Abstract
The interleukin-1β-mitogen-activated protein kinase (MAPK) and NF-κB signaling pathways are involved in the pathogenesis of rheumatoid arthritis. Ebosin, a novel exopolysaccharide (EPS), exhibits anti-inflammatory activity in rat collagen-induced arthritis by suppressing the production of tumor necrosis factor-α, interleukin-6 and interleukin-1β. The aim of the present study was to assess the effects of ebosin on NF-κB and MAPK signaling pathways mediated through interleukin-1β in rat fibroblast-like synoviocytes (FLSs). Western blotting showed decreased production of phosphorylated p38, JNK1, JNK2, IKKα, IKKβ and IκB in the cytoplasm and NF-κB in the nucleus upon ebosin treatment. The DNA-binding activity of NF-κB in the cell nucleus was also inhibited by ebosin treatment, as demonstrated using an electrophoresis mobility gel shift assay. Analysis of the results of the immunofluorescence assay also showed a reduced amount of NF-κB in the nucleus of cells affected by ebosin. These results provided evidence for the effects of ebosin on both interleukin-1β-mediated MAPK and NF-κB signaling pathways in rat FLSs. In addition, enzyme-linked immunosorbent assay demonstrated that ebosin reduces the levels of matrix metalloproteinases MMP-1 and MMP-3 and the chemokines, interleukin-8 and RANTES. Thus, the results of the present study provide further evidence for understanding the medicinal activity of ebosin at a molecular level, therefore nominating this EPS as a potential therapeutic candidate for the treatment of rheumatic arthritis.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic polyarticular inflammatory joint disease that eventually causes the destruction of cartilage and bone.1 Proinflammatory cytokines produced in fibroblast-like synoviocytes (FLSs), such as interleukin-1β (IL-1β), play a key role in the pathogenesis of RA. Antagonists to the IL-1 receptor (IL-1R) have been demonstrated to be effective in ameliorating RA during clinical studies.2
Upon binding to IL-1, IL-1R heterodimerizes with the accessory protein IL-1RAcP, which then binds to the adaptor protein MyD88 (myeloid differentiation marker 88). MyD88 recruits the IL-1R-associated serine/threonine kinase, IRAK-1, which subsequently interacts with TRAF6, a tumor necrosis factor receptor-associated factor for the activation of mitogen-activated protein kinase (MAPK) pathways and the transcription factors AP-1 and NF-κB. Additional pro-inflammatory cytokines produced by these pathways mediate joint destruction.3
Based on the current knowledge of the signal transduction mechanisms and gene regulation involved in inflammation, MAPK signaling pathways have been considered to be molecular targets for anti-inflammatory therapy. The p38 MAPK and JNK pathway inhibitors have attracted much attention, as these molecules reduce both the synthesis of and intracellular signaling of pro-inflammatory cytokines.4 Transcription factors play key roles in RA processes, leading to cartilage and bone destruction.5 An anti-inflammatory therapy that inhibits NF-κB signaling has been well-recognized.6 Lee et al. demonstrated that guggulsterone suppresses NF-κB activation in FLS and block the inflammatory responses mediated through IL-1β.7 Turner-Brannen et al. demonstrated the effect of the IDR-1002 peptide on inhibiting IL-1β-induced NF-κB, JNK and p38 MAPK activation in FLS.8
Ebosin, a novel exopolysaccharide (EPS) comprising rhamnose, fucose, arabinose, mannose, xylose, glucose, galactose and galacturonic acid, was isolated from the fermentation culture of Streptomyces sp. 1399 and shown to significantly suppress the development of rat collagen-induced arthritis (CIA).10 The remarkable anti-inflammatory effect of ebosin on CIA is mediated through the inhibition of IL-1β, IL-6 and TNF-α production at both transcriptional and posttranscriptional levels.10
The aim of the present study was to assess the effects of ebosin on IL-1β-mediated MAPK and NF-κB signaling pathways and the levels of matrix metalloproteinases (MMP-1 and MMP-3) and chemokines (IL-8 and RANTES) in rat FLSs.
MATERIALS AND METHODS
Animals
Wistar rats (male, 180 ± 20 g, Certificate No.: SCXK 2005-0013) were purchased from the Institute of Experimental Animals, Chinese Academy of Medical Sciences, Beijing. All rats were housed under standard laboratory conditions as previously described.10
Collagen-induced arthritis (CIA) model
Chicken type II collagen (CII, Sigma, St. Louis, Missouri, USA) was dissolved in 0.1 M acetic acid at 4 mg/ml after stirring overnight at 4 °C and emulsified with an equal volume of complete Freund's adjuvant (CFA, Sigma), prepared by adding Freund's incomplete adjuvant to heat-inactivated BGG (bovine gamma globulin, Invitrogen, Carlsbad, CA, USA) at a final concentration of 2 mg/ml. The rats were injected intradermally at the base of the tail with 100 μl of the emulsion. After 7 days, the rats were injected again in the same manner (the first injection was in the right hind metatarsal footpad; the second injection was in the base of the tail).11 The day of the first immunization was defined as day 0.
Preparation of FLSs and MTT assay
FLSs were prepared and cultured according to the protocol of Zhang et al.10
FLSs were seeded at 1 × 104/ml onto 96-well plates in DMEM and cultivated at 37 °C overnight. Ebosin (400, 80, 16, 3.2 and 0.64 μg/ml) was added to each well, and the cells were cultivated at 37 °C for 48 h. MTT solution (at a final concentration of 500 μg/mL) (Boster, Wuhan, China) was added to each well, and the MTT assay was performed as previously reported.10 The absorbance was measured at 570 nm, and the % survival was determined after comparison with the control group.
