Abstract
The expression of immunogenic markers after differentiation of umbilical cord blood (UCB)-derived mesenchymal stem cells (MSC) has been poorly investigated and requires extensive in vitro and in vivo testing for clinical application. The expression of human leukocyte antigen (HLA) classes on UCB-derived MSC was tested by Fluorescence-activated cell sorting analysis and immunocytochemical staining. The undifferentiated MSC were moderately positive for HLA-ABC, but almost completely negative for HLA-DR. The MSC differentiated to chondrocytes expressed neither HLA-ABC nor HLA-DR. The proliferation of MSC was not significantly affected by the allogeneic lymphocytes stimulated with concanavalin A. The responder lymphocytes showed no significant decrease in proliferation in the presence of the MSC, but the apoptosis rate of the lymphocytes was increased in the presence of MSC. Taken together, these findings indicate that UCB-derived MSC differentiated to chondrocytes expressed less HLA class I and no class II antigens. The MSC showed an immunomodulatory effect on the proliferation and apoptosis of allogeneic lymphocytes. These data suggest that the differentiated and undifferentiated allogeneic MSC derived from umbilical cord blood can be a useful candidate for allogeneic cell therapy and transplantation without a major risk of rejection.
Keywords: apoptosis, human leukocyte antigen, human umbilical cord blood, immunophenotypes, mesenchymal stem cells
Introduction
Mesenchymal stem cells (MSC) can be isolated from a variety of tissues including bone marrow, skin, adipose tissue, skeletal muscle, liver, fetal tissues, amniotic fluid, placenta, peripheral blood and umbilical cord blood. MSC also have a capacity to expand and differentiate into tissues of three germ layers, in vivo and in vitro. These cells have various beneficial characteristics, such as self-renewal, multipotential differentiation, easy isolation, plastic-adherence under standard culture condition and high expansive potential [2,26,28]. Besides these beneficial characteristics of multipotency, the immunogenicity and immunomodulatory ability of MSC before and after differentiation has raised a great deal of interest [5,8,39]. Moreover, their immunosuppressive potential on lymphocytes has been investigated [16].
MSC from bone marrow have been widely employed as the main source in various experimental and clinical studies. However, the use of bone marrow-derived cells is not always acceptable owing to the high degree of viral exposure and the significant decrease in cell number and the proliferative/differentiation capacity along with age [25]. In addition, their extraction from bone marrow requires a painful, invasive procedure.
Therefore, umbilical cord blood (UCB) has often been used as an alternative source of hematopoietic stem cells and mesenchymal stem cells. Human UCB-derived MSC also have a practical advantage over bone marrow-derived MSC in that they are obtained by non-invasive methods that do not harm either the mother or the infant. Furthermore, the cord blood stem cells are more immature than adult MSC, expand readily in vitro [15] and have potent differentiation potential [41].
One of the most important factors regarding the clinical use of the MSC in cell therapies and transplantation is their immune reaction within the host. The low immunogenicity of MSC is advantageous for clinical applications. Although the expansion and differentiation potentials of UCB-derived MSC have already been well characterized, the immunological properties have not been as thoroughly investigated as bone marrow-derived MSC.
It has been suggested that UCB-derived MSC expanded in vitro retained the characteristics of their low immunogenicity and immunomodulatory effects [27,40]. The autologous, allogeneic and xenogenic transplantation of MSC from adult tissues and umbilical cord blood has also been shown to exhibit considerable therapeutic potential in spinal cord injury [13,23,24,35], and in an animal model, the immunomodulation potential of MSC was found to prolong skin graft survival in vitro [2]. The MSC derived from bone marrow were shown to be poorly immunogenic and suppress allogeneic or autologous T-cell responses [6,19,22,38].
These specific characteristics of MSC make them an attractive tool for cell therapies, tissue engineering, regenerative medicine and transplantation of tissues and solid organs [3,30]. However, the mechanism underlying the MSC-mediated inhibition of T-lymphocyte proliferation has not been fully elucidated to date.
Therefore, this study was conducted to investigate the immunological properties of the UCB-derived MSC to assess their potential usefulness in allogeneic transplantation and cellular therapies. We examined the expression pattern of HLA class I and II antigens on the UCB-derived MSC before and after differentiation to chondrocytes. Furthermore, the effects of immunologic reactivity of MSC with allogeneic lymphocytes on proliferation and apoptosis were tested in vitro.
Materials and Methods
Isolation, cultivation and identification of MSC from umbilical cord blood
UCB samples were obtained during Cesarean section after receiving informed consent from patients. This study was approved by the Institutional Review Board at Seoul National University Hospital and Gyeongsang National University Hospital. Blood was collected into 250 mL standard blood collection bags (Green Cross, Korea) that contained citrate-phosphate dextrose anticoagulant.
