Abstract
Aims
Metal absorbable scaffolds constitute a conceptually attractive alternative to polymeric scaffolds. Promising 6-month outcomes of a second-generation drug-eluting absorbable metal scaffold (DREAMS 2G), consisting of an absorbable magnesium scaffold backbone, have been reported. We assessed the 12-month safety and performance of this novel device.
Methods and results
The prospective, international, multi-centre, first-in-man BIOSOLVE-II trial enrolled 123 patients with up to two de novo lesions with a reference diameter between 2.2 and 3.7 mm. All patients were scheduled for angiographic follow-up at 6 months, and—if subjects consented—at 12 months. Dual antiplatelet therapy was recommended for 6 months. Quantitative coronary angiography (QCA) parameters remained stable from 6 to 12 months [paired data of 42 patients: in-segment late lumen loss 0.20 ± 0.21 mm vs. 0.25 ± 0.22 mm, P = 0.117, Δ 0.05 ± 0.21 mm (95% CI: −0.01;0.12); in-scaffold late lumen loss 0.37 ± 0.25 mm vs. 0.39 ± 0.27 mm, P = 0.446, Δ 0.03 ± 0.22 (95% CI: −0.04;0.10), respectively]. Intravascular ultrasound and optical coherence tomography findings corroborated the QCA results. Target lesion failure occurred in four patients (3.4%), consisting of one death of unknown cause, one target-vessel myocardial infarction, and two clinically driven target lesion revascularization. No additional event occurred beyond the 6-month follow-up. During the entire follow-up of 12 months, none of the patients experienced a definite or probable scaffold thrombosis.
Conclusion
The novel drug-eluting metal absorbable scaffold DREAMS 2G showed a continuous favourable safety profile up to 12 months and stable angiographic parameters between 6 and 12 months.
ClinicalTrials.gov identifier
Keywords: Coronary artery disease, Bioresorbable, Scaffold, DREAMS, Magnesium, PLLA
Introduction
Bioresorbable scaffolds (BRS) have been designed to overcome problems related to the long-term persistence of metallic stents implanted in coronary arteries.1,2 Several randomized controlled trials (RCTs) comparing polymeric BRS with new-generation drug-eluting stents (DES) found similar clinical outcomes at 1 year. A recent meta-analysis, though, showed an increased risk of definite or probable scaffold thrombosis for BRS (1.3% compared with 0.5%).3 As the true benefit of BRS is expected to ensue later, long-term data of RCTs are awaited. So far, non-randomized studies up to 5 years showed promising outcomes.4,5
Absorbable metal scaffolds constitute an attractive alternative to polymeric BRS, as they conform more to the technique of percutaneous coronary intervention with DES.6 We assessed a novel second-generation drug-eluting absorbable metal scaffold (DREAMS 2G), which has been redesigned iteratively by (i) improving the design of the magnesium backbone of its previous version7,8 and (ii) using the same drug–polymer combination (sirolimus/poly-l-lactide) as the new-generation biodegradable polymer stent Orsiro (Biotronik AG, Buelach, Switzerland).9,10 Six-month outcomes were recently published, showing favourable angiographic and clinical outcomes with a low rate of target lesion failure (TLF) and no definite or probable scaffold thrombosis.6 We aimed to evaluate if those results are sustained at 12 months, a time point when the magnesium scaffold is expected to be degraded.
Methods
Study design and population
A detailed description of the study has been recently published.6 In brief, BIOSOLVE-II is a prospective, multi-centre, first-in-man study to evaluate the safety and performance of DREAMS 2G (Biotronik AG, Buelach, Switzerland). The study is in compliance with the Declaration of Helsinki, Good Clinical Practice, ISO14155, and was approved by the institutional ethics committees at the participating 13 institutions in Europe, South America, and Asia. All patients provided written informed consent.
Eligible patients had stable or unstable angina or documented silent ischaemia, a maximum of two single de novo lesions in two separate coronary arteries, with a reference vessel diameter between 2.2 and 3.7 mm, a lesion length of ≤21 mm, and a diameter stenosis between 50 and 99%. Exclusion criteria included thrombus in the target vessel, severe calcification, three-vessel disease, ostial lesion, bifurcation lesion involving a side branch >2.0 mm in diameter, target lesion located in or supplied by an arterial or venous bypass graft, and unsuccessful pre-dilatation. The full list of inclusion and exclusion criteria can be accessed at clinicaltrials.gov (NCT01960504).
Clinical follow-up was planned at 1, 6, 12, 24, and 36 months. Angiographic follow-up was scheduled at 6 months for all patients, an additional pre-specified imaging follow-up was scheduled at 12 months if subjects consented. A subgroup of 30 patients underwent intravascular ultrasound (IVUS), optical coherence tomography (OCT), and vasomotion testing at 6 months. At this time point, these patients were also asked to consent for voluntary IVUS, OCT, and vasomotion assessment at 12 months. Only patients without target lesion revascularization (TLR) were asked for additional 12-month imaging assessment.