Isolation of ebosin
The ebosin-producing strain Streptomyces sp. 139 was collected from a soil sample in China and maintained in the China General Microbiology Culture Collection Center (No. 0405). This strain was cultured at 28 °C for 96 h with shaking (250 rpm). Ebosin was isolated from the supernatant of Streptomyces sp. 139 fermentation culture using the previously described protocol.9
Western blot analysis
FLSs were seeded at 1 × 105/ml onto 6-well plates in DMEM and cultivated at 37 °C for 24 h, and various concentrations of ebosin in DMEM (assay for MAPK and NF-κB: 400, 80, 16, 3.2, 0.64, and 0.128 μg/ml; assay for IKK, IκB: 80, 16 and 3.2 μg/ml) were added and the cells were cultivated at 37 °C for an additional 12 h. IL-1β (10 ng/ml) was then added for 15 min (assay for MAPK) or 3 h (assay for IKK/NF-κB). After removing the supernatant through centrifugation (800g, 10 min), the cells were lysed in RIPA lysis buffer (Applygen) at 4 °C for 30 min. The supernatants were collected through centrifugation (12 000g, 10 min), and the protein concentration was identified using a BCA protein assay kit (Applygen). The supernatant of the lysed cells was separated through 12% sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently transferred onto PVDF membranes (Millipore, Billerica, Massachusetts, USA). The membranes were blocked with 5% skim milk in TBS buffer at room temperature for 1 h, followed by washing with TBST (TBS + 0.1% Tween 20). The membranes were first probed with antibodies, including rabbit anti-rat phosphorylated (or non-phosphorylated) p38, JNK1, JNK2, ERK, IKKα, IKKβ, IκB and NF-κB p65 (Cell Signaling Technology, Danvers, Massachusetts, USA), at 4 °C for 12 h. After washing with TBST, the membranes were incubated with secondary antibodies (goat anti rabbit HRP-IgG) at room temperature for 2 h. The proteins were visualized using a chemiluminescent HRP substrate (Millipore). The densities of the protein bands from the western blot analysis were scanned using ImageQuant 300 (GE Healthcare, Pittsburgh, Pennsylvania, USA) and subsequently analyzed using Image J software.
Assays for chemokines and metalloproteinases
The levels of chemokines (IL-8 and RANTES) and metalloproteinases (MMP-1 and MMP-3) were measured using enzyme-linked immunosorbent assays (ELISAs). FLSs were seeded at 1 × 106/ml onto 24-well plates and cultivated at 37 °C for 24 h. Ebosin (80, 16 and 3.2 μg/ml in DMEM) was subsequently added to each well, and the samples were cultivated at 37 °C for an additional 12 h, followed by the addition of IL-1β (10 ng/ml) and further incubation for 24 h. After centrifugation (1000g, 20 min), the supernatant was collected. The total amounts of MMP-1 and MMP-3 in the supernatant were determined using ELISA kits obtained from USCN Life Science Inc (Wuhan, China), while the levels of the chemokines RANTES and IL-8 were quantified using ELISA kits obtained from Boster, Elab.
Preparation of nuclear extracts
The cells were seeded onto 6-well plates (at 1 × 106/ml) and cultivated at 37 °C for 24 h, followed by the addition of ebosin (80, 16 and 3.2 μg/ml in DMEM) and IL-1β (10 ng/ml). After 3 h, the cells were treated with 0.25% trypsin–EDTA (HyClone, Logan, Utah, USA) and subsequently harvested and centrifuged at 500g for 5 min. After washing with PBS (pH 7.4), the cell pellet was resuspended in ice-cold PBS (pH 7.4) and transferred to a 1.5 ml microcentrifuge tube and centrifuged again at 500g for 2–3 min. The cell pellet was resuspended in 200 μl of cold CER I (cytoplasmic extraction reagent I, Thermo, Waltham, Massachusetts, USA), vortexed for 15 s and then incubated on ice for 10 min. The cells were harvested after centrifugation, resuspended in 11 μl of cold CRE II (Thermo), vortexed for 5 s, and incubated on ice for 1 min. The nuclear fraction was precipitated by centrifugation at 16 000g for 5 min at 4 °C. The supernatants (cytoplasmic extract) were collected and stored at −80 °C. The pelleted nuclei were resuspended in 100 μl of ice-cold NER (nuclear extraction reagent, Thermo), vortexed for 15 s, and incubated on ice for 40 min with shaking for 15 s per 10 min. The sample was then centrifuged for 10 min at 16 000g, and aliquots of the nuclear extracts were stored at −80 °C. The protein concentration was determined using the Bradford method.12
Electrophoretic mobility shift analysis (EMSAs)
FLSs were seeded at 1 × 106/ml onto 6-well plates and cultivated at 37 °C for 24 h. Subsequently, ebosin (80, 16 and 3.2 μg/ml in DMEM) was added to each well, and the cells were cultivated at 37 °C for 12 h, followed by the addition of IL-1β (10 ng/ml) and cultivation for a further 3 h. To evaluate the effects of ebosin on NF-κB binding to DNA in the nucleus, nuclear extracts from control and treated cells were used in an EMSA.13 The biotin-labeled oligonucleotide probe was synthesized (Beyotime, Shanghai, China): 5′-AGTTGAGGGGACTTTCCCAGGC-3′; 3′-TCAACTCCCCTGAAAGGGTCCG-5′, containing the κ-chain binding site (κB, 5′-GGGACTTTCC-3′). An unlabeled oligonucleotide with an identical sequence was also synthesized as a control. A LightShift Chemiluminescent EMSA Kit (Thermo) was used according to the manufacturer's instructions. Specific binding was controlled through competition with a 50-fold excess of cold κB oligonucleotide.
