Abstract
The differentiation of dendritic cells (DC) is affected by the aging process. However, the molecular mechanisms responsible for the alteration of DC development in aged mice have not been clarified. Recently, Wnt5a was reported to be an important aging-related molecule in hematopoietic systems. Here, we hypothesized that the increased expression of Wnt5a in aged hematopoietic precursors led to deficient DC differentiation in aged mice. The percentages and cell numbers of plasmacytoid DC (pDC) and CD172a−CD8α+conventional DC (cDC) were decreased in aged mice compared to young mice. Further analysis indicated that the hematopoietic precursors that gave rise to DC, including Flt3+ lymphoid-primed multipotent precursors (LMPP), common lymphoid progenitors (CLP) and common DC precursors (CDP), were all decreased in the bone marrow of aged mice. Overexpression of Wnt5a in hematopoietic precursors strongly affected the differentiation of cDC and pDC in vivo. Treatment of hematopoietic stem cells (HSC) with Wnt5a led to a significant decrease in the differentiation of the LMPP, CLP and CDP populations that was similar to the decrease observed in the bone marrow (BM) HSC of aged mice. Molecular studies demonstrated that Wnt5a negatively regulated the expression of an array of genes important for DC differentiation, including Flt3, Gfi-1, Ikaros, Bcl11a, and IL-7R, by activating the Wnt5a-Cdc42 pathway. Finally, we rejuvenated DC differentiation from aged precursors by blocking the non-canonical Wnt pathway. Our study identified the key roles of the non-canonical Wnt pathway in DC differentiation and DC aging.
Keywords: aging, dendritic cell, Wnt5a
Introduction
Dendritic cells (DC) are bone marrow (BM)-derived antigen-presenting cells (APC) of the immune system that play important roles in the regulation of immune responses.1 Multiple DC subtypes have been identified,2,3,4 including plasmacytoid DC (pDC) that generate type-1 interferon (IFN-I) and antigen-presenting conventional DC (cDC). Both types are present in lymphoid and non-lymphoid tissues. At steady-state, cDC can be further divided into CD172a−CD8α+and CD172a+CD8α− subsets that possess different capacities for promoting responses to pathogens as a result of their different abilities to cross-present antigens and secrete cytokines.2,3,4 DC development shows remarkable plasticity, and DC have been shown to develop from both lymphoid and myeloid compartments via fms-related tyrosine kinase (Flt) 3-expressing progenitors.3 The lymphoid and myeloid branches are produced from the lymphoid-primed multipotent progenitor (LMPP), with both branches subsequently producing DC.5 The common myeloid progenitors (CMP) can generate the common M-CSFR+ DC precursors (CDP), the immediate precursors of both pre-pDC and pre-cDC, which are restricted to their respective lineages but not yet fully mature.6,7 The common lymphoid progenitors (CLP) can generate pDC and limited numbers of cDC, although the intrinsic deficiency of the IFN-I receptor in CLP greatly reduces their differentiation into pDC.8,9 Recently, a distinct M-CSFR−DC-restricted precursor that predominately produces pDC and represents a parallel stream of DC development branching off from the myeloid pathway has also been identified.10 These M-CSFR−DC-restricted precursors are produced within one division from the upstream LMPP population, indicating that multiple pathways are involved in DC differentiation.10,11
Initial in vitro studies of the functions of cytokines in DC development revealed distinct and important roles for the Flt3 ligand (Flt3L), GM-CSF, and M-CSF in generating various DC populations. Specifically, Flt3L and M-CSF have been shown to influence a number of discrete DC subsets.12,13,14,15 Flt3L-supplemented cultures could induce the differentiation of the phenotypic and functional equivalents of spleen CD8α+and CD8α− cDC, as well as pDC, from multiple precursor populations in mice.16 Targeted deletion of Flt3 or Flt3L in mice led to significantly reduced numbers of DC progenitors and impaired DC development, indicating that the Flt3 pathway was essential for steady-state DC differentiation.15,17 M-CSF-supplemented cultures also generated the equivalents of splenic CD8α+and CD8α− cDC in addition to pDC, albeit with lower efficiency than the Flt3L cultures.13 Moreover, IL-7 signaling was demonstrated to be required for the development of DC, and all DC subsets were found to be decreased in IL-7−/− mice and IL-7Rα−/− mice.18
The Wnt signaling pathway is an evolutionarily conserved pathway that regulates crucial aspects of cell fate determination, cell migration, and cell polarity.19,20 The Wnt proteins are secreted glycoproteins and comprise a large family with 19 members in humans and mice. To date, two signaling pathways downstream of the Wnt ligand receptors(the Frizzled (Fz) receptors) have been identified, including the canonical or Wnt/β-catenin-dependent pathway and the non-canonical pathway, which can be further divided into the Planar Cell Polarity and Wnt/Ca2+ pathways.19,20 The Wnt signaling pathways have been implicated as the signaling cascades involved in the regulation of hematopoietic stem cell (HSC) function and other stages during hematopoiesis.21,22 Hematopoiesis proceeds in a stepwise manner from primordial long-term (LT)-HSCs that give rise to short-term (ST)-HSCs; in turn, (ST)-HSC can differentiate into a multipotent progenitor (MPP) population.23 The canonical Wnt signaling pathway has been demonstrated to regulate the differentiation of HSC, myeloid precursors, and T lymphoid precursors during hematopoiesis in a dose-dependent manner.21 Mild, intermediate, and intermediate–high levels of canonical Wnt pathway activation facilitate HSC function, myeloid development, and early T-cell development, respectively.21 However, the non-canonical Wnt pathway was reported to inhibit canonical Wnt signaling in HSC and increased the numbers of short-term (ST-HSC) and long-term HSC (LT-HSC) populations by maintaining HSC in a quiescent G0 state.22,24,25 Both the canonical and non-canonical Wnt pathways can induce BM-derived tolerogenic DC induced by GM-CSF and IL-4 (GM-DC).26 Activation of the canonical Wnt signaling pathway during Flt3L-induced DC (FL-DC) differentiation resulted in a significant increase in the proportion of conventional CD11c+ CD11b+B220−DC, and the proportion of CD11c+ CD11b−B220+pDC was dramatically reduced.27 In contrast with the canonical Wnt pathway, very little is known about the potential role of non-canonical Wnt signaling in DC differentiation.
The development and function of DC populations are altered during the process of aging.28,29,30 However, the molecular mechanisms responsible for these changes in aged mice have not been thoroughly investigated. Considering that Wnt5a expression was elevated in aged hematopoietic precursors and functioned as an important molecule in the aging of hematopoietic systems25, the deficiency in DC development in aged mice may also have been elevated due to the expression of Wnt5a. In this study, we investigated the role of Wnt5a in DC differentiation under steady-state conditions. We found that the numbers of pDC and CD172a−CD8α+cDC declined in aged mice, while the expression of Wnt5a increased in aged hematopoietic precursor cells. The overexpression of Wnt5a in BM chimeric mice could inhibit the differentiation of cDC and pDC in vivo, with a more significant decrease in pDC. Furthermore, our data suggested that Wnt5a controlled DC development by inhibiting the expansion of Flt3+ LMPP. Importantly, the expression of Flt3, IL-7R, and the lymphoid-biased transcription factors Gfi-1, Ikaros, and Bcl11a, which were shown to be important for DC differentiation31,32,33,34, were also decreased in LT-HSC and LMPP upon Wnt5a treatment. Finally, we reversed the deficiency in DC differentiation in aged mice by pharmacologically blocking the non-canonical Wnt pathway. Therefore, our study identified a key role for the non-canonical Wnt signaling pathway in DC differentiation during aging.