Study device
DREAMS 2G is a drug-eluting absorbable metal scaffold system comprising of an absorbable magnesium scaffold pre-mounted on a balloon-expandable delivery system. The scaffold is laser-polished and its surface is completely coated with bioresorbable poly-l-lactide, which incorporates sirolimus.6
Endpoints and definitions
The primary endpoint was in-segment late lumen loss (LLL) at 6-month follow-up. Secondary endpoints at 12 months were TLF, a composite of cardiac death,11 target-vessel myocardial infarction,12 and clinically TLR11; scaffold thrombosis11; in-scaffold and in-segment binary restenosis; diameter stenosis; and in-scaffold LLL.13
A clinical event committee adjudicated all adverse events, and an independent core laboratory (Cardialysis B.V., Rotterdam, The Netherlands) performed the quantitative coronary angiography (QCA), IVUS, and OCT analyses. Imaging analysis methods and definitions have been published previously.6
Procedure
The device was available to operators in sizes 2.5 mm × 20 mm, 3.0 mm × 20 mm, or 3.5 mm × 25 mm. Pre-dilatation was mandatory; post-dilatation was left to the operator's discretion. Only one study device per lesion was allowed, although in bailout situations, a second DREAMS 2G could be used, and, in case of failure, an Orsiro DES. Dual antiplatelet therapy was recommended for a minimum of 6 months. The procedural details of vasomotion testing and imaging procedure are described elsewhere.6
Statistical analysis
The sample size was calculated based on the primary endpoint in-segment LLL at 6 months and has been previously reported.6 The null hypothesis was a mean in-segment LLL at 6 months of ≥0.5 mm. An overall of 121 (101 + 20) subjects were scheduled to be enrolled, assuming an expected in-segment LLL mean of 0.45 mm, standard deviation of 0.2 mm, one-sided, significance level of 0.05, and 80% power.
The intention-to-treat analysis population was defined as patients for whom an investigational scaffold entered the guide catheter following the diagnostic angiogram. Patients not receiving a study device were included in the calculation of procedural success, but excluded from further analysis as defined in the study protocol. For serial imaging analysis, paired data were used.
Descriptive statistical methods were used. Means and standard deviations, medians and interquartile ranges (IQR), and 95% CIs were calculated as appropriate. For categorical data, absolute and relative frequencies were calculated and 95% CIs for proportions. P-values were calculated using paired t-test, Fisher's exact test, χ2 test, and Wilcoxon signed-rank test, where applicable. All statistical analyses were performed with SAS (version 9.3).
Role of the funding source
The study was sponsored by Biotronik AG, Buelach, Switzerland. The sponsor was involved in the design and conduction of the study, data collection, monitoring, and data analysis.
Results
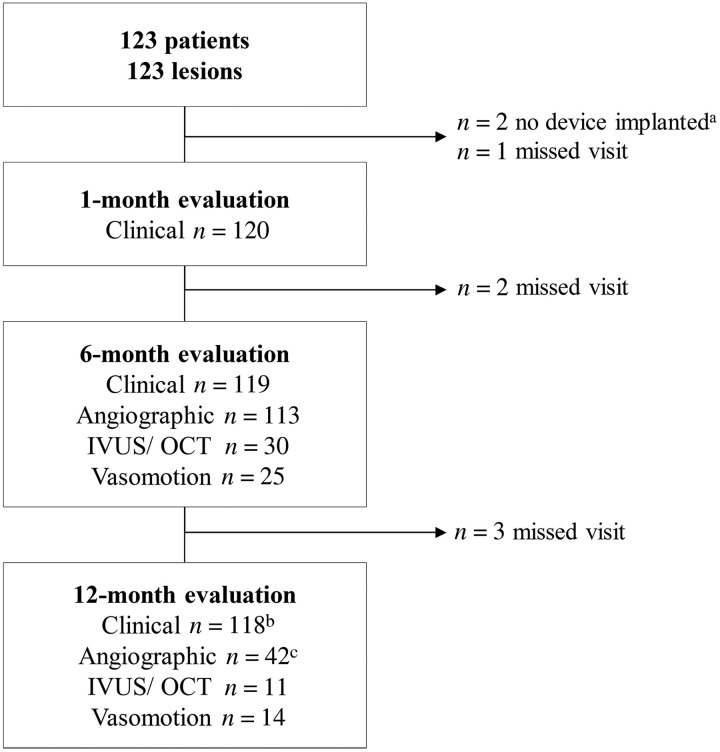
Between October 2013 and May 2015, 123 subjects were enrolled. Clinical follow-up at 12 months was 98% (Figure 1). Baseline parameters are listed in Table 1. A comparison of baseline parameters of the angiographic and IVUS/OCT subgroups with serial 6- and 12-month evaluation compared with the overall population is provided in the Supplementary material online, Table S1. In two lesions, DREAMS 2G could not be implanted due to insufficient pre-dilatation; follow-up of these two subjects was consequently not included in this analysis.
Figure 1.
Patient flow chart. IVUS, intravascular ultrasound; OCT, optical coherence tomography. aTwo patients who did not receive an implant were used for calculation of device and procedural success only. bThirty-four visits were conducted by phone. cForty-five angiographic assessments of which two were excluded as they did not have matched projections recorded, one did not have a 6-month but only 12-month angiography.
Table 1.