Immunofluorescence analysis
The cells were seeded onto 96-well plates (at 1 × 104/ml) and cultivated at 37 °C for 12 h, followed by the addition of 80 μg/ml of ebosin in DMEM and cultivation at 37 °C for an additional 12 h. Subsequently, IL-1β (10 ng/ml) was added and the cells were cultivated for an additional 3 h. The cells were fixed in PBS containing 5% paraformaldehyde at room temperature for 10 min and permeabilized in 100 μl of PBS containing 0.5% Triton X-100 for 20 min at room temperature. The cells were incubated with rabbit antibody against NF-κB p65 (Cell Signaling Technology) at a 1:500 dilution overnight at 4 °C. After washing with PBS, the cells were incubated with donkey anti-rabbit IgG labeled with Alexa Fluor 546 (10 μg/ml, Molecular Probes, Eugene, Oregon, USA) and Hoechst 33528 (10 μg/ml, Beyotime) for 1 h at 37 °C under dark conditions. For analysis using fluorescence microscopy, the cells were counterstained with Hoechst 33528 for nuclear staining.14
Statistical analysis
The quantitative results presented were obtained from at least three independent experiments, and the data are presented as the mean ± SD. Student's t-test was applied to evaluate the significance of differences between samples, and P-values less than 0.05 were considered significant.
RESULTS
Effect of ebosin on the production of p38 MAPK
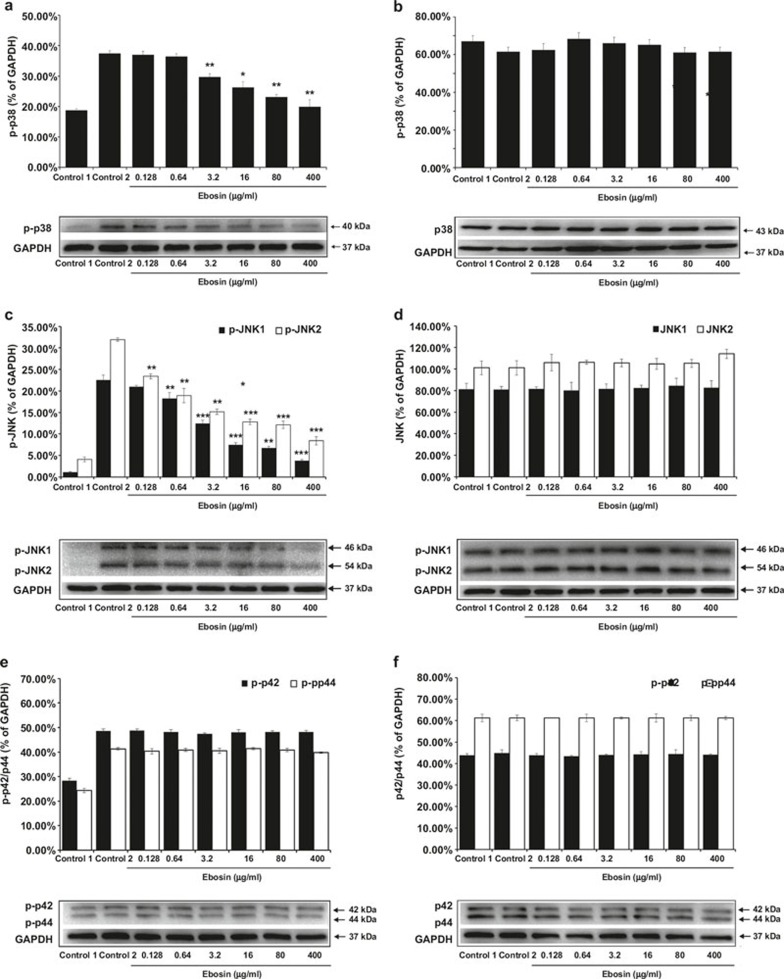
Several cytokines (IL-1β, TNF-α, etc.) act as extracellular stimuli of the p38 MAP kinase pathway, which is associated with inflammation and the growth, differentiation, and death of cells.15 To evaluate the effects of ebosin on p38 MAP kinase production in FLSs, western blot analysis was performed. The results showed that ebosin at dosages of 400, 80, 16, 3.2, 0.64, and 0.128 μg/ml abated the phosphorylated p38 expression levels by 46.74% (P < 0.01), 38.33% (P < 0.01), 29.71% (P < 0.05), 21.37% (P < 0.01), 2.67%, and 1.15%, respectively but had no effect on the production of non-phosphorylated p38 in FLSs at the same dosages (Figure 1a and b). These results suggest that ebosin has reduces phosphorylated p38 protein levels in FLSs in a dose-dependent manner. No significant effect on cell viability was observed at a test concentration up to 400 μg/ml of ebosin, as determined by the MTT assay (>85% cell survival), indicating that the inhibition of phosphorylated p38 production through ebosin was not mediated by a cytotoxic effect (data not shown).
Figure 1.
The effects of ebosin on the MAPK signaling pathway mediated by IL-1β in FLSs. (a, b) Effect of ebosin on the levels of phosphorylated and non-phosphorylated p38, as determined using western blotting. (c, d) Effect of ebosin on the levels of phosphorylated and non-phosphorylated JNK1 and JNK2. (e, f) Effect of ebosin on the levels of phosphorylated and non-phosphorylated ERK (P42) and ERK (P44). These data are expressed as the mean ± SD from at least three independent experiments. P < 0.001, P < 0.01, and P < 0.05 compared with the control 2 (FLS incubated with IL-1β).
Ebosin suppresses the expression of JNK1 and JNK2 MAPK
JNK is one of the major groups of MAPK cascades in humans, leading to altered gene expression16; therefore, we investigated the impact of ebosin on the IL-1β-induced expression of JNK in FLSs. The cells were treated with IL-1β in the presence of ebosin, and cell lysates were analyzed using western blotting. The results showed that ebosin significantly inhibited the production of phosphorylated JNK1 and JNK2 in a dose-dependent manner. At dosages of 400, 80, 16, 3.2, 0.64, and 0.128 μg/ml of ebosin, the expression levels of phosphorylated JNK1 were diminished by 83.21% (P < 0.001), 70.22% (P < 0.01), 66.97% (P < 0.001), 44.85% (P < 0.001), 19.06% (P < 0.01), and 6.99%, respectively (Figure 1c), and the levels of phosphorylated JNK2 were diminished by 73.68% (P < 0.001), 61.99% (P < 0.001), 59.94% (P < 0.001), 52.47% (P < 0.01), 40.77% (P < 0.01), and 26.71% (P < 0.01), respectively. Notably, ebosin did not affect the levels of unphosphorylated forms of JNK1 and JNK2 (Figure 1d).