Materials and Methods
Mice
C57BL/6J (B6) and B6.SJL-Ptprca Pepcb/BoyJ (CD45.1-congenic) mice were bred in our animal facilities. All mice were maintained under specific pathogen-free conditions in the animal facility of Tsinghua University and used in accordance with governmental and institutional guidelines for animal welfare.
Plasmid construction and retrovirus production
The coding region of Wnt5a was cloned from the cDNA template from C57BL/6J mice using the primers upper, 5′-CGGGATCCGCCACCATGTCTTCCAAGTTCTTCCT-3′ and lower, 5′-GGAATTCCTATTTGCACACGAACTGAT-3′. Then, the Wnt5a coding region was inserted into the pMYs-EGFP retrovirus plasmid for overexpression. The retrovirus was transfected into a Plat-E cell line to produce retrovirus particles.
Lin− cell separation and retroviral transduction
BM cells were flushed and red blood cells were lysed using: Red cell removing buffer was 0.168 M NH4Cl in Milli Q water. Then, BM cells were subjected to gradient centrifugation using Nycodenz (Axis Shield Poc, Scotland, UK) at a density of 1.086 g/cm.3 Lin+ cells were removed using antibody cocktails including anti-CD2, anti-CD3, anti-CD8, anti-CD45R, anti-CD11b, anti-TER119, and anti-Ly6G. Before retroviral transduction, Lin− cells were stimulated with 30 ng/mL of Flt3L (Peprotech, Rocky Hill, NJ, USA) plus 30 ng/mL SCF (Peprotech, Rocky Hill, NJ, USA) overnight; then, the cells were plated into a 24-well plate and transfected with the retrovirus using Retronectin (Takara Bio Inc, Otsu, Shiga, Japan).
Preparation of hematopoietic precursors and DC
cDC and pDC were enriched from the spleen by digesting the tissue fragments with 1 mg/mL collagenase III (Worthington Biochemical Corp, Lakewood, NJ, USA) and 0.1 mg/mL DNase I (Roche Diagnostics Corporation, Indianapolis, IN,USA), followed by light density separation and immune-magnetic bead depletion using a procedure described elsewhere.34 Briefly, spleen cells were suspended in 1.077 g/cm3 Nycodenz medium and gradient centrifuged at 1700g for 10 minutes. The light density cells were collected and labeled with antibodies against lineage antigens, including anti-CD3 (clone KT3-1.1), anti-Thy-1 (clone T24/31.7), anti-Ly6G (clone 1A8), anti-CD19 (clone ID3), and anti-erythrocyte (clone TER119); non-DC cells were removed using anti-rat Ig conjugated Biomag beads. Red cells were removed for BM progenitor enrichment, followed by gradient density separation and immune-magnetic bead depletion using a procedure described elsewhere.35 Briefly, BM cells were suspended in 1.086 g/cm3 Nycodenz medium and gradient centrifuged at 1700g for 10 minutes. The light density cells were collected and labeled with antibodies against lineage antigens, including anti-CD2 (clone RM2.1), anti-CD3 (clone KT3-1.1), anti-CD8 (clone 53–6.7), anti-CD45R (clone RA36B2), anti-CD11b (clone M1/70), anti-erythrocyte (clone TER119), and anti-Ly6G (clone 1A8); lineage cells were removed using anti-rat Ig conjugated Biomag beads. For B cells, granulocytes and pDC in BM, total BM cells were collected and the red cells were removed using red-cell removing buffer. Then, the cells were subjected to FACS analysis.
Flow cytometric analysis
Antibodies used for colonic staining included: fluorochrome- or biotin-conjugated monoclonal antibodies (mAbs) specific for mouse CD115 (clone AFS98), CD45.2 (clone 104), CD45 (clone 30-F11), CD45.1 (clone A20), Ly6G (clone 1A8), CD11c (clone N418), CD34 (clone RAM34), CD135 (clone A2F10), CD117 (clone ACK2), Ly6A/E (clone D7), CD127 (clone A7R34), Ly6C (clone H1.4), Siglec-H (clone eBio440c), CD8α (clone 53–6.7), CD172a (clone P84), CD11b (clone M1/70), CD19 (clone) CD16/32 (clone 2.4G2), and B220 (clone RA3-6B2) and the corresponding isotype controls. The secondary reagents included PE-Cy7, APC, BV605, BV421, PE, FITC-conjugated streptavidin antibodies. All of the antibodies were purchased either from (BD Biosciences, Palo Alto, CA, USA) or eBioscience (eBioscience, San Diego, CA, USA). Cells were analyzed with a LSRII flowcytometer (Becton Dickinson, Franklin Lakes, New Jersey, USA) or sorted with a FACSAria III machine (Becton Dickinson, Franklin Lakes, New Jersey, USA). Flow cytometric analysis was performed with the FlowJo software (TreeStar, Ashland, USA).
Cell culture and stimulation
Cells were maintained under standard cell culture conditions in complete RPMI 1640 medium (RPMI 1640, Gibco, Invitrogen, Carlsbad, CA, USA) containing 10% fetal calf serum, 100 U/mL penicillin (Gibco), 100 g/mL streptomycin (Gibco) and 50 μM 2-ME. Isolated LSK, LMPP, and LT-HSC were cultured in 24-well flat-bottom plates at a concentration of 1 × 105 cells, 1 × 105 cells, and 1 × 104 cells, respectively. The cells were stimulated with 200 ng/mL Wnt5a (R&D Systems, Minneapolis, MN, USA) or 5 μM CASIN (Tocris Bioscience, Bristol, UK).
Quantitative real-time RT-PCR
Total cellular RNA was isolated with the TRIzol Reagent (Invitrogen, Grand Island, NY, USA). cDNA was prepared by reverse transcription (PrimeScript RT Reagent Kit, Takara, Shiga, Japan) and amplified by real-time quantitative PCR (qPCR) with the primers shown in Supplemental Table 1. Equal amounts of cDNA were used for the subsequent qPCR performed with the SYBR PrimeScript RT-PCR Kit (Takara Bio Inc, Otsu, Shiga, Japan). Amplification was performed in an ABI PRISM 7900 Real Time PCR System (Applied Biosystem, Grand Island, NY, USA). The amplification efficiency of these genes was the same as the efficiency of β-actin, as indicated by the standard curves for amplification. Thus, we used the formula: fold difference = 2–(ΔCtA − ΔCtB), where Ct is the cycle threshold.
Construction of BM chimeras
For the Wnt5a overexpression experiments, CD45.1 recipient mice were lethally irradiated by X-ray (5 Gy × 2) and intravenously transferred with a combination of 5 × 104 sorted GFP+ CD45.2 Lin− cells and 3 × 105 BM leukocytes from CD45.1 mice. For the precursor study, LSKs were pretreated with or without Wnt5a, CASIN, or Wnt5a+CASIN. Then, 8000 LSK from CD45.2 mice were mixed with 3 × 105 BM leukocytes from CD45.1 mice and intravenously transferred into lethally irradiated CD45.1 recipient mice.
Statistical analysis
Data were analyzed by ANOVA followed by Bonferroni's multiple comparison test or an unpaired, two-tailed t-test with GraphPad Prism 5. Data are presented as the mean (±SEM). P < 0.05 was considered statistically significant.
