3043–3051. doi: 10.1002/art.34468. [DOI] [PubMed] [Google Scholar]
- 6.Mateen FJ, Muralidharan R, Carone M, van de Beek D, Harrison DM, Aksamit AJ, Gould MS, Clifford DB, Nath A. Progressive multifocal leukoencephalopathy in transplant recipients. Ann Neurol. 2011;70:305–322. doi: 10.1002/ana.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmedt N, Andersohn F, Garbe E. Signals of progressive multifocal leukoencephalopathy for immunosuppressants: a disproportionality analysis of spontaneous reports within the US Adverse Event Reporting System (AERS) Pharmacoepidemiol Drug Saf. 2012;21:1216–1220. doi: 10.1002/pds.3320. [DOI] [PubMed] [Google Scholar]
- 8.Tan IL, McArthur JC, Clifford DB, Major EO, Nath A. Immune reconstitution inflammatory syndrome in natalizumab-associated PML. Neurology. 2011;77:1061–1067. doi: 10.1212/WNL.0b013e31822e55e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wijburg MT, van Oosten BW, Murk JL, Karimi O, Killestein J, Wattjes MP. Heterogeneous imaging characteristics of JC virus granule cell neuronopathy (GCN): a case series and review of the literature. J Neurol. 2015;262:65–73. doi: 10.1007/s00415-014-7530-5. [DOI] [PubMed] [Google Scholar]
- 10.Wijburg MT, Siepman D, van Eijk JJ, Killestein J, Wattjes MP. Concomitant granule cell neuronopathy in patients with natalizumab-associated PML. J Neurol. 2016;263:649–656. doi: 10.1007/s00415-015-8001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eichstaedt KE, Kovatch K, Maroof DA. A less conservative method to adjust for familywise error rate in neuropsychological research: the Holm’s sequential Bonferroni procedure. NeuroRehabilitation. 2013;32:693–696. doi: 10.3233/NRE-130893. [DOI] [PubMed] [Google Scholar]
- 12.Riskind PN, Richardson EP. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 20–1995. A 66-year-old man with a history of rheumatoid arthritis treated with adrenocorticosteroids, with the development of aphasia and right-sided weakness. N Engl J Med. 1995;332:1773–1780. doi: 10.1056/NEJM199506293322609. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed F, Aziz T, Kaufman LD. Progressive multifocal leukoencephalopathy in a patient with systemic lupus erythematosus. J Rheumatol. 1999;26:1609–1612. [PubMed] [Google Scholar]
- 14.Aksamit AJ, Jr, de Groen PC. Cyclosporine-related leukoencephalopathy and PML in a liver transplant recipient. Transplantation. 1995;60:874–876. doi: 10.1097/00007890-199510270-00019. [DOI] [PubMed] [Google Scholar]
- 15.Aksamit AJ., Jr Progressive multifocal leukoencephalopathy. Continuum (Minneap Minn) 2012;18:1374–1391. doi: 10.1212/01.CON.0000423852.70641.de. [DOI] [PubMed] [Google Scholar]
- 16.Al-Tawfiq JA, Banda RW, Daabil RA, Dawamneh MF. Progressive multifocal leukoencephalopathy (PML) in a patient with lymphoma treated with rituximab: a case report and literature review. J Infect Public Health. 2015;8:493–497. doi: 10.1016/j.jiph.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Arbusow V, Strupp M, Pfister HW, Seelos KC, Bruckmann H, Brandt T. Contrast enhancement in progressive multifocal leukoencephalopathy: a predictive factor for long-term survival? J Neurol. 2000;247:306–308. doi: 10.1007/s004150050590. [DOI] [PubMed] [Google Scholar]
- 18.Arnaud FX, Hissene A, Metivier D, Dutasta F, Berets O, N’guema B, A’teriitehau C, Baccialone J, Potet J. Gadolinium enhancement in brain magnetic resonance imaging in progressive multifocal leukoencephalopathy after natalizumab monotherapy: is it really atypical? J Neuroradiol. 2012;39:267–270. doi: 10.1016/j.neurad.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Ayzenberg I, Lukas C, Trampe N, Gold R, Hellwig K. Value of MRI as a surrogate marker for PML in natalizumab long-term therapy. J Neurol. 2012;259:1732–1733. doi: 10.1007/s00415-012-6426-5. [DOI] [PubMed] [Google Scholar]
- 20.Baehring JM, Vives K, Bannykh S. Progressive multifocal leukoencephalopathy in a patient with marginal zone B-cell lymphoma. J Neurooncol. 2007;85:289–290. doi: 10.1007/s11060-007-9452-x. [DOI] [PubMed] [Google Scholar]
- 21.Bartsch T, Rempe T, Wrede A, Leypoldt F, Bruck W, Adams O, Rohr A, Jansen O, Wuthrich C, Deuschl G, Koralnik IJ. Progressive neurologic dysfunction in a psoriasis patient treated with dimethyl fumarate. Ann Neurol. 2015;78:501–514. doi: 10.1002/ana.24471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belli LS, De CL, Romani F, Rondinara GF, Rimoldi P, Alberti A, Bettale G, Dughetti L, Ideo G, Sberna M. Dysarthria and cerebellar ataxia: late occurrence of severe neurotoxicity in a liver transplant recipient. Transpl Int. 1993;6:176–178. doi: 10.1111/j.1432-2277.1993.tb00642.x. [DOI] [PubMed] [Google Scholar]
- 23.Beppu M, Kawamoto M, Nukuzuma S, Kohara N. Mefloquine improved progressive multifocal leukoencephalopathy in a patient with systemic lupus erythematosus. Intern Med. 2012;51:1245–1247. doi: 10.2169/internalmedicine.51.6810. [DOI] [PubMed] [Google Scholar]
- 24.Berghoff M, Schanzer A, Hildebrandt GC, Dassinger B, Klappstein G, Kaps M, Gizewski ER, Acker T, Grams A. Development of progressive multifocal leukoencephalopathy in a patient with non-Hodgkin lymphoma 13 years after treatment with cladribine. Leuk Lymphoma. 2013;54:1340–1342. doi: 10.3109/10428194.2012.740669. [DOI] [PubMed] [Google Scholar]
- 25.Berghoff M, Dassinger B, Iwinska-Zelder J, Giraldo M, Bilgin S, Kaps M, Gizewski ER. A case of natalizumab-associated progressive multifocal leukoencephalopathy-role for advanced MRI? Clin Neuroradiol. 2014;24:173–176. doi: 10.1007/s00062-013-0216-z. [DOI] [PubMed] [Google Scholar]
- 26.Berner B, Krieter DH, Rumpf KW, Grunewald RW, Beuche W, Weber T, Muller GA. Progressive multifocal leukoencephalopathy in a renal transplant patient diagnosed by JCV-specific DNA amplification and an intrathecal humoral immune response to recombinant virus protein 1. Nephrol Dial Transplant. 1999;14:462–465. doi: 10.1093/ndt/14.2.462. [DOI] [PubMed] [Google Scholar]
- 27.Blair NF, Brew BJ, Halpern JP. Natalizumab-associated PML identified in the presymptomatic phase using MRI surveillance. Neurology. 2012;78:507–508. doi: 10.1212/WNL.0b013e318246d6d8. [DOI] [PubMed] [Google Scholar]
- 28.Boster AL, Nicholas JA, Topalli I, Kisanuki YY, Pei W, Morgan-Followell B, Kirsch CF, Racke MK, Pitt D. Lessons learned from fatal progressive multifocal leukoencephalopathy in a patient with multiple sclerosis treated with natalizumab. JAMA Neurol. 2013;70:398–402. doi: 10.1001/jamaneurol.2013.1960. [DOI] [PubMed] [Google Scholar]
- 29.Boulton-Jones JR, Fraser-Moodie C, Ryder SD. Long term survival from progressive multifocal leucoencephalopathy after liver transplantation. J Hepatol. 2001;35:828–829. doi: 10.1016/S0168-8278(01)00202-1. [DOI] [PubMed] [Google Scholar]
- 30.Bronster DJ, Lidov MW, Wolfe D, Schwartz ME, Miller CM. Progressive multifocal leukoencephalopathy after orthotopic liver transplantation. Liver Transpl Surg. 1995;1:371–372. doi: 10.1002/lt.500010606. [DOI] [PubMed] [Google Scholar]
- 31.Bruggemann N, Gottschalk S, Kortke D, Marxsen JH, Moser A. Excessively increased CSF tau in progressive multifocal leukoencephalopathy. Clin Neurol Neurosurg. 2012;114:762–764. doi: 10.1016/j.clineuro.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 32.Buckanovich RJ, Liu G, Stricker C, Luger SM, Stadtmauer EA, Schuster SJ, Duffy K, Tsai D, Pruitt A, Porter DL. Nonmyeloablative allogeneic stem cell transplantation for refractory Hodgkin’s lymphoma complicated by interleukin-2 responsive progressive multifocal leukoencephalopathy. Ann Hematol. 2002;81:410–413. doi: 10.1007/s00277-002-0481-4. [DOI] [PubMed] [Google Scholar]
- 33.Buttmann M, Stoll G. Case reports of PML in patients treated for psoriasis. N Engl J Med. 2013;369:1081. doi: 10.1056/NEJMc1307680. [DOI] [PubMed] [Google Scholar]
- 34.Calvi A, De RM, Pietroboni AM, Ghezzi L, Maltese V, Arighi A, Fumagalli GG, Jacini F, Donelli C, Comi G, Galimberti D, Scarpini E. Partial recovery after severe immune reconstitution inflammatory syndrome in a multiple sclerosis patient with progressive multifocal leukoencephalopathy. Immunotherapy. 2014;6:23–28. doi: 10.2217/imt.13.155. [DOI] [PubMed] [Google Scholar]
- 35.Carson KR, Newsome SD, Kim EJ, Wagner-Johnston ND, von Geldern G, Moskowitz CH, Moskowitz AJ, Rook AH, Jalan P, Loren AW, Landsburg D, Coyne T, Tsai D, Raisch DW, Norris LB, Bookstaver PB, Sartor O, Bennett CL. Progressive multifocal leukoencephalopathy associated with brentuximab vedotin therapy: a report of 5 cases from the Southern Network on Adverse Reactions (SONAR) project. Cancer. 2014;120:2464–2471. doi: 10.1002/cncr.28712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cei M, Mumoli N, Ferrito G, Scazzeri F. Progressive multifocal leukoencephalopathy. South Med J. 2010;103:1074–1075. doi: 10.1097/SMJ.0b013e3181f070a1. [DOI] [PubMed] [Google Scholar]