Baseline clinical and lesion characteristics
| n = 123 | |
|---|---|
| Mean age, years | 65.2 ± 10.3 |
| Male gender | 78 (63.4) |
| Hypertension | 101 (82.1) |
| Hyperlipidaemia | 74 (60.2) |
| Diabetes | 36 (29.3) |
| History of smoking | 67 (54.5) |
| Previous percutaneous coronary interventions | 44 (35.8) |
| CABG | 8 (6.5) |
| History of myocardial infarction | 29 (23.6) |
| Renal failure | 4 (3.3) |
| Congestive heart failure | 8 (6.5) |
| History of stroke or TIA | 7 (5.7) |
| Mean lesion length, mma | 12.61 ± 4.53 |
| Mean reference vessel diameter, mm2a | 2.68 ± 0.40 |
| AHA/ACC classification type B2/Cb | 53 (43.4) |
| Moderate-to-severe calcification | 13 (10.6) |
| Thrombus | 3 (2.4) |
Data are shown as mean ± SD or n (%).
AHA/ACC, American Heart Association/American College of Cardiology; CABG, coronary artery bypass graft; TIA, transient ischaemic attack.
a n = 120, in 3 subjects, images were not analysable.
b n = 122, 1 subject not analysable.
At baseline, 88 (71.5%) patients had stable angina, 17 (13.8%) unstable angina, and 18 (14.6%) silent ischaemia. At 12-month follow-up, 100 (86.2%) patients were symptom-free, 14 (12.1%) had stable angina (9 CCS class I and 5 CCS class II), 1 (0.9%) had silent ischaemia, and none had unstable angina. Fifty-seven patients (48.3%) were still on dual antiplatelet therapy at 12 months.
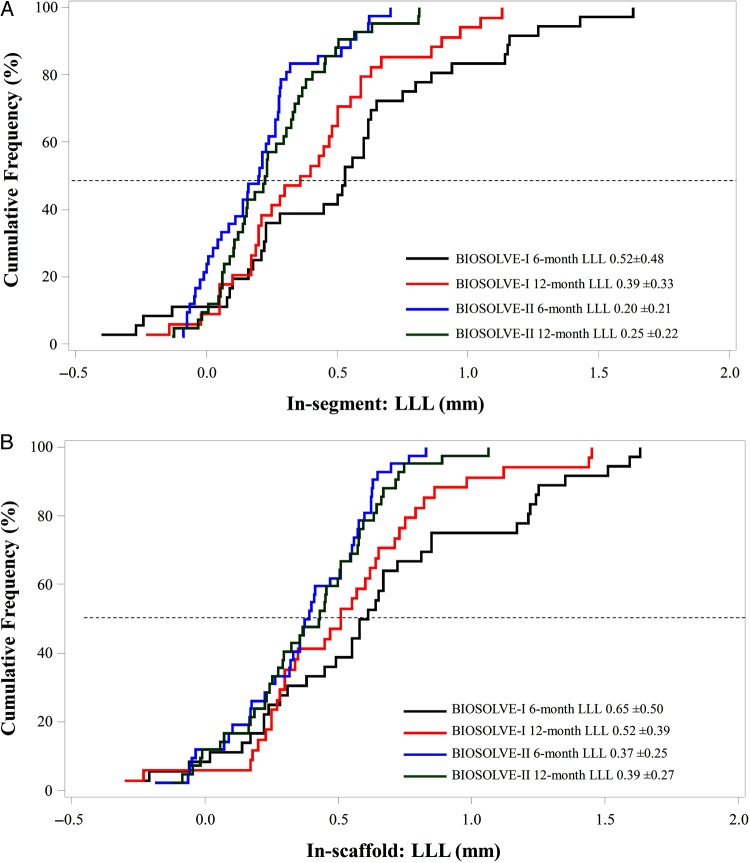
Paired QCA data could be obtained in 42 patients from seven centres at a mean follow-up time of 181 ± 17 days and 367 ± 17 days for 6- and 12-month assessments, respectively. Paired in-segment LLL at 6 and 12 months was 0.20 ± 0.21 mm (95% CI: 0.13;0.26) and 0.25 ± 0.22 mm (95% CI: 0.18;0.32), P = 0.117, Δ 0.05 ± 0.21 mm (95% CI: −0.01;0.12), and in-scaffold LLL 0.37 ± 0.25 mm (95% CI: 0.29;0.45) and 0.39 ± 0.27 mm (95% CI: 0.31;0.48), P = 0.446, Δ 0.03 ± 0.22 mm (95% CI: −0.04;0.10) (Table 2, Figure 2). The in-segment and in-scaffold LLL of the overall population at 6 months were 0.27 ± 0.37 and 0.44 ± 0.36 mm for the overall population (0.21 ± 0.28 and 0.37 ± 0.28 mm in patients without TLR). There was no statistically significant difference in baseline characteristics between this subgroup and the overall patient population.
Table 2.