Results
Decreased DC numbers in aged mice
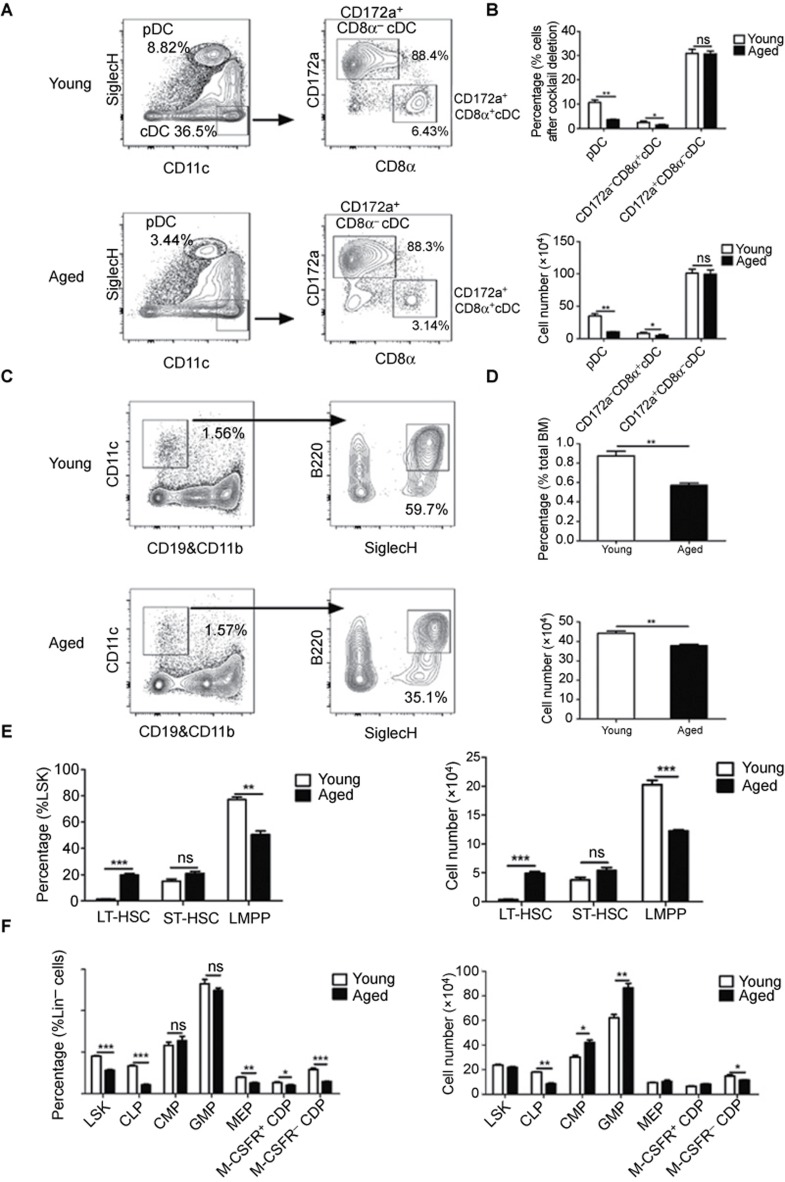
To explore the effects of aging on the composition of DC populations, we analyzed cDC and pDC in the spleens from aged (16–18 months) or young mice (6–8 weeks). Phenotypic characterization revealed a reduction in the number of pDC and CD172a−CD8α+ cDC, with a more significant change in the pDC (Figure 1a and b). Furthermore, both the frequency and total cell number of pDC were decreased in the BM of aged mice (Figure 1c and d).
Figure 1.
PDC and CD172a−CD8α+cDC were decreased in aged mice. (A) DC from the spleens of young and aged mice were enriched, and the DC subsets were analyzed by flow cytometry. The plots show the percentages of DC subsets in the spleens of young and aged mice. (B) The percentages and cell numbers of DC subsets in the spleens of young and aged mice. (C) The plots show the percentages of pDC in the BM of young and aged mice. (D) The percentages and cell numbers of pDC in the BM of young and aged mice. (E) Lin− cells enriched from the BM of young and aged mice were analyzed by flow cytometry. The graphs show the percentages and cell numbers of HSC populations in young and aged mice. (F) The percentages and cell numbers of LSK, CMP, CLP, GMP, MEP, M-CSFR+ CDP, and M-CSFR− CDP in young and aged mice. Data are representative of three independent experiments. Error bars represent SEM (*P < 0.05, **P < 0.01, ***P < 0.001).
Both pDC and cDC originate from HSC in BM via different restricted progenitors.2,3,4 To elucidate the mechanisms behind the reduction in pDC and cDC in aged mice, we examined the presence of distinct precursor populations at different differentiation stages from lineage marker-negative (Lin−) Scal-1+c-kit+ progenitors (LSK, containing LT-HSC, ST-HSC, and LMPP) to DC. The results demonstrated that both the frequency and total cell number of Flt3+ LMPP within the LSK population was decreased significantly in aged mice, whereas LT-HSC was increased (Figure 1e and Supplemental Figure 1a). This result suggested that the differentiation of HSCs was hampered at the LT-HSC stage in aged mice. Furthermore, the number of CLP was also decreased (Figure 1f and Supplemental Figure 1b), whereas the number of granulocyte-macrophage precursors (GMP) was increased (Figure 1f and Supplemental Figure 1c), indicating the myeloid-biased differentiation of aged HSC. In agreement with this result, a decreased number of B cells and an increased number of granulocytes were observed in the BM of aged mice (Supplemental Figure 1d). Additionally, the number of M-CSFR− CDP was slightly decreased amongst the DC restricted precursor CPD, whereas the number of M-CSFR+ CDP remained unchanged (Supplemental Figure 1e). Because CLP and M-CSFR− CDP predominately produce pDC, the decrease in the numbers of CLP and M-CSFR− CDP may result in the substantial reduction of pDC in aged mice. Therefore, we observed altered DC differentiation in aged mice.
