Abstract
The androgen testosterone is essential for the Sertoli cell to support the maturation of male germ cells and the production of spermatozoa (spermatogenesis). In the classical view of androgen action, binding of androgen to the intracellular androgen receptor (AR) produces a conformational change in AR such that the receptor–steroid complex has high affinity for specific DNA regulatory elements and is able to stimulate gene transcription. Here, we demonstrate that testosterone can act by means of an alternative, rapid, and sustainable mechanism in Sertoli cells that is independent of AR–DNA interactions. Specifically, the addition of physiological levels of testosterone to Sertoli cells stimulates the mitogen-activated protein kinase signaling pathway and causes phosphorylation of the cAMP response element binding protein transcription factor on serine 133, a modification known to be required for Sertoli cells to support spermatogenesis. Androgen-mediated activation of mitogen-activated protein kinase and cAMP response element binding protein occurs within 1 min, extends for at least 12 h and requires AR. Furthermore, androgen induces endogenous cAMP response element binding protein-mediated transcription in Sertoli cells. These newly identified mechanisms of androgen action in Sertoli cells suggest new targets for developing male contraceptive agents.
Keywords: testis, spermatogenesis, nongenomic, signal transduction
Androgen actions are critical in the testis for the maturation of male germ cells into spermatozoa (spermatogenesis). In the absence of relatively high levels of testosterone (>70 nM) in the testis, spermatogenesis is halted before the completion of meiosis so that few if any spermatozoa are produced (1, 2). The molecular mechanisms by which testosterone regulates spermatogenesis are not well understood, but like other steroid hormones, androgens exert many of their actions by diffusing into target cells and binding specific intracellular receptor proteins that are located in the cytoplasm and the nucleus (3). The importance of the androgen receptor (AR) for maintaining spermatogenesis is confirmed by mutations that eliminate AR activity. These mutations result in the testicular feminization phenotype (tfm) and the absence of mature male germ cells (4). In the classical view of androgen action, binding of androgen to its receptor produces a conformational change in AR such that the receptor–steroid complex has high affinity for specific DNA regulatory elements and is able to stimulate gene transcription (5). The entire process required to initiate gene expression by means of this classical mechanism takes at least 30–45 min (6, 7), and the length of time required to produce significant levels of nascent proteins is in the order of hours.
Numerous genes and proteins are up-regulated in response to testosterone (8–10), but few genes are known to be induced by androgens through AR binding to promoter elements in Sertoli cells (11, 12). In contrast, at least two observations support the hypothesis that testosterone may act through alternative mechanisms to complement classical AR actions in Sertoli cells. First, studies using rats demonstrated that Sertoli cells require testicular testosterone levels >70 nM to support spermatogenesis, even though testosterone binding to AR and gene expression responses to testosterone are saturated at 1 nM (1, 2, 13). Second, intracellular calcium levels are elevated in primary Sertoli cells within seconds of androgen stimulation and thus cannot be dependent on AR–DNA interactions and initiation of gene expression (14–16). Together, these observations suggest that testosterone may act in Sertoli cells through alternative pathways as well as classical mechanisms to regulate spermatogenesis.
Recent studies have demonstrated that androgen can directly activate cellular signaling pathways independent of AR binding to DNA (17–19). Evidence for androgen stimulation of the mitogen-activated protein (MAP) kinase pathway includes the finding that the nonhydrolyzable androgen agonist R1881 (1 nM) activates extracellular-regulated kinase (ERK) in human PMC42 breast cancer cells (20). Similarly, dihydrotestosterone rapidly and transiently (2–60 min) increased ERK phosphorylation in primary prostate stroma cells (21). Phosphorylation of ERK kinases was also elevated by dihydrotestosterone concentrations as low as 0.1 nM in LNCaP cells that contain AR and in PC3 cells stably transfected with AR, but not in wild-type PC3 cells that are AR deficient.
We noted that MAP kinase or calcium-regulated pathways can cause the cAMP response element binding protein (CREB) transcription factor to be phosphorylated on serine 133 (22). Once phosphorylated on serine 133, CREB bound to cAMP response element motifs (TGACGTCA) in gene promoters is able to associate with the CREB binding protein coactivator (23), which facilitates the recruitment of RNA polymerase to the transcription initiation site (23–25). The potential relevance of testosterone regulation of CREB phosphorylation is highlighted by our recent studies demonstrating that phosphorylated CREB in Sertoli cells is an essential factor that is required for Sertoli cells to support spermatogenesis (26). Specifically, spermatocyte germ cells undergo apoptosis, and spermatozoa are not produced if CREB cannot be phosphorylated in Sertoli cells.
In this study, we test the hypothesis that androgen can regulate Sertoli cell processes, and therefore spermatogenesis, by means of nonclassical mechanisms. We explore androgen stimulation of ERK MAP kinases as well as the CREB transcription factor and investigate signaling pathways by which testosterone actions are transduced in Sertoli cells. We determine whether AR is required for nonclassical actions of androgens in Sertoli cells. Finally, we assay androgen induction of CREB-mediated transcription in Sertoli cells.