The isolation and cultivation of MSC from UCB were performed as previously described [18]. Briefly, low-density mononuclear cells (MNC) were isolated using Ficoll-Paque Plus (< 1.077 g/mL; Amersham Biosciences, Sweden). The cells were then resuspended in Dulbeco's Modified Eagle low glucose medium (Gibco-BRL, USA) that was supplemented with 10% fetal bovine serum (FBS; Gibco-BRL) and antibiotics. The total numbers of nucleated and viable cells were determined by vital staining using tryphan blue stain.
Cells were cultured in low glucose Dulbeco's Modified Eagle Media (DMEM) with 10% FBS, 2 mmol/L L-glutamine (Gibco-BRL) and 0.3% penicillin-streptomycin (Gibco-BRL) under 5% CO2 at 37℃.
For identification and characterization of the cultured MSC, periodic acid-Schiff (PAS) treatment for glycogen in hUCB-MSC and FACS analysis using FACS Calibur (BD Biosciences, USA) were performed as previously described [18]. Mouse monoclonal antibodies were conjugated to the fluorescein isothiocyanate (FITC; BD Biosciences).
Following immunophenotyping of surface antigens of MSC, cells positively expressing the surface markers of CD29, CD44, CD73, CD90, and CD105 and negatively expressing the surface markers of CD14, CD31, CD34, CD45, and CD133 were used for further experiments.
Induction and identification of chondrogenic differentiation
Chondrogenic differentiation of MSC was induced as previously described [18]. Briefly, the third- to fourth-passage MSC were transferred into 60 mm dishes or 15 mL polypropylene tubes at a concentration of 5 × 105 cells/mL. The cells in the tubes were centrifuged at 400 × g for 5 min to form a pelleted micromass at the bottom of the tube, then treated with a chondrogenic medium (Cambrex Bio Science MycoAlert; BMG LABTECH, USA). The chondrogenic medium consists of differentiation basal medium supplemented with dexamethasone, ascorbate, ITS+supplement, sodium pyruvate, proline, penicillin/streptomycin, L-glutamine and TGF-β3. Media were changed twice weekly for 3 to 4 weeks and chondrogenesis was assessed at intervals of one week.
For histological assessment of chondrogenesis, the Safranin-O staining for proteoglycan and immunocytochemical assay of collagen type-II were performed. For Safranin-O staining, the cells were collected and fixed in 4% paraformaldehyde and embedded in paraffin. Embedded blocks were sectioned into 5 µm slices. After deparaffinization, the specimens were stained with 0.1% aqueous Safranin-O solution for 1 min. The stained specimens were washed in distilled water and dehydrated, after which they were coverslipped for observation under light microscopy.
For the immunocytochemical assay of chondrogenesis, dried specimens were fixed in formalin-ethanol fixative solution for 1 min, then rinsed with dH2O. Following three washes with distilled water, 1% normal goat serum was applied for 1 h at room temperature. Polyclonal antibody to human-collagen type II (Millipore, USA), diluted 1 : 20 in goat serum, was reacted overnight at 4℃. After washing in PBS, FITC-conjugated anti-rabbit IgG (Santa Cruz Biotechnology, USA) was applied for 2 h.
Fluorescence-activated cell sorting (FACS) analysis
Undifferentiated MSC in passage 3 to 5 and MSC differentiated along chondrogenic lineages were analyzed for the expression of surface-bound HLA class I and II with monoclonal mouse anti-human HLA-ABC and HLA-DR antibodies using FACS Calibur (BD Biosciences). The mouse monoclonal antibodies were conjugated to the FITC (BD Biosciences). The peripheral lymphocytes served as a positive control.
Immunocytochemical staining for HLA expression
The undifferentiated and differentiated MSC cultured on cover slips in the dish were washed with PBS to remove medium, then fixed with 4% paraformaldehyde in PBS for 20 min. Following treatment of the cells and lymphocytes with 0.5% triton X-100 for 5 min, cells were washed three times with PBS and then unspecific epitopes were blocked by incubation with 5% goat serum in PBS for 2 h at room temperature. The primary antibodies against HLA class I and II were goat monoclonal anti-HLA-ABC or anti-HLA-DR antibodies (Santa Cruz Biotechnology). The secondary antibodies against HLA class I and II were FITC-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology). Specimens were incubated with primary antibody at 4℃ overnight. After washing in PBS, specimens were incubated with secondary antibody for 2 h at room temperature. Nuclei were then counterstained with 4', 6-diamidino-2-phenylindol (1 : 2,500; Sigma, USA) for 10 min.