Ebosin did not inhibit the expression of p42/44 MAPK (ERK1/2)
Similar to p38 MAPK and JNK, p42/44 MAPK (ERK1/2) also participates in protein kinase cascades that play an essential role in the regulation of the growth and differentiation of cells and control cellular responses to cytokines and stress.15 To examine the influence of ebosin on p42/44 MAPK expression, the cells were treated with IL-1β in the presence of ebosin, and the cell lysates were analyzed through western blotting using antibodies raised against phosphorylated (or non-phosphorylated) p42/44. The results (Figure 1e and f) did not show any effects of ebosin at 400, 80, 16, 3.2, 0.64, and 0.128 μg/ml on either the phosphorylated or non-phosphorylated p42/44 MAPK.
Influence of ebosin on the expression of IKKα and IKKβ
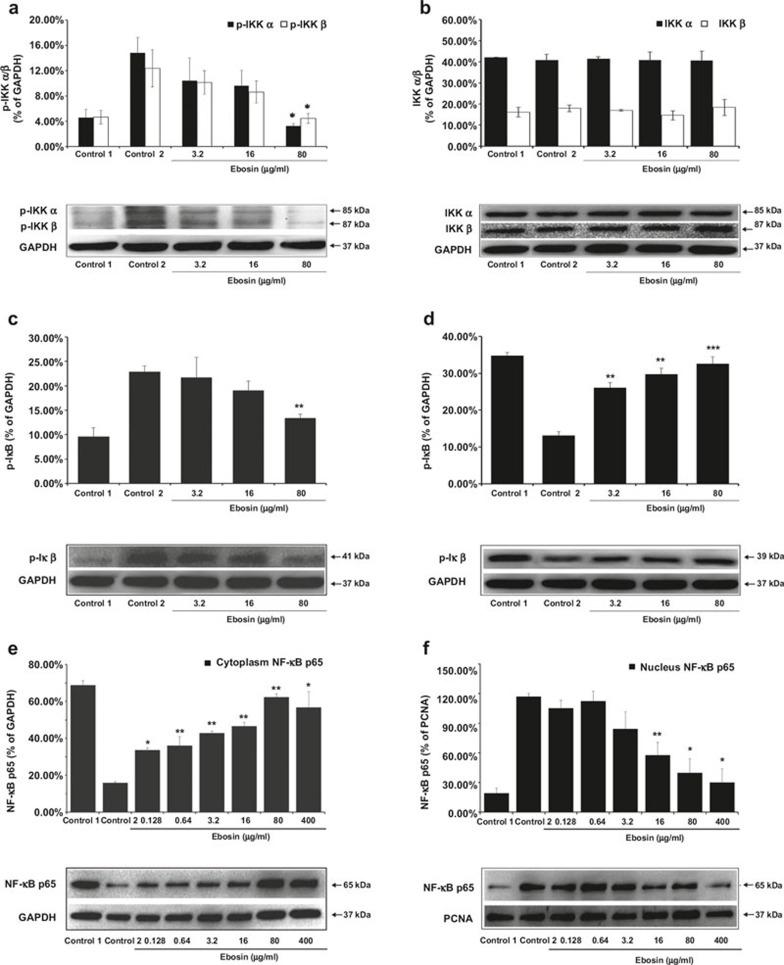
NF-κB signaling ultimately leads to the activation of the IκB kinase (IKK) complex, which mediates the phosphorylation of the inhibitory IκB protein. The IKK complex comprises the regulatory subunit IKKγ and two catalytic subunits IKKα and IKKβ.17 To assess whether ebosin influences the production of IKKα and IKKβ, the cells were treated with IL-1β, and the lysates were subjected to western blot analysis. The results (Figure 2a and b) indicated that 80, 16 and 3.2 μg/ml of ebosin decreased the levels of phosphorylated IKKα by 78.13% (P < 0.05), 35.08%, and 29.79%, respectively, and IKKβ by 64.06% (P < 0.05), 30.26%, and 18.11%, respectively, while the production of non-phosphorylated IKKα and IKKβ was not affected.
Figure 2.
The effects of ebosin on the NF-κB signaling pathway mediated by IL-1β in FLS. (a, b) Influence on the expression of phosphorylated and non-phosphorylated IKKα and IKKβ. The expression levels were analyzed by western blotting. (c, d) Effect on the production of phosphorylated and non-phosphorylated IκBα. (e, f) Effect on the nuclear and cytoplasmic levels of NF-κB in FLSs. These data are expressed as the mean ± SD from at least three independent experiments. P < 0.001, P < 0.01, and P < 0.05 compared with the control (FLS incubated with IL-1β) for NF-κB, IKKα, IKKβ and IκBα, respectively.
Effect of ebosin on the production of IκBα
IκBs mask the DNA-binding and nuclear localization domains of NF-κB, thereby restricting the migration of this transcription factor into the nucleus.18 We performed western blot analyses to determine whether ebosin also effects the levels of IκBα expression in FLSs. As shown in Figure 2c, 80, 16, and 3.2 μg/ml of ebosin reduced the levels of phosphorylated IκBα by 41.64% (P < 0.01), 16.97% and 5.36%, respectively, but the levels of non-phosphorylated IκBα were increased by 59.71% (P < 0.001), 55.97% (P < 0.01), and 49.58% (P < 0.01) (Figure 2d). Because the phosphorylation of IκBα was inhibited through ebosin, the levels of non-phosphorylated IκBα were indeed increased in FLSs.