Materials and Methods
Isolation of Primary Sertoli Cells and Cell Culture. Sertoli cells were isolated from 15-day-old Sprague–Dawley rats and cultured in serum-free media as described previously (26). Sertoli cells were routinely >95% pure as determined by phase microscopy and alkaline phosphatase staining (27). Animals used in these studies were maintained and killed according to the principles and procedures described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. These studies were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Preparation of Whole Cell Extracts, Western Blots, and Statistical Analysis. Three days after isolation, Sertoli cells were treated for 1 min to 24 h with potential regulators of CREB phosphorylation including estradiol (100 nM), RU 5020 (100 nM), testosterone (10–250 nM), follicle-stimulating hormone (FSH, 100 ng/ml), or R1881 (100 nM). In some cases, cells were pretreated for 4 h with actinomycin D (50 μg/ml), puromycin (10 μg/ml), ICI 182,780 (1 μM), or flutamide (1 μM) or 1 h with the signaling pathway inhibitors PD 98059 (50 μM) or wortmannin (100 nM). Cells were washed once with PBS and then lysed on the plates by using boiling Laemmli sample buffer to minimize phosphatase activity (28) and assayed by Western immunoblot (29). Primary antisera used include those against the CREB phosphorylated on serine 133 (Upstate Biotechnology, Lake Placid, NY), the phosphorylated, active forms of ERK1/2 and protein kinase B phosphorylated on serine 473 (Cell Signaling Technology), as well as all forms of CREB and ERK (Upstate Biotechnology). Digitized autoradiograms were quantified by using nih image 1.6 software. Phosphorylated (P)-CREB and P-ERK levels were normalized to overall CREB and ERK expression. Comparisons of androgen-stimulated levels of P-CREB and P-ERK to that of vehicle-treated controls were performed by using ANOVA and Fisher's post hoc analysis with statview 4.5 software (Abacus Concepts, Berkeley, CA).
cAMP RIA. Sertoli cells were stimulated for 15 min with ethanol (vehicle), FSH (100 ng/ml), or testosterone (100 nM). Media was collected and boiled for 10 min before analysis. cAMP concentrations in culture medium were analyzed by RIA by using 125I-cAMP-TME (2–0′ monosuccinlyl cAMP tyrosine methyl ester) and anti-cAMP in accordance to the instructions provided by the National Hormone and Pituitary Program (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health). Antiserum to cAMP (Lot CV-27) was generously provided by the National Hormone and Pituitary Program.
Small Inhibitory RNA (siRNA) and PCR Primer Oligonucleotides. The sense strands used to construct AR and Luciferase siRNAs were 5′-AGGAGCGUUCCAGdTdT-3′ and 5′-Cy3-CGUACGCGGAAUACUUCGAdTdT-3′, respectively. PCR primers used include: AR, 5′-AGATGGCATGCATTCAGTATTCCTGGA-3′ and 5′-CATGCGATACTCATTGAAAACCAGGTC-3′; SRY, 5′-ACCTGCTGCAATGGGACAACAACC-3′ and 5′-CTGCTGGTGCTGCTGTTTCTGCTG-3′; CREB, 5′-AGGGGTGCCAAGGATTGAAGAAG-3′ and 5′-GCTTTTAGCTCCTCAATCAATGT-3′; LDH-A, 5′-ATGAAGGACTTGGCTGATGAGCT-3′ and 5′-TTAGAACTGCAGCTCCTTCTGGA-3′; EGR1, 5′-TTCGCTCACTCCACTATCCAC-3′ and 5′-GGGGGATGGGTAGGAGGTAGC-3′; or GAPDH, 5′-GCATGGCCTTCCGTGTTCCTA-3′ and 5′-GTAGGCCATGAGGTCCACCAC-3′.
RNA Interference, Culture of tfm-Derived Sertoli Cells, and Adenoviral Infection. Two days after plating, Sertoli cells were transfected with siRNAs (20 nM) by using TransIT-TKO (Mirus, Madison, WI) according to the manufacturer's instructions. The duplex siRNAs corresponded to +60 to +78 of AR or +153 to +171 of the luciferase gene. Three days after transfection, the Sertoli cells were stimulated with ethanol or testosterone (100 nM), and whole cell extracts were collected in boiling Laemmli sample buffer.
Pups from matings of wild-type males and tfm carrier females were sexed after analysis of genomic DNA isolated from tail clips. Positive results of PCR reactions employing SRY gene primers were indicative of the presence of the Y chromosome and male pups. To distinguish wild-type and tfm males, a 130-bp region encompassing the AR mutation was amplified from genomic DNA and sequenced. Separate Sertoli cell cultures were prepared from each wild-type and tfm pup at 15 days of age. In some cases, the Sertoli cells were infected 2 days after plating with adenoviral constructs (1 × 1010 particles per ml) expressing either β-galactosidase or wild-type AR. Sertoli cells were harvested for assays of CREB phosphorylation 3 days after plating or infection with adenovirus.
Androgen Receptor Binding Activity. AR binding assays to determine AR binding activity in Sertoli cells were carried out as previously described (30). Briefly, Sertoli cells (1 × 105) were plated onto 60-mm2 dishes in serum-free media. After 3 days in culture, the cells were washed with PBS and incubated in media containing 5 nM 3H-R1881 in the presence or absence of 5 μM cold R1881 for 4 h at 37°C. Cells were then washed five times with PBS and resuspended in ethanol for 30 min. Total radioactivity was determined by using a liquid scintillation counter (Beckman Coulter), and AR levels were expressed as fmol/mg protein.
RT-PCR Analysis of Gene Expression. Three days after transfection, Sertoli cells were stimulated with vehicle (ethanol) or R1881 (100 nM) for 6 or 24 h, and RNA was isolated by using RNA STAT60 (Tel-Test, Friendswood, TX). After digestion with RNase-free DNase, the RNA was subjected to reverse transcription by using gene-specific primers for CREB, LDH-A, EGR1, and GAPDH. The resulting cDNAs were amplified in separate nested PCR reactions by using a predetermined number of cycles found to amplify the cDNAs within a linear range (25 cycles for CREB, LDH-A, and EGR1 and 20 cycles for GAPDH). Ten percent of the PCR yields were resolved on 1% agarose gels.