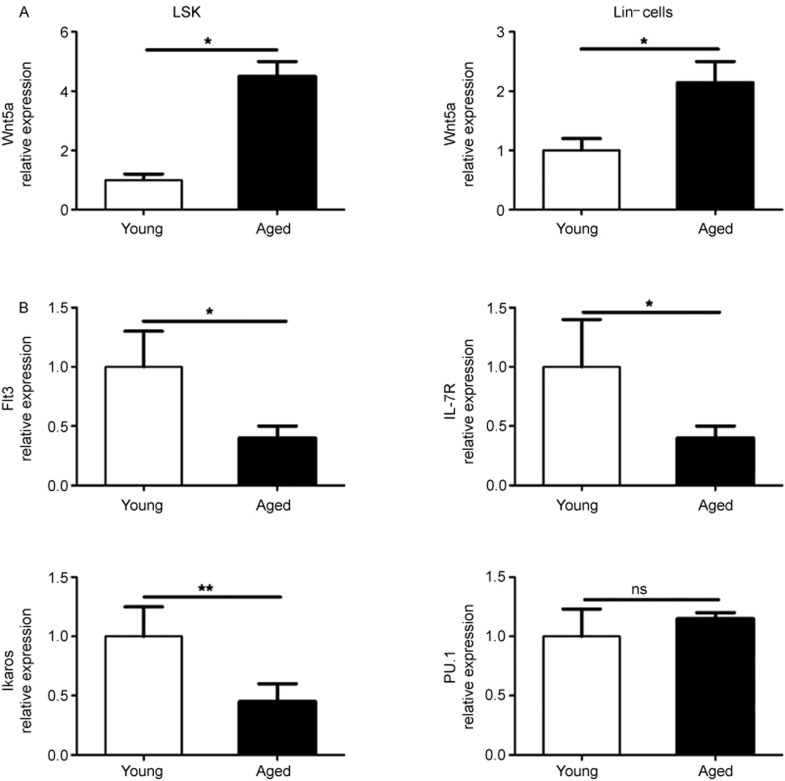
Increased expression of Wnt5a in aged hematopoietic precursor cells
The elevated expression of Wnt5a in aged HSC was demonstrated to cause stem cell aging and aging-like myeloid–lymphoid differentiation skewing via activation of the non-canonical Wnt signaling pathway.25 In view of this, we compared the expression levels of Wnt5a in LSK and Lin− cells from the BM of young and aged mice and found that Wnt5a expression was significantly increased in LSK and Lin− cells from aged mice (Figure 2a). Next, we examined the expression of several DC differentiation-related genes, including Flt3, IL7R, Ikaros, and PU.1.31,32,33,34 A significant reduction in Flt3, IL7R, and Ikaros expression was observed in aged LSK compared with young LSK (Figure 2b).
Figure 2.
Wnt5a expression was increased in aged hematopoietic precursors. (A) Graphical summary of qPCR analysis showing the mRNA levels of Wnt5a in different hematopoietic precursors isolated from young and aged mice. (B) The mRNA expression level of Flt3, IL-7R, Ikaros, and PU.1 in LSK isolated from young and aged mice. Data are representative of two independent experiments. Error bars represent SEM (*P < 0.05, **P < 0.01).
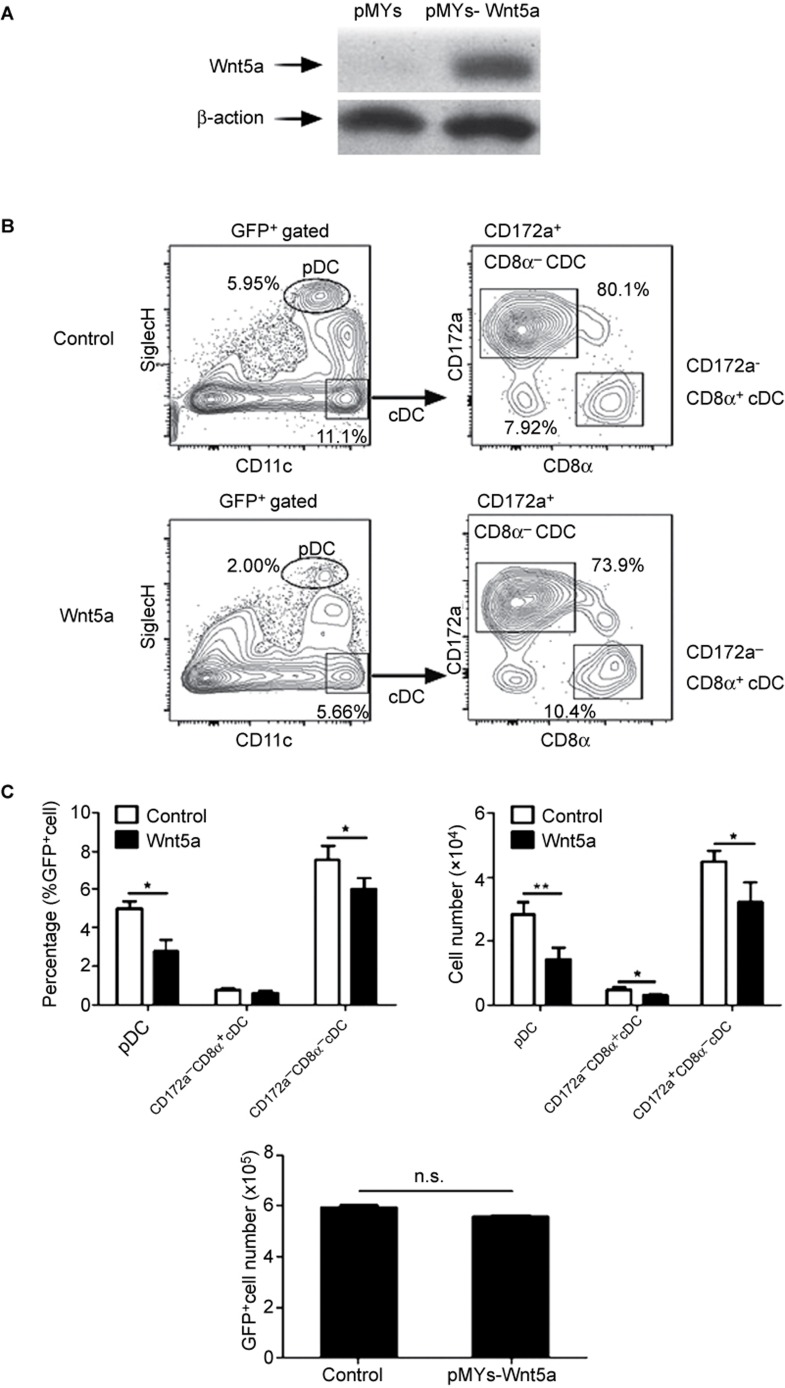
Overexpression of Wnt5a in young adult hematopoietic precursors resulted in a defect in DC development
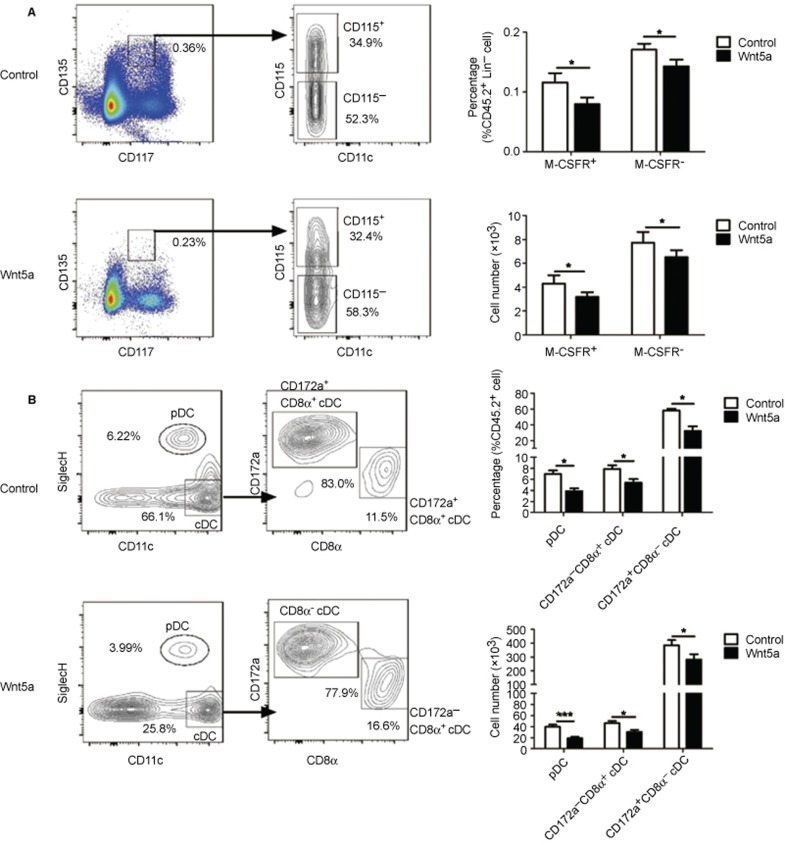
To identify the role of Wnt5a in DC development, we constructed chimeric mice by transferring Wnt5a-overexpressing Lin−BM cells into lethally X-ray-irradiated mice. Western blot experiments showed efficient overexpression of Wnt5a in GFP+BM cells (Figure 3a). Four weeks after transfer, the DC populations in the spleens of the mice were analyzed. The percentages of both pDC and CD172a+CD8α−cDC were decreased (Figure 3b and c). Although the percentages of CD172a−CD8α+ were not decreased, the numbers of all three DC subsets were decreased due to the reduction in the total numbers of GFP+ cells derived from the Wnt5a-overexpressing precursors (Figure 3c). Therefore, this result indicated that Wnt5a negatively regulated the process of DC development.