- 37.Chakraborty S, Tarantolo SR, Treves J, Sambol D, Hauke RJ, Batra SK. Progressive multifocal leukoencephalopathy in a HIV-negative patient with small lymphocytic leukemia following treatment with Rituximab. Case Rep Oncol. 2011;4:136–142. doi: 10.1159/000326851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chihara D, Takeoka T, Shirase T, Kishimoto W, Arimoto-Miyamoto K, Tsuji M, Ohno T. Progressive multifocal leukoencephalopathy in myelodysplastic syndrome involving pure red cell aplasia. Intern Med. 2010;49:2347–2352. doi: 10.2169/internalmedicine.49.4081. [DOI] [PubMed] [Google Scholar]
- 39.Choy DS, Weiss A, Lin PT. Progressive multifocal leukoencephalopathy following treatment for Wegener’s granulomatosis. JAMA. 1992;268:600–601. doi: 10.1001/jama.1992.03490050048011. [DOI] [PubMed] [Google Scholar]
- 40.Cid J, Revilla M, Cervera A, Cervantes F, Munoz E, Ferrer I, Montserrat E. Progressive multifocal leukoencephalopathy following oral fludarabine treatment of chronic lymphocytic leukemia. Ann Hematol. 2000;79:392–395. doi: 10.1007/s002779900149. [DOI] [PubMed] [Google Scholar]
- 41.Clerico M, Schiavetti I, De Mercanti SF, Piazza F, Gned D, Brescia MV, Lanzillo R, Ghezzi A, Bianchi A, Salemi G, Realmuto S, Sola P, Vitetta F, Cavalla P, Paolicelli D, Trojano M, Sormani MP, Durelli L. Treatment of relapsing-remitting multiple sclerosis after 24 doses of natalizumab: evidence from an Italian spontaneous, prospective, and observational study (the TY-STOP Study) JAMA Neurol. 2014;71:954–960. doi: 10.1001/jamaneurol.2014.1200. [DOI] [PubMed] [Google Scholar]
- 42.Clifford DB, Ances B, Costello C, Rosen-Schmidt S, Andersson M, Parks D, Perry A, Yerra R, Schmidt R, Alvarez E, Tyler KL. Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol. 2011;68:1156–1164. doi: 10.1001/archneurol.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coppo P, Laporte JP, Aoudjhane M, Lebon P, Isnard F, Lesage S, Gorin NC, Najman A. Progressive multifocal leucoencephalopathy with peripheral demyelinating neuropathy after autologous bone marrow transplantation for acute myeloblastic leukemia (FAB5) Bone Marrow Transplant. 1999;23:401–403. doi: 10.1038/sj.bmt.1701555. [DOI] [PubMed] [Google Scholar]
- 44.Crowder CD, Gyure KA, Drachenberg CB, Werner J, Morales RE, Hirsch HH, Ramos E. Successful outcome of progressive multifocal leukoencephalopathy in a renal transplant patient. Am J Transplant. 2005;5:1151–1158. doi: 10.1111/j.1600-6143.2005.00800.x. [DOI] [PubMed] [Google Scholar]
- 45.Cuevas LA, Fuchs HA. Progressive multifocal leucoencephalopathy and immunosuppression. Ann Rheum Dis. 2004;63:112–113. [PMC free article] [PubMed] [Google Scholar]
- 46.D’Souza A, Wilson J, Mukherjee S, Jaiyesimi I. Progressive multifocal leukoencephalopathy in chronic lymphocytic leukemia: a report of three cases and review of the literature. Clin Lymphoma Myeloma Leuk. 2010;10:E1–E9. doi: 10.3816/CLML.2010.n.009. [DOI] [PubMed] [Google Scholar]
- 47.Daibata M, Hatakeyama N, Kamioka M, Nemoto Y, Hiroi M, Miyoshi I, Taguchi H. Detection of human herpesvirus 6 and JC virus in progressive multifocal leukoencephalopathy complicating follicular lymphoma. Am J Hematol. 2001;67:200–205. doi: 10.1002/ajh.1108. [DOI] [PubMed] [Google Scholar]
- 48.Damasceno A, von Glehn F, Martinez AR, Longhini AL, Deus-Silva L, Brandao CO, Santos LM, Damasceno BP. Early onset of natalizumab-related progressive multifocal leukoencephalopathy. Mult Scler. 2011;17:1397–1398. doi: 10.1177/1352458511422929. [DOI] [PubMed] [Google Scholar]
- 49.Dammeier N, Schubert V, Hauser TK, Bornemann A, Bischof F. Case report of a patient with progressive multifocal leukoencephalopathy under treatment with dimethyl fumarate. BMC Neurol. 2015;15:108. doi: 10.1186/s12883-015-0363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dastmalchi M, Laki J, Lundberg IE, Iacobaeus E. Progressive multifocal leukoencephalopathy in a patient with polymyositis: case report and literature review. J Rheumatol. 2012;39:1299–1303. doi: 10.3899/jrheum.111126. [DOI] [PubMed] [Google Scholar]
- 51.Dawson DM. Progressive multifocal leukoencephalopathy in myasthenia gravis. Ann Neurol. 1982;11:218–219. doi: 10.1002/ana.410110227. [DOI] [PubMed] [Google Scholar]
- 52.del Pilar Martin M, Cravens PD, Winger R, Kieseier BC, Cepok S, Eagar TN, Zamvil SS, Weber MS, Frohman EM, Kleinschmidt-DeMasters BK, Montine TJ, Hemmer B, Marra CM, Stuve O. Depletion of B lymphocytes from cerebral perivascular spaces by rituximab. Arch Neurol. 2009;66:1016–1020. doi: 10.1001/archneurol.2009.157. [DOI] [PubMed] [Google Scholar]
- 53.Desmond R, Lynch K, Gleeson M, Farrell M, Murphy P. Progressive multifocal leukoencephalopathy and cerebral toxoplasmosis in a patient with CLL. Am J Hematol. 2010;85:607. doi: 10.1002/ajh.21589. [DOI] [PubMed] [Google Scholar]
- 54.Dima D, Tomuleasa C, Irimie A, Florian IS, Petrushev B, Berindan-Neagoe I, Cucuianu A. Magnetic resonance imaging-based diagnosis of progressive multifocal leukoencephalopathy in a patient with non-Hodgkin lymphoma after therapy with cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab. Cancer. 2014;120:4005–4006. doi: 10.1002/cncr.28948. [DOI] [PubMed] [Google Scholar]
- 55.Dominguez-Mozo MI, Garcia-Montojo M, De Las Heras V, Garcia-Martinez A, Arias-Leal AM, Casanova I, Arroyo R, Alvarez-Lafuente R. Anti-JCV antibodies detection and JCV DNA levels in PBMC, serum and urine in a cohort of Spanish Multiple Sclerosis patients treated with natalizumab. J Neuroimmune Pharmacol. 2013;8:1277–1286. doi: 10.1007/s11481-013-9496-y. [DOI] [PubMed] [Google Scholar]
- 56.Egan JD, Ring BL, Reding MJ, Wells IC, Shuman RM. Reticulum cell sarcoma and progressive multifocal leukoencephalopathy following renal transplantation. Transplantation. 1980;29:84–86. doi: 10.1097/00007890-198001000-00021. [DOI] [PubMed] [Google Scholar]
- 57.Elster MJ. Natalizumab (tysabri)-associated progressive multifocal leukoencephalopathy: insights from perfusion magnetic resonance imaging. J Comput Assist Tomogr. 2013;37:694–697. doi: 10.1097/RCT.0b013e318298aa0d. [DOI] [PubMed] [Google Scholar]
- 58.Embrey JR, Silva FG, Helderman JH, Peters PC, Sagalowsky AI. Long-term survival and late development of bladder cancer in renal transplant patient with progressive multifocal leukoencephalopathy. J Urol. 1988;139:580–581. doi: 10.1016/s0022-5347(17)42533-x. [DOI] [PubMed] [Google Scholar]
- 59.Epker JL, van Biezen P, van Daele PL, van Gelder T, Vossen A, van Saase JL. Progressive multifocal leukoencephalopathy, a review and an extended report of five patients with different immune compromised states. Eur J Intern Med. 2009;20:261–267. doi: 10.1016/j.ejim.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 60.Ermis U, Weis J, Schulz JB. PML in a patient treated with fumaric acid. N Engl J Med. 2013;368:1657–1658. doi: 10.1056/NEJMc1211805. [DOI] [PubMed] [Google Scholar]
- 61.Felli V, Di SA, Anselmi M, Gennarelli A, Sucapane P, Splendiani A, Catalucci A, Marini C, Gallucci M. Progressive multifocal leukoencephalopathy following treatment with rituximab in an HIV-negative patient with non-hodgkin lymphoma. A case report and literature review. Neuroradiol J. 2014;27:657–664. doi: 10.15274/NRJ-2014-10087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fianchi L, Colosimo C, De LA, Pompucci A, Cattani P, Voso MT, LaRocca LM, Leone G, Pagano L. Atypical presentation of progressive multifocal leukoencephalopathy in a multiple myeloma patient after auto-SCT successfully treated with combination therapy. Bone Marrow Transplant. 2010;45:1668–1670. doi: 10.1038/bmt.2010.33. [DOI] [PubMed] [Google Scholar]
- 63.Fine AJ, Sorbello A, Kortepeter C, Scarazzini L. Progressive multifocal leukoencephalopathy after natalizumab discontinuation. Ann Neurol. 2014;75:108–115. doi: 10.1002/ana.24051. [DOI] [PubMed] [Google Scholar]
- 64.Fleischmann RM. Progressive multifocal leukoencephalopathy following rituximab treatment in a patient with rheumatoid arthritis. Arthritis Rheum. 2009;60:3225–3228. doi: 10.1002/art.24906. [DOI] [PubMed] [Google Scholar]
- 65.Flomenbaum MA, Jarcho JA, Schoen FJ. Progressive multifocal leukoencephalopathy fifty-seven months after heart transplantation. J Heart Lung Transplant. 1991;10:888–893. [PubMed] [Google Scholar]
- 66.Focosi D, Fazzi R, Montanaro D, Emdin M, Petrini M. Progressive multifocal leukoencephalopathy in a haploidentical stem cell transplant recipient: a clinical, neuroradiological and virological response after treatment with risperidone. Antiviral Res. 2007;74:156–158. doi: 10.1016/j.antiviral.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 67.Fredericks CA, Kvam KA, Bear J, Crabtree GS, Josephson SA. A case of progressive multifocal leukoencephalopathy in a lupus patient treated with belimumab. Lupus. 2014;23:711–713. doi: 10.1177/0961203314524292. [DOI] [PubMed] [Google Scholar]
