Abstract
Tick-borne rickettsial diseases (TBRD) are commonly encountered in medical and veterinary clinical settings. The control of these diseases is difficult, requiring disruption of a complex transmission chain involving a vertebrate host and ticks. The geographical distribution of the diseases is related to distribution of the vector, which is an indicator of risk for the population. A total of 1,107 ticks were collected by tick dragging from forests, ecotourism parks and hosts at 101 sites in 22 of the 32 states of Mexico. Collected ticks were placed in 1.5 mL cryovials containing 70% ethanol and were identified to species. Ticks were pooled according to location/host of collection, date of collection, sex, and stage of development. A total of 51 ticks were assayed by polymerase chain reaction (PCR) to confirm species identification using morphological methods. A total of 477 pools of ticks were assayed using PCR techniques for selected tick-borne pathogens. Anaplasma phagocytophilum was the most commonly detected pathogen (45 pools), followed by, Ehrlichia (E.) canis (42), Rickettsia (R.) rickettsii (11), E. chaffeensis (8), and R. amblyommii (1). Rhipicephalus sanguineus was the tick most frequently positive for selected pathogens. Overall, our results indicate that potential tick vectors positive for rickettsial pathogens are distributed throughout the area surveyed in Mexico.
Keywords: Anaplasma phagocytophilum, Ehrlichia canis, Ehrlichia chaffeensis, Rickettsia rickettsia, ticks
Introduction
Ticks transmit more pathogens than any other group of blood-feeding arthropods worldwide, affecting humans, livestock and domestic animals [12]. Ixodid ticks of the genera Rhipicephalus, Dermacentor, Ixodes, and Amblyomma are the most important vectors for Rickettsiaceae, including pathogens known to cause disease in humans [27,28].
Tick-borne diseases (TBD) caused by Anaplasma (A.) phagocytophilum, Rickettsia (R.) rickettsii, Ehrlichia (E.) chaffeensis, E. canis and other rickettsial pathogens affect humans and/or wild and domestic animals. These pathogens are maintained in natural cycles involving wild mammals and hard-ticks or domestic cycles [11]. The distribution and epidemiology of vector-borne diseases reflects the geographic distribution and seasonal activities of the vectors, reservoirs, animal care, and human behavior. Tick-borne diseases are commonly encountered in medical and veterinary clinical settings, and have increased in recent years, gaining more attention from physicians and veterinarians. Early signs and symptoms of these illnesses are notoriously nonspecific, mimicking benign viral illnesses, which often leads to misdiagnosis and treatment failure with unfortunate, and sometimes severe outcomes [9]. With the application of molecular methods, new species, strains, and genetic variants of microorganisms are being detected in ticks worldwide, and the list of potential tick-borne pathogens continues to grow [30]. The case fatality rate ranges from 4–25% in patients with rickettsioses, 3% in ehrlichioses and 1% in anaplasmoses if treatment is not received [39].
Screening ticks for disease-causing pathogens provides useful epidemiological information on their distribution and the prevalence of pathogens that pose veterinary and medical health risks [6]. This study was conducted to estimate the frequency of Ehrlichia, Anaplasma and R. rickettsii in ticks collected from forests, eco-tourist parks, and hosts, including humans, to determine the geographic distribution and potential exposure to humans and domestic animals in Mexico.
Materials and Methods
Sample collection
Ticks were collected from 1997–2013 from forests, eco-tourist parks, vegetation and wild animals in 22 states of Mexico. In the grass, we used a tick drag consisting of a 1.0 m wide × 1.5 m long white cotton cloth attached to a wooden dowel and a rope attached to each end of the dowel as described by Chong et al. [37]. Drags were pulled at a slow to moderate walking pace behind the collector over vegetation and ground cover. Additionally, ticks were collected from various species of live wild caught rodents and other small mammals by grasping the mouthparts with a fine tweezers and gently pulling them off the host, after which they were placed in 1.5 mL cryovials with 70% ethanol. Ticks were stored until identification and extraction of DNA in the Emerging Infectious Diseases Lab in the National Medical Center XXI Century. Identification was based on their morphological characteristics using relevant taxonomic keys [15,17,37]. A data form that included the collection site, date of collection, species, and stage of development was prepared for each collection.