Mixed lymphocyte culture and apoptosis assay
Allogeneic peripheral blood was collected from healthy adults. After dilution of the blood with D-PBS solution, peripheral blood lymphocytes were prepared by Ficoll-Paque density gradient centrifugation and suspended in RPMI-1640 medium (Gibco/Invitrogen, USA) supplemented with 20 mmol/L HEPES, 100 U/mL penicillin, 100 µg/mL streptomycin, 20 mmol/L L-glutamine and 10% FBS. For activation, lymphocytes were pretreated for three days by addition of 5 µg/mL concanavalin A (Con A; Sigma) in the medium.
To determine the effects of allogeneic reactivity of MSC with activated lymphocytes, the third-passage MSC at a density of 1 × 105 cells/mL and pooled allogeneic peripheral blood lymphocytes at a density of 1 to 5 × 105 cells/mL were used for co-cultivation in RPMI-1640 medium (Gibco/Invitrogen) supplemented with 20 mmol/L HEPES, 100 U/mL penicillin, 100 µg/mL streptomycin, 20 mmol/L L-glutamine and 10% FBS under 5% CO2 at 37℃ for 3 days. For negative control of experiments, the wells were set for the MSC only group and the non-Con A treated lymphocyte group. After three days, cells were collected and their proliferation activity and viability were measured.
To determine the apoptosis of the MSC and activated lymphocytes, cells were suspended at a concentration of 1.0 × 105/mL to 5.0 × 105/mL in the RPMI-1640 medium. Following co-culture for 3 days they were collected and washed in PBS. The cells were stained with an FITC Annexin-V kit (BD Biosciences) according to the manual and their apoptosis was analyzed using a FACS Calibur cell analyzer (BD Biosciences).
Statistical analysis
One-way ANOVA was conducted to identify significant differences among groups using SPSS Statistics 21 (IBM, USA). Differences were considered statistically significant at a p < 0.05.
Results
Expression of HLA antigens on undifferentiated and differentiated MSC
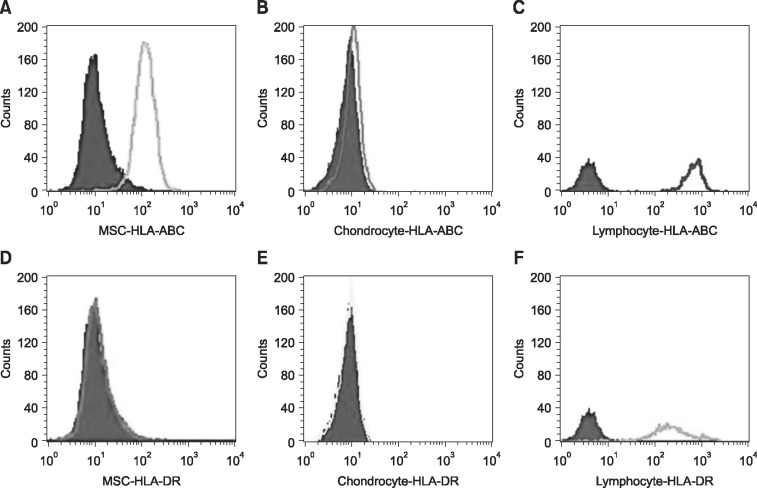
Immunophenotypes of undifferentiated MSC, chondrocytes differentiated from the MSC and peripheral allogeneic lymphocytes as a positive control were determined by FACS analysis. Evaluation of the expression of HLA-ABC antigen revealed that MSC (panel A in Fig. 1) showed moderate expression, chondrocytes (panel B in Fig. 1) showed negative expression, and lymphocytes (panel C in Fig. 1) showed stronger expression. Evaluation of the expression of HLA-DR antigen revealed that MSC (panel D in Fig. 1) and chondrocytes (panel E in Fig. 1) showed negative expression, but lymphocytes (panel F in Fig. 1) showed moderate expression.
Fig. 1. FACS analysis of immunophenotypes of human leukocyte antigen (HLA)-ABC and HLA-DR on the undifferentiated mesenchymal stem cells (MSC) (A and D), chondrocytes differentiated from the MSC (B and E) and allogeneic peripheral lymphocytes (C and F).