- 68.Freim Wahl SG, Folvik MR, Torp SH. Progressive multifocal leukoencephalopathy in a lymphoma patient with complete remission after treatment with cytostatics and rituximab: case report and review of the literature. Clin Neuropathol. 2007;26:68–73. doi: 10.5414/NPP26068. [DOI] [PubMed] [Google Scholar]
- 69.Gajofatto A, Bianchi MR, Deotto L, Benedetti MD. Are natalizumab and fingolimod analogous second-line options for the treatment of relapsing-remitting multiple sclerosis? A clinical practice observational study. Eur Neurol. 2014;72:173–180. doi: 10.1159/000361044. [DOI] [PubMed] [Google Scholar]
- 70.Gaman A, Bold A, Gaman G. The unexpected evolution of a case of diffuse large B-cell non-Hodgkin lymphoma. Rom J Morphol Embryol. 2011;52:719–722. [PubMed] [Google Scholar]
- 71.Garcia JH, Pearson J, Bonnin J, Gupta KL. Deteriorating neurologic function in a 28-year-old renal transplant recipient. Ala J Med Sci. 1985;22:208–214. [PubMed] [Google Scholar]
- 72.Garrote H, de la Fuente A, Ona R, Rodriguez I, Echevarria JE, Sepulveda JM, Garcia JF. Long-term survival in a patient with progressive multifocal leukoencephalopathy after therapy with rituximab, fludarabine and cyclophosphamide for chronic lymphocytic leukemia. Exp Hematol Oncol. 2015;4:8. doi: 10.1186/s40164-015-0003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gedizlioglu M, Coban P, Ce P, Sivasli IE. An unusual complication of immunosuppression in myasthenia gravis: progressive multifocal leukoencephalopathy. Neuromuscul Disord. 2009;19:155–157. doi: 10.1016/j.nmd.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 74.Gentile S, Sacerdote I, Roccatello D, Giordana MT. Progressive multifocal leukoencephalopathy during cyclosporine treatment. A case report. Ital J Neurol Sci. 1996;17:363–366. doi: 10.1007/BF01999899. [DOI] [PubMed] [Google Scholar]
- 75.Giacomini PS, Rozenberg A, Metz I, Araujo D, Arbour N, Bar-Or A. Maraviroc and JC virus-associated immune reconstitution inflammatory syndrome. N Engl J Med. 2014;370:486–488. doi: 10.1056/NEJMc1304828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldberg SL, Pecora AL, Alter RS, Kroll MS, Rowley SD, Waintraub SE, Imrit K, Preti RA. Unusual viral infections (progressive multifocal leukoencephalopathy and cytomegalovirus disease) after high-dose chemotherapy with autologous blood stem cell rescue and peritransplantation rituximab. Blood. 2002;99:1486–1488. doi: 10.1182/blood.V99.4.1486. [DOI] [PubMed] [Google Scholar]
- 77.Gonzalez H, Bolgert F, Camporo P, Leblond V. Progressive multifocal leukoencephalitis (PML) in three patients treated with standard-dose fludarabine (FAMP) Hematol Cell Ther. 1999;41:183–186. doi: 10.1007/s00282-999-0183-7. [DOI] [PubMed] [Google Scholar]
- 78.Govindappa V, Hicks S, Wichter M, Jolly M. Progressive multifocal leukoencephalopathy in systemic lupus erythematosus. Arthritis Rheum. 2007;57:352–354. doi: 10.1002/art.22545. [DOI] [PubMed] [Google Scholar]
- 79.Graff-Radford J, Robinson MT, Warsame RM, Matteson EL, Eggers SD, Keegan BM. Progressive multifocal leukoencephalopathy in a patient treated with etanercept. Neurologist. 2012;18:85–87. doi: 10.1097/NRL.0b013e318247b868. [DOI] [PubMed] [Google Scholar]
- 80.Grinyo J, Charpentier B, Pestana JM, Vanrenterghem Y, Vincenti F, Reyes-Acevedo R, Apanovitch AM, Gujrathi S, Agarwal M, Thomas D, Larsen CP. An integrated safety profile analysis of belatacept in kidney transplant recipients. Transplantation. 2010;90:1521–1527. doi: 10.1097/TP.0b013e3182007b95. [DOI] [PubMed] [Google Scholar]
- 81.Haghikia A, Perrech M, Pula B, Ruhrmann S, Potthoff A, Brockmeyer NH, Goelz S, Wiendl H, Linda H, Ziemssen T, Baranzini SE, Kall TB, Bengel D, Olsson T, Gold R, Chan A. Functional energetics of CD4+ -cellular immunity in monoclonal antibody-associated progressive multifocal leukoencephalopathy in autoimmune disorders. PLoS One. 2011;6:e18506. doi: 10.1371/journal.pone.0018506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hall WA, Martinez AJ, Dummer JS. Progressive multifocal leukoencephalopathy after cardiac transplantation. Neurology. 1988;38:995–996. doi: 10.1212/WNL.38.6.995-a. [DOI] [PubMed] [Google Scholar]
- 83.Harris HE. Progressive multifocal leucoencephalopathy in a patient with systemic lupus erythematosus treated with rituximab. Rheumatology (Oxford) 2008;47:224–225. doi: 10.1093/rheumatology/kem299. [DOI] [PubMed] [Google Scholar]
- 84.Hasan MM, Taylor P. Progressive multifocal leucoencephalopathy in a case of chronic lymphocytic leukaemia. Br J Haematol. 2005;130:808. doi: 10.1111/j.1365-2141.2005.05587.x. [DOI] [PubMed] [Google Scholar]
- 85.Havla J, Berthele A, Kumpfel T, Krumbholz M, Jochim A, Kronsbein H, Ryschkewitsch C, Jensen P, Lippmann K, Hemmer B, Major E, Hohlfeld R. Co-occurrence of two cases of progressive multifocal leukoencephalopathy in a natalizumab “infusion group’. Mult Scler. 2013;19:1213–1215. doi: 10.1177/1352458512466165. [DOI] [PubMed] [Google Scholar]
- 86.Heine A, Schmiedel A, Menschik T, Held SA, Erdmann C, Brossart P. Regression of liver metastases after treatment with oxaliplatin/capecitabine and development of a progressive multifocal leukoencephalopathy in a patient with advanced thymoma. J Clin Oncol. 2013;31:e203–e205. doi: 10.1200/JCO.2012.43.8150. [DOI] [PubMed] [Google Scholar]
- 87.Hendel-Chavez H, de Goer de Herve MG, Giannesini C, Mazet AA, Papeix C, Louapre C, Chardain A, Boutarfa N, Theaudin M, Adams D, Gasnault J, Stankoff B, Taoufik Y. Immunological hallmarks of JC virus replication in multiple sclerosis patients on long-term natalizumab therapy. J Virol. 2013;87:6055–6059. doi: 10.1128/JVI.00131-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Herold T, Seiler T, Egensperger R, Trumm C, Bergmann M, Franke D, Mumm FF, Schinwald N, Buske C, Dreyling M. Progressive multifocal leukoencephalopathy after treatment with rituximab, fludarabine and cyclophosphamide in a patient with chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53:169–172. doi: 10.3109/10428194.2011.608446. [DOI] [PubMed] [Google Scholar]
- 89.Ho K, Garancis JC, Paegle RD, Gerber MA, Borkowski WJ. Progressive multifocal leukoencephalopathy and malignant lymphoma of the brain in a patient with immunosuppressive therapy. Acta Neuropathol. 1980;52:81–83. doi: 10.1007/BF00687233. [DOI] [PubMed] [Google Scholar]
- 90.Hoepner R, Faissner S, Klasing A, Schneider R, Metz I, Bellenberg B, Lukas C, Altmeyer P, Gold R, Chan A. Progressive multifocal leukoencephalopathy during fumarate monotherapy of psoriasis. Neurol Neuroimmunol Neuroinflamm. 2015;2:e85. doi: 10.1212/NXI.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Holmen C, Piehl F, Hillert J, Fogdell-Hahn A, Lundkvist M, Karlberg E, Nilsson P, Dahle C, Feltelius N, Svenningsson A, Lycke J, Olsson T. A Swedish national post-marketing surveillance study of natalizumab treatment in multiple sclerosis. Mult Scler. 2011;17:708–719. doi: 10.1177/1352458510394701. [DOI] [PubMed] [Google Scholar]
- 92.Isidoro L, Pires P, Rito L, Cordeiro G. Progressive multifocal leukoencephalopathy in a patient with chronic lymphocytic leukaemia treated with alemtuzumab. BMJ Case Rep. 2014 doi: 10.1136/bcr-2013-201781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Itoh K, Kano T, Nagashio C, Mimori A, Kinoshita M, Sumiya M. Progressive multifocal leukoencephalopathy in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:1020–1022. doi: 10.1002/art.21721. [DOI] [PubMed] [Google Scholar]
- 94.Jones HR, Jr, Hedley-Whyte ET, Freidberg SR, Kelleher JE, Jr, Krolikowski J. Primary cerebellopontine progressive multifocal leukoencephalopathy diagnosed premortem by cerebellar biopsy. Ann Neurol. 1982;11:199–202. doi: 10.1002/ana.410110218. [DOI] [PubMed] [Google Scholar]
- 95.Kalisch A, Wilhelm M, Erbguth F, Birkmann J. Progressive multifocal leukoencephalopathy in patients with a hematological malignancy: review of therapeutic options. Chemotherapy. 2014;60:47–53. doi: 10.1159/000368072. [DOI] [PubMed] [Google Scholar]
- 96.Kaufman GP, Aksamit AJ, Klein CJ, Yi ES, Delone DR, Litzow MR. Progressive multifocal leukoencephalopathy: a rare infectious complication following allogeneic hematopoietic cell transplantation (HCT) Eur J Haematol. 2014;92:83–87. doi: 10.1111/ejh.12208. [DOI] [PubMed] [Google Scholar]
- 97.Kharfan-Dabaja MA, Ayala E, Greene J, Rojiani A, Murtagh FR, Anasetti C. Two cases of progressive multifocal leukoencephalopathy after allogeneic hematopoietic cell transplantation and a review of the literature. Bone Marrow Transplant. 2007;39:101–107. doi: 10.1038/sj.bmt.1705548. [DOI] [PubMed] [Google Scholar]
- 98.Khoury S, Shapira S, Zilberman T, Mekori YA, Hershko AY. Progressive multifocal leukoencephalopathy in an HIV-negative patient following treatment with rituximab. Isr Med Assoc J. 2013;15:321–322. [PubMed] [Google Scholar]
- 99.Kiewe P, Seyfert S, Korper S, Rieger K, Thiel E, Knauf W. Progressive multifocal leukoencephalopathy with detection of JC virus in a patient with chronic lymphocytic leukemia parallel to onset of fludarabine therapy. Leuk Lymphoma. 2003;44:1815–1818. doi: 10.1080/1042819031000116625. [DOI] [PubMed] [Google Scholar]