DNA preparation
DNA from the tissues of ticks was prepared individually. Briefly, ticks were disinfected by immersion in 70% ethanol solution for five minutes, rinsed with sterile water, and then dried on filter paper. Adult ticks were dissected using an aseptic technique and a sterile scalpel to obtain intestinal tissue, while whole larvae and nymphs were used for DNA extraction. Pools were made by stage of development and species after identification. Overall, DNA from 68 individual adult ticks, as well as pools of adults (2 ticks, 286 pools), nymphs (3–4 ticks, 103 pools) and larvae (4–5 ticks, 20 pools) were analyzed. DNA extraction from ticks was performed with a DNeasy Tissue Kit (Qiagen, Germany) according to the manufacturer's instructions. The pathogens detected in the pools were expressed as the percentage and minimum infection rate based on the assumption that each polymerase chain reaction (PCR)-positive pool contained a least one positive tick [13].
PCR for tick identification
We selected 5% of the ticks (51 ticks) from small wild mammals and vegetation (7 adults, 32 nymphs, 12 larvae) for molecular identification using the 12S rRNA gene to verify the accuracy of the morphological identification and then stored species by stage of development. Specifically, larvae (4 pools), nymphs (11 pools), and adults (7) were stored in 1.5 mL tubes containing 70% ethanol until assay.
PCR for R. rickettsii, A. phagocytophilum, E. chaffeensis and E. canis: Tick DNA was amplified with species-specific primers as previously described. The 16S rRNA gene fragment was amplified from all the targeted genes, except R. rickettsii, for which the glta2 and 17kDa genes were amplified. PCR was performed as previously described [30,33] using primers Ge3A-Ge10-Ge9F for A. phagocytophilum (16S rRNA gene), HE1-HE3 for E. chaffeensis (16S rRNA gene), ECA-HE1 for E. canis (16S rRNA gene), CS78-CS323 (gltA gene) and Tz15-Tz16 [33] (17 kDa protein-encoding gene) for Rickettsia spp. Positive controls consisted of DNA form the spleens or hearts of Peromyscus leucopus naturally infected with E. chaffeensis and A. phagocytophilum, DNA from a dog infected with E. canis [30,32,33], and DNA from kidney tissue of a patient infected with R. rickettsii. PCR reaction mixtures consisted of 2 pmol of each primer, 200 µM concentration of each deoxynucleoside triphosphate, PCR buffer, 1 U of Taq DNA polymerase (Invitrogen, Brazil) and 50–100 ng of sample DNA for each PCR in a 50 µL mixture reaction.
The PCR products of expected sizes were purified using a QIAquick Gel Extraction Kit (Qiagen, USA), after which forward and reverse nucleotide sequences were determined using a DNA sequencer (Applied Biosystems, USA). Sequence data were collected using the Chromas Lite software (ver. 2.1.1; Technelysium, Australia), and sequences were aligned using MEGA 5.0 [35] and compared with genetic sequences using BLAST (National Center for Biotechnology Information, USA).
Analyses of results were performed using a chi squared or Fisher's exact test, and the odds ratio calculated with 95% confidence intervals. A p value < 0.05 was considered significant. All analyses were conducted using the Epi Info program ver. 6 (Centers for Disease Control and Prevention, USA).