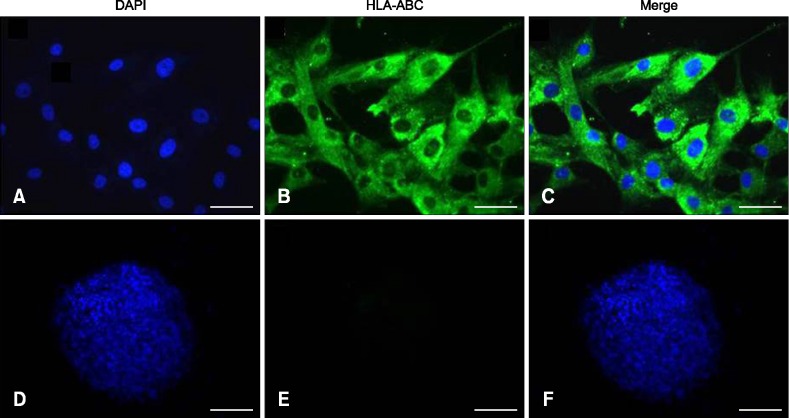
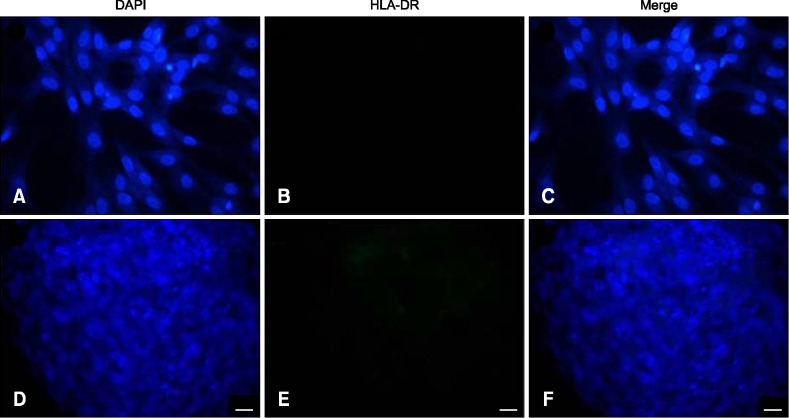
When the immunophenotypes of undifferentiated and differentiated MSC were determined by immunocytochemistry, the undifferentiated MSC (panels A-C in Fig. 2) showed moderate expression of HLA-ABC antigen compared to chondrocytes (panels D-F in Fig. 2). Evaluation of the expression of HLA-DR antigen revealed negative expression by MSC (panels A-C in Fig. 3) and chondrocytes (panels D-F in Fig. 3).
Fig. 2. Expression of human HLA-ABC on the surface of human undifferentiated (A–C) and differentiated (D–F) MSC by immunocytochemical stain (green color). The nuclei of the MSC were counterstained with DAPI (blue color). (A and D) DAPI stain. (B and E) FITC stain. (C and F) Merged. 200× (A–C), 40× (D–F). Sale bars = 20 µm (A–C), 100 µm (D–F).
Fig. 3. Expression of human HLA-DR on the surface of human undifferentiated (A–C) and differentiated (D–F) MSC by immunocytochemical stain (green color). The nuclei of the MSC were counterstained with DAPI (blue color). The HLA-DR antigen was negatively expressed on the differentiated and undifferentiated MSC. (A and D) DAPI stain. (B and E) FITC stain. (C and F) Merged. 200× (A–F). Scale bars = 20 µm.
Effect of allogeneic reactivity on proliferation of MSC with activated peripheral lymphocytes
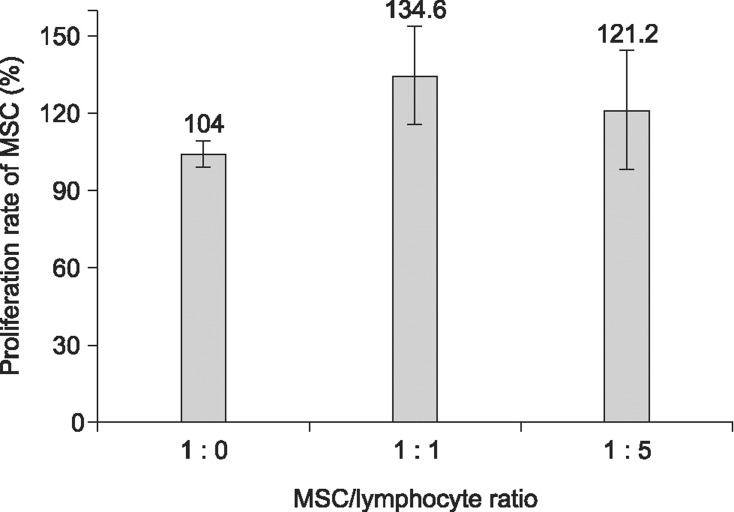
To determine if the proliferation of MSC was affected by allogeneic lymphocytes, MSC were co-cultured with Con A-activated allogeneic lymphocytes at a ratio of 1 : 1 to 1 : 5 in vitro for three days. As shown in Fig. 4, the proliferation rate of MSC at a ratio of 1 : 1 to 1 : 5 was increased to 134.6 ± 18.9% and 120.0 ± 23.2% relative to the control (104.0 ± 5.1%), respectively. The MSC did not show any significant interference with proliferation by the allogeneic Con A-activated lymphocytes (Fig. 4), and apoptosis analysis by flow cytometry did not show any induction of apoptosis in MSC by allogeneic lymphocytes (Fig. 5).