Inhibition of NF-κB DNA-binding activity through ebosin
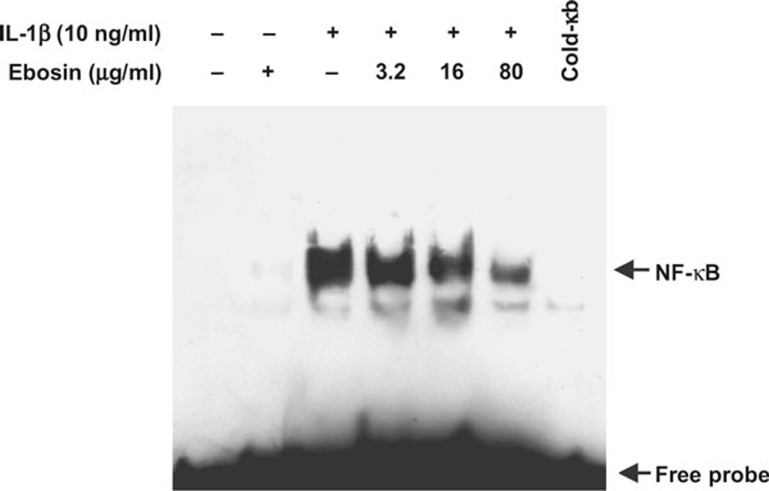
To assess whether ebosin affects the specific binding of NF-κB to DNA, an EMSA was conducted using various concentrations of ebosin in vitro. The nuclear extracts of FLSs were prepared as described earlier. Figure 3 showed that 80, 16, and 3.2 μg/ml of ebosin inhibited the specific binding of NF-κB to DNA in a dose-dependent manner.
Figure 3.
Inhibition of NF-κB DNA-binding activity by ebosin in FLSs mediated via IL-1β. The DNA-binding activity of the nuclear extracts obtained from FLSs was examined in an EMSA using a specific biotin-labeled oligonucleotide probe. The LightShift Chemiluminescent EMSA kit was used according to the manufacturer's instructions. Specific binding was controlled through competition with a 50-fold excess of cold κB oligonucleotide.
Effect of ebosin on nuclear translocation of NF-κB
To evaluate the effect of ebosin on NF-κB expression in both the cytoplasm and the nucleus of FLSs, western blot analysis was performed using an NF-κB p65 antibody. As depicted in Figure 2e, 400, 80, 16, 3.2, 0.64, and 0.128 μg/ml of ebosin increased the levels of NF-κB in the cytoplasm of FLSs by 72.13% (P < 0.05), 74.61% (P < 0.01), 65.93% (P < 0.01), 62.94% (P < 0.01), 55.93% (P < 0.01), and 52.76% (P < 0.05), respectively; however, NF-κB in the nucleus was suppressed by 74.38% (P < 0.05), 65.99% (P < 0.05), 50.70% (P < 0.01), 27.98%, 4.08%, and 10.14%, respectively (Figure 2f). The results indicated that ebosin markedly inhibited the nuclear translocation of NF-κB in FLSs.
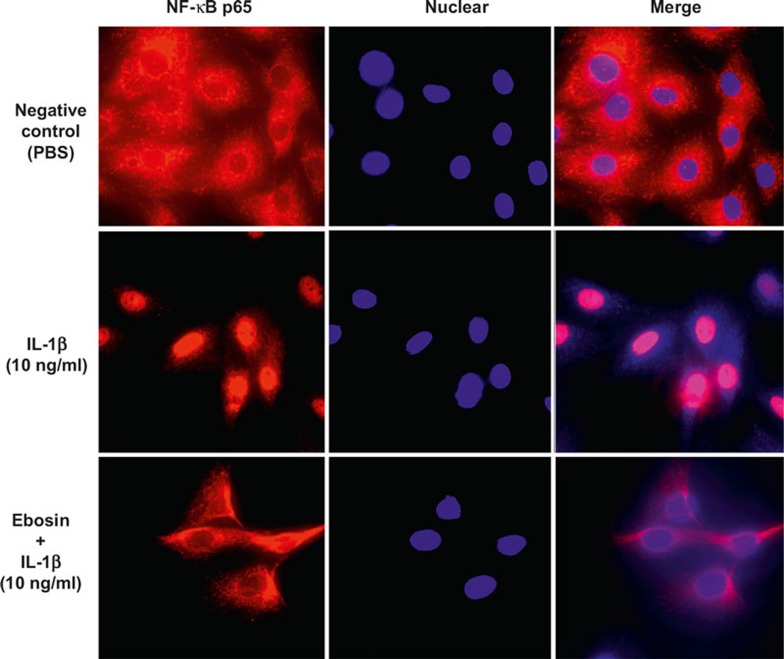
To further examine the effect of ebosin on NF-κB translocation from the cytoplasm into the nucleus of FLSs, the cells were treated with IL-1β in the presence of ebosin at 80 μg/ml and subsequently incubated with rabbit antibody against NF-κB p65. For fluorescence microscopy, the cells were counterstained with Hoechst 33528 for nuclear staining, showing that NF-κB translocation from the cytoplasm to the nucleus in FLSs was indeed inhibited through ebosin (Figure 4).
Figure 4.
Effect of ebosin on the nuclear translocation of NF-κB mediated by IL-1β in FLSs. Indirect immunofluorescence using the specific anti-NF-κB p65 antibody was performed. For fluorescence microscopy, the cells were counterstained with Hoechst 33528 for nuclear staining.