Results
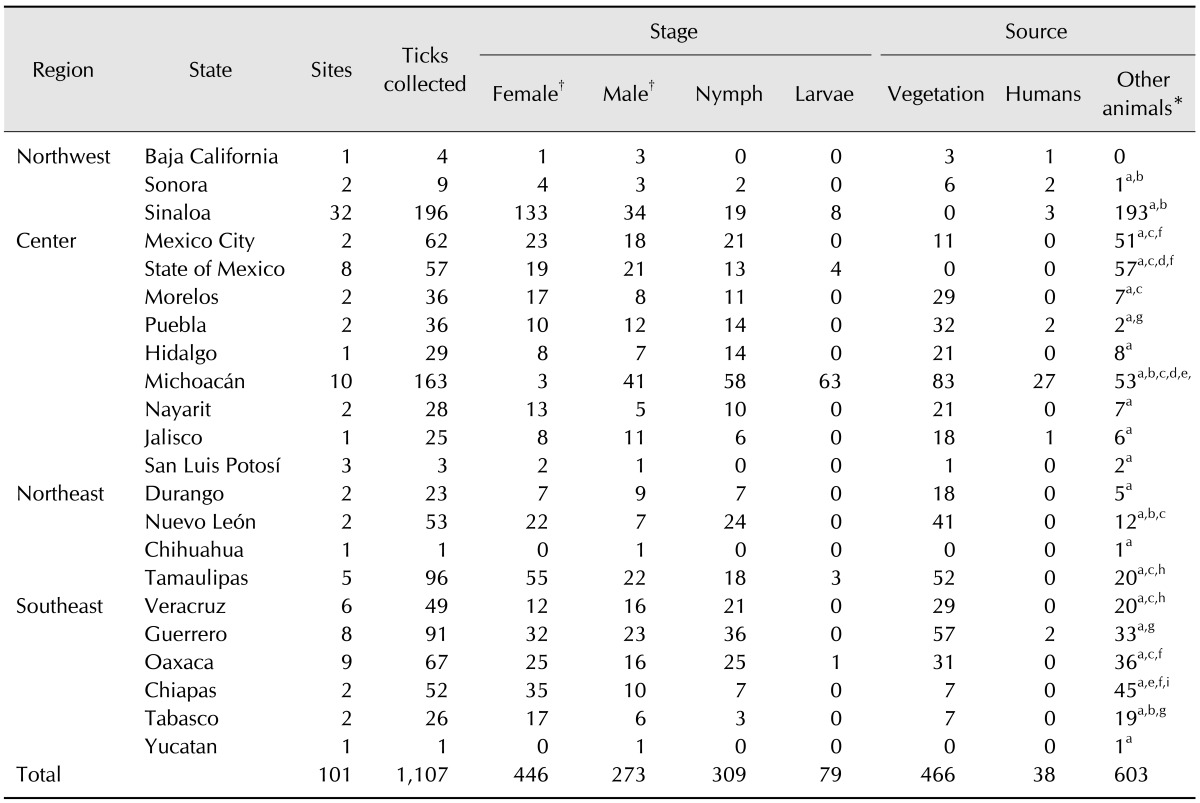
A total of 1,107 ticks were collected 641 ticks from host, while 466 ticks were collected from forests, grass and herbaceous vegetation. The ticks on hosts were collected from nine humans (38 ticks), 148 domestic dogs (262), three deer (8), 183 mice (229), one turtle (6), four sheep (5), two goats (3), 11 cattle (83), two toads (3), three horses (4), and one rabbit (2). The 101 sites include national parks, ecotourism parks, and recreational sites distributed in 22 states of Mexico (Table 1). A total of 1,107 ticks, including 16 species belonging to six genera were collected (Table 2). Rhipicephalus sanguineus was the most frequently collected (43.4%), followed by Amblyomma cajennense (12.2%), Amblyomma maculatum (10.9%), R. microplus (9.0%), Amblyomma dissimile (6.1%), Ixodes (I.) texanus (1.3%), R. annulatus (2.0%), Amblyomma americanum (1.5%), Dermacentor (D.) nitens (1.8%), I. scapularis (1.5%), D. andersoni (1.3%), and Haemaphysalis (H.) leporispalustris (1.3%), while the remaining species only accounted for 1.4% (Fig. 1).
Table 1. Sites and stages of development in ticks collected from Mexico.
*Type of host: a, human; b, dog; c, deer; d, mice; e, turtles; f, sheep; g, goats; h, cattle and i, horse. †Adults.