Fig. 4. Proliferation of UCB-derived MSC when co-cultured with or without allogeneic lymphocytes at a ratio of 1 : 0 and 1 : 5 in vitro (n = 5). The MSC and Con A-activated lymphocytes were co-cultured in RPMI-1640 medium with 10% FBS, L-glutamine and antibiotics in a CO2 incubator at 37℃ for 3 days. There was no significant (p = 0.05) difference between groups. Allogeneic lymphocytes were pre-activated with 5 µg/mL of Con A for 3 days.
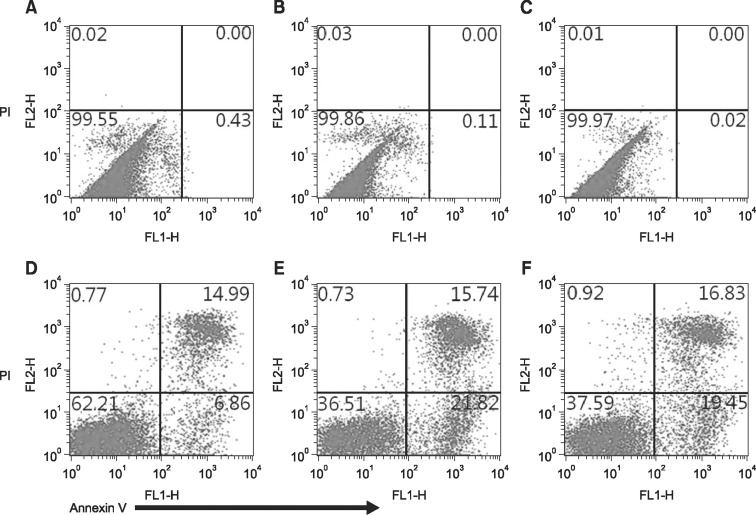
Fig. 5. Effect of MSC on apoptosis of allogeneic lymphocytes following co-culture at ratios of 1 : 1 and 1 : 5 for 3 days. The test was conducted by Annexin-V and PI double staining and analyzed by flow cytometry. Apoptosis of MSC gated on size (A–C) and apoptosis of lymphocytes gated on size (D–F) were analyzed following MSC and activated lymphocytes were cultured alone (A and D) or cocultured at a ratio of 1 : 1 (B and E) and 1 : 5 (C and F).
Growth inhibitory effect of MSC on proliferation of allogeneic lymphocytes
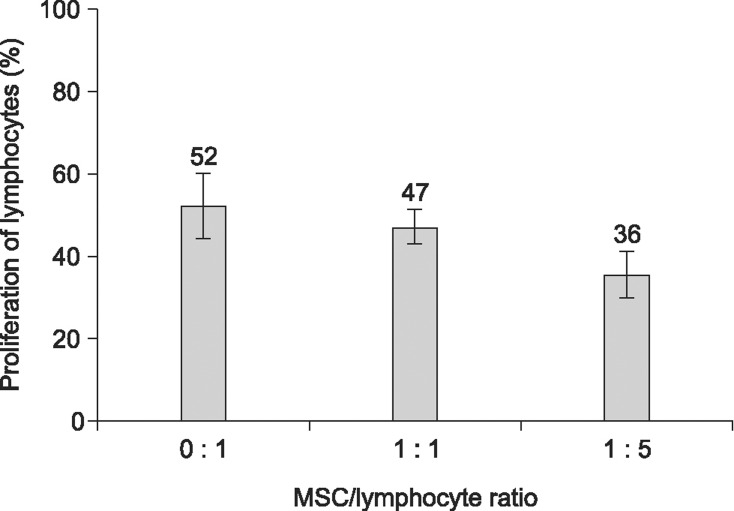
To determine if MSC could affect the proliferation of allogeneic lymphocytes, MSC were co-cultured with Con A activated allogeneic lymphocytes at ratios of 1 : 1 to 1 : 5 in vitro for three days. As shown in Fig. 6, at a MSC/lymphocyte ratio of 1 : 1 and 1 : 5, the proliferation ratio of activated lymphocytes (47.20 ± 4.21% and 35.60 ± 5.80) was not significantly (p > 0.05) decreased relative to the lymphocytes only group (52.20 ± 7.88).
Fig. 6. Proliferation of allogeneic lymphocytes when co-cultured with UCB-derived MSC at ratios of 0 : 1, 1 : 1 and 1 : 5 in vitro (n = 5). The Con A-activated allogeneic lymphocytes were cultured with or without MSC at ratios of 0 : 1, 1 : 1 and 1 : 5 in a RPMI-1640 medium with 10% FBS, L-glutamine and antibiotics in a CO2 incubator at 37℃ for 3 days. There was no significant (p > 0.05) inhibition of the proliferation of lymphocytes when they were co-cultured with MSC.