- 100.Kinoshita M, Iwana K, Shinoura H, Aotsuka S, Sumiya M. Progressive multifocal leukoencephalopathy resembling central nervous system systemic lupus erythematosus. Clin Exp Rheumatol. 1998;16:313–315. [PubMed] [Google Scholar]
- 101.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 102.Kleinschmidt-DeMasters BK, Miravalle A, Schowinsky J, Corboy J, Vollmer T. Update on PML and PML-IRIS occurring in multiple sclerosis patients treated with natalizumab. J Neuropathol Exp Neurol. 2012;71:604–617. doi: 10.1097/NEN.0b013e31825caf2c. [DOI] [PubMed] [Google Scholar]
- 103.Kleiter I, Schroder M, Lurding R, Schuierer G, Clifford DB, Bogdahn U, Steinbrecher A, Poschl P. Early changes on electroencephalography in natalizumab-associated progressive multifocal leucoencephalopathy. Mult Scler. 2010;16:749–753. doi: 10.1177/1352458510367718. [DOI] [PubMed] [Google Scholar]
- 104.Klintmalm GB, Feng S, Lake JR, Vargas HE, Wekerle T, Agnes S, Brown KA, Nashan B, Rostaing L, Meadows-Shropshire S, Agarwal M, Harler MB, Garcia-Valdecasas JC. Belatacept-based immunosuppression in de novo liver transplant recipients: 1-year experience from a phase II randomized study. Am J Transplant. 2014;14:1817–1827. doi: 10.1111/ajt.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ko MY, Stefoski D, Balabanov R. Indolent course of progressive multifocal leukoencephalopathy during natalizumab treatment in MS. Neurology. 2011;77:1020. doi: 10.1212/WNL.0b013e31822946d0. [DOI] [PubMed] [Google Scholar]
- 106.Kobayashi K, Okamoto Y, Inoue H, Usui T, Ihara M, Kawamata J, Miki Y, Mimori T, Tomimoto H, Takahashi R. Leukoencephalopathy with cognitive impairment following tocilizumab for the treatment of rheumatoid arthritis (RA) Intern Med. 2009;48:1307–1309. doi: 10.2169/internalmedicine.48.1926. [DOI] [PubMed] [Google Scholar]
- 107.Kobayashi Z, Akaza M, Numasawa Y, Ishihara S, Tomimitsu H, Nakamichi K, Saijo M, Morio T, Shimizu N, Sanjo N, Shintani S, Mizusawa H. Failure of mefloquine therapy in progressive multifocal leukoencephalopathy: report of two Japanese patients without human immunodeficiency virus infection. J Neurol Sci. 2013;324:190–194. doi: 10.1016/j.jns.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 108.Koralnik IJ, Schellingerhout D, Frosch MP. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 14-2004. A 66-year-old man with progressive neurologic deficits. N Engl J Med. 2004;350:1882–1893. doi: 10.1056/NEJMcpc030038. [DOI] [PubMed] [Google Scholar]
- 109.Kothary N, Diak IL, Brinker A, Bezabeh S, Avigan M, Dal PG. Progressive multifocal leukoencephalopathy associated with efalizumab use in psoriasis patients. J Am Acad Dermatol. 2011;65:546–551. doi: 10.1016/j.jaad.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 110.Kranick SM, Mowry EM, Rosenfeld MR. Progressive multifocal leukoencephalopathy after rituximab in a case of non-Hodgkin lymphoma. Neurology. 2007;69:704–706. doi: 10.1212/01.wnl.0000267325.06000.d9. [DOI] [PubMed] [Google Scholar]
- 111.Kuhle J, Gosert R, Buhler R, Derfuss T, Sutter R, Yaldizli O, Radue EW, Ryschkewitsch C, Major EO, Kappos L, Frank S, Hirsch HH. Management and outcome of CSF-JC virus PCR-negative PML in a natalizumab-treated patient with MS. Neurology. 2011;77:2010–2016. doi: 10.1212/WNL.0b013e31823b9b27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kumar D, Bouldin TW, Berger RG. A case of progressive multifocal leukoencephalopathy in a patient treated with infliximab. Arthritis Rheum. 2010;62:3191–3195. doi: 10.1002/art.27687. [DOI] [PubMed] [Google Scholar]
- 113.Lach B, Connolly B, Wuthrich C, Koralnik IJ. Inflammatory infratentorial progressive multifocal leukoencephalopathy in a patient with rheumatoid arthritis. Neuropathology. 2014;34:39–44. doi: 10.1111/neup.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lalive PH, Bridel C, Ferfoglia RI, Kaiser L, Du PR, Barkhof F, Haller S. Minimal supportive treatment in natalizumab-related PML in a MS patient. J Neurol Neurosurg Psychiatry. 2015;86:354–355. doi: 10.1136/jnnp-2014-308154. [DOI] [PubMed] [Google Scholar]
- 115.Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 116.Leblanc-Trudeau C, Masetto A, Bocti C. Progressive multifocal leukoencephalopathy associated with belimumab in a patient with systemic lupus erythematosus. J Rheumatol. 2015;42:551–552. doi: 10.3899/jrheum.140577. [DOI] [PubMed] [Google Scholar]
- 117.Lee DH, Waschbisch A, Lammer AB, Doerfler A, Schwab S, Linker RA. Immunological and clinical consequences of splenectomy in a multiple sclerosis patient treated with natalizumab. J Neuroinflammation. 2013;10:123. doi: 10.1186/1742-2094-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lefevre G, Queyrel V, Maurage CA, Laurent C, Launay D, Lacour A, Charlanne H, Morell-Dubois S, Lambert M, Maillard H, Vermersch P, Hachulla E, Hatron PY. Effective immune restoration after immunosuppressant discontinuation in a lupus patient presenting progressive multifocal leukoencephalopathy. J Neurol Sci. 2009;287:246–249. doi: 10.1016/j.jns.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 119.Lejniece S, Murovska M, Chapenko S, Breiksa B, Jaunmuktane Z, Feldmane L, Ziedina I, Gomez-Roman J, Garcia-Cabeza M, Lejnieks A. Progressive multifocal leukoencephalopathy following fludarabine treatment in a chronic lymphocytic leukemia patient. Exp Oncol. 2011;33:239–241. [PubMed] [Google Scholar]
- 120.Lewis AR, Kline LB, Pinkard NB. Visual loss due to progressive multifocal leukoencephalopathy in a heart transplant patient. J Clin Neuroophthalmol. 1993;13:237–241. [PubMed] [Google Scholar]
- 121.Lima MA, Hanto DW, Curry MP, Wong MT, Dang X, Koralnik IJ. Atypical radiological presentation of progressive multifocal leukoencephalopathy following liver transplantation. J Neurovirol. 2005;11:46–50. doi: 10.1080/13550280590900742. [DOI] [PubMed] [Google Scholar]
- 122.Linda H, von Heijne A, Major EO, Ryschkewitsch C, Berg J, Olsson T, Martin C. Progressive multifocal leukoencephalopathy after natalizumab monotherapy. N Engl J Med. 2009;361:1081–1087. doi: 10.1056/NEJMoa0810316. [DOI] [PubMed] [Google Scholar]
- 123.Linda H, von Heijne A. Presymptomatic diagnosis with MRI and adequate treatment ameliorate the outcome after natalizumab-associated progressive multifocal leukoencephalopathy. Front Neurol. 2013;4:11. doi: 10.3389/fneur.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lobo LJ, Reynolds JM, Snyder LD. Rituximab-associated progressive multifocal leukoencephalopathy after lung transplantation. J Heart Lung Transplant. 2013;32:752–753. doi: 10.1016/j.healun.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 125.Loyaga-Rendon RY, Taylor DO, Koval CE. Progressive multifocal leukoencephalopathy in a heart transplant recipient following rituximab therapy for antibody-mediated rejection. Am J Transplant. 2013;13:1075–1079. doi: 10.1111/ajt.12153. [DOI] [PubMed] [Google Scholar]
- 126.Maillart E, Louapre C, Lubetzki C, Papeix C. Fingolimod to treat severe multiple sclerosis after natalizumab-associated progressive multifocal leukoencephalopathy: a valid option? Mult Scler. 2014;20:505–509. doi: 10.1177/1352458513516530. [DOI] [PubMed] [Google Scholar]
- 127.Malas D, Weiss S. Progressive multifocal leukoencephalopathy and cryptococcal meningitis with systemic lupus erythematosus and thymoma. Ann Neurol. 1977;1:188–191. doi: 10.1002/ana.410010216. [DOI] [PubMed] [Google Scholar]
- 128.Manfro RC, Vedolin L, Cantarelli M, Oppitz P, Antunes AC, Rieder CR. Progressive multifocal leukoencephalopathy in a kidney transplant recipient after conversion to mycophenolic acid therapy. Transpl Infect Dis. 2009;11:189–190. doi: 10.1111/j.1399-3062.2009.00368.x. [DOI] [PubMed] [Google Scholar]
- 129.Manz HJ, Dinsdale HB, Morrin PA. Progressive multifocal leukoencephalopathy after renal transplantation. Demonstration of Papova-like virions. Ann Intern Med. 1971;75:77–81. doi: 10.7326/0003-4819-75-1-77. [DOI] [PubMed] [Google Scholar]
- 130.Marie I, Guegan-Massardier E, Levesque H. Progressive multifocal leukoencephalopathy in refractory polymyositis treated with rituximab. Eur J Intern Med. 2011;22:e13–e14. doi: 10.1016/j.ejim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 131.Marriott PJ, O’Brien MD, Mackenzie IC, Janota I. Progressive multifocal leucoencephalopathy: remission with cytarabine. J Neurol Neurosurg Psychiatry. 1975;38:205–209. doi: 10.1136/jnnp.38.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Martin SI, Marty FM, Fiumara K, Treon SP, Gribben JG, Baden LR. Infectious complications associated with alemtuzumab use for lymphoproliferative disorders. Clin Infect Dis. 2006;43:16–24. doi: 10.1086/504811. [DOI] [PubMed] [Google Scholar]