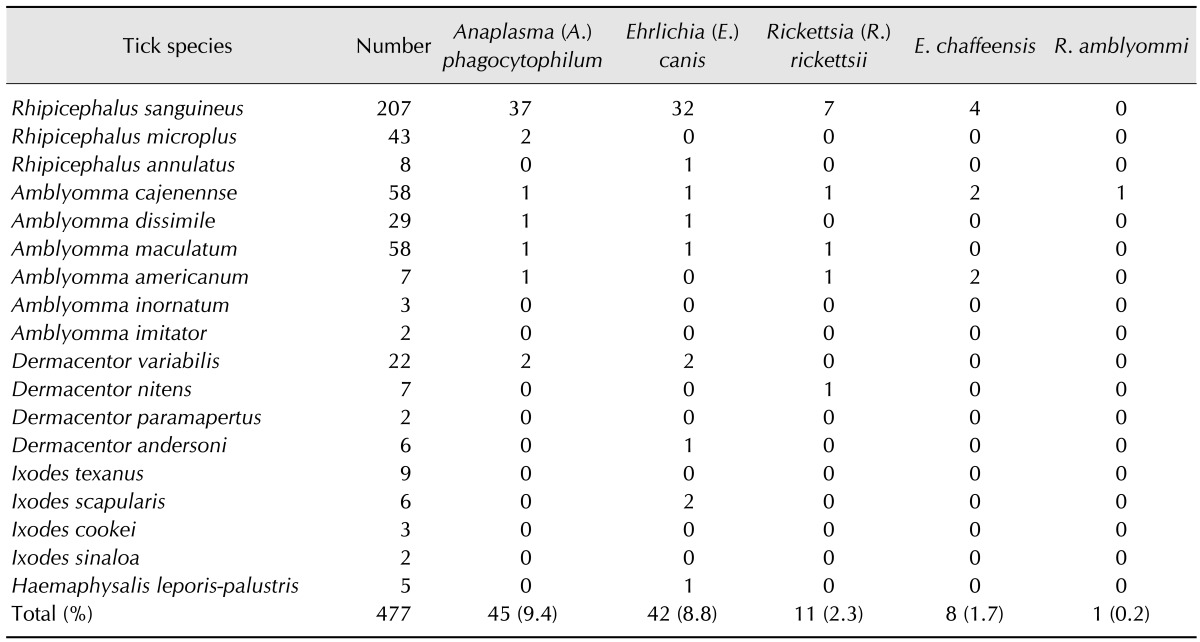
Table 2. Tick species, number of pools assayed by PCR for selected pathogens, and number of pools positive by PCR for selected pathogens.
Fig. 1. Distribution of selected tick species collected in Mexico.
Of the 38 ticks collected from humans, (6.3%) of Amblyomma cajenennse were positive for A. phagocytophilum and (10%) of D. variabilis were positive for R. rickettsii, while no R. sanguineus were positive for the selected tick-borne pathogens. A total of 466 ticks collected from vegetation were combined in 158 pools. Of these, 13 were positive for A. phagocytophilum (3.8%), while six were positive for E. chaffeensis (3.8%), five for E. canis (3.2%) and two for R. rickettsii (1.3%).
A total of 603 ticks were collected from various wild and domestic animals and placed in 281 pools. Assuming one positive tick/pool, E. canis (12.8%) was the most commonly detected tick-borne pathogen by PCR, followed by A. phagocytophilum (11.0%), R. rickettsii (2.9%), E. chaffeensis (2, 0.8%), and R. amblyommii (0.4%) (GenBank accession No. KP844658, KP8446589, KP84663, KP84664 and KP84665).
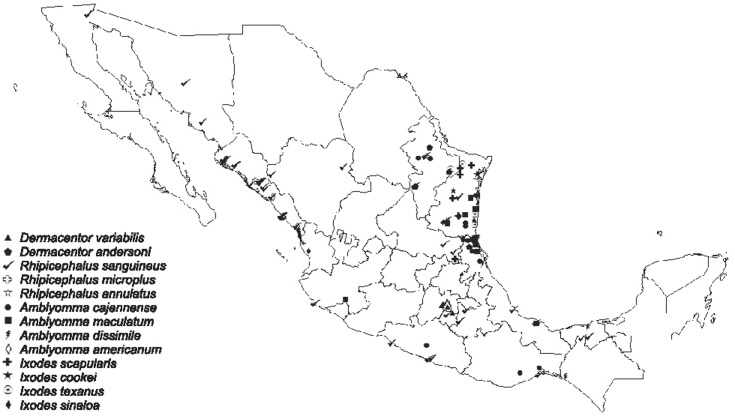
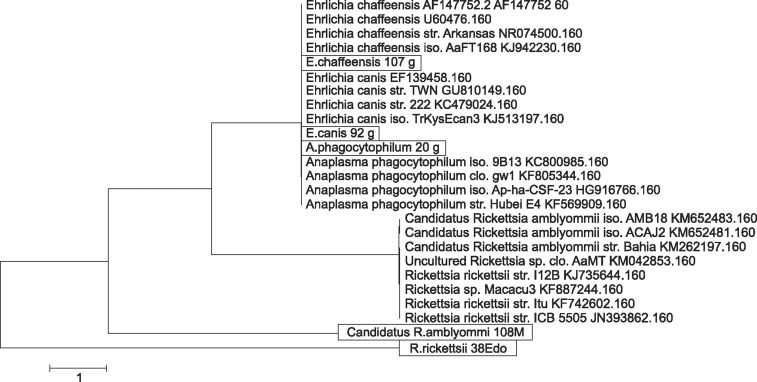
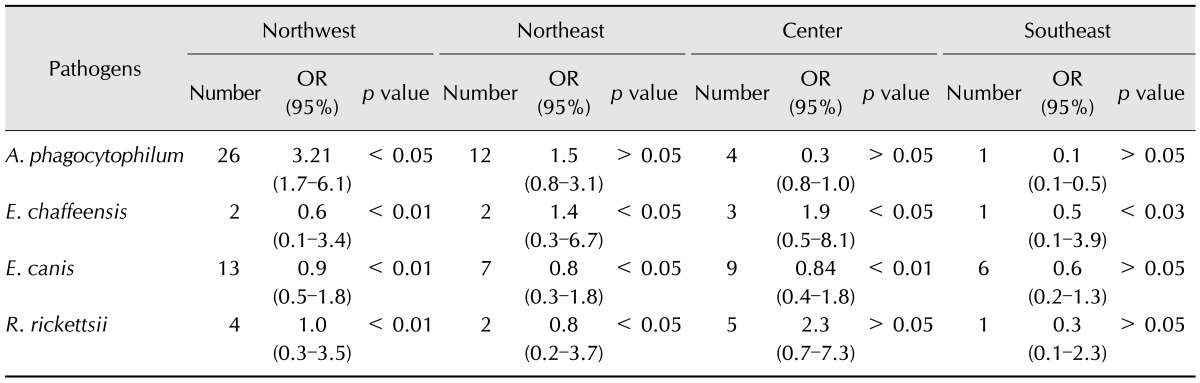