Effect of MSC on apoptosis of allogeneic lymphocytes
We found that MSC from umbilical cord blood could significantly affect apoptosis of allogeneic lymphocytes through co-culture of the two cell types for 3 days (Fig. 5). During the in vitro culture of Con A-stimulated allogeneic lymphocytes alone, 6.86% showed early apoptosis. When lymphocytes were co-cultured with MSC at ratios of 1 : 1 and 1 : 5, the percentage of early apoptotic lymphocytes increased to 21.82% and 19.45%, respectively, whereas apoptosis of MSC was not induced. These results suggest that the early apoptosis of activation-induced lymphocytes appeared to be enhanced by the effect of UCB-derived MSC.
Discussion
MSC are known to be capable of renewing themselves and to undergo multilineage differentiation into tissues of all three germ layers. In addition to these beneficial characteristics, MSC are known to have immunomodulation capacity. In recent years, MSC have been used in various preclinical and clinical studies, including investigations of their use for attenuation of graft-versus-host disease [33], osteogenesis imperfecta [12], intracoronary transplantation in patients with acute myocardial infarction [4], and support of haematopoiesis [20].
MSC can be isolated from umbilical cord blood, fetal tissues and placenta in new born babies, and from adult tissues of bone marrow, fat, liver, lung and spleen. Among these, cord blood may be an ideal source because of its accessibility, lack of risk to the mother or infant and ethical barriers, as well as the fact that it is a painless procedure to donors, promising source for autologous cell therapy and has a lower risk of viral contamination [7,34].
In this experiment, the morphology of hUCB-derived mesenchymal progenitor cells showed spindle-shaped fibroblastoid appearance and plastic-adherence. Moreover, UCB-derived MSC positively expressing the MSC-specific surface markers of CD29, CD44, CD90 and CD105 were used successfully [18].
Moreover, we confirmed whether these cells have MSC specific characters and if they can differentiate into chondrocytes during in vitro development under appropriate culture conditions. Our results showed that culture of human MSCs in chondrogenic differentiation medium led to the expression of type II collagen, one of the chondrocyte specific markers. Furthermore, chondrocytes were stained positively with Safranin-O dye and immunofluorescence stain of type II collagen protein.
Immunophenotypes of undifferentiated and differentiated MSC were determined by flow cytometric analysis and immunocyometric staining. Evaluation of the HLA-ABC antigen revealed moderate expression for undifferentiated MSC, but MSC that had differentiated to chondrocytes showed negligible expression. Upon evaluation of HLA-DR antigen, undifferentiated and differentiated MSC showed negative expression, but lymphocytes showed higher expression after Con A activation (Fig. 1).
Le Blanc et al. [22] and Klyushenenkova et al. [17] demonstrated that undifferentiated MSC derived from adult bone marrow expressed intermediate levels of HLA class I antigen on the surface by flow cytometry, but negligibly low levels of class II antigen were expressed on the surface, even though HLA class II antigen could be demonstrated intracellularly by Western blot analysis of whole-cell lysates. MSC from umbilical cord blood [27,40] and adipose tissues [37] were also found to express an intermediate level of HLA-ABC and major histocompatibility complex (MHC) class I molecules, with negligible levels of HLA-DR.
Previous studies have shown that MSC from other tissues; namely, bone marrow [1,21,38], adipose tissue [31] and fetal lung [9], also inhibit lymphocyte proliferation and inflammatory cytokine production. Yen et al. [42] demonstrated that human embryonic stem cell-derived mesenchymal progenitor cells also possessed strong immunosuppresive properties toward lymphocytes. In particular, it was suggested that UCB-derived MSC had the characteristics of low immunogenicity and suppression of alloreactive T cell responses [27,40].
While the immunosuppressive properties of MSC have been extensively investigated, the mechanism of suppression remains to be clarified for further clinical applications. In this experiment, the UCB-derived MSC suppressed the alloreactive proliferation of lymphocytes at a ratio of 1 : 1 to 1 : 5. These results were expected since several studies had suggested that the bone marrow- and adipose tissue-derived MSCs not only suppressed alloreactive T lymphocytes, but also greatly reduced the proliferation of lymphocytes activated by the potent T-lymphocyte mitogens PHA, Con A or SpA [22,31,43]. While UCB-derived MSC were cultured with activated lymphocytes, their proliferation was not significantly affected by allogeneic lymphocytes at ratios up to 1 : 5.