Results
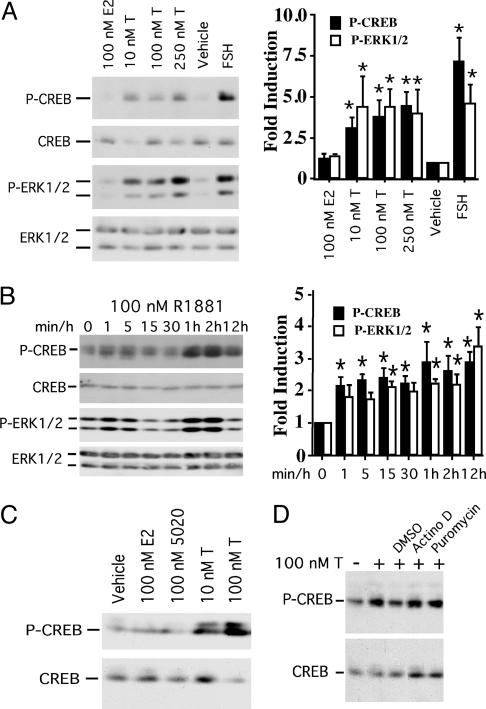
Androgen Stimulates CREB and ERK Phosphorylation in Sertoli Cells. To evaluate nonclassical androgen activation of regulatory factors in Sertoli cells, we first assessed the phosphorylation status of both the CREB transcription factor, a known target of numerous signaling kinases (22) that is required for germ-cell survival (26), and the MAP kinase ERK, a target of androgen in breast cancer and prostate cells (20, 21). Exposure to testosterone (10–250 nM), the major androgen effector in the testis, rapidly (within 15 min) induced the phosphorylation of CREB (3.1–4.5-fold) as well as both forms of ERK (ERK1/2, 4.0–4.4-fold) (Fig. 1A). Overall CREB and ERK1/2 protein expression was not altered by testosterone stimulation. Notably, the levels of testosterone used are at the lower range of in vivo testicular interstitial levels (200–825 nM) (2, 31, 32). Testosterone actions were specific, as the steroid hormone estradiol did not induce phosphorylation of CREB or ERK1/2. Addition of FSH, a known activator of ERK and inducer of CREB phosphorylation in Sertoli cells (28, 33), increased P-CREB and P-ERK1/2 levels as expected. Together, these data demonstrate that testosterone stimulation is capable of inducing the phosphorylation of ERK1/2 and CREB within 15 min. These actions of testosterone are not likely to require AR–DNA interactions because the initiation of gene expression by means of this classical mechanism takes at least 30–45 min (7), and hours are needed to produce significant levels of nascent proteins.
Fig. 1.
Testosterone induces phosphorylation of CREB and ERK in primary rat Sertoli cells. (A) Sertoli cells were stimulated with ethanol (vehicle), estradiol (E2), and testosterone (T) at the concentrations shown or ovine FSH (100 ng/ml) for 15 min. Western immunoblotting of whole cell extracts was performed sequentially with antisera specifically recognizing CREB phosphorylated on serine 133 (P-CREB), P-ERK1/2, and all forms of ERK. Fractionation and probing of the samples was repeated by using an antiserum recognizing all forms of CREB. P-CREB and P-ERK1/2 levels were normalized to overall CREB and ERK1/2 expression, and the normalized P-CREB and P-ERK1/2 levels for vehicle-treated cells were arbitrarily set equal to 1. The mean fold induction (±SE) of hormone-stimulated P-CREB (▪) and P-ERK1/2 (□) over vehicle-treated cells for five (P-CREB) and four (P-ERK1/2) independent experiments is reported in the graph to the right. Values that are significantly different from vehicle-treated controls (P < 0.05) are indicted with an asterisk (*). (B) Sertoli cells were stimulated with ethanol for 15 min (0) or 100 nM R1881 for the times shown. Western analyses were performed as in A. The mean (±SE) relative levels of P-CREB (▪) and P-ERK1/2 (□) normalized for CREB and ERK1/2 expression are provided for four (P-CREB) and six (P-ERK1/2) independent experiments with statistical analysis as described in A. No changes in protein expression were observed over 12 h with vehicle (EtOH) controls (data not shown). (C) Sertoli cells were treated for 60 min with EtOH (vehicle), estradiol (E2), RU 5020, or testosterone (T). Western analyses were performed for P-CREB and CREB. The data shown are representative of three independent experiments. (D) Sertoli cells were pretreated with DMSO, actinomycin D (Actino D, 50 μg/ml), or puromycin (10 μg/ml) for 2 h before stimulation with EtOH vehicle (–) or 100 nM testosterone (+). Western blot analyses of P-CREB and CREB levels are shown. The blot shown is representative of at least three experiments.
Androgen Induces an Immediate and Sustained Increase in CREB and ERK Phosphorylation. We used the nonhydrolyzable androgen agonist R1881 (100 nM) to stimulate Sertoli cells for longer-term studies to determine the kinetics of CREB phosphorylation after androgen stimulation. CREB phosphorylation increased an average of 2.2- to 2.4-fold within 1–15 min after R1881 stimulation (Fig. 1B); 1–2 h after the initial R1881 stimulation, P-CREB levels increased 2.6- to 2.9-fold. ERK1/2 phosphorylation followed similar kinetics, supporting the hypothesis that androgen-induced CREB phosphorylation is mediated by means of the MAP kinase pathway. Again, the induction of CREB phosphorylation 1 h after androgen stimulation was specific for androgen, as stimulation with estradiol or the progestin agonist R5020 did not enhance CREB phosphorylation (Fig. 1C). Androgen-mediated activation of CREB was independent of RNA or protein synthesis because the levels of phosphorylated CREB were not reduced by either the RNA synthesis inhibitor actinomycin D or the protein synthesis inhibitor puromycin (Fig. 1D).