Quantitative coronary angiographic analysis (paired data, n = 42)
| Pre-procedure | Post-procedure | 6 months | 12 months | |
|---|---|---|---|---|
| Reference vessel diameter in-segment | 2.74 ± 0.35 | 2.75 ± 0.35 | 2.60 ± 0.38 | 2.60 ± 0.41 |
| Reference vessel diameter in-scaffold | NA | 2.84 ± 0.37 | 2.66 ± 0.34 | 2.64 ± 0.44 |
| Minimum lumen diameter in-segment | 1.22 ± 0.33 | 2.25 ± 0.41 | 2.01 ± 0.38 | 1.96 ± 0.41 |
| Minimum lumen diameter in-scaffold | NA | 2.54 ± 0.33 | 2.14 ± 0.38 | 2.10 ± 0.41 |
| Acute gain in-segment | NA | 1.00 ± 0.38 | NA | NA |
| Acute gain in-scaffold | NA | 1.29 ± 0.34 | NA | NA |
| Diameter stenosis in-segment | 55.2 ± 10.9 | 18.7 ± 6.8 | 22.6 ± 9.2 | 24.7 ± 10.6 |
| Diameter stenosis in-scaffold | NA | 10.4 ± 6.0 | 19.6 ± 8.4 | 20.4 ± 8.6 |
| Binary restenosis in-segment, % | NA | NA | 0 (0) | 2 (4.8) |
| Binary restenosis in-scaffold, % | NA | NA | 0 (0) | 0 (0) |
| Late lumen loss in-segment | NA | NA | 0.20 ± 0.21 | 0.25 ± 0.22 |
| Late lumen loss in-scaffold | NA | NA | 0.37 ± 0.25 | 0.39 ± 0.27 |
Data are shown as mean ± SD or n (%). All measurements are in mm if not otherwise specified. There was no significant difference in outcomes between 6 and 12 months.
NA, not applicable.
Figure 2.
Cumulative frequency curves for in-segment (A) and in-scaffold (B) late lumen loss. Six- and twelve-month serial analysis of late lumen loss observed in BIOSOLVE-II compared with BIOSOLVE-I using the precursor device DREAMS first generation.
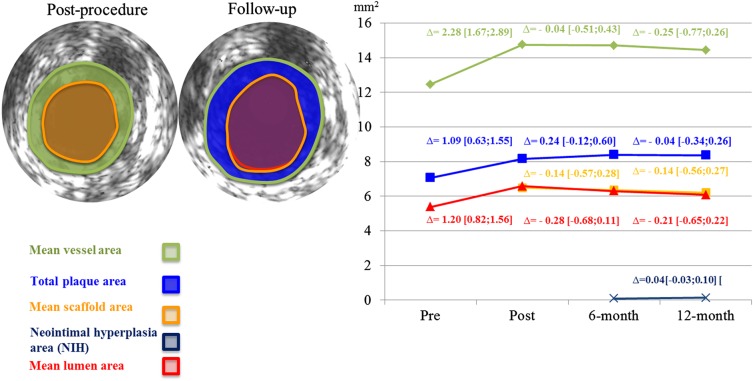
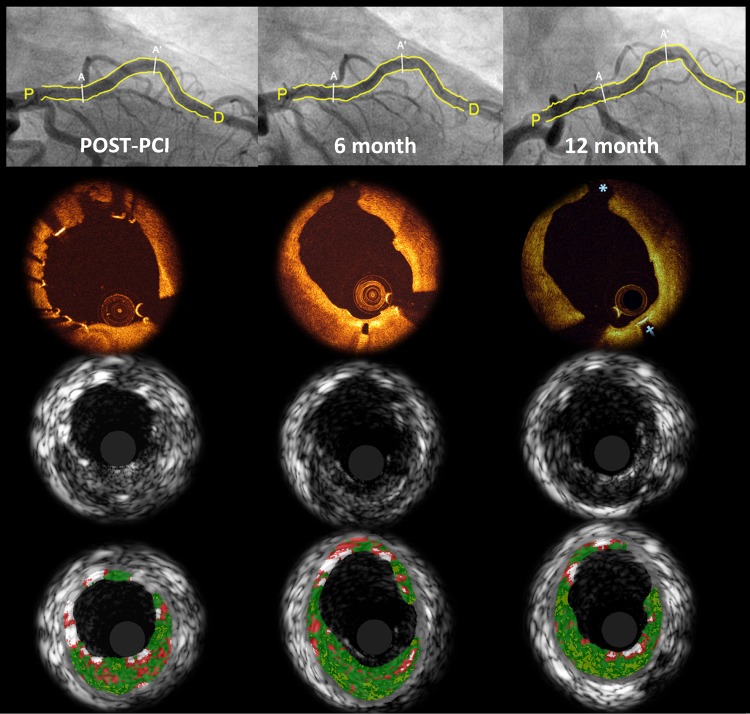
Serial IVUS and OCT analyses at 6 and 12 months were performed in 11 patients (respective data listings are provided in the Supplementary material online, Tables S2 and S3). Paired IVUS parameters did not differ significantly between 6 and 12 months (median minimum lumen area of 4.80 vs. 4.69 mm2, P = 0.700), except for the number of patients with incomplete strut apposition, which was reduced to zero at 12 months (Supplementary material online, Table S4; Figure 3). By OCT, the median minimal lumen area decreased from 4.58 mm2 at 6 months to 4.19 mm2 at 12 months, P = 0.032. No intraluminal mass was observed at any time. Figure 4 shows that at 6 and 12 months, strut-like remnants are only visible by IVUS but not by OCT.
Figure 3.
Serial changes of intravascular ultrasound parameters in 11 patients. Only a very small neo-intimal hyperplasia area is visible (7 o'clock). Δ indicates the difference between follow-ups in mm2 [95% CI].