Previous studies have suggested that MSC derived from bone marrow could inhibit T-cell proliferation by inducing apoptosis of activated T cells [8,11,29]. Interestingly, apoptosis of lymphocytes was induced in a co-culture system with UCB-derived MSC. However, MSC themselves showed no apoptosis in the co-culture system. Although the described mechanisms of MSC-mediated T cell suppression are rather complex, a number of secreted factors have been implicated, including transforming growth factor-β, hepatocyte growth factor, prostaglandin E2, FasL, interleukin 10, indoleamine 2,3 dioxygenase, interferon-γ, and agalectins [8,10,14,29,32,36].
We were able to not only confirm the results of these studies, but also demonstrate that the UCB-derived MSC exhibit immunomodulatory effects and induction of apoptosis on mitogen-activated allogeneic lymphocytes. Thus, the UCB-derived MSC could serve as a potential alternative source of MSC for allogeneic transplantation in the future. Further investigations of appropriate in vivo models are required to evaluate the clinical relevance of MSC-mediated lymphocyte suppression.
This study was conducted to investigate the immunological properties of MSC derived from umbilical cord blood to assess their potential usefulness in allogeneic transplantation and cellular therapies. We examined the expression of class I and II HLA in undifferentiated and differentiated MSC. Furthermore, the effects of immunologic reactivity of UCB-derived MSC with allogeneic lymphocytes on proliferation and induction of apoptosis were tested in vitro.
Immunophenotypes of undifferentiated MSC, chondrocytes differentiated from the MSC and peripheral allogeneic lymphocytes for positive control were determined by flow cytometric analysis. Flow cytometric analysis and immunocytochemical staining for HLA-ABC antigen revealed that the undifferentiated MSC showed moderate expression, MSC differentiated to chondrocytes showed negative expression, and lymphocytes showed stronger expression. Upon expression of HLA-DR antigen, the undifferentiated MSC and MSC differentiated to chondrocytes showed negative expression, but lymphocytes showed stronger expression in both analyses.
When MSC were co-cultured with ConA-activated allogeneic lymphocytes at ratios of 1 : 1 to 1 : 5 in vitro for three days, the proliferation of the MSC themselves was not affected by Con A-activated lymphocytes at ratios up to 1 : 5, and the growth inhibitory effect of MSC on proliferation of allogeneic lymphocytes was not significant. Apoptosis tests revealed that UCB-derived MSC could significantly enhance the apoptosis of Con A-activated allogeneic lymphocytes, thus inhibiting the proliferation of allogeneic lymphocytes by inducing apoptosis.
These results demonstrate that MSC derived from UCB, as well as other tissues, could also suppress the allogeneic response of lymphocytes and serve as a useful source for cell therapies and allogeneic stem cell transplantation between HLA-incompatible recipients. However, further investigations are warranted to determine the mechanism of immunomodulation activity and develop appropriate in vivo models to determine their full clinical applicability.
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2008-313-E00644).
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 2.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 3.Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci. 2009;1176:101–117. doi: 10.1111/j.1749-6632.2009.04607.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen SL, Fang WW, Qian J, Ye F, Liu YH, Shan SJ, Zhang JJ, Lin S, Liao LM, Zhao RC. Improvement of cardiac function after transplantation of autologous bone marrow mesenchymal stem cells in patients with acute myocardial infarction. Chin Med J (Engl) 2004;117:1443–1448. [PubMed] [Google Scholar]
- 5.Chen X, Armstrong MA, Li G. Mesenchymal stem cells in immunoregulation. Immunol Cell Biol. 2006;84:413–421. doi: 10.1111/j.1440-1711.2006.01458.x. [DOI] [PubMed] [Google Scholar]
- 6.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 7.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 8.Gao K, Chen Y, Wei L, Li S, Jin X, Cong C, Yuan Y, Long D, Li Y, Cheng J, Lu Y. Inhibitory effect of mesenchymal stem cells on lymphocyte proliferation. Cell Biochem Funct. 2008;26:900–907. doi: 10.1002/cbf.1523. [DOI] [PubMed] [Google Scholar]
- 9.Götherström C, Ringdén O, Tammik C, Zetterberg E, Westgren M, Le Blanc K. Immunologic properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol. 2004;190:239–245. doi: 10.1016/j.ajog.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Groh ME, Maitra B, Szekely E, Koç ON. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol. 2005;33:928–934. doi: 10.1016/j.exphem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Han Q, Sun Z, Liu L, Chen B, Cao Y, Li K, Zhao RC. Impairment in immuno-modulatory function of Flk1+CD31-CD34- MSCs from MDS-RA patients. Leuk Res. 2007;31:1469–1478. doi: 10.1016/j.leukres.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WWK, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 13.Jung DI, Ha J, Kang BT, Kim JW, Quan FS, Lee JH, Woo EJ, Park HM. A comparison of autologous and allogenic bone marrow-derived mesenchymal stem cell transplantation in canine spinal cord injury. J Neurol Sci. 2009;285:67–77. doi: 10.1016/j.jns.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 14.Keating A. How do mesenchymal stromal cells suppress T cells? Cell Stem Cell. 2008;2:106–108. doi: 10.1016/j.stem.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 16.Keyser KA, Beagles KE, Kiem HP. Comparison of mesenchymal stem cells from different tissues to suppress T-cell activation. Cell Transplant. 2007;16:555–562. doi: 10.3727/000000007783464939. [DOI] [PubMed] [Google Scholar]