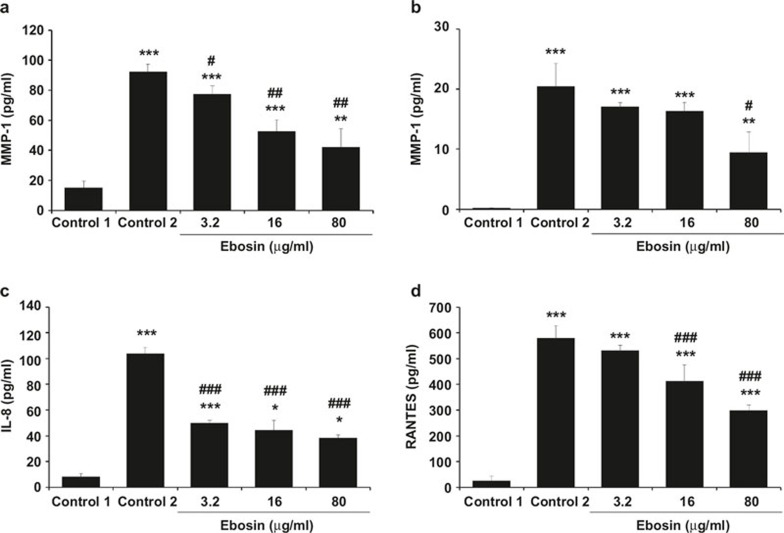
Suppression of the expression of MMP1 and MMP3 by ebosin
NF-κB activation contributes to the pathogenesis of RA by the activation of matrix metalloproteinases (MMPs).19 MMP-1 and MMP-3 are involved in tissue destruction, as they are detected at high levels in the synovial fluids of RA patients.20 To examine the effects of ebosin on the expression of MMP-1 and MMP-3 in FLSs, ELISA analyses were performed. As shown in Figure 5a, at 80, 16, and 3.2 μg/ml of ebosin, MMP-1 expression levels in FLSs were decreased by 54.29% (P < 0.01), 42.99% (P < 0.01), and 16.32% (P < 0.05), respectively, and MMP-3 production in FLSs was suppressed by 53.61% (P < 0.05), 20.12%, and 16.49%, respectively (Figure 5b).
Figure 5.
Effect of ebosin on the production of MMPs and chemokines mediated by IL-1β in FLSs. (a, b) Inhibition of ebosin on the production of MMP1 and MMP3. The levels of MMP1 and MMP3 were determined using ELISA. (c, d) Effects of ebosin on the expression of chemokines, including RANTES and IL-8. The expression levels of RANTES and IL-8 were determined through ELISA. These data are expressed as the mean ± SD from at least three independent experiments. P < 0.001, P < 0.01 and P < 0.05 compared with the respective control (FLS incubated with IL-1β).
Effect of ebosin on the production of chemokines
IL-1β stimulates chemokine production in FLSs.21 The influence of ebosin on the expression levels of chemokines, including RANTES and IL-8, in FLSs was assessed using ELISA. At 80, 16, and 3.2 μg/ml of ebosin the production of IL-8 in FLS was reduced by 63.05% (P < 0.001), 57.09% (P < 0.001), and 51.96% (P < 0.001), respectively (Figure 5c), while RANTES expression levels were diminished by 48.53% (P < 0.001), 28.85% (P < 0.001), and 8.39%, respectively (Figure 5d).
DISCUSSION
As a key process in the host defense system, inflammation is highly regulated, and many diseases, including RA and chronic inflammation, result from the loss of control over this process.22,23 Inflammatory responses are varied in different diseases, characterized by a common spectrum of genes and endogenous mediators, including growth factors; inflammatory cytokines, such as IL-1β, TNF-α, and IL-6, chemokines (Macrophage Inflammatory Factor-MIP-1α,β, IL-8); MMPs; and toxic molecules, such as nitric oxide or free radicals.24,25
EPSs possess branched repeating units of sugar or sugar derivatives as long-chain polysaccharides, which are secreted into the surrounding environment of the bacteria during growth,26 and these molecules play vital roles in a variety of biological processes. EPSs, including a range of diverse polymers, also have significant industrial applications, particularly as biothickeners in foods.27 Notably, the EPSs secreted from lactic acid bacteria essentially contribute to the structure and viscosity of fermented milk products.28 Certain EPSs also confer health benefits, such as cholesterol-lowering properties,29 antitumor activity,30 anti-diabetic activity, and so on.31 Trichoderma erinaceum DG-312 produced EPSs that exhibited strong anti-inflammatory activity in inflamed mice.32 Nowak et al.33 reported that EPS from Lactobacillus rhamnosus inhibits the production of arthritogenic antibodies, thereby suppressing active CIA.
A novel EPS isolated from the supernatant of the fermentation culture of Streptomyces sp. 139 showed an anti-inflammatory effect on rat CIA through the suppression of the production of IL-1β, interleukin-6 and tumor necrosis factor α at both transcriptional and posttranslational levels.10 In the present study, we further demonstrated that ebosin affects the IL-1β-induced inflammatory responses in FLSs by inhibiting IL-1β-induced MAPK and NF-κB signaling pathways.
The results of the current study indicate that ebosin markedly reduces the levels of phosphorylated JNK1, JNK2, and p38 MAPK in FLSs in a dose-dependent manner. Previous studies34,35 have shown that IL-1β-induced p38 MAPK and JNK play critical roles in MMP induction and subsequent tissue destruction in arthritis, suggesting that both p38 MAPK and JNK are valuable therapeutic targets for arthritis.36,37,38 Additionally, these results showed that ebosin does not decrease the levels of p42/44 MAPK (ERK1/2), which play a role in cell proliferation, cell differentiation, and cell migration.39,40
IL-1β exerts its primary effects through NF-κB, which is initially located in the cytoplasm complexed with the inhibitory factor IκB in an inactive form. A number of factors induce the dissociation of this complex, presumably through the phosphorylation of IκB, to release NF-κB. Subsequently, NF-κB translocates to the nucleus to mediate gene transcription through interactions at specific DNA recognition sites.41 In the present study, we focused on the capacity of ebosin to counteract NF-κB production after IL-1β treatment of FLSs. The results showed that ebosin treatment decreased the levels of phosphorylated IKKα and IKKβ. In addition, ebosin induced the downregulation of phosphorylated IκBα. EMSA revealed that ebosin inhibits the DNA-binding activity of NF-κB in the nucleus, likely through NF-κB phosphorylation. Furthermore, western blot analysis and fluorescence microscopy indicated that the translocation of NF-κB from the cytoplasm into the nucleus (nuclear translocation) was indeed inhibited by ebosin in FLSs. Taken together, these results showed that ebosin inhibits NF-κB signaling pathways, which are critical for the development of RA and cancer.18
MMPs are primary products of FLSs upon cytokine stimulation, and these molecules efficiently degrade the collagenous components of cartilage and bone, causing joint deformity and severe pain in patients with RA. In the present study, the ELISA results showed that the expression of MMP-1 and MMP-3 was suppressed by ebosin in FLSs. These results indicate that the suppression of NF-κB by ebosin might also reduce MMP production, potentially leading to protection from joint destruction in RA patients.