A phylogenetic tree that included the selected tick-borne pathogens was constructed (Fig. 2). A map depicting the distribution of selected tick-borne pathogens detected by PCR from ticks collected by tick drag, humans and wild/domestic animals is shown in Fig. 3. A. phagocytophilum was the most frequent tick infection in the northwest (Table 3). We also identified the following associations: A. phagocytophilum infecting Amblyomma cajennense, Amblyomma dissimile, Amblyomma maculatum and D. variabilis; E. canis infecting Amblyomma cajennense, Amblyomma dissimile, Amblyomma maculatum, I. scapularis and H. leporis-palustris; R. rickettsii infecting D. nitens; and E. chaffeensis infecting Amblyomma cajennense.
Fig. 2. Distribution of selected tick-borne pathogens detected by PCR in ticks collected via tick drag and from human and animal hosts in Mexico.
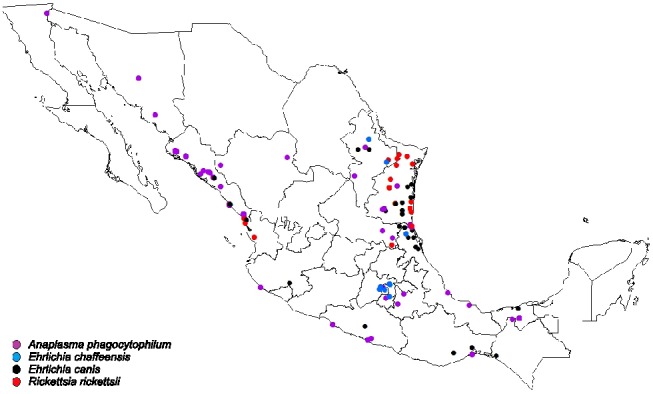
Fig. 3. Phylogenetic tree of selected tick-borne pathogens detected in ticks collected via tick drag and from human and animal hosts in Mexico.
Table 3. The overall risk of selected tick-borne pathogens in ticks collected by tick drag and from various hosts from northwestern, northeastern, central and southeastern areas of Mexico from 1997–2013.
OR, odds ratio with a 95% of confidence interval.