Figure 4.
Serial angiographic, optical coherence tomography, intravascular ultrasound, and virtual histology of a patient implanted with DREAMS 2G. Matched images show that at 6 months, struts are hardly discernible by optical coherence tomography and the scaffold strut that had covered the side branch post-procedure (12 o'clock) had disappeared. At 12 months, the vessel surface appears even smoother than at 6 months (2 o'clock). On intravascular ultrasound, strut remnants are still discernible at follow-up. Assessed by virtual histology, the white colour coding of the scaffold struts disappears over time demonstrating the absorption process. A–A′, scaffolded segment; P, proximal reference; D, distal reference.
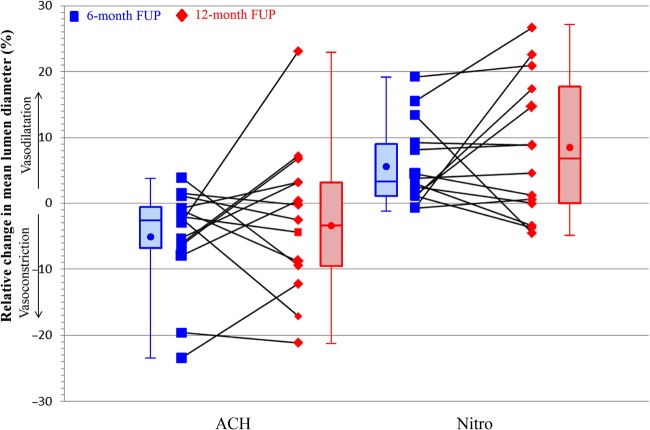
Serial vasomotion at 6 and 12 months was tested in 14 patients; thereof 11 (79%) had a change of >3% to the mean lumen diameter after infusion or injection of acetylcholine (Figure 5). The median percentage change in mean lumen diameter between pre-and post-acetylcholine was −2.6% (IQR: −6.4 to −0.6%, mean −5.1 ± 7.7%) at 6 months and −3.4% (IQR: −9.4 to 3.2%, mean −3.4 ± 11.0%) at 12 months; the percentage change in mean lumen diameter between post-acetylcholine and nitroglycerine was 3.4% (IQR: 1.1–9.2%, mean 5.8 ± 6.4%) and 6.7% (IQR: 0.0–17.4%, mean 8.2 ± 10.6%), respectively.
Figure 5.
Change in vasomotion between 6 and 12 months. Percentage change in mean lumen diameter after acetylcholine and after subsequent intracoronary injection of nitroglycerine at 6 and 12 months in 14 patients. Each line reflects one patient. The ends of the boxes represent the first and third quartiles, the band the median, and the crystal the mean. There was no statistical significant difference between 6 and 12 months (P = 0.808 for acetylcholine and P = 0.626 for nitroglycerine). ACH, acetylcholine; Nitro, nitroglycerine.
Target lesion failure at 12 months was observed in 4/118 patients (3.4%, 95% CI: 0.9–8.4). No events beyond 6 months occurred. Specifically, one death (0.8%) of unknown cause on day 134 post-procedure was classified as cardiac death. One target-vessel myocardial infarction (0.8%) was due to temporary no-reflow after scaffold implantation. Clinically driven TLR was performed in two patients (1.7%). One patient died of cancer prior to 6-month follow-up. No definite or probable scaffold thrombosis was observed. Further details are provided in the Supplementary material online, Table S5.
Discussion
The main findings of our study are stability of the angiographic and clinical outcomes of the second-generation device DREAMS 2G between 6 and 12 months. There was no definite or probable scaffold thrombosis up to 12 months and no TLF beyond 6 months. These findings are relevant as ∼95% of the magnesium scaffold is expected to be absorbed within 12 months.6 The importance of this time point for DREAMS 2G is reflected in the recently published European Society of Cardiology–European Association of Percutaneous Cardiovascular Interventions report on the evaluation on coronary stents, which states that ‘for bioresorbable stents, critical time points of follow-up will depend on the pace of biodegradation and should cover complete resorption’.13
Compared with the first generation, DREAMS 2G has markedly improved angiographic performance parameters also at 12 months (in-segment LLL of 0.25 ± 0.22 mm vs. 0.39 ± 0.33 mm and in-scaffold LLL of 0.39 ± 0.27 vs. 0.52 ± 0.39 mm).7 There are reports that found a significant relationship between LLL and TLR even in patients with a LLL of <0.5mm, but to a lesser extent than above 0.5 mm.14 In other studies with permanent metallic stents, an in-segment LLL of ≤0.5 mm has been associated with a maximal TLR rate of 5%15 and a binary restenosis rate of 5%.16 Furthermore, in an evaluation from 11 RCTs, Pocock et al.17 pointed out that a 12-month in-segment LLL of <0.3 mm and in-stent LLL of <0.4 mm were associated with very low TLR rates and that further reductions in LLL are unlikely to reduce clinical restenosis. Correspondingly, a meta-analysis of RCTs showed that BRS had similar 12-month TLR rates as new-generation DES despite inferior LLL.3 This might also explain the good clinical outcomes in our series despite a higher LLL. Specifically, compared with recently established objective performance criteria for coronary stent trials at 9–12 months,13 our series had inferior LLL [mean in-scaffold LLL of 0.39 ± 0.27 mm compared with a median of 0.18 mm (IQR: 0.13–0.25) for new-generation DES], but comparable clinical outcomes [TLR of 1.7% compared with 2.91% (IQR: 1.67–5.94) for new-generation DES, definitive stent thrombosis of 0.0% compared with 0.47% (IQR:0.28 to 0.72), respectively].