Figure 3.
Wnt5a negatively regulated DC differentiation. Control or Wnt5a-overexpressing plasmids were transfected into CD45.2+Lin− cells. CD45.1+ mice were lethally irradiated and reconstituted with a mixture of CD45.1+ BM cells and CD45.2+GFP+Lin− cells. (A) The overexpression of Wnt5a in Lin− cells. (B) The plots show the percentages of donor-derived DC subsets in the spleens of control and Wnt5a-overexpressing chimeric mice 4 weeks after Lin− cell transplantation. (C) The percentages and numbers of donor-derived DC subsets, and the total numbers of GFP+ donor-derived splenocytes. Data are representative of three independent experiments, each including 5–8 mice. Error bars represent SEM (*P < 0.05, **P < 0.01).
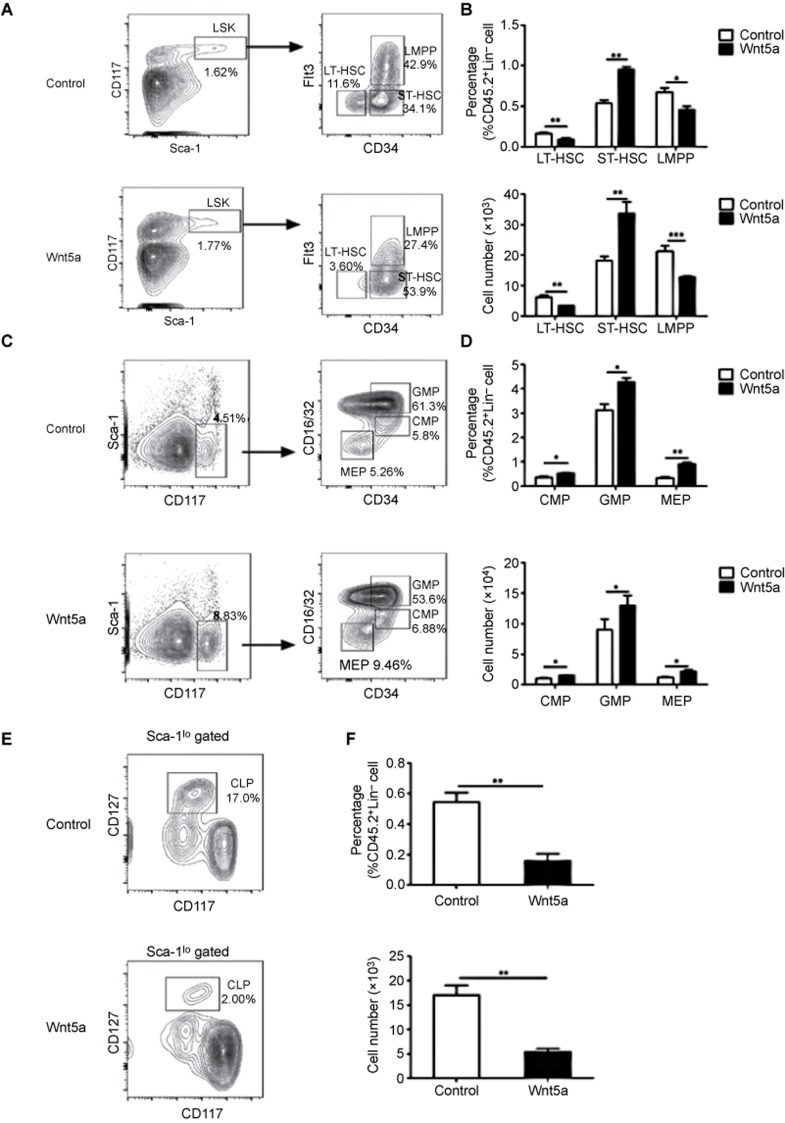
Wnt5a negatively regulated DC development by affecting LSK differentiation
Because DC can be generated from HSC and Flt3+ myeloid- or lymphoid-committed precursors2,3,4, the defect in DC development after Wnt5a overexpression may have occurred at different precursor stages. To determine which developmental stage was the target of Wnt5a, we pretreated LSK from CD45.2 mice with Wnt5a for 24 hours prior to transplantation into CD45.1 recipient mice. The effect of Wnt5a pretreatment on HSC has been shown to be maintained for more than 24 weeks after transplantation.25 Therefore, this strategy provided us with a good opportunity to monitor the function of Wnt5a treatment during the differentiation of LSK to the different DC-restricted precursor stages in vivo. The differentiation of LSK was analyzed 4 weeks after transplantation. A significant decrease was observed in the number of Flt3+ LMPP derived from Wnt5a-treated LSK (Figure 4a and b). An increase in the percentage of ST-HSC indicated the hampered differentiation of Wnt5a-treated LSK. Moreover, the percentage and number of CLP progeny in the Wnt5a-treated LSK was dramatically decreased, while the percentage of GMP was increased compared with untreated LSK (Figure 4c–f). These findings indicated that Wnt5a promoted the myeloid-biased differentiation of LSK at the expense of lymphoid differentiation. Finally, we examined the CDP in the BM and DC subsets in the spleens of the chimeric mice and found a decrease in the percentage and cell number of both M-CSFR+ CDP and M-CSFR− CDP derived from Wnt5a-treated LSK compared with the precursors derived from the non-treated LSK (Figure 5a and b). Consistent with this finding, both the percentage and cell number of cDC and pDC progeny derived from Wnt5a-treated LSK was also decreased (Figure 5c and d), which was similar to the results obtained from the Wnt5a-overexpressing chimeric mice. Therefore, Wnt5a could affect DC development at a stage as early as the LSK stage in vivo.
Figure 4.
Wnt5a regulated the differentiation of DC at the LSK stage. CD45.2+LSK with or without Wnt5a treatment were transferred into lethally irradiated CD45.1+ mice. Precursor cells in the BM of chimeric mice were analyzed 4 weeks after transplantation. (A) The plots show the percentages of HSC populations in the BM of chimeric mice. (B) The graphs show the percentages and cell numbers of LT-HSC, ST-HSC, and LMPP. (C) The representative FACS plots of GMP, CMP, and MEP in the BM of chimeric mice. (D) The graphs show the percentages and cell numbers of GMP, CMP, and MEP. (E) The representative FACS plots of CLP in the BM of chimeric mice. (F) The graphs show the percentages and cell numbers of CLP. Data are representative of three independent experiments, each including 5–8 mice. Error bars represent SEM (*P < 0.05, **P < 0.01, ***P < 0.001).