- 133.Marzocchetti A, Wuthrich C, Tan CS, Tompkins T, Bernal-Cano F, Bhargava P, Ropper AH, Koralnik IJ. Rearrangement of the JC virus regulatory region sequence in the bone marrow of a patient with rheumatoid arthritis and progressive multifocal leukoencephalopathy. J Neurovirol. 2008;14:455–458. doi: 10.1080/13550280802356837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Matijaca M, Vlasic-Matas J, Jankovic S, Pintaric I, Marovic A. Neurotoxicity that may mimic progressive multifocal leukoencephalopathy in patient with transplanted kidney. Coll Antropol. 2007;31:349–353. [PubMed] [Google Scholar]
- 135.Matteucci P, Magni M, Di NM, Carlo-Stella C, Uberti C, Gianni AM. Leukoencephalopathy and papovavirus infection after treatment with chemotherapy and anti-CD20 monoclonal antibody. Blood. 2002;100:1104–1105. doi: 10.1182/blood-2002-04-1271. [DOI] [PubMed] [Google Scholar]
- 136.Mazda ME, Brosch JR, Wiens AL, Bonnin JM, Kamer AP, Mattson DH, Snook RJ. A case of natalizumab-associated progressive multifocal leukoencephalopathy with repeated negative CSF JCV testing. Int J Neurosci. 2013;123:353–357. doi: 10.3109/00207454.2012.760561. [DOI] [PubMed] [Google Scholar]
- 137.Mc Govern EM, Hennessy MJ. Asymptomatic progressive multifocal leukoencephalopathy associated with natalizumab. J Neurol. 2013;260:665–667. doi: 10.1007/s00415-012-6759-0. [DOI] [PubMed] [Google Scholar]
- 138.McCormick WF, Schochet SS, Jr, Sarles HE, Calverley JR. Progressive multifocal leukoencephalopathy in renal transplant recipients. Arch Intern Med. 1976;136:829–834. doi: 10.1001/archinte.1976.03630070067020. [DOI] [PubMed] [Google Scholar]
- 139.McNally PG, Taylor JM, Wood JK. Progressive multifocal leucoencephalopathy associated with chronic lymphocytic leukaemia. Clin Lab Haematol. 1988;10:229–233. doi: 10.1111/j.1365-2257.1988.tb01177.x. [DOI] [PubMed] [Google Scholar]
- 140.Mesquita R, Parravicini C, Bjorkholm M, Ekman M, Biberfeld P. Macrophage association of polyomavirus in progressive multifocal leukoencephalopathy: an immunohistochemical and ultrastructural study. Case report. APMIS. 1992;100:993–1000. doi: 10.1111/j.1699-0463.1992.tb04031.x. [DOI] [PubMed] [Google Scholar]
- 141.Metivier D, Arnaud FX, Dutasta F, Nguema B, Teriitehau C, Berets O, Baccialone J, Potet J. Immune reconstitution inflammatory syndrome in a patient treated with natalizumab presenting progressive multifocal leukoencephalopathy. Diagn Interv Imaging. 2013;94:101–103. doi: 10.1016/j.diii.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 142.Montoto S, Moreno C, Domingo-Domenech E, Estany C, Oriol A, Altes A, Besalduch J, Pedro C, Gardella S, Escoda L, Asensio A, Vivancos P, Galan P, de Sevilla AF, Ribera JM, Briones J, Colomer D, Campo E, Montserrat E, Lopez-Guillermo A. High clinical and molecular response rates with fludarabine, cyclophosphamide and mitoxantrone in previously untreated patients with advanced stage follicular lymphoma. Haematologica. 2008;93:207–214. doi: 10.3324/haematol.11671. [DOI] [PubMed] [Google Scholar]
- 143.Moreno C, Montillo M, Panayiotidis P, Dimou M, Bloor A, Dupuis J, Schuh A, Norin S, Geisler C, Hillmen P, Doubek M, Trneny M, Obrtlikova P, Laurenti L, Stilgenbauer S, Smolej L, Ghia P, Cymbalista F, Jaeger U, Stamatopoulos K, Stavroyianni N, Carrington P, Zouabi H, Leblond V, Gomez-Garcia JC, Rubio M, Marasca R, Musuraca G, Rigacci L, Farina L, Paolini R, Pospisilova S, Kimby E, Bradley C, Montserrat E. Ofatumumab in poor-prognosis chronic lymphocytic leukemia: a phase IV, non-interventional, observational study from the European Research Initiative on Chronic Lymphocytic Leukemia. Haematologica. 2015;100:511–516. doi: 10.3324/haematol.2014.118158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Morgenstern LB, Pardo CA. Progressive multifocal leukoencephalopathy complicating treatment for Wegener’s granulomatosis. J Rheumatol. 1995;22:1593–1595. [PubMed] [Google Scholar]
- 145.Nagayama S, Gondo Y, Araya S, Minato N, Fujita-Nakata M, Kaito M, Nakanishi M, Tanaka K, Yamaya H, Yokoyama H, Nakamichi K, Saijo M, Okamoto K, Toyoshima Y, Kakita A, Matsui M. Progressive multifocal leukoencephalopathy developed 26 years after renal transplantation. Clin Neurol Neurosurg. 2013;115:1482–1484. doi: 10.1016/j.clineuro.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 146.Newton P, Aldridge RD, Lessells AM, Best PV. Progressive multifocal leukoencephalopathy complicating systemic lupus erythematosus. Arthritis Rheum. 1986;29:337–343. doi: 10.1002/art.1780290305. [DOI] [PubMed] [Google Scholar]
- 147.Ng C, Slavin MA, Seymour JF. Progressive multifocal leukoencephalopathy complicating Waldenstrom’s macroglobulinaemia. Leuk Lymphoma. 2003;44:1819–1821. doi: 10.1080/1042819031000111071. [DOI] [PubMed] [Google Scholar]
- 148.Nieuwkamp DJ, Murk JL, van Oosten BW, Cremers CH, Killestein J, Viveen MC, Van HW, Frijlink DW, Wattjes MP. PML in a patient without severe lymphocytopenia receiving dimethyl fumarate. N Engl J Med. 2015;372:1474–1476. doi: 10.1056/NEJMc1413724. [DOI] [PubMed] [Google Scholar]
- 149.Nived O, Bengtsson AA, Jonsen A, Sturfelt G. Progressive multifocal leukoencephalopathy–the importance of early diagnosis illustrated in four cases. Lupus. 2008;17:1036–1041. doi: 10.1177/0961203308089445. [DOI] [PubMed] [Google Scholar]
- 150.O’Shaughnessy D, Goldman JM, Roddie M, Schofield JB. Dizziness and confusion after bone marrow transplantation. BMJ. 1994;309:262–265. doi: 10.1136/bmj.309.6949.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ohta K, Obara K, Sakauchi M, Obara K, Takane H, Yogo Y. Lesion extension detected by diffusion-weighted magnetic resonance imaging in progressive multifocal leukoencephalopathy. J Neurol. 2001;248:809–811. doi: 10.1007/s004150170099. [DOI] [PubMed] [Google Scholar]
- 152.Osorio S, de la Camara R, Golbano N, Marti E, Fedele CG, Nieto S, Manzanares R, Fernandez-Ranada JM. Progressive multifocal leukoencephalopathy after stem cell transplantation, unsuccessfully treated with cidofovir. Bone Marrow Transplant. 2002;30:963–966. doi: 10.1038/sj.bmt.1703704. [DOI] [PubMed] [Google Scholar]
- 153.Outteryck O, Ongagna JC, Duhamel A, Zephir H, Collongues N, Lacour A, Fleury MC, Berteloot AS, Blanc F, Giroux M, Vermersch P, de Seze J. Anti-JCV antibody prevalence in a French cohort of MS patients under natalizumab therapy. J Neurol. 2012;259:2293–2298. doi: 10.1007/s00415-012-6487-5. [DOI] [PubMed] [Google Scholar]
- 154.Outteryck O, Ongagna JC, Brochet B, Rumbach L, Lebrun-Frenay C, Debouverie M, Zephir H, Ouallet JC, Berger E, Cohen M, Pittion S, Laplaud D, Wiertlewski S, Cabre P, Pelletier J, Rico A, Defer G, Derache N, Camu W, Thouvenot E, Moreau T, Fromont A, Tourbah A, Labauge P, Castelnovo G, Clavelou P, Casez O, Hautecoeur P, Papeix C, Lubetzki C, Fontaine B, Couturier N, Bohossian N, Clanet M, Vermersch P, de Seze J, Brassat D. A prospective observational post-marketing study of natalizumab-treated multiple sclerosis patients: clinical, radiological and biological features and adverse events. The BIONAT cohort. Eur J Neurol. 2014;21:40–48. doi: 10.1111/ene.12204. [DOI] [PubMed] [Google Scholar]
- 155.Ouwens JP, Haaxma-Reiche H, Verschuuren EA, Timens W, Steenhuis LH, de Boer WJ, van der Bij W. Visual symptoms after lung transplantation: a case of progressive multifocal leukoencephalopathy. Transpl Infect Dis. 2000;2:29–32. doi: 10.1034/j.1399-3062.2000.020106.x. [DOI] [PubMed] [Google Scholar]
- 156.Owczarczyk K, Hilker R, Brunn A, Hallek M, Rubbert A. Progressive multifocal leucoencephalopathy in a patient with sarcoidosis–successful treatment with cidofovir and mirtazapine. Rheumatology (Oxford) 2007;46:888–890. doi: 10.1093/rheumatology/kem049. [DOI] [PubMed] [Google Scholar]
- 157.Owen RG, Patmore RD, Smith GM, Barnard DL. Cytomegalovirus-induced T-cell proliferation and the development of progressive multifocal leucoencephalopathy following bone marrow transplantation. Br J Haematol. 1995;89:196–198. doi: 10.1111/j.1365-2141.1995.tb08930.x. [DOI] [PubMed] [Google Scholar]
- 158.Pagnoux C, Hayem G, Roux F, Rouidi SA, Palazzo E, Henin D, Meyer O. JC virus leukoencephalopathy complicating Wegener’s granulomatosis. Joint Bone Spine. 2003;70:376–379. doi: 10.1016/S1297-319X(03)00062-9. [DOI] [PubMed] [Google Scholar]
- 159.Palmieri A, Valentinis L, Bazzano S, Baldi A, Orlando F, Tenaglia S, D’Anna S. Progressive multifocal leukoencephalopathy following chemotherapy for lung cancer. Neurol Sci. 2011;32:683–685. doi: 10.1007/s10072-011-0494-7. [DOI] [PubMed] [Google Scholar]
- 160.Pavlovic AM, Bonaci-Nikolic B, Kozic D, Ostojic J, Abinun M, Svabic-Medjedovic T, Nikolic M, Sternic N. Progressive multifocal leukoencephalopathy associated with mycophenolate mofetil treatment in a woman with lupus and CD4+ T-lymphocyte deficiency. Lupus. 2012;21:100–102. doi: 10.1177/0961203311416693. [DOI] [PubMed] [Google Scholar]