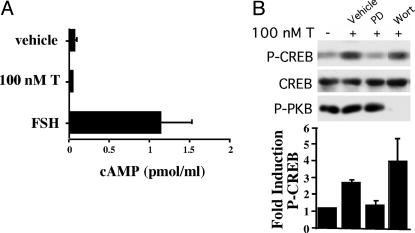
Testosterone Stimulates CREB Phosphorylation by Means of MAP Kinase but Not cAMP. Testosterone is capable of elevating cAMP levels by binding to sex hormone binding globulin (SHBG) associated with its receptor, RSHBG (34, 35). We investigated the possibility that testosterone could cause CREB to be phosphorylated by means of a cAMP-dependent pathway in Sertoli cells. Testosterone stimulation for 15 min actually reduced cAMP levels by 33% to 0.04 ± 0.02 pmol/ml from vehicle-treated levels of 0.06 ± 0.04 pmol/ml (Fig. 2A). In contrast, FSH stimulation increased cAMP levels by at least 18-fold to 1.1 ± 0.4 pmol/ml. These data indicate that activation of the MAP kinase pathway and CREB by testosterone is not associated with increases in cAMP levels and that steroid hormone binding globulin is not required for testosterone actions in Sertoli cells. In contrast, pretreatment of Sertoli cells with the MAP kinase pathway inhibitor PD 98059 reduced testosterone-induced phosphorylation of CREB to basal levels (Fig. 2B). Pretreatment with wortmannin resulted in no inhibition of CREB phosphorylation but eliminated phosphorylation of protein kinase B, a kinase that can be activated by wortmannin-sensitive phosphoinositide 3-kinase. These data confirm that androgen-induced phosphorylation of CREB is mediated by means of the MAP kinase signaling cascade.
Fig. 2.
Testosterone-induced phosphorylation of CREB is mediated by means of MAP kinase. (A) Sertoli cells were treated with FSH (100 ng/ml), testosterone (100 nM), or ethanol (vehicle) for 15 min. cAMP secreted into the media was measured by RIA. The data shown represent the mean levels of cAMP (±SE) for five observations. (B) DMSO (vehicle), PD98059 (PD, 50 μM), or wortmannin (Wort, 100 nM) were added to Sertoli cell cultures 1 h before stimulation with EtOH (–) or testosterone (+) (100 nM) for 15 min. Western immunoblotting was performed as in Fig. 1. Fractionization and probing of samples was repeated by using an antiserum recognizing phosphorylated protein kinase B, a target for wortmannin-sensitive phosphoinositide 3-kinase. The mean fold induction (±SE) of P-CREB levels over that of vehicle-treated cells is shown for three experiments.
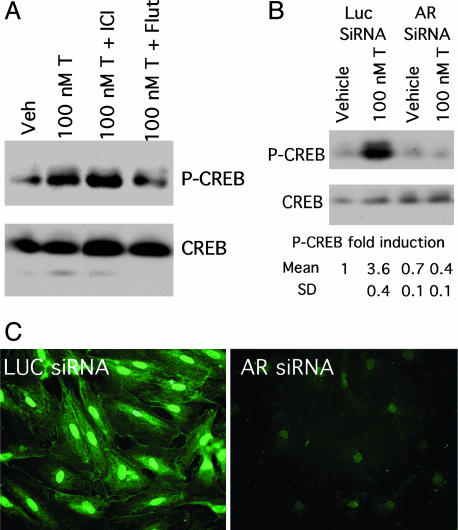
AR Is Required for Androgen-Mediated CREB Phosphorylation. To determine whether AR is required to propagate rapid androgen actions, Sertoli cells were first pretreated with steroid receptor antagonists before stimulation with testosterone. Flutamide, an AR antagonist, decreased phosphorylation of CREB in response to testosterone, whereas the estrogen receptor antagonist ICI 182,780 had no effect (Fig. 1 A). An RNA interference strategy was then used to knock-down AR expression (36). Duplex siRNA oligonucleotides corresponding to either AR or luciferase (control) transcripts were used in transfections of rat Sertoli cells. Initial immunofluorescence studies demonstrated that transfection efficiency of Sertoli cells was >90% by using a fluorescently tagged luciferase siRNA (data not shown). Transfection of Sertoli cells with AR siRNA abolished testosterone-mediated induction of CREB phosphorylation (Fig. 3B). Specifically, testosterone (100 nM, 15 min) stimulation resulted in a 3.6 ± 0.4-fold increase in P-CREB levels in Sertoli cells transfected with the control luciferase siRNA, but P-CREB levels were not elevated by testosterone (0.7 ± 0.4-fold induction) in cells transfected with AR siRNA. As expected, expression of AR (Fig. 3C), but not the transcription factor Sp1 (data not shown), was reduced dramatically in cells transfected with AR siRNA. Together, these data provide evidence that AR is required for testosterone-meditated induction of CREB phosphorylation.
Fig. 3.