Discussion
Since 1947, R. sanguineus has been reported as the tick vector that transmitted R. rickettsi in Mexico [4], while in the United States D. variabilis and D. andersoni tick are the competent vectors for the same pathogen [29]. In 2002, R. rickettsii was identified in R. sanguineus ticks from eastern Arizona as the tick-borne vector of Rocky Mountain spotted fever (RMSF) [10]. In South America, Amblyomma spp. has been reported to transmit other Rickettsia sp. [24,26,27]. In the last ten years, an epidemic outbreak of RMSF has been reported on the northern border of Mexico and into 30 states within Mexico [3,4],
The tick species reported to transmit R. rickettsii in Mexico include R. sanguineus, D. variabilis and Amblyomma imitator [4,28], while H. leporis-palustris has been described as a potential vector in northwestern Mexico [37].
We found that R. sanguineus was the most frequently encountered tick, accounting for over 52.2% of all ticks examined in all regions of Mexico. These findings are consistent with the fact that R. sanguineus is considered a global tick capable of transmitting pathogens such as R. rickettsii, Ehrlichia species and even Leishmania infantum [2,23,27,31]. The existence of co-infections in individual R. sanguineus adults and nymph ticks was important, and it is known that more than one pathogen may coexist in a single vector [7].
Although the role of R. sanguineus in the transmission cycle of R. rickettsii, the agent of spotted fever rickettsia in humans, has been known in Mexico since the 1940s [3,4], R. rickettsii was not identified in R. sanguineus in the United States until 2002, when it was identified as an important vector RMSF in eastern Arizona [10]. Our results are similar to reports from Panama, Argentina, and Brazil, where R. sanguineus is a competent vector for most of the tick-borne rickettsial diseases pathogens [8,24], and was found as the tick most frequently infected with these pathogens.
We found that A. phagocytophilum and E. canis were the most commonly observed TBD pathogens infecting ticks in Mexico, whereas E. chaffeensis and R. rickettsii were less frequent. A previous study in northeastern Mexico reported a prevalence of 50% Rickettsia spp. infection in ticks of the Amblyomma species [24]. Although this frequency is higher than that observed in our study, we performed a more thorough collection, with 16 different tick species. In Texas, Amblyomma cajennense ticks collected from people were found to have multiple infections, including Borrelia spp., Ehrlichia spp., and Rickettsia spp., while Amblyomma maculatum was infected with Ehrlichia spp. and Rickettsia spp. [41].
We report here the presence of A. phagocytophilum, the agent of human granulocytic anaplasmoses, in Mexico. The disease was recognized in humans in the United States in 1994, and subsequently in Europe in 1995 [28]. The Ixodes group is predicted to be present in all of the central and northeastern states of Mexico [13,15,17,22]. The main distribution predicted for Amblyomma cajennense is the northeast coast of Mexico, which is characterized by lowlands and warm temperatures. In contrast, Ixodes species are predicted to be present primarily in all of the northern states, which are characterized by high altitudes with a temperate climate and vegetation [13,22]. In the United States, I. scapularis and I. pacificus have been found to be the principal vectors for A. phagocytophilum [8].
The detection of the agent of human monocytic ehrlichiosis (E. chaffeensis) in R. sanguineus, Amblyomma americanum and Amblyomma cajennense ticks could indicate that it plays a role in transmission in Mexico. The disease was reported for the first time in Mexico in a patient from Yucatan, who was diagnosed serologically by immunofluorescence assay [14]. In our study, we collected in Yucatan only from R. sanguineus ticks that contained E. canis; however, our results indicate that this infection may occur in other areas in Mexico, including states from the south and north, where the competent vector is predicted to inhabit [13,15,17,22].
Amblyomma imitator ticks were found on Silvilagus spp. This tick is a potential vector for R. rickettsii and R. prowasekii, which suggests the participation of other mammals in enzootic cycles in Mexico [28]. We found R. amblyommii in an Amblyomma cajennense tick collected in a northeastern state of Mexico, making this the first report of Amblyomma cajennense as a potential vector for the agent in Mexico. R. amblyommii infection was described in Amblyomma cajennense from the Amazonian region in Brazil [14,27] and in Amblyomma neumani in Argentina [26]. Although the pathogenicity of R. amblyommii is still unknown, a study in North Carolina in the United States reported that 11 out of 25 Amblyomma americanum ticks were infected with R. amblyommii, and that three out of six patients had antibodies reactive with R. amblyommii [1].