- 161.Pelosini M, Focosi D, Rita F, Galimberti S, Caracciolo F, Benedetti E, Papineschi F, Petrini M. Progressive multifocal leukoencephalopathy: report of three cases in HIV-negative hematological patients and review of literature. Ann Hematol. 2008;87:405–412. doi: 10.1007/s00277-007-0411-6. [DOI] [PubMed] [Google Scholar]
- 162.Phan-Ba R, Lommers E, Tshibanda L, Calay P, Dubois B, Moonen G, Clifford D, Belachew S. MRI preclinical detection and asymptomatic course of a progressive multifocal leucoencephalopathy (PML) under natalizumab therapy. J Neurol Neurosurg Psychiatry. 2012;83:224–226. doi: 10.1136/jnnp-2011-300511. [DOI] [PubMed] [Google Scholar]
- 163.Phillips T, Jacobs R, Ellis EN. Polyoma nephropathy and progressive multifocal leukoencephalopathy in a renal transplant recipient. J Child Neurol. 2004;19:301–304. doi: 10.1177/088307380401900412. [DOI] [PubMed] [Google Scholar]
- 164.Piehl F, Holmen C, Hillert J, Olsson T. Swedish natalizumab (Tysabri) multiple sclerosis surveillance study. Neurol Sci. 2011;31(Suppl 3):289–293. doi: 10.1007/s10072-010-0345-y. [DOI] [PubMed] [Google Scholar]
- 165.Piola M, Di PF, Mascoli N, Binda S, Arnaboldi M, Rezzonico M. Atypical MRI features at early onset natalizumab-associated progressive multifocal leukoencephalopathy: a case report. J Neurol Sci. 2014;340:213–214. doi: 10.1016/j.jns.2014.02.037. [DOI] [PubMed] [Google Scholar]
- 166.Przepiorka D, Jaeckle KA, Birdwell RR, Fuller GN, Kumar AJ, Huh YO, McCutcheon I. Successful treatment of progressive multifocal leukoencephalopathy with low-dose interleukin-2. Bone Marrow Transplant. 1997;20:983–987. doi: 10.1038/sj.bmt.1701010. [DOI] [PubMed] [Google Scholar]
- 167.Pugnet G, Pagnoux C, Bezanahary H, Ly KH, Vidal E, Guillevin L. Progressive multifocal encephalopathy after cyclophosphamide in granulomatosis with polyangiitis (Wegener) patients: case report and review of literature. Clin Exp Rheumatol. 2013;31:S62–S64. [PubMed] [Google Scholar]
- 168.Rahmlow M, Shuster EA, Dominik J, Deen HG, Jr, Dickson DW, Aksamit AJ, Jr, Robles HA, Freeman WD. Leflunomide-associated progressive multifocal leukoencephalopathy. Arch Neurol. 2008;65:1538–1539. doi: 10.1001/archneur.65.11.1538. [DOI] [PubMed] [Google Scholar]
- 169.Rankin E, Scaravilli F. Progressive multifocal leukoencephalopathy in a patient with rheumatoid arthritis and polymyositis. J Rheumatol. 1995;22:777–779. [PubMed] [Google Scholar]
- 170.Ray M, Curtis JR, Baddley JW. A case report of progressive multifocal leucoencephalopathy (PML) associated with adalimumab. Ann Rheum Dis. 2014;73:1429–1430. doi: 10.1136/annrheumdis-2013-204978. [DOI] [PubMed] [Google Scholar]
- 171.Re D, Bamborschke S, Feiden W, Schroder R, Lehrke R, Diehl V, Tesch H. Progressive multifocal leukoencephalopathy after autologous bone marrow transplantation and alpha-interferon immunotherapy. Bone Marrow Transplant. 1999;23:295–298. doi: 10.1038/sj.bmt.1701568. [DOI] [PubMed] [Google Scholar]
- 172.Reddy N, Abel TW, Jagasia M, Morgan D, Weaver K, Greer J. Progressive multifocal leukoencephalopathy in a patient with follicular lymphoma treated with multiple courses of rituximab. Leuk Lymphoma. 2009;50:460–462. doi: 10.1080/10428190802695827. [DOI] [PubMed] [Google Scholar]
- 173.Rey J, Belmecheri N, Bouayed N, Ivanov V, Coso D, Gastaut JA, Bouabdallah R. JC papovavirus leukoencephalopathy after first line treatment with CHOP and rituximab. Haematologica. 2007;92:e101. doi: 10.3324/haematol.11259. [DOI] [PubMed] [Google Scholar]
- 174.Reznik M, Halleux J, Urbain E, Mouchette R, Castermans P, Beaujean M. Two cases of progressive multifocal leukoencephalopathy after renal transplantation. Acta Neuropathol Suppl. 1981;7:189–191. doi: 10.1007/978-3-642-81553-9_57. [DOI] [PubMed] [Google Scholar]
- 175.Ripellino P, Comi C, Mula M, Varrasi C, Conconi A, Stecco A, Brustia D, Nasuelli N, Savio K, De PL, Cantello R, Gaidano G, Monaco F. Progressive multifocal leucoencephalopathy after autologous bone marrow transplantation: a treatment option. BMJ Case Rep. 2011 doi: 10.1136/bcr.11.2010.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rodriguez L, Ribera JM, Batlle M, Xicoy B, Mate JL, Milla F, Feliu E. Progressive multifocal leukoencephalopathy shortly after the diagnosis of follicular lymphoma in a patient treated with fludarabine. Haematologica. 2002;87:ECR26. [PubMed] [Google Scholar]
- 177.Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med. 2015;372:1476–1478. doi: 10.1056/NEJMc1415408. [DOI] [PubMed] [Google Scholar]
- 178.Saad ED, Thomas DA, O’Brien S, Fuller GN, Medeiros LJ, Forman A, Albitar M, Schomer D, Kantarjian HM, Keating MJ. Progressive multifocal leukoencephalopathy with concurrent Richter’s syndrome. Leuk Lymphoma. 2000;38:183–190. doi: 10.3109/10428190009060332. [DOI] [PubMed] [Google Scholar]
- 179.Salmaggi A, Maccagnano E, Castagna A, Zeni S, Fantini F, Cinque P, Savoiardo M. Reversal of CSF positivity for JC virus genome by cidofovir in a patient with systemic lupus erythematosus and progressive multifocal leukoencephalopathy. Neurol Sci. 2001;22:17–20. doi: 10.1007/s100720170031. [DOI] [PubMed] [Google Scholar]
- 180.Sanders JS, Riezebos-Brilman A, Homan van der Heide JJ. Progressive multifocal leuko-encephalopathy after ABO-incompatible kidney transplantation. Transpl Int. 2012;25:e104–e105. doi: 10.1111/j.1432-2277.2012.01511.x. [DOI] [PubMed] [Google Scholar]
- 181.Sano Y, Nakano Y, Omoto M, Takao M, Ikeda E, Oga A, Nakamichi K, Saijo M, Maoka T, Sano H, Kawai M, Kanda T. Rituximab-associated progressive multifocal leukoencephalopathy derived from non-Hodgkin lymphoma: neuropathological findings and results of mefloquine treatment. Intern Med. 2015;54:965–970. doi: 10.2169/internalmedicine.54.2308. [DOI] [PubMed] [Google Scholar]
- 182.Saumoy M, Castells G, Escoda L, Mares R, Richart C, Ugarriza A. Progressive multifocal leukoencephalopathy in chronic lymphocytic leukemia after treatment with fludarabine. Leuk Lymphoma. 2002;43:433–436. doi: 10.1080/10428190290006297. [DOI] [PubMed] [Google Scholar]
- 183.Saxton CR, Gailiunas P, Jr, Helderman JH, Farkas RA, McCoy R, Diehl J, Sagalowsky A, Murphy FK, Ross ED, Silva FR. Progressive multifocal leukoencephalopathy in a renal transplant recipient. Increased diagnostic sensitivity of computed tomographic scanning by double-dose contrast with delayed films. Am J Med. 1984;77:333–337. doi: 10.1016/0002-9343(84)90715-0. [DOI] [PubMed] [Google Scholar]
- 184.Schroder A, Lee DH, Hellwig K, Lukas C, Linker RA, Gold R. Successful management of natalizumab-associated progressive multifocal leukoencephalopathy and immune reconstitution syndrome in a patient with multiple sclerosis. Arch Neurol. 2010;67:1391–1394. doi: 10.1001/archneurol.2010.157. [DOI] [PubMed] [Google Scholar]
- 185.Schwab N, Hohn KG, Schneider-Hohendorf T, Metz I, Stenner MP, Jilek S, Du Pasquier RA, Gold R, Meuth SG, Ransohoff RM, Bruck W, Wiendl H. Immunological and clinical consequences of treating a patient with natalizumab. Mult Scler. 2012;18:335–344. doi: 10.1177/1352458511421919. [DOI] [PubMed] [Google Scholar]
- 186.Schwab N, Schneider-Hohendorf T, Posevitz V, Breuer J, Gobel K, Windhagen S, Brochet B, Vermersch P, Lebrun-Frenay C, Posevitz-Fejfar A, Capra R, Imberti L, Straeten V, Haas J, Wildemann B, Havla J, Kumpfel T, Meinl I, Niessen K, Goelz S, Kleinschnitz C, Warnke C, Buck D, Gold R, Kieseier BC, Meuth SG, Foley J, Chan A, Brassat D, Wiendl H. L-selectin is a possible biomarker for individual PML risk in natalizumab-treated MS patients. Neurology. 2013;81:865–871. doi: 10.1212/WNL.0b013e3182a351fb. [DOI] [PubMed] [Google Scholar]
- 187.Seong D, Bruner JM, Lee KH, Mirza N, Kwon BD, Lee JH, Lee YY, Ro J, Talpaz M, Champlin R, Deisseroth AB. Progressive multifocal leukoencephalopathy after autologous bone marrow transplantation in a patient with chronic myelogenous leukemia. Clin Infect Dis. 1996;23:402–403. doi: 10.1093/clinids/23.2.402. [DOI] [PubMed] [Google Scholar]
- 188.Shin JW, Jung KH, Lee ST, Moon J, Lim JA, Byun JI, Park KI, Lee SK, Chu K. Mefloquine improved progressive multifocal leukoencephalopathy in a patient with immunoglobulin A nephropathy. J Clin Neurosci. 2014;21:1661–1664. doi: 10.1016/j.jocn.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 189.Shitrit D, Nirit L, Shiran SI, Izbicki G, Sofer D, Eldad M, Kramer MR. Progressive multifocal leukoencephalopathy in a lung transplant recipient. J Heart Lung Transplant. 2003;22:946–950. doi: 10.1016/S1053-2498(02)00804-5. [DOI] [PubMed] [Google Scholar]
- 190.Shprecher D, Frech T, Chin S, Eskandari R, Steffens J. Progressive multifocal leucoencephalopathy associated with lupus and methotrexate overdose. Lupus. 2008;17:1029–1032. doi: 10.1177/0961203308089435. [DOI] [PubMed] [Google Scholar]
- 191.Sikkema T, Schuiling WJ, Hoogendoorn M. Progressive multifocal leukoencephalopathy during treatment with rituximab and CHOP chemotherapy in a patient with a diffuse large B-cell lymphoma. BMJ Case Rep. 2013 doi: 10.1136/bcr-2012-008142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Smolle E, Trojan A, Schuster SJ, Haybaeck J. Progressive multifocal leukoencephalopathy–a case report and review of the literature. In Vivo. 2014;28:941–948. [PubMed] [Google Scholar]