RNA interference knock-down of AR expression inhibits androgen-mediated CREB phosphorylation. (A) Sertoli cells were pretreated with ICI 182,780 (ICI) or flutamide (Flut) (1 μM) for 4 h as indicated before stimulation with EtOH (Veh) or 100 nM testosterone (T) for 60 min. The western analysis shown of phosphorylated CREB and all forms of CREB is representative of three independent experiments. (B) Sertoli cells transfected with control luciferase (Luc) or AR siRNA oligonucleotides (20 nM) were stimulated 3 days after transfection with EtOH (vehicle) or 100 nM testosterone (T) for 15 min. Whole cell lysates were subjected to Western analysis as in Fig. 1. For each condition, the mean fold induction and SD for P-CREB expression over that of vehicle-treated cells transfected with luciferase siRNA is reported (n = 2). (C) Sertoli cells were transfected with luciferase (Left) or AR siRNA oligonucleotides (Right) (20 nM). Three days after transfection, immunofluorescence analysis was performed with AR antiserum and Alexa 488-conjugated secondary antiserum.
To confirm that AR is required to transduce nonclassical actions of testosterone, Sertoli cells were isolated from rats having an R734Q mutation in AR that reduces affinity for androgen and causes a tfm phenotype (37). DNA sequence analysis of the genomic AR genes was used to distinguish the genotypes of littermates (Fig. 4A), and segregated cultures of Sertoli cells were isolated from wild-type or tfm mutant rats. Because Sertoli cells have not been cultured from tfm rats previously, we confirmed that our cultures contained <5% peritubular cells, the major contaminating testicular cell type (27) (Fig. 4B). In agreement with earlier studies (37), androgen binding capacity of Sertoli cells from tfm rats was reduced nearly 80% as compared to wild type (60 ± 14 fmol/mg vs. 250 ± 15 fmol/mg) (Fig. 4C). Testosterone stimulation of tfm Sertoli cells did not induce CREB phosphorylation over vehicle-stimulated levels (1.0 ± 0.3-fold induction), whereas testosterone stimulation (100 nM, 15 min) of wild-type Sertoli cells increased CREB phosphorylation 2.9 ± 0.4-fold over vehicle-treated levels (Fig. 4 D and E). Finally, androgen-mediated CREB phosphorylation was restored after infection of tfm-derived Sertoli cells with adenoviral constructs expressing wild-type AR, thus demonstrating that the testosterone–AR complex is required to induce the phosphorylation of CREB (Fig. 4F).
Fig. 4.
Testosterone induces CREB phosphorylation in Sertoli cells derived from wild-type but not AR-defective tfm mutant rats. (A) A portion of the sequencing results after PCR amplification of AR genomic DNA from wild-type and tfm rats is shown. The arrows denote the 1-bp difference in the sequences for AR. (B) A DIC image of Sertoli cells from 15-day-old tfm rats stained for peritubular cell-specific alkaline phosphatase activity after 3 days in culture shows that peritubular cells account for <5% of the culture. (C) The relative [3H]R1881-binding activities of wild-type (wt) and tfm Sertoli cells were determined by hormone-binding assay. The data shown represent the means (±SE) of three independent experiments. (D) Sertoli cell cultures from wild-type or tfm rats were stimulated with ethanol vehicle (V) or 100 nM testosterone (T) for 15 min. Whole cell extracts were subjected to Western analysis with antisera specific for P-CREB followed by reprobing with antisera against all CREB isoforms. (E) The relative mean fold inductions (±SE) of P-CREB levels for testosterone-stimulated cells versus ethanol-treated cells are shown for the studies performed in D (n = 4). (F) Sertoli cells derived from tfm rats were infected in duplicate with adenoviral vectors expressing β-galactosidase or AR and then stimulated 3 days later with EtOH (V) or 100 nM testosterone (T). CREB and P-CREB levels were determined by Western blot.
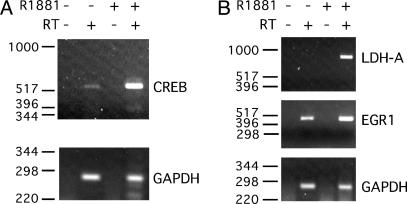
Androgen Stimulates Endogenous CREB-Mediated Transcription in Sertoli Cells. We previously showed that the CREB gene is autopositively regulated by P-CREB binding to the CREB promoter (28). Therefore, the CREB gene was used as a first model to test the assumption that androgen-induced phosphorylation of CREB can stimulate CREB-regulated genes. Sertoli cells were stimulated with vehicle or 100 nM R1881 for 6 or 24 h, followed by semiquantitative RT-PCR analysis of CREB mRNA expression. R1881 stimulation for 6 h did not alter CREB mRNA levels (data not shown), but CREB mRNA levels were elevated after 24 h of stimulation (Fig. 5A). The observed timing of CREB gene induction by androgen is similar to the previously reported, delayed induction of the CREB gene promoter by means of a cAMP and P-CREB-mediated mechanism (28). Two other CREB-regulated genes that lack known AR response elements, lactate dehydrogenase A (LDH-A) and early growth response 1 (Egr1), were induced more rapidly within 6 h of stimulation with R1881 (Fig. 5B). The kinetics of the androgen-mediated induction of LDH-A and EGR1 is similar to more rapidly induced, CREB-regulated genes as exemplified by proenkephalin and c-fos (38, 39). In contrast, the negative control GAPDH housekeeping gene was not induced after either 6 or 24 h of R1881 stimulation. Together, these results demonstrate that androgen treatment can induce expression of endogenous CREB-regulated genes in Sertoli cells.
Fig. 5.