Figure 5.
LSK pretreated with Wnt5a gave rise to reduced DC in vivo. CD45.2+ LSK with or without Wnt5a treatment were transferred into lethally irradiated CD45.1+ mice. CDP in the BM and DC in the spleens of chimeric mice were analyzed 4 weeks after transplantation. (A) The plots show the percentages of CDP in the BM of chimeric mice, and the graphs summarize the percentages and cell numbers of CDP. (B) The plots show the percentages of DC subsets in the spleens of chimeric mice, and the graphs summarize the percentages and cell numbers of DC subsets. Data are representative of three independent experiments, each including 5–8 mice. Error bars represent SEM (*P < 0.05, **P < 0.01, ***P < 0.001).
Wnt5a inhibited the expression of genes critical for DC differentiation via Cdc42 activation
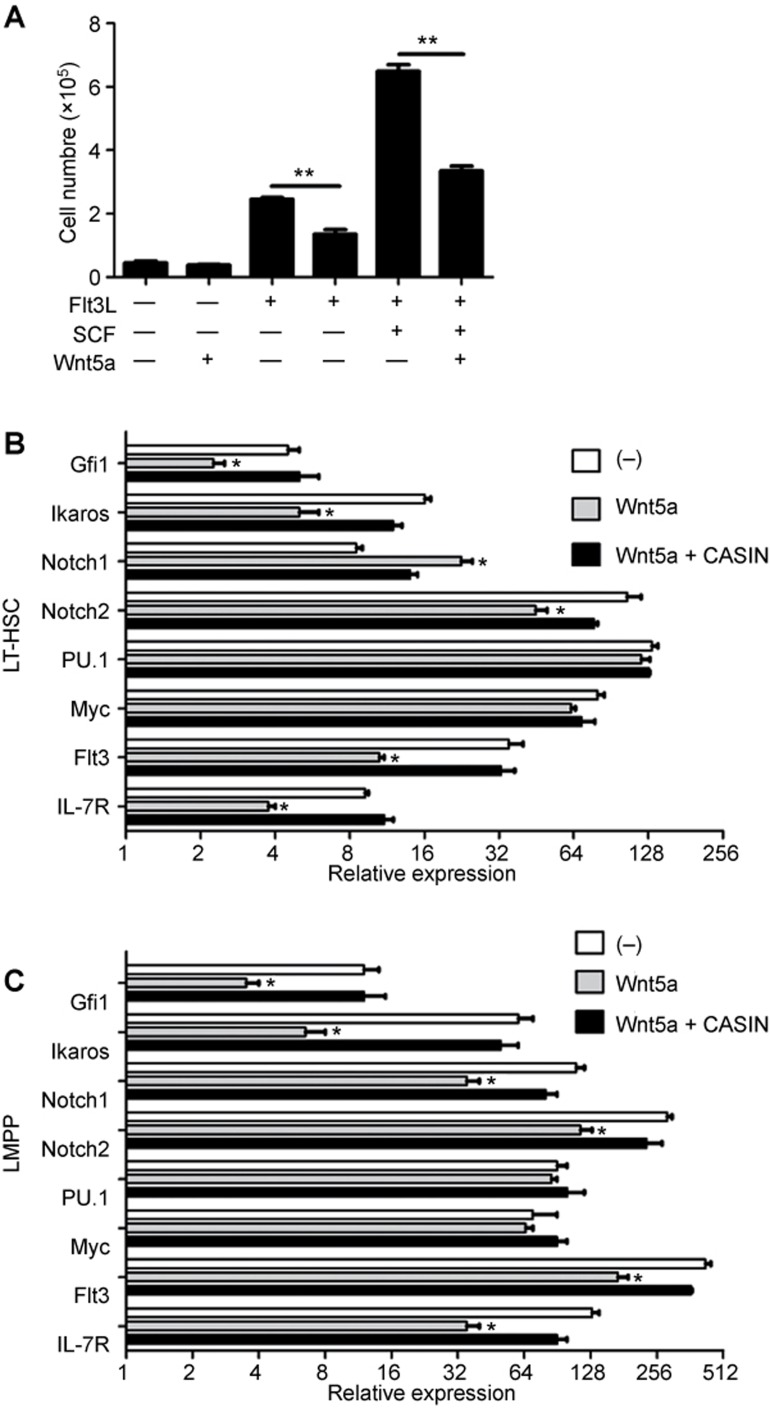
The above results suggested that Wnt5a inhibited DC differentiation at the LSK stage. Because the number of Flt3+ LMPP was decreased upon Wnt5a treatment, we hypothesized that Wnt5a might affect the expansion of Flt3+ LMPP. To test this hypothesis, we stimulated the LMPP with Wnt5a with or without Flt3L for 4 days. The Flt3L cultures led to an expansion of LMPP in vitro, while the addition of Wnt5a impaired this expansion (Figure 6a). A similar effect of Wnt5a on the expansion of LMPP was also observed when the LMPP were cultured in Flt3L plus SCF medium.
Figure 6.
Wnt5a negatively regulated the expansion of LMPP. (A) LMPP were isolated from the BM and treated with the indicated cytokines. The graph shows the proliferation of LMPP upon different stimulations. (B, C) LT-HSC or LMPP were treated with Wnt5a or Wnt5a and CASIN. (B) Graphical summary of qPCR analysis showing the mRNA levels of Gfi-1, Ikaros, Notch1, Notch2, PU.1, Myc, Flt3, and IL7R in LT-HSC. (C) Graphical summary of qPCR analysis showing the mRNA levels of Gfi-1, Ikaros, Notch1, Notch2, PU.1, Myc, Flt3, and IL7R in LMPP. Data are representative of two independent experiments. Error bars represent SEM (*P < 0.05, **P < 0.01).
LMPP are derived from LT-HSC that express Flt3 mRNA despite testing negative for its surface expression.36 To study the mechanisms underlying the inhibition of LMPP expansion by Wnt5a, we examined the changes in the expression of relevant genes in LT-HSC and LMPP upon Wnt5a treatment. We found that Flt3 mRNA levels were downregulated by Wnt5a in LT-HSC and LMPP (Figure 6b and c). To determine the mechanism of Flt3 downregulation by Wnt5a, we examined the effects of Wnt5a on the expression of key molecules that directly regulates Flt3 expression. The expression of Gfi-1, Ikaros, and Bcl11a were all suppressed by Wnt5a, while PU.1 was not affected (Figure 6b and c). Moreover, IL7R, which was reported to be important for DC homeostasis18, was also downregulated in LMPP after Wnt5a treatment. Therefore, the Wnt5a pathway might inhibit the differentiation of DC by repressing the expression of key transcription factors and cytokine receptors during the early hematopoietic precursor stages.
The Wnt5a-driven non-canonical signaling pathway was reported to exert an effect on LT-HSC via regulating the activity of the small Rho GTPase Cdc42.25 To test whether Cdc42 was involved in the suppressive effects of Wnt5a on the expression of genes important for DC differentiation, we used a specific inhibitor for Cdc42 (CASIN) during treatment of LT-HSC and LMPP with Wnt5a.25,37,38 We found that blocking Cdc42 activity could completely reverse the inhibitory effects of Wnt5a on the expression of Gfi-1, Ikaros, and Bcl11a in these precursors (Figure 6b and c). As a result, the expression of Flt3 mRNA also recovered from the Wnt5a-induced inhibition following treatment with CASIN (Figure 6b and c). These results indicated that the Wnt5a-Cdc42 pathway was involved in the suppression of DC differentiation.