One of the encouraging finding is the persisting absence of definite or probable scaffold thrombosis in this trial and any other trial with precursor devices of DREAMS 2G, tested in the PROGRESS or BIOSOLVE-I study.7,8 However, this has to be interpreted with the caveat that (i) only 123 patients were enrolled, (ii) the results were not obtained in an all-comers patient population, (iii) randomized controlled data are missing yet, and (iv) dual antiplatelet therapy was recommended for at least 6 months, and nearly half of the patients were still on dual antiplatelet therapy at 12 months. Still, these results are promising in the context of a recent meta-analyses where higher scaffold thrombosis rates (mostly acute and subacute) were reported for patients treated with polymeric BRS compared with DES.3,18 Notably, in ABSORB cohort A and B, there was also no scaffold thrombosis except one iatrogenic scaffold thrombosis with subsequent myocardial infarction.4,5 Nevertheless, there are several factors indicating that DREAMS 2G might have a reduced risk for thrombosis: At 12 months, struts were not discernible by OCT, but IVUS showed strut-like remnants. In the case example of Figure 4, the white colour coding of the scaffold struts disappears over time demonstrating the absorption process. This is most likely explained by the biodegradation of magnesium. Approximately 95% of the magnesium is converted at 12 months.6 In brief, the biodegradation of magnesium occurs by means of anions and cations exchange, magnesium hydroxide is formed, and in a second step, magnesium hydroxide is converted to amorphous calcium phosphate.22,23 Hence, at 12 months, elution of magnesium from the metallic backbone is usually completed, and the strut areas are mostly filled by amorphous calcium phosphate with high water content, which is not discernible by OCT but to some extent by IVUS. Amorphous calcium phosphate should not be misinterpreted as calcification that is associated with an echo shadowing that was not documented behind the scaffold remnants in IVUS.
In 30 patients assessed up to 6 months6 and 11 up to 12 months, no intraluminal mass was detected by OCT. At 6 months, no mal-apposed struts were present, because the scaffold struts were already embedded into the vessel wall. Furthermore, in the presence of nearly full absorption of the magnesium scaffold at 12 months,6 late acquired mal-apposition appears to be impossible. This is important since recent observations found four very late scaffold thrombosis in 171 patients treated with a polymeric BRS with a longer degradation time compared with DREAMS 2G. All of those were associated with scaffold discontinuity, mal-apposed, and/or uncovered scaffold struts potentially promoting thrombotic events.19
Magnesium has a negatively charged surface promoting anti-thrombotic properties in vivo.1,20 However, this advantage might be hypothetical as the scaffold has been shown to be covered and embedded in the vessel prior to the absorption of the drug–polymer coating.
DREAMS 2G is laser-polished, leading to a very smooth surface.6
While struts of absorbable scaffolds are thicker than DES struts, the strut cross section of DREAMS 2G is rectangular with rounded edges,1 which might result in better embedding into the vessel wall.
DREAMS 2G does not require stepwise inflation as required with polymeric scaffolds,21 which may result in better expansion and apposition.
Vasodilatation or vasoconstriction >3% was already observed at 6 months, and continued to increase at 12 months. In general, angiographic, IVUS, and OCT parameters remained stable between 6 and 12 months, yet there was a small, but significant decrease in median minimal lumen area when serially measured by OCT in 11 patients (−0.25 mm2, IQR: −0.64 to −0.09, P = 0.032). Given the very small number of patients included in the intracoronary imaging sub-study, these findings should be interpreted with caution. Since DREAMS 2G was designed to provide a longer scaffolding time compared with DREAMS 1G, it may be assumed that late lumen enlargement is postponed and unlikely to be seen as early as after 1 year. Additional longer-term follow-up evaluation is required to confirm luminal enlargement, as shown for other polymeric scaffolds or previous versions of the magnesium scaffold implanted in the PROGRESS and BIOSOLVE-I studies.5,8,24
Limitations
As with most first-in-man trials, BIOSOLVE-II was not randomized and included a limited population with simple lesions. Consequently, comparisons with other devices have to be interpreted with caution.
The clinical follow-up rate was high, but—attributed to the fact that it was voluntary per protocol—only a relatively small cohort was available for serial QCA, IVUS, and OCT assessments. The demographics of these patients were comparable to those of the overall study cohort, but nevertheless A further limitation is that long-term data are pending to document lumen enlargement—as seen in BIOSOLVE-I at 3 years.24 Finally, the favourable outcomes of BIOSOLVE-II need to be confirmed in more complex patient and lesion scenarios.
the data were only powered for in-segment LLL at 6 month,
eventually, a bias is introduced as symptomatic patients may be more prone to agree to 12-month imaging assessments than asymptomatic patients, and
12-month imaging assessments were not performed in all participating centres.