Androgen induces expression of endogenous CREB mRNA. RNA isolated from Sertoli cells stimulated with EtOH vehicle (–) or 100 nM R1881 (+) for 24 h (A) or 6 h (B) as indicated was used in reverse transcriptase reactions employing gene-specific primers. Reverse transcriptase reactions were performed in the absence (–) and presence (+) of reverse transcriptase enzyme (RT). PCR analysis was performed by using primers designed to amplify portions of CREB, LDH-A, Egr1, or GAPDH cDNAs. The cDNAs from the reverse transcriptase reactions were amplified for 25 cycles (CREB, LDH, and EGR1) or 20 cycles (GAPDH), and the resulting products were fractionated on agarose gels. The data shown are representative of more than three experiments. The migration of DNA size markers is shown to the left.
Discussion
Our findings suggest a mechanism for androgen action in Sertoli cells, that being the rapid activation of the MAP kinase pathway and the CREB transcription factor. This mechanism seems to be limited to androgens in Sertoli cells as neither estradiol nor a progestin agonist was capable of activating ERK1/2 or CREB. Together, the similar kinetics of ERK1/2 and CREB activation in response to androgen and the finding that a MAP kinase pathway inhibitor abolished androgen-mediated phosphorylation of CREB indicate that androgen signals in Sertoli cells are transmitted through the MAP kinase pathway. These results are similar to those observed in studies of breast cancer and prostate stroma cells in which androgen activates ERKs and the MAP kinase pathway within minutes (20, 21).
Androgen-induced phosphorylation of ERK1/2 and CREB was observed within 1 min of androgen stimulation and extended for at least 12 h. The prolonged stimulation of CREB phosphorylation by androgen is significant because CREB-induced alterations in gene expression patterns are thought to require long-term (>20 min) elevations in P-CREB (22). Furthermore, CREB phosphorylation has been linked to the activation of numerous Sertoli cell genes that potentially contribute to germ-cell development and survival (26).
Evidence for the nonclassical actions of androgens in Sertoli cells being transmitted by means of the classical AR was provided by three models systems (AR antagonist, RNA interference, and the tfm mutant rats) in which AR activity is reduced. The mechanism by which the androgen–AR complex initiates signaling in Sertoli cells is currently under investigation. However, recently AR has been localized to the plasma membrane in Xenopus oocytes and in hypothalamic cell lines (40, 41), just as estrogen and progesterone receptors were found to be associated with the plasma membrane (42–44). Localization of steroid hormone receptors to the membrane is believed to facilitate interactions with their cognate ligands and place the complex near membrane-associated signaling factors, such as G proteins, or kinases, such as Src or phosphoinositide 3-kinase.
As a result of intracellular signals originating from androgen–AR interactions, the CREB-regulated LDH-A and CREB genes are induced in Sertoli cells (28, 45). Androgen stimulation also induced the Egr1 gene that has been shown to be activated by means of MAP kinase as well as CREB (46). None of the genes activated by androgen in this study have been shown to be regulated by androgen response elements in their promoters, a finding that is consistent with the paradigm that androgen can activate Sertoli cell gene expression by means of the MAP kinase pathway and CREB. Presently, only two genes (Pem and c-Myc) expressed in Sertoli cells are known to be induced by means of the classical model of direct interactions of AR with regulatory regions of gene promoter (11, 12). It is possible that additional genes will be found that are regulated by the classical mechanism. However, further study of the signaling pathways resulting from androgen-induced activation of the MAP kinase cascade will likely identify additional transcription factors and genes that are activated in addition to CREB. Examples of transcription factors that might be activated by androgen by means of MAP kinase include STATs, NF-κB, Elk-1, fos, and jun (47). This potential mechanism for regulating the expression of numerous genes would explain how androgen is capable of sustaining the many processes required to support spermatogenesis.
The significance of testosterone regulation of the CREB transcription factor for the support of spermatogenesis is highlighted by studies demonstrating that spermatozoa are not produced if CREB phosphorylation is inhibited in Sertoli cells (26). CREB phosphorylation has been linked to the activation of Sertoli cell genes that potentially contribute to germ-cell development and survival including c-fos and C/EBP, AR, transferrin, and insulin-like growth factor (48–56). Previously, CREB was thought to be phosphorylated in Sertoli cells predominately by means of cAMP and Ca2+-mediated signaling pathways, initiated by the binding of FSH to G protein-coupled receptors on the Sertoli cell membrane (57). However, androgen stimulation of Sertoli cells does not result in elevated cAMP levels, suggesting that androgen acts by a pathway that is complimentary to that used by FSH.
Although androgen does not elevate cAMP as FSH does, the two hormones have a common mechanism of action because FSH is one of the few known regulators of the MAP kinase pathway in Sertoli cells, and FSH has been shown to regulate Sertoli cell proliferation through the temporal control of MAP kinase (33). Together, FSH and testosterone hormonal signals provide for maximal spermatozoa production; however, in the absence of FSH, androgen is capable of maintaining spermatogenesis (2). The finding that androgen also stimulates phosphorylation of MAP kinase and CREB provides a potential mechanism by which androgens could support spermatogenesis in the absence of FSH. Most importantly, the expansion of androgen responsibilities to the activation of MAP kinase, CREB, and potentially other downstream factors will provide new targets for male contraceptive agents and may explain how testosterone is capable of sustaining the many processes required for spermatogenesis.
Acknowledgments
We thank A. Zeleznik, P. Auren, and S. Schlatt for critical reading of the manuscript. This research was supported by National Institutes of Health Grants RO1-HD43143 (to W.H.W.) and NS045195 (to C.J.).
Abbreviations: AR, androgen receptor; tfm, testicular feminization mutation; ERK, extracellular-regulated kinase; CREB, cAMP response element binding protein; FSH, follicle-stimulating hormone; siRNA, small inhibitory RNA; MAP, mitogen-activated protein; P-CREB, phosphorylated CREB; P-ERK, phosphorylated ERK.