Taken together, these results suggested that Wnt5a suppressed Flt3 expression by inhibiting key molecules that directly regulated Flt3 expression.
Pharmacological inhibition of the non-canonical Wnt pathway rejuvenated DC differentiation in aged LSK
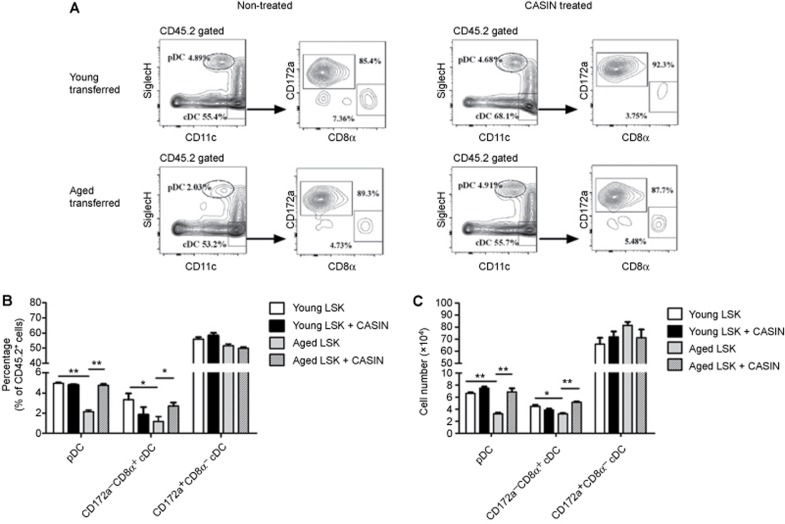
Our data suggested that the non-canonical Wnt signaling pathway could inhibit DC differentiation. The differentiation of DC was impaired in aged mice, whereas the expression of Wnt5a was markedly increased in the hematopoietic precursors. Therefore, we hypothesized that the inhibition of the non-canonical pathway in aged LSK by pharmacological means might represent a potential approach to revert the deficiency of aged DC differentiation, at least in part. Although CASIN acts transiently on Cdc42 activity, the transient reduction in Cdc42 activity in aged LSK has been maintained for more than 24 weeks after transplantation.38 To explore the effect of Wnt5a-Cdc42 pathway blockage on aged DC differentiation, the LSK from aged or young mice were treated in vitro with CASIN for16 hours, then transplanted into young recipients and compared with untreated LSK. DC subsets in the spleens of recipient mice were analyzed 16 weeks' post-transplantation. CASIN treatment of aged LSK resulted in an increase in the proportion of pDC that was comparable to the proportion derived from young LSK (Figure 7a and b). These findings indicated that blockage of the Wnt5a-Cdc42 pathway significantly improved DC differentiation from aged precursors.
Figure 7.
Blocking of the Wnt5a-Cdc42 pathway rejuvenated the differentiation of aged DC. CD45.2+ LSK were isolated from the BM of young and aged mice treated with or without CASIN for 16 hours and transferred into lethally irradiated CD45.1+ mice. DC subsets in the spleens of chimeric mice were analyzed 16 weeks after transplantation. (A) The FACS plots show the percentages of DC subsets in the chimeric mice. (B) The graph summarizes the percentages of DC subsets in the chimeric mice. (C) The numbers of DC subsets. Data are representative of two independent experiments. Error bars represent SEM (*P < 0.05, **P < 0.01).
Discussion
Both the canonical and non-canonical Wnt signaling pathways have been found to negatively regulate the differentiation and activation of moDC.26,27 The function of the canonical Wnt signaling pathway in the differentiation of cDC and pDC has also been extensively studied.27 However, the effects of the non-canonical Wnt pathway on the differentiation of cDC and pDC are not clear. In this study, our results suggested that the non-canonical Wnt pathway (particularly Wnt5a) negatively regulated the differentiation of cDC and pDC in aged mice in vivo. Activation of the Wnt5a-Cdc42 pathway can inhibit an array of genes that regulate Flt3 expression and lead to a reduction in the number of Flt3+ LMPP. As a result, the differentiation of DC from these hematopoietic precursors was also affected. Using an aged mouse model in which the expression of Wnt5a was markedly increased in the hematopoietic precursors, we verified that the deficiency in DC differentiation in aged mice was mainly due to the activation of the non-canonical Wnt signaling pathway in aged HSC.
Florian et al. showed that Wnt5a levels were significantly elevated in aged long-term HSC (LT-HSC), while the expression of canonical Wnt ligands remained unaltered during aging.25 Increasing or decreasing Wnt5a levels led to an induction or reversal of aging-related HSC phenotypes, respectively.25 Exposure of HSCs to Wnt5a induced lymphoid to myeloid skewing of donor cells in blood after transplantation, while conversely Wnt5a downregulation increased lymphoid chimerism from aged HSCs.25 In our study, we also found that the numbers of B cells decreased while the numbers of CD11b+Ly6G+myeloid cells increased in the BM of Wnt5a-overexpressing mice (Supplemental Figure 2). Additionally, the percentages of pDC, which were considered to have partly originated from lymphoid progenitors1,2,3,4,6, decreased more significantly than the myeloid-originated CD172a−CD8α+cDC and CD172a+CD8α−cDC in the Wnt5a-overexpressing chimeric mice. A more obvious decrease in the number of pDC was also observed in aged mice. Therefore, the changes in the corresponding DC subsets further supported the function of the non-canonical Wnt signaling pathway in inhibiting the lymphoid-biased differentiation of HSC.
The differentiation of DC is regulated by different transcription factors during different developmental stages. During the LSK stage, lymphoid lineage-biased transcription factors including Gfi-1, Ikaros, and Bcl11a and the myeloid lineage-biased transcription factor PU.1 have been demonstrated to regulate DC differentiation.31,32,33,34 All of these molecules were shown to regulate Flt3 expression in a direct or indirect manner. Interestingly, both Ikaros and Flt3 were decreased in aged LSK, which might lead to the decreased differentiation of DC subsets in aged mice. In this study, we attributed this deficiency to the elevation of Wnt5a in aged LSK by demonstrating that the expression of these critical transcriptional factors and cytokine receptors were downregulated by Wnt5a. Blockage of the Wnt5a-Cdc42 pathway in aged mice resulted in the rejuvenation of the DC differentiation potential of aged BM hematopoietic precursors, further supporting our conclusion.
Wnt5a might also regulate the differentiation of DC by affecting other pathways in addition to Cdc42. The non-canonical Wnt pathway has been reported to inhibit the canonical Wnt pathway in HSCs.24,25 We also found that the expression of downstream genes of the Wnt/β-catenin pathway could be inhibited by Wnt5a treatment in LSK (unpublished data).
In addition to the effect of the non-canonical Wnt pathway on DC differentiation, we also investigated its role in DC activation. Our results showed that the production of type I IFN and IL12p70 by pDC was inhibited upon Wnt5a pretreatment (Supplemental Figure 3), suggesting that Wnt5a could inhibit the differentiation and function of pDC. However, pharmacological inhibition of the non-canonical Wnt pathway could not reverse pDC activation, indicating that the Wnt pathway may not be the only pathway affected by aging. Therefore, other molecular pathways in aged pDC might also be affected and contribute to the impaired function of pDC in aged mice.