In response to proinflammatory cytokines, FLSs produce chemokines, which further enhance inflammation, hyperplasia, and cartilage destruction.42 Chemokines comprise two major subfamilies, the CC and CXC subfamilies. RANTES, as a member of the CC subfamilies, has been implicated in chronic inflammatory disease. Herein, we showed that ebosin inhibited the production of chemokines, including RANTES and IL-8, constitutively produced by FLSs, and the expression of these chemokines typically precedes the onset of clinical symptoms in animal models of RA.43
In summary, the results obtained in the present study demonstrated the inhibitory effect of ebosin on IL-1β-induced inflammatory responses in isolated FLSs. Ebosin is an effective in vitro inhibitor of the MAPK and NF-κB signaling pathways, MMPs secretion and chemokine production. Compared with guggulsterone,7 a plant sterol, and IDR peptide,8 ebosin has similar modulating effects on IL-1β-induced inflammatory responses in FLSs. This study provides the first evidence demonstrating that the anti-inflammatory activity of ebosin, at least in part, reflects the inhibition of these pathways. Hence, ebosin is a promising candidate for development as a novel therapeutic agent for RA. Further studies are needed to determine the clinical usefulness of this compound.
CONFLICT OF INTEREST
All authors declare no conflicts of interest.
Acknowledgments
This research was financially supported through a grant from the Natural Science Foundation of China (NSFC 30530830) and a grant from the National Key Project of New Drug Study of China (2012ZX09301002-001-023-02).
References
- Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell 1996; 85: 307–310. [DOI] [PubMed] [Google Scholar]
- Burger D, Dayer JM, Palmer G, Gabay C. Is IL-1 a good therapeutic target in the treatment of arthritis? Best Pract Res Clin Rheumatol 2006; 20: 879–896. [DOI] [PubMed] [Google Scholar]
- Piecyk M, Anderson P. Signal transduction in rheumatoid arthritis. Best Pract Res Clin Rheumatol 2001; 15: 789–803. [DOI] [PubMed] [Google Scholar]
- Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy. Biochim Biophys Acta 2005; 1754: 253–262. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Cujec TP, Yamanaka H, Kamatani N. Molecular aspects of rheumatoid arthritis: role of transcription factors. FEBS J 2008; 275: 4463–4470. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Yoshio T, Kaneko H, Yamanaka H. Inhibition of NF-κB signaling by Fasudil as a potential therapeutic strategy for rheumatoid arthritis. Arthritis Rheum 2010; 62: 82–92. [DOI] [PubMed] [Google Scholar]
- Lee YR, Lee JH, Noh EM, Kim EK, Song MY, Jung WS. Guggulsterone blocks IL-1β-mediated inflammatory responses by suppressing NF-kappaB activation in fibroblast-like synoviocytes. Life Sci 2008; 82: 1203–1209. [DOI] [PubMed] [Google Scholar]
- Turner-Brannen E, Choi KY, Lippert DN, Cortens JP, Hancock RE, El-Gabalawy H. Modulation of interleukin-1β-induced inflammatory responses by a synthetic cationic innate defence regulator peptide, IDR-1002, in synovial fibroblasts. Arthritis Res Ther 2011; 13: R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing C, Jianbo W, Yuan L, Rong J, Baoyi L. A new IL-1 receptor inhibitor 139A: fermentation, isolation, physico-chemical properties and structure. J Antibiot (Tokyo) 2003; 56: 87–90. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang LF, Bai JY, Guan MJ, Jiang R, Li Y. Anti-inflammatory effect of Ebosin on rat collagen-induced arthritis through suppression production of interferon-1β, interferon-6 and tumor necrosis factor-α. Eur J Inflamm 2013; 11: 677–688. [Google Scholar]
- Cuzzocrea S, Mazzon E, Bevilaqua C, Costantino G, Britti D, Mazzulo G. Cloricromene, a coumarine derivative, protects against collagen-induced arthritis in Lewis rats. Br J Pharmacol 2000; 131: 1399–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Yang JP, Merin JP, Nakano T, Kato T, Kitade Y, Okamoto T. Inhibition of the DNA-binding activity of NF-κB by gold compounds in vitro. FEBS Lett 1995; 361: 89–96. [DOI] [PubMed] [Google Scholar]
- Turner-Brannen E, Choi KY, Lippert DN, Cortens JP, Hancock RE, El-Gabalawy H. Modulation of interleukin-1α-induced inflammatory responses by a synthetic cationic innate defence regulator peptide, IDR-1002, in synovial fibroblasts. Arthritis Res Ther 2011; 13: R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 2001; 81: 807–869. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 2001; 22: 153–183. [DOI] [PubMed] [Google Scholar]
- van Loo G, Beyaert R. Negative regulation of NF-κB and its involvement in rheumatoid arthritis. Arthritis Res Ther 2011; 13: 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev 2012; 246: 379–400. [DOI] [PubMed] [Google Scholar]
- Vincenti MP, Coon CI, Brinckerhoff CE. Nuclear factor κB/p50 activates an element in the distal matrix metalloproteinase 1 promoter in interleukin-1β stimulated synovial fibroblasts. Arthritis Rheum 1998; 41: 1987–1994. [DOI] [PubMed] [Google Scholar]