References
- 1.Rommerts, F. F. G. (1988) J. Endocrinol. 116, 7–9. [DOI] [PubMed] [Google Scholar]
- 2.Sharpe, R. M. (1994) in The Physiology of Reproduction, eds. Knobil, E. & Neil, J. D. (Raven, New York), pp. 1363–1434.
- 3.Tsai, M. J. & O'Malley, B. W. (1994) Annu. Rev. Biochem. 63, 451–486. [DOI] [PubMed] [Google Scholar]
- 4.Quigley, C. A., De Bellis, A., Marschke, K. B., El-awady, M. K., Wilson, E. M. & French, F. S. (1995) Endocr. Rev. 16, 271–321. [DOI] [PubMed] [Google Scholar]
- 5.Bagchi, M. K., Tsai, M. J., O'Malley, B. W. & Tsai, S. Y. (1992) Endocr. Rev. 13, 525–535. [DOI] [PubMed] [Google Scholar]
- 6.Shang, Y., Hu, X., DiRenzo, J., Lazar, M. A. & Brown, M. (2000) Cell 103, 843–852. [DOI] [PubMed] [Google Scholar]
- 7.Shang, Y., Myers, M. & Brown, M. (2002) Mol. Cell 9, 601–610. [DOI] [PubMed] [Google Scholar]
- 8.Roberts, K. & Griswold, M. D. (1989) Endocrinology 125, 1174–1179. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, C. Y., Mather, J. P., Byer, A. L. & Bardin, C. W. (1986) Endocrinology 118, 480–488. [DOI] [PubMed] [Google Scholar]
- 10.Kokontis, J. & Liao, S. (1999) Vitam. Horm. (San Francisco) 55, 219–307. [DOI] [PubMed] [Google Scholar]
- 11.Lindsey, J. S. & Wilkinson, M. F. (1996) Dev. Biol. 179, 471–484. [DOI] [PubMed] [Google Scholar]
- 12.Lim, K., Yoo, J. H., Kim, K. Y., Kweon, G. R., Kwak, S. T. & Hwang, B. D. (1994) J. Androl. 15, 543–550. [PubMed] [Google Scholar]
- 13.Veldscholte, J., Berrevoets, C. A., Ris-Stalpers, C., Kuiper, G. G., Jenster, G., Trapman, J., Brinkmann, A. O. & Mulder, E. (1992) J. Steroid Biochem. Mol. Biol. 41, 665–669. [DOI] [PubMed] [Google Scholar]
- 14.Steinsapir, J., Socci, R. & Reinach, P. (1991) Biochem. Biophys. Res. Commun. 179, 90–96. [DOI] [PubMed] [Google Scholar]
- 15.Gorczynska, E. & Handelsman, D. J. (1995) Endocrinology 136, 2052–2059. [DOI] [PubMed] [Google Scholar]
- 16.Lyng, F. M., Jones, G. R. & Rommerts, F. F. G. (2000) Biol. Reprod. 63, 736–747. [DOI] [PubMed] [Google Scholar]
- 17.Falkenstein, E., Tillmann, H. C., Christ, M., Feuring, M. & Wehling, M. (2000) Pharmacol. Rev. 52, 513–555. [PubMed] [Google Scholar]
- 18.Heinlein, C. A. & Chang, C. (2002) Mol. Endocrinol. 16, 2181–2187. [DOI] [PubMed] [Google Scholar]
- 19.Losel, R. M., Falkenstein, E., Feuring, M., Schultz, A., Tillmann, H. C., Rossol-Haseroth, K. & Wehling, M. (2003) Physiol. Rev. 83, 965–1016. [DOI] [PubMed] [Google Scholar]
- 20.Zhu, X., Li, H., Liu, J. P. & Funder, J. W. (1999) Mol. Cell. Endocrinol. 152, 199–206. [DOI] [PubMed] [Google Scholar]
- 21.Peterziel, H., Mink, S., Schonert, A., Becker, M., Klocker, H. & Cato, A. C. (1999) Oncogene 18, 6322–6329. [DOI] [PubMed] [Google Scholar]
- 22.Shaywitz, A. J. & Greengerg, M. E. (1999) Annu. Rev. Biochem. 68, 821–861. [DOI] [PubMed] [Google Scholar]
- 23.Kwok, R. P. S., Lundbland, J. R., Chrivia, J. C., Richards, J. P., Bachinger, H. P., Brennan, R. G., Roberts, S. G. E., Green, M. R. & Goodman, R. H. (1994) Nature 370, 223–226. [DOI] [PubMed] [Google Scholar]
- 24.Chrivia, J. C., Kwok, R. P., Lamb, N., Hagiwara, M., Montminy, M. R. & Goodman, R. H. (1993) Nature 365, 855–859. [DOI] [PubMed] [Google Scholar]
- 25.Meyer, T. E. & Habener, J. F. (1993) Endocr. Rev. 14, 269–290. [DOI] [PubMed] [Google Scholar]
- 26.Scobey, M. J., Bertera, S., Somers, J. P., Watkins, S. C., Zeleznik, A. J. & Walker, W. H. (2001) Endocrinology 142, 948–954. [DOI] [PubMed] [Google Scholar]
- 27.Chapin, R. E., Phelps, J. L., Miller, B. E. & Gary, T. J. B. (1987) J. Androl. 8, 155–161. [DOI] [PubMed] [Google Scholar]
- 28.Walker, W. H., Fucci, L. & Habener, J. F. (1995) Endocrinology 136, 3534–3545. [DOI] [PubMed] [Google Scholar]
- 29.Delfino, F. J. & Walker, W. H. (1999) J. Biol. Chem. 274, 35607–35613. [DOI] [PubMed] [Google Scholar]