Because DC play central roles in linking innate and adaptive immune responses, DC aging has been considered to be an important contributor to aging-related diseases, including infectious diseases, autoimmune diseases, and cancers.39,40,41,42 Here, we rejuvenated the differentiation of aged DC via pharmacological inhibition of the non-canonical Wnt pathway, providing a new strategy for the treatment of DC aging-related immune disorders.
Acknowledgments
This study was supported by a Key Project Grant from the National Natural Science Foundation of China (No. 31330027), a Tsinghua University Initiative Scientific Research Program Research Fund (No. 20111080963) and the Tsinghua-Peking Center for Life Sciences.
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website (http://www.nature.com/cmi).
Supplementary Information
References
- Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 2013; 31: 563–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity 2014; 40: 642–656. [DOI] [PubMed] [Google Scholar]
- Wu L, Liu YJ. Development of dendritic-cell lineages. Immunity 2007; 26: 741–750. [DOI] [PubMed] [Google Scholar]
- Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol 2007; 7: 19–30. [DOI] [PubMed] [Google Scholar]
- Adolfsson J, Månsson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell 2005; 121: 295–306. [DOI] [PubMed] [Google Scholar]
- Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev 2010; 234: 45–54. [DOI] [PubMed] [Google Scholar]
- Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol 2007; 8: 1207–1216. [DOI] [PubMed] [Google Scholar]
- Sathe P, Vremec D, Wu L, Corcoran L, Shortman K. Convergent differentiation: myeloid and lymphoid pathways to murine plasmacytoid dendritic cells. Blood 2013; 121: 11–19. [DOI] [PubMed] [Google Scholar]
- Chen YL, Chen TT, Pai LM, Wesoly J, Bluyssen HA, Lee CK. A type I IFN-Flt3 ligand axis augments plasmacytoid dendritic cell development from common lymphoid progenitors. J Exp Med 2013; 210: 2515–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onai N, Kurabayashi K, Hosoi-Amaike M, Toyama-Sorimachi N, Matsushima K, Inaba K et al. A clonogenic progenitor with prominent plasmacytoid dendritic cell developmental potential. Immunity 2013; 38: 943–957. [DOI] [PubMed] [Google Scholar]
- Shortman K, Sathe P. Another heritage for plasmacytoid dendritic cells. Immunity 2013; 38: 845–846. [DOI] [PubMed] [Google Scholar]
- Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M et al. Origin of the lamina propria dendritic cell network. Immunity 2009; 31: 513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancke B, Suter M, Hochrein H, O'Keeffe M. M-CSF: a novel plasmacytoid and conventional dendritic cell poietin. Blood 2008; 111: 150–159. [DOI] [PubMed] [Google Scholar]
- Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J Exp Med 2003; 198: 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskow C, Liu K, Darrasse-Jèze G, Guermonprez P, Ginhoux F, Merad M et al. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol 2008; 9: 676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasel K, De Smedt T, Smith JL, Maliszewski CR. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood 2000; 96: 3029–3039. [PubMed] [Google Scholar]
- McKenna HJ1, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 2000; 95: 3489–3497. [PubMed] [Google Scholar]
- Vogt TK, Link A, Perrin J, Finke D, Luther SA. Novel function for interleukin-7 in dendritic cell development. Blood 2009; 113: 3961–3968. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol 2008; 8: 581–593. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Nat Rev Immunol 2008; 8: 581–593.18617885 [Google Scholar]
- Luis TC, Naber BA, Roozen PP, Brugman MH, de Haas EF, Ghazvini M et al. Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell 2011; 9: 345–356. [DOI] [PubMed] [Google Scholar]
- Sugimura R, He XC, Venkatraman A, Arai F, Box A, Semerad C et al. Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche. Cell 2012; 150: 351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semerad CL, Mercer EM, Inlay MA, Weissman IL, Murre C. E2A proteins maintain the hematopoietic stem cell pool and promote the maturation of myelolymphoid and myeloerythroid progenitors. Proc Natl Acad Sci U S A 2009; 106: 1930–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth MJ, Topol L, Anderson SM, Yang Y, Bodine DM. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci U S A 2007; 104: 15436–15441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian MC, Nattamai KJ, Dörr K, Marka G, Uberle B, Vas V et al. A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature 2013; 503: 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oderup C, LaJevic M, Butcher EC. Canonical and noncanonical Wnt proteins program dendritic cell responses for tolerance. J Immunol 2013; 190: 6126–6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Cheng P, Youn JI, Cotter MJ, Gabrilovich DI. Notch and wingless signaling cooperate in regulation of dendritic cell differentiation. Immunity 2009; 30: 845–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CP, Magnusson KR, Ho E. Aging is associated with altered dendritic cells subset distribution and impaired proinflammatory cytokine production. Exp Gerontol 2010; 45: 163–169. [DOI] [PubMed] [Google Scholar]
- Jing Y, Shaheen E, Drake RR, Chen N, Gravenstein S, Deng Y. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum Immunol 2009; 70: 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Gupta S. Impact of aging on dendritic cell functions in humans. Ageing Res Rev 2011; 10: 336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam C, Geffers R, Yücel R, Buer J, Welte K, Möröy T et al. The transcriptional repressor Gfi1 controls STAT3-dependent dendritic cell development and function. Immunity 2005; 22: 717–728. [DOI] [PubMed] [Google Scholar]
- Wu L, Nichogiannopoulou A, Shortman K, Georgopoulos K. Cell-autonomous defects in dendritic cell populations of Ikaros mutant mice point to a developmental relationship with the lymphoid lineage. Immunity 1997; 7: 483–492. [DOI] [PubMed] [Google Scholar]
- Wu X, Satpathy AT, Kc W, Liu P, Murphy TL, Murphy KM. Bcl11a controls Flt3 expression in early hematopoietic progenitors and is required for pDC development in vivo. PLoS One 201; 8: e64800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carotta S, Dakic A, D'Amico A, Pang SH, Greig KT, Nutt SL et al. The transcription factor PU.1 controls dendritic cell development and Flt3 cytokine receptor expression in a dose-dependent manner. Immunity 2010; 32: 628–641. [DOI] [PubMed] [Google Scholar]
- Sathe P, Metcalf D, Vremec D, Naik SH, Langdon WY, Huntington ND et al. Lymphoid tissue and plasmacytoid dendritic cells and macrophages do not share a common macrophage-dendritic cell-restricted progenitor. Immunity 2014; 41: 104–115. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A 2005; 102: 9194–9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JR, Lebensohn AM, Pelish HE, Kirschner MW. Biochemical suppression of small-molecule inhibitors: a strategy to identify inhibitor targets and signaling pathway components. Chem Biol 2006; 13: 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian MC, Dörr K, Niebel A, Daria D, Schrezenmeier H, Rojewski M et al. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell 2012; 10: 520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurin MR, Shurin GV, Chatta GS. Aging and the dendritic cell system: implications for cancer. Crit Rev Oncol Hematol 2007; 64: 90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Sridharan A, Prakash S, Agrawal H. Dendritic cells and aging: consequences for autoimmunity. Expert Rev Clin Immunol 2012; 8: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grolleau-Julius A, Harning EK, Abernathy LM, Yung RL. Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res 2008; 68: 6341–6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest 2013; 123: 958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.