Conclusion
This novel absorbable metal scaffold showed a sustained favourable safety profile up to 12 months and no increase in LLL between 6 and 12 months in a subgroup of 42 patients. No additional TLF event occurred beyond 6 months. These findings suggest that DREAMS 2G can be considered as an alternative to current polymeric BRS.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors’ contributions
M.H. handled funding and supervision, conceived and designed the research, and drafted the manuscript. M.H., H.I., A.A., R.T., P.A.L., C.v.B., E.H.C., W.W., F.-J.N., C.K., E.E., S.T.L., and J.E. acquired the data. All authors made critical revision of the manuscript for key intellectual content.
Funding
This work was supported by Biotronik AG, Buelach, Switzerland. Funding to pay the Open Access publication charges for this article was provided by Biotronik AG, Buelach, Switzerland.
Conflict of interest: M.H. reports study grants and lecture fees from Biotronik, Abbott Vascular, Cardiac dimensions, Medtronic, Volcano, and Lilly. R.T. reports personal fees from Biotronik and Abbott Vascular. P.A.L. reports grants from Biotronik, Boston Scientific, and Scitech. C.v.B. reports institutional research grants from Biotronik, Medtronic, Boston Scientific, and AstraZeneca; and personal fees from Medtronic, Boston Scientific, and Astra Zeneca. W.W. reports grants from Abbott Vascular, Biotronik, and Terumo; and being a non-executive board member and shareholder of Argonauts Partners, Celyad. F.-J.N. reports grants from Biotronik; and personal fees and non-financial support from Abbott Vascular, Boston Scientific, Medtronic, and Biotronik. E.E. reports speakers’ honoraria and research grants from Biotronik. S.T.L. reports institutional research grants from Biotronik, Medtronic, Biosensors, Bayer, and Actelion; and non-financial support from Abbott Vascular, Orbus Neich, Alvimedica, Philips, Asahi Intecc, and Terumo. Y.O. reports being a member of the advisory board of Abbott Vascular. R.W. reports personal fees from Biotronik, Medtronic, and Abbott Vascular; grants and personal fees from Astra Zeneca and Boston Scientific; and grants from The Medicines Company and Edwards Lifesciences.
Supplementary Material
Acknowledgement
We thank Beatrix Doerr for her expert medical writing assistance, reimbursed by Biotronik AG.
References
- 1. Campos CM, Muramatsu T, Iqbal J, Zhang YJ, Onuma Y, Garcia-Garcia HM, Haude M, Lemos PA, Warnack B, Serruys PW. Bioresorbable drug-eluting magnesium-alloy scaffold for treatment of coronary artery disease. Int J Mol Sci 2013;14:24492–24500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iqbal J, Onuma Y, Ormiston J, Abizaid A, Waksman R, Serruys P. Bioresorbable scaffolds: rationale, current status, challenges, and future. Eur Heart J 2014;35:765–776. [DOI] [PubMed] [Google Scholar]
- 3. Cassese S, Byrne RA, Ndrepepa G, Kufner S, Wiebe J, Repp J, Schunkert H, Fusaro M, Kimura T, Kastrati A. Everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: a meta-analysis of randomised controlled trials. Lancet 2016;387:537–544. [DOI] [PubMed] [Google Scholar]
- 4. Onuma Y, Dudek D, Thuesen L, Webster M, Nieman K, Garcia-Garcia HM, Ormiston JA, Serruys PW. Five-year clinical and functional multislice computed tomography angiographic results after coronary implantation of the fully resorbable polymeric everolimus-eluting scaffold in patients with de novo coronary artery disease: the ABSORB cohort A trial. JACC Cardiovasc Interv 2013;6:999–1009. [DOI] [PubMed] [Google Scholar]
- 5. Serruys PW, Onuma Y, Garcia-Garcia HM, Muramatsu T, van Geuns RJ, de Bruyne B, Dudek D, Thuesen L, Smits PC, Chevalier B, McClean D, Koolen J, Windecker S, Whitbourn R, Meredith I, Dorange C, Veldhof S, Hebert KM, Rapoza R, Ormiston JA. Dynamics of vessel wall changes following the implantation of the Absorb everolimus-eluting bioresorbable vascular scaffold: a multi-imaging modality study at 6, 12, 24 and 36 months. EuroIntervention 2014;9:1271–1284. [DOI] [PubMed] [Google Scholar]
- 6. Haude M, Ince H, Abizaid A, Toelg R, Lemos PA, von BC, Christiansen EH, Wijns W, Neumann FJ, Kaiser C, Eeckhout E, Lim ST, Escaned J, Garcia-Garcia HM, Waksman R. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet 2013;381:836–844. [DOI] [PubMed] [Google Scholar]
- 7. Haude M, Erbel R, Erne P, Verheye S, Degen H, Bose D, Vermeersch P, Wijnbergen I, Weissman N, Prati F, Waksman R, Koolen J. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. Lancet 2013;381:836–844. [DOI] [PubMed] [Google Scholar]