- 30.Hicks, L. L. & Walsh, P. C. (1979) Steroids 33, 389–406. [DOI] [PubMed] [Google Scholar]
- 31.Turner, T. T., Jones, C. E., Howards, S. S., Ewing, L. L., Zegeye, B. & Gunsalus, G. L. (1984) Endocrinology 115, 1925–1932. [DOI] [PubMed] [Google Scholar]
- 32.Comhaire, F. H. & Vermeulen, A. (1976) J. Endocrinol. 70, 229–235. [DOI] [PubMed] [Google Scholar]
- 33.Crepieux, P., Marion, S., Martinat, N., Fafeur, V., Vern, Y. L., Kerboeuf, D., Guillou, F. & Reiter, E. (2001) Oncogene 20, 4696–4709. [DOI] [PubMed] [Google Scholar]
- 34.Nakhla, A. M., Khan, M. S. & Rosner, W. (1990) J. Clin. Endocrinol. Metab. 71, 398–404. [DOI] [PubMed] [Google Scholar]
- 35.Nakhla, A. M., Leonard, J., Hryb, D. J. & Rosner, W. (1999) Steroids 64, 213–216. [DOI] [PubMed] [Google Scholar]
- 36.Fire, A. (1999) Trends Genet. 15, 358–363. [DOI] [PubMed] [Google Scholar]
- 37.Yarbrough, W. G., Quarmby, V. E., Simental, J. A., Joseph, D. R., Sar, M., Lubahn, D. B., Olsen, K. L., French, F. S. & Wilson, E. M. (1990) J. Biol. Chem. 265, 8893–8900. [PubMed] [Google Scholar]
- 38.Yoshikawa, K. & Azawa, T. (1988) FEBS Lett. 237, 183–186. [DOI] [PubMed] [Google Scholar]
- 39.Sassone-Corsi, P., Visvader, J., Ferland, L., Mellon, P. L. & Verma, I. M. (1988) Genes Dev. 2, 1529–1538. [DOI] [PubMed] [Google Scholar]
- 40.Lutz, L. B., Jamnongjit, M., Yang, W. H., Jahani, D., Gill, A. & Hammes, S. R. (2003) Mol. Endocrinol. 17, 1106–1116. [DOI] [PubMed] [Google Scholar]
- 41.Shakil, T., Hoque, A. N., Husain, M. & Belsham, D. D. (2002) Mol. Endocrinol. 16, 2592–2602. [DOI] [PubMed] [Google Scholar]
- 42.Razandi, M., Oh, P., Pedram, A., Schnitzer, J. & Levin, E. R. (2002) Mol. Endocrinol. 16, 100–115. [DOI] [PubMed] [Google Scholar]
- 43.Peluso, J. J., Fernandez, G., Pappalardo, A. & White, B. A. (2001) Biol. Reprod. 65, 94–101. [DOI] [PubMed] [Google Scholar]
- 44.El-Hefnawy, T., Manna, P. R., Luconi, M., Baldi, E., Slotte, J. P. & Huhtaniemi, I. (2000) Endocrinology 141, 247–255. [DOI] [PubMed] [Google Scholar]
- 45.Short, M. L., Huang, D., Milkowski, D. M., Short, S., Kunstman, K., Soong, C. J., Chung, K. C. & Jungmann, R. A. (1994) Biochem. J. 304, 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwon, E. M., Raines, M. A., Blenis, J. & Sakamoto, K. M. (2000) Blood 95, 2552–2558. [PubMed] [Google Scholar]
- 47.Hazzalin, C. A. & Mahadevan, L. C. (2002) Nat. Rev. Mol. Cell Biol. 3, 30–40. [DOI] [PubMed] [Google Scholar]
- 48.Niehof, M., Manns, M. P. & Trautwein, C. (1997) Mol. Cell. Biol. 17, 3600–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lidzey, J., Grossmann, M., Kumar, M. V. & Tindall, D. J. (1993) Mol. Endocrinol. 7, 1530–1540. [DOI] [PubMed] [Google Scholar]
- 50.Mizokami, A., Yeh, S.-Y. & Chang, C. (1994) Mol. Endocrinol. 8, 77–88. [DOI] [PubMed] [Google Scholar]
- 51.Sanborn, B. M., Caston, L. A., Chang, C., Liao, S., Speller, R., Porter, L. D. & Ku, C. Y. (1991) Biol. Reprod. 45, 634–641. [DOI] [PubMed] [Google Scholar]
- 52.Verhoven, G. & Cailleau, J. (1988) Endocrinology 122, 1541–1550. [DOI] [PubMed] [Google Scholar]
- 53.Blok, L. J., Hoogenbrugge, J. W., Themmen, A. P. N., Baarends, W. M., Post, M. & Grootegoed, A. (1992) Endocrinology 131, 1343–1349. [DOI] [PubMed] [Google Scholar]
- 54.Suire, S., Fontaine, I. & Guillou, F. (1995) Mol. Endocrinol. 9, 756–766. [DOI] [PubMed] [Google Scholar]
- 55.Chaudhary, J. & Skinner, M. K. (1999) Endocrinology 140, 1262–1271. [DOI] [PubMed] [Google Scholar]
- 56.Suwanichkul, A., DePaolis, L. A., Lee, P. D. & Powell, D. R. (1993) J. Biol. Chem. 268, 9730–9736. [PubMed] [Google Scholar]
- 57.Simoni, M., Gromoli, J. & Niesclag, E. (1997) Endocr. Rev. 18, 739–773. [DOI] [PubMed] [Google Scholar]