- 193.Sottini A, Capra R, Zanotti C, Chiarini M, Serana F, Ricotta D, Caimi L, Imberti L. Pre-existing T- and B-cell defects in one progressive multifocal leukoencephalopathy patient. PLoS One. 2012;7:e34493. doi: 10.1371/journal.pone.0034493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sponzilli EE, Smith JK, Malamud N, McCulloch JR. Progressive multifocal leukoencephalopathy: a complication of immunosuppressive treatment. Neurology. 1975;25:664–668. doi: 10.1212/WNL.25.7.664. [DOI] [PubMed] [Google Scholar]
- 195.Stahl NI. Progressive multifocal leukoencephalopathy in a minimally immunosuppressed patient with systemic lupus erythematosus treated with dapsone. J Rheumatol. 2008;35:725–727. [PubMed] [Google Scholar]
- 196.Steurer M, Clausen J, Gotwald T, Gunsilius E, Stockhammer G, Gastl G, Nachbaur D. Progressive multifocal leukoencephalopathy after allogeneic stem cell transplantation and posttransplantation rituximab. Transplantation. 2003;76:435–436. doi: 10.1097/01.TP.0000078897.11633.5F. [DOI] [PubMed] [Google Scholar]
- 197.Stoppe M, Thoma E, Liebert UG, Major EO, Hoffmann KT, Classen J, Then BF. Cerebellar manifestation of PML under fumarate and after efalizumab treatment of psoriasis. J Neurol. 2014;261:1021–1024. doi: 10.1007/s00415-014-7311-1. [DOI] [PubMed] [Google Scholar]
- 198.Taieb G, Renard D, Thouvenot E, Servillo G, Castelnovo G. Transient punctuate enhancing lesions preceding natalizumab-associated progressive multifocal leukoencephalopathy. J Neurol Sci. 2014;346:364–365. doi: 10.1016/j.jns.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 199.Thaker AA, Schmitt SE, Pollard JR, Dubroff JG. Natalizumab-induced progressive multifocal leukoencephalopathy. Clin Nucl Med. 2014;39:e365–e366. doi: 10.1097/RLU.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 200.Tomura N, Watanabe M, Kato T, Nishino K, Kowada M. Case report: progressive multifocal leukoencephalopathy with prominent medullary veins on angiogram. Clin Radiol. 1994;49:66–68. doi: 10.1016/S0009-9260(05)82920-4. [DOI] [PubMed] [Google Scholar]
- 201.Tortorella C, Direnzo V, D’Onghia M, Trojano M. Brainstem PML lesion mimicking MS plaque in a natalizumab-treated MS patient. Neurology. 2013;81:1470–1471. doi: 10.1212/WNL.0b013e3182a84179. [DOI] [PubMed] [Google Scholar]
- 202.Travasarou M, Marousi S, Papageorgiou E, Karageorgiou CE. JCV-negative natalizumab-associated progressive multifocal leukoencephalopathy: a clinico-radiological diagnosis. Clin Neurol Neurosurg. 2013;115:827–829. doi: 10.1016/j.clineuro.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 203.Tubridy N, Wells C, Lewis D, Schon F. Unsuccessful treatment with cidofovir and cytarabine in progressive multifocal leukoencephalopathy associated with dermatomyositis. J R Soc Med. 2000;93:374–375. doi: 10.1177/014107680009300710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tuccori M, Focosi D, Maggi F, Cosottini M, Meini B, Lena F, Blandizzi C, Del TM, Petrini M. Progressive multifocal leukoencephalopathy: a report of three cases in HIV-negative patients with non-Hodgkin’s lymphomas treated with rituximab. Ann Hematol. 2010;89:519–522. doi: 10.1007/s00277-009-0819-2. [DOI] [PubMed] [Google Scholar]
- 205.Uppenkamp M, Engert A, Diehl V, Bunjes D, Huhn D, Brittinger G. Monoclonal antibody therapy with CAMPATH-1H in patients with relapsed high- and low-grade non-Hodgkin’s lymphomas: a multicenter phase I/II study. Ann Hematol. 2002;81:26–32. doi: 10.1007/s00277-001-0394-7. [DOI] [PubMed] [Google Scholar]
- 206.van Oosten BW, Killestein J, Barkhof F, Polman CH, Wattjes MP. PML in a patient treated with dimethyl fumarate from a compounding pharmacy. N Engl J Med. 2013;368:1658–1659. doi: 10.1056/NEJMc1215357. [DOI] [PubMed] [Google Scholar]
- 207.Van AG, Van RM, Sciot R, Dubois B, Vermeire S, Noman M, Verbeeck J, Geboes K, Robberecht W, Rutgeerts P. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353:362–368. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- 208.van Pesch V, Bartholome E, Bissay V, Bouquiaux O, Bureau M, Caekebeke J, Debruyne J, Declercq I, Decoo D, Denayer P, De Smet E, D’hooghe M, Dubois B, Dupuis M, Sankari SE, Geens K, Guillaume D, van Landegem W, Lysandropoulos A, de Noordhout AM, Medaer R, Melin A, Peeters K, Ba RP, Retif C, Seeldrayers P, Symons A, Urbain E, Vanderdonckt P, Van Ingelghem E, Vanopdenbosch L, Vanroose E, Van Wijmeersch B, Willekens B, Willems C, Sindic C. Safety and efficacy of natalizumab in Belgian multiple sclerosis patients: subgroup analysis of the natalizumab observational program. Acta Neurol Belg. 2014;114:167–178. doi: 10.1007/s13760-014-0308-9. [DOI] [PubMed] [Google Scholar]
- 209.Vennegoor A, Wattjes MP, van Munster ET, Kriekaart RL, van Oosten BW, Barkhof F, Killestein J, Polman CH. Indolent course of progressive multifocal leukoencephalopathy during natalizumab treatment in MS. Neurology. 2011;76:574–576. doi: 10.1212/WNL.0b013e31820b7644. [DOI] [PubMed] [Google Scholar]
- 210.Verhelst X, Vanhooren G, Vanopdenbosch L, Casselman J, Laleman W, Pirenne J, Nevens F, Orlent H. Progressive multifocal leukoencephalopathy in liver transplant recipients: a case report and review of the literature. Transpl Int. 2011;24:e30–e34. doi: 10.1111/j.1432-2277.2010.01190.x. [DOI] [PubMed] [Google Scholar]
- 211.Vidarsson B, Mosher DF, Salamat MS, Isaksson HJ, Onundarson PT. Progressive multifocal leukoencephalopathy after fludarabine therapy for low-grade lymphoproliferative disease. Am J Hematol. 2002;70:51–54. doi: 10.1002/ajh.10085. [DOI] [PubMed] [Google Scholar]
- 212.Volker HU, Kraft K, Arnold E, Steinhoff S, Kolios G, Sommer S. Progressive multifocal leukoencephalopathy developing in advanced pulmonal sarcoidosis. Clin Neurol Neurosurg. 2007;109:624–630. doi: 10.1016/j.clineuro.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 213.Vollmer-Haase J, Young P, Ringelstein EB. Efficacy of camptothecin in progressive multifocal leucoencephalopathy. Lancet. 1997;349:1366. doi: 10.1016/S0140-6736(05)63201-1. [DOI] [PubMed] [Google Scholar]
- 214.von Geldern G, Pardo CA, Calabresi PA, Newsome SD. PML-IRIS in a patient treated with brentuximab. Neurology. 2012;79:2075–2077. doi: 10.1212/WNL.0b013e3182749f17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Vulliemoz S, Lurati-Ruiz F, Borruat FX, Delavelle J, Koralnik IJ, Kuntzer T, Bogousslavsky J, Picard F, Landis T, Du Pasquier RA. Favourable outcome of progressive multifocal leucoencephalopathy in two patients with dermatomyositis. J Neurol Neurosurg Psychiatry. 2006;77:1079–1082. doi: 10.1136/jnnp.2006.092353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Waggoner J, Martinu T, Palmer SM. Progressive multifocal leukoencephalopathy following heightened immunosuppression after lung transplant. J Heart Lung Transplant. 2009;28:395–398. doi: 10.1016/j.healun.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wagner-Johnston ND, Bartlett NL, Cashen A, Berger JR. Progressive multifocal leukoencephalopathy in a patient with Hodgkin lymphoma treated with brentuximab vedotin. Leuk Lymphoma. 2012;53:2283–2286. doi: 10.3109/10428194.2012.676170. [DOI] [PubMed] [Google Scholar]
- 218.Warnatz K, Peter HH, Schumacher M, Wiese L, Prasse A, Petschner F, Vaith P, Volk B, Weiner SM. Infectious CNS disease as a differential diagnosis in systemic rheumatic diseases: three case reports and a review of the literature. Ann Rheum Dis. 2003;62:50–57. doi: 10.1136/ard.62.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Warsch S, Hosein PJ, Morris MI, Teomete U, Benveniste R, Chapman JR, Lossos IS. Progressive multifocal leukoencephalopathy following treatment with bendamustine and rituximab. Int J Hematol. 2012;96:274–278. doi: 10.1007/s12185-012-1118-6. [DOI] [PubMed] [Google Scholar]
- 220.Wattjes MP, Verhoeff L, Zentjens W, Killestein J, van Munster ET, Barkhof F, van Eijk JJ. Punctate lesion pattern suggestive of perivascular inflammation in acute natalizumab-associated progressive multifocal leukoencephalopathy: productive JC virus infection or preclinical PML-IRIS manifestation? J Neurol Neurosurg Psychiatry. 2013;84:1176–1177. doi: 10.1136/jnnp-2013-304986. [DOI] [PubMed] [Google Scholar]
- 221.Wattjes MP, Vennegoor A, Mostert J, van Oosten BW, Barkhof F, Killestein J. Diagnosis of asymptomatic natalizumab-associated PML: are we between a rock and a hard place? J Neurol. 2014;261:1139–1143. doi: 10.1007/s00415-014-7336-5. [DOI] [PubMed] [Google Scholar]
- 222.Weber SC, Uhlenberg B, Raile K, Querfeld U, Muller D. Polyoma virus-associated progressive multifocal leukoencephalopathy after renal transplantation: regression following withdrawal of mycophenolate mofetil. Pediatr Transplant. 2011;15:E19–E24. doi: 10.1111/j.1399-3046.2010.01437.x. [DOI] [PubMed] [Google Scholar]