- Konttinen YT, Ainola M, Valleala H, Ma J, Ida H, Mandelin J. Analysis of 16 different matrix metalloproteinases (MMP-1 to MMP-20) in the synovial membrane: different profiles in trauma and rheumatoid arthritis. Ann Rheum Dis 1999; 58: 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Pakozdi A, Koch AE. Regulation of interleukin-1β-induced chemokine production and matrix metalloproteinase 2 activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 2006; 54: 2393–2401. [DOI] [PubMed] [Google Scholar]
- Monaco C, Andreakos E, Kiriakidis S, Feldmann M, Paleolog E. T-cell-mediated signalling in immune, inflammatory and angiogenic processes: the cascade of events leading to inflammatory diseases. Curr Drug Targets Inflamm Allergy 2004; 3: 35–42. [DOI] [PubMed] [Google Scholar]
- Schwartsburd PM. Chronic inflammation as inductor of procancer microenvironment: pathogenesis of dysregulated feedback control. Cancer Metastasis Rev 2003; 22: 95–102. [DOI] [PubMed] [Google Scholar]
- Heller RA, Schena M, Chai A, Shalon D, Bedilion T, Gilmore J. Discovery and analysis of inflammatory disease-related genes using cDNA microarrays. Proc Natl Acad Sci U S A 1997; 94: 2150–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M, Maini RN. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med 2003; 9: 1245–1250. [DOI] [PubMed] [Google Scholar]
- Laws A, Gu Y, Marshall V. Biosynthesis, characterization and design of bacterial exopolysaccharides from lactic acid bacteria. Biotechnol Adv 2001; 19: 597–625. [DOI] [PubMed] [Google Scholar]
- Boels IC, Ramos A, Kleerebezem M, de Vos WM. Functional analysis of the Lactoccus lactis GalU and GalE genes and their impact on sugar nucleotide and exopolysaccharide biosynthesis. Appl Environ Microbiol 2001; 67: 3033–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boels IC, Kleerebezem M, de Vos WM. Engineering of carbon distribution between glycolysis and sugar nucleotide biosynthesis in Lactococcus lactis. Appl Environ Microbiol 2003; 69: 1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima LF, Habu S, Gern JC, Nascimento BM, Parada JL, Noseda MD. Production and characterization of the exopolysaccharides produced by Agaricus brasiliensis in submerged fermentation. Appl Biochem Biotechnol 2008; 151: 283–294. [DOI] [PubMed] [Google Scholar]
- Fernandes MB, Habu S, de Lima MA, Thomaz-Soccol V, Soccol CR. Influence of drying methods over in vitro antitumoral effects of exopolysaccharides produced by Agaricus blazei LPB 03 on submerged fermentation. Bioprocess Biosyst Eng 2011; 34: 253–261. [DOI] [PubMed] [Google Scholar]
- Jin M, Lu Z, Huang M, Wang Y, Wang Y. Effects of Se-enriched polysaccharides produced by Enterobacter cloacae Z0206 on alloxan-induced diabetic mice. Int J Biol Macromol 2012; 50: 348–352. [DOI] [PubMed] [Google Scholar]
- Joo JH, Yun JW. Structure and molecular characterization of extracellular polysaccharides produced by Trichoderma erinaceum DG-312. J Mol Biotechnol 2005; 15: 1250–1257. [Google Scholar]
- Nowak B, Ciszek-Lenda M, Sróttek M, Gamian A, Kontny E, Górska-Fraczek S. Lactobacillus rhamnosus exopolysaccharide ameliorates arthritis induced by the systemic injection of collagen and lipopolysaccharide in DBA/1 mice. Arch Immunol Ther Exp (Warsz) 2012; 60: 211–220. [DOI] [PubMed] [Google Scholar]
- Sweeney SE, Firestein GS. Signal transduction in rheumatoid arthritis. Curr Opin Rheumatol 2004; 16: 231–237. [DOI] [PubMed] [Google Scholar]
- Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest 2001; 108: 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Hammaker D, Boyle DL, Firestein GS. Regulation of JNK by MKK-7 in fibroblast-like synoviocytes. Arthritis Rheum 2006; 54: 2127–2135. [DOI] [PubMed] [Google Scholar]
- Shin M, Yan C, Boyd D. An inhibitor of c-jun aminoterminal kinase (SP600125) represses c-Jun activation, DNA-binding and PMA-inducible 92-kDa type IV collagenase expression. Biochim Biophys Acta 2002; 1589: 311–316. [DOI] [PubMed] [Google Scholar]
- Triantaphyllopoulos K, Madden L, Rioja I, Essex D, Buckton J, Malhotra R. In vitro target validation and in vivo efficacy of p38 MAP kinase inhibition in established chronic collagen-induced arthritis model: a pre-clinical study. Clin Exp Heumatol 2010; 28: 176–185. [PubMed] [Google Scholar]
- Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 2010; 1802: 396–405. [DOI] [PubMed] [Google Scholar]
- Beloueche-Babari M, Jackson LE, Al-Saffar NM, Workman P, Leach MO, Ronen SM. Magnetic resonance spectroscopy monitoring of mitogen-activated protein kinase signaling inhibition. Cancer Res 2005; 65: 3356–3563. [DOI] [PubMed] [Google Scholar]
- May MJ, Ghosh S. Signal transduction through NF-kappa B. Immunol Today 1998; 19: 80–88. [DOI] [PubMed] [Google Scholar]
- Sweeney SE, Firestein GS. Rheumatoid arthritis: regulation of synovial inflammation. Int J Biochem Cell Biol 2004; 36: 372–378. [DOI] [PubMed] [Google Scholar]
- Thornton S, Duwel LE, Boivin GP, Ma Y, Hirsch R. Association of the course of collagen-induced arthritis with distinct patterns of cytokine and chemokine messenger RNA expression. Arthritis Rheum 1999; 42: 1109–1118. [DOI] [PubMed] [Google Scholar]