- 8. Erbel R, Di MC, Bartunek J, Bonnier J, de BB, Eberli FR, Erne P, Haude M, Heublein B, Horrigan M, Ilsley C, Bose D, Koolen J, Luscher TF, Weissman N, Waksman R. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet 2007;369:1869–1875. [DOI] [PubMed] [Google Scholar]
- 9. Windecker S, Haude M, Neumann FJ, Stangl K, Witzenbichler B, Slagboom T, Sabate M, Goicolea J, Barragan P, Cook S, Piot C, Richardt G, Merkely B, Schneider H, Bilger J, Erne P, Waksman R, Zaugg S, Juni P, Lefevre T. Comparison of a novel biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: results of the randomized BIOFLOW-II trial. Circ Cardiovasc Interv 2015;8:e001441. [DOI] [PubMed] [Google Scholar]
- 10. Pilgrim T, Heg D, Roffi M, Tuller D, Muller O, Vuilliomenet A, Cook S, Weilenmann D, Kaiser C, Jamshidi P, Fahrni T, Moschovitis A, Noble S, Eberli FR, Wenaweser P, Juni P, Windecker S. Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): a randomised, single-blind, non-inferiority trial. Lancet 2014;384:2111–2122. [DOI] [PubMed] [Google Scholar]
- 11. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 12. Moussa ID, Klein LW, Shah B, Mehran R, Mack MJ, Brilakis ES, Reilly JP, Zoghbi G, Holper E, Stone GW. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol 2013;62:1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Byrne RA, Serruys PW, Baumbach A, Escaned J, Fajadet J, James S, Joner M, Oktay S, Juni P, Kastrati A, Sianos G, Stefanini GG, Wijns W, Windecker S. Report of a European Society of Cardiology-European Association of Percutaneous Cardiovascular Interventions task force on the evaluation of coronary stents in Europe: executive summary. Eur Heart J 2015;36:2608–2620. [DOI] [PubMed] [Google Scholar]
- 14. Moreno R, Fernandez C, Sanchez-Recalde A, Galeote G, Calvo L, Alfonso F, Hernandez R, Sanchez-Aquino R, Angiolillo DJ, Villarreal S, Macaya C, Lopez-Sendon JL. Clinical impact of in-stent late loss after drug-eluting coronary stent implantation. Eur Heart J 2007;28:1583–1591. [DOI] [PubMed] [Google Scholar]
- 15. Ellis SG, Popma JJ, Lasala JM, Koglin JJ, Cox DA, Hermiller J, O'shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, Stone GW. Relationship between angiographic late loss and target lesion revascularization after coronary stent implantation: analysis from the TAXUS-IV trial. J Am Coll Cardiol 2005;45:1193–1200. [DOI] [PubMed] [Google Scholar]
- 16. Mauri L, Orav EJ, Kuntz RE. Late loss in lumen diameter and binary restenosis for drug-eluting stent comparison. Circulation 2005;111:3435–3442. [DOI] [PubMed] [Google Scholar]
- 17. Pocock SJ, Lansky AJ, Mehran R, Popma JJ, Fahy MP, Na Y, Dangas G, Moses JW, Pucelikova T, Kandzari DE, Ellis SG, Leon MB, Stone GW. Angiographic surrogate end points in drug-eluting stent trials: a systematic evaluation based on individual patient data from 11 randomized, controlled trials. J Am Coll Cardiol 2008;51:23–32. [DOI] [PubMed] [Google Scholar]
- 18. Lipinski MJ, Escarcega RO, Baker NC, Benn HA, Gaglia MA Jr., Torguson R, Waksman R. Scaffold thrombosis after percutaneous coronary intervention with ABSORB bioresorbable vascular scaffold: a systematic review and meta-analysis. JACC Cardiovasc Interv 2016;9:12–24. [DOI] [PubMed] [Google Scholar]
- 19. Raber L, Brugaletta S, Yamaji K, O'Sullivan CJ, Otsuki S, Koppara T, Taniwaki M, Onuma Y, Freixa X, Eberli FR, Serruys PW, Joner M, Sabate M, Windecker S. Very late scaffold thrombosis: intracoronary imaging and histopathological and spectroscopic findings. J Am Coll Cardiol 2015;66:1901–1914. [DOI] [PubMed] [Google Scholar]
- 20. Moravej M, Mantovani D. Biodegradable metals for cardiovascular stent application: interests and new opportunities. Int J Mol Sci 2011;12:4250–4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robaei D, Back L, Ooi SY, Pitney M, Jepson N. Twelve-month outcomes with a bioresorbable everolimus-eluting scaffold: results of the ESHC-BVS registry at two Australian centers. J Invasive Cardiol 2015; pii:JIC20151115-2. [PubMed] [Google Scholar]
- 22. Wittchow E, Adden N, Riedmuller J, Savard C, Waksman R, Braune M. Bioresorbable drug-eluting magnesium-alloy scaffold: design and feasibility in a porcine coronary model. EuroIntervention 2013;8:1441–1450. [DOI] [PubMed] [Google Scholar]
- 23. Zheng YF, Gu XN, Witte F. Biodegradable metals. Mater Sci Eng R 2014;77:1–34. [Google Scholar]
- 24. Haude M. Safety and performance of the Drug-Eluting Absorbable Metal Scaffold (DREAMS) in patients with de-novo coronary lesions: 3-year results of the prospective, multi-centre, first-in-man BIOSOLVE-I trial. EuroIntervention 2016; 10.4244/EIJY16M06_01. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.