- 223.Wenning W, Haghikia A, Laubenberger J, Clifford DB, Behrens PF, Chan A, Gold R. Treatment of progressive multifocal leukoencephalopathy associated with natalizumab. N Engl J Med. 2009;361:1075–1080. doi: 10.1056/NEJMoa0810257. [DOI] [PubMed] [Google Scholar]
- 224.White RP, Abraham S, Singhal S, Manji H, Clarke CR. Progressive multifocal leucoencephalopathy isolated to the posterior fossa in a patient with systemic lupus erythematosus. Rheumatology (Oxford) 2002;41:826–827. doi: 10.1093/rheumatology/41.7.826. [DOI] [PubMed] [Google Scholar]
- 225.Worthmann F, Turker T, Muller AR, Patt S, Stoltenburg-Didinger G. Progressive multifocal leukoencephalopathy after orthotopic liver transplantation. Transplantation. 1994;57:1268–1271. doi: 10.1097/00007890-199404270-00023. [DOI] [PubMed] [Google Scholar]
- 226.Wuthrich C, Popescu BF, Gheuens S, Marvi M, Ziman R, Denq SP, Tham M, Norton E, Parisi JE, Dang X, Lucchinetti CF, Koralnik IJ. Natalizumab-associated progressive multifocal leukoencephalopathy in a patient with multiple sclerosis: a postmortem study. J Neuropathol Exp Neurol. 2013;72:1043–1051. doi: 10.1097/NEN.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yehia B, Davison A, Sisson S. Transplant troubles. Am J Med. 2009;122:629–631. doi: 10.1016/j.amjmed.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 228.Yokoyama H, Watanabe T, Maruyama D, Kim SW, Kobayashi Y, Tobinai K. Progressive multifocal leukoencephalopathy in a patient with B-cell lymphoma during rituximab-containing chemotherapy: case report and review of the literature. Int J Hematol. 2008;88:443–447. doi: 10.1007/s12185-008-0168-2. [DOI] [PubMed] [Google Scholar]
- 229.Zabernigg A, Maier H, Thaler J, Gattringer C. Late-onset fatal neurological toxicity of fludarabine. Lancet. 1994;344:1780. doi: 10.1016/S0140-6736(94)92922-X. [DOI] [PubMed] [Google Scholar]
- 230.Zivadinov R, Dwyer MG, Hussein S, Carl E, Kennedy C, Andrews M, Hojnacki D, Heininen-Brown M, Willis L, Cherneva M, Bergsland N, Weinstock-Guttman B. Voxel-wise magnetization transfer imaging study of effects of natalizumab and IFNbeta-1a in multiple sclerosis. Mult Scler. 2012;18:1125–1134. doi: 10.1177/1352458511433304. [DOI] [PubMed] [Google Scholar]
- 231.ZuRhein GM, Varakis J. Letter: progressive multifocal leukoencephalopathy in a renal-allograft recipient. N Engl J Med. 1974;291:798. doi: 10.1056/nejm197410102911524. [DOI] [PubMed] [Google Scholar]
- 232.Gheuens S, Smith DR, Wang X, Alsop DC, Lenkinski RE, Koralnik IJ. Simultaneous PML-IRIS after discontinuation of natalizumab in a patient with MS. Neurology. 2012;78:1390–1393. doi: 10.1212/WNL.0b013e318253d61e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Havla J, Hohlfeld R, Kumpfel T. Unusual natalizumab-associated progressive multifocal leukoencephalopathy starting in the brainstem. J Neurol. 2014;261:232–234. doi: 10.1007/s00415-013-7191-9. [DOI] [PubMed] [Google Scholar]
- 234.Metz I, Radue EW, Oterino A, Kumpfel T, Wiendl H, Schippling S, Kuhle J, Sahraian MA, Gray F, Jakl V, Hausler D, Bruck W. Pathology of immune reconstitution inflammatory syndrome in multiple sclerosis with natalizumab-associated progressive multifocal leukoencephalopathy. Acta Neuropathol. 2012;123:235–245. doi: 10.1007/s00401-011-0900-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Eisele P, Szabo K, Hornberger E, Griebe M, Hennerici MG, Kieseier BC, Gass A. Presumptive progressive multifocal leukoencephalopathy in multiple sclerosis after natalizumab therapy. J Neuroimaging. 2014;24:425–428. doi: 10.1111/jon.12010. [DOI] [PubMed] [Google Scholar]
- 236.Blinkenberg M, Sellebjerg F, Leffers AM, Madsen CG, Sorensen PS. Clinically silent PML and prolonged immune reconstitution inflammatory syndrome in a patient with multiple sclerosis treated with natalizumab. Mult Scler. 2013;19:1226–1229. doi: 10.1177/1352458513481010. [DOI] [PubMed] [Google Scholar]
- 237.Paues J, Vrethem M. Fatal progressive multifocal leukoencephalopathy in a patient with non-Hodgkin lymphoma treated with rituximab. J Clin Virol. 2010;48:291–293. doi: 10.1016/j.jcv.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 238.Belhassen-Garcia M, Rabano-Gutierrez A, Velasco-Tirado V, Romero-Alegria A, Perez-Garcia ML, Martin-Oterino JA. Atypical progressive multifocal leukoencephalopathy in a patient with antisynthetase syndrome. Intern Med. 2015;54:519–524. doi: 10.2169/internalmedicine.54.2748. [DOI] [PubMed] [Google Scholar]
- 239.Hopfinger G, Plessl A, Grisold W, Klimpfinger M, Hoftberger R, Bernt R, Mostl M, Waldner R, Pittermann-Hocker E. Progressive multifocal leukoencephalopathy after rituximab in a patient with relapsed follicular lymphoma and low IgG levels and a low CD4+ lymphocyte count. Leuk Lymphoma. 2008;49:2367–2369. doi: 10.1080/10428190802404048. [DOI] [PubMed] [Google Scholar]
- 240.Boster A, Hreha S, Berger JR, Bao F, Penmesta R, Tselis A, Endress C, Zak I, Perumal J, Caon C, Vazquez J, Tyler KL, Racke MK, Millis S, Khan O. Progressive multifocal leukoencephalopathy and relapsing-remitting multiple sclerosis: a comparative study. Arch Neurol. 2009;66:593–599. doi: 10.1001/archneurol.2009.31. [DOI] [PubMed] [Google Scholar]
- 241.Kappos L, Bates D, Edan G, Eraksoy M, Garcia-Merino A, Grigoriadis N, Hartung HP, Havrdova E, Hillert J, Hohlfeld R, Kremenchutzky M, Lyon-Caen O, Miller A, Pozzilli C, Ravnborg M, Saida T, Sindic C, Vass K, Clifford DB, Hauser S, Major EO, O’Connor PW, Weiner HL, Clanet M, Gold R, Hirsch HH, Radu EW, Sorensen PS, King J. Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring. Lancet Neurol. 2011;10:745–758. doi: 10.1016/S1474-4422(11)70149-1. [DOI] [PubMed] [Google Scholar]
- 242.Sinnecker T, Othman J, Kuhl M, Mekle R, Selbig I, Niendorf T, Kunkel A, Wienecke P, Kern P, Paul F, Faiss J, Wuerfel J. 7T MRI in natalizumab-associated PML and ongoing MS disease activity: a case study. Neurol Neuroimmunol Neuroinflamm. 2015;2:e171. doi: 10.1212/NXI.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wattjes MP, Rovira À, Miller D, Yousry TA, Sormani MP, de Stefano MP, Tintoré M, Auger C, Tur C, Filippi M, Rocca MA, Fazekas F, Kappos L, Polman C, Barkhof F, Montalban X; MAGNIMS study group (2015) Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-establishing disease prognosis and monitoring patients. Nat Rev Neurol 11:597–606 [DOI] [PubMed]
- 244.McGuigan C, Craner M, Guadagno J, Kapoor R, Mazibrada G, Molyneux P, Nicholas R, Palace J, Pearson OR, Rog D, Young CA. Stratification and monitoring of natalizumab-associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group. J Neurol Neurosurg Psychiatry. 2015;87:117–125. doi: 10.1136/jnnp-2015-311100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Warnke C, von Geldern G, Markwerth P, Dehmel T, Hoepner R, Gold R, Pawlita M, Kumpfel T, Maurer M, Stangel M, Wegner F, Hohlfeld R, Straeten V, Limmroth V, Weber T, Hermsen D, Kleinschnitz C, Hartung HP, Wattjes MP, Svenningson A, Major E, Olsson T, Kieseier BC, Adams O. Cerebrospinal fluid JC virus antibody index for diagnosis of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2014;76:792–801. doi: 10.1002/ana.24153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Schneider-Hohendorf T, Rossaint J, Mohan H, Boning D, Breuer J, Kuhlmann T, Gross CC, Flanagan K, Sorokin L, Vestweber D, Zarbock A, Schwab N, Wiendl H. VLA-4 blockade promotes differential routes into human CNS involving PSGL-1 rolling of T cells and MCAM-adhesion of TH17 cells. J Exp Med. 2014;211:1833–1846. doi: 10.1084/jem.20140540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Berger JR, Pall L, Lanska D, Whiteman M. Progressive multifocal leukoencephalopathy in patients with HIV infection. J Neurovirol. 1998;4:59–68. doi: 10.3109/13550289809113482. [DOI] [PubMed] [Google Scholar]
- 248.Khoury MN, Alsop DC, Agnihotri SP, Pfannl R, Wuthrich C, Ho ML, Hackney D, Ngo L, Anderson MP, Koralnik IJ. Hyperintense cortical signal on magnetic resonance imaging reflects focal leukocortical encephalitis and seizure risk in progressive multifocal leukoencephalopathy. Ann Neurol. 2014;75:659–669. doi: 10.1002/ana.24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lima MA, Drislane FW, Koralnik IJ. Seizures and their outcome in progressive multifocal leukoencephalopathy. Neurology. 2006;66:262–264. doi: 10.1212/01.wnl.0000194227.16696.11. [DOI] [PubMed] [Google Scholar]
- 250.Davis MJ, Khan A, Royal W., III Progressive multifocal leukoencephalopathy as the first manifestation of occult sarcoidosis: case report and review of the literature. Neurologist. 2013;19:26–29. doi: 10.1097/NRL.0b013e31827c6c3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy in patients with rheumatic diseases: are patients with systemic lupus erythematosus at particular risk? Autoimmun Rev. 2008;8:144–146. doi: 10.1016/j.autrev.2008.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.