- 17.Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 18.Koh PO, Cho JH, Nho KH, Cha YI, Kim YK, Cho EH, Lee HC, Jung TS, Yeon SC, Kang KS, Lee HJ. Chondrogenesis of mesenchymal stem cells derived from human umbilical cord blood. J Vet Clin. 2009;26:528–533. [Google Scholar]
- 19.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 20.Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, Shpall EJ, McCarthy P, Atkinson K, Cooper BW, Gerson SL, Laughlin MJ, Loberiza FR, Jr, Moseley AB, Bacigalupo A. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11:389–398. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 22.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Chang HS, Kang EH, Chung DJ, Choi CB, Lee JH, Hwang SH, Han H, Kim HY. Percutaneous transplantation of human umbilical cord blood-derived multipotent stem cells in a canine model of spinal cord injury. J Neurosurg Spine. 2009;11:749–757. doi: 10.3171/2009.6.SPINE08710. [DOI] [PubMed] [Google Scholar]
- 24.Lim JH, Byeon YE, Ryu HH, Jeong YH, Lee YW, Kim WH, Kang KS, Kweon OK. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J Vet Sci. 2007;8:275–282. doi: 10.4142/jvs.2007.8.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82:583–590. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- 26.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 27.Oh W, Kim DS, Yang YS, Lee JK. Immunological properties of umbilical cord blood-derived mesenchymal stromal cells. Cell Immunol. 2008;251:116–123. doi: 10.1016/j.cellimm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 29.Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005;19:1597–1604. doi: 10.1038/sj.leu.2403871. [DOI] [PubMed] [Google Scholar]
- 30.Prigozhina TB, Khitrin S, Elkin G, Eizik O, Morecki S, Slavin S. Mesenchymal stromal cells lose their immunosuppressive potential after allotransplantation. Exp Hematol. 2008;36:1370–1376. doi: 10.1016/j.exphem.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 31.Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, Casteilla L, Blancher A. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 32.Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305:33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Ringdén O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lönnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, Omazic B, Aschan J, Barkholt L, Le Blanc K. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 34.Rubinstein P, Rosenfield RE, Adamson JW, Stevens CE. Stored placental blood for unrelated bone marrow reconstitution. Blood. 1993;81:1679–1690. [PubMed] [Google Scholar]
- 35.Ryu HH, Lim JH, Byeon YE, Park JR, Seo MS, Lee YW, Kim WH, Kang KS, Kweon OK. Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. J Vet Sci. 2009;10:273–284. doi: 10.4142/jvs.2009.10.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sioud M. New insights into mesenchymal stromal cell-mediated T-cell suppression through galectins. Scand J Immunol. 2011;73:79–84. doi: 10.1111/j.1365-3083.2010.02491.x. [DOI] [PubMed] [Google Scholar]
- 37.Technau A, Froelich K, Hagen R, Kleinsasser N. Adipose tissue-derived stem cells show both immunogenic and immunosuppressive properties after chondrogenic differentiation. Cytotherapy. 2011;13:310–317. doi: 10.3109/14653249.2010.504769. [DOI] [PubMed] [Google Scholar]
- 38.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 39.Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566–2573. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 40.Wang M, Yang Y, Yang D, Luo F, Liang W, Guo S, Xu J. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009;126:220–232. doi: 10.1111/j.1365-2567.2008.02891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang SE, Ha CW, Jung M, Jin HJ, Lee M, Song H, Choi S, Oh W, Yang YS. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy. 2004;6:476–486. doi: 10.1080/14653240410005041. [DOI] [PubMed] [Google Scholar]
- 42.Yen BL, Chang CJ, Liu KJ, Chen YC, Hu HI, Bai CH, Yen ML. Brief report—human embryonic stem cell-derived mesenchymal progenitors possess strong immunosuppressive effects toward natural killer cells as well as T lymphocytes. Stem cells. 2009;27:451–456. doi: 10.1634/stemcells.2008-0390. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y, Huang Z, Qi M, Lazzarini P, Mazzone T. Immune regulation of T lymphocyte by a newly characterized human umbilical cord blood stem cell. Immunol Lett. 2007;108:78–87. doi: 10.1016/j.imlet.2006.10.007. [DOI] [PubMed] [Google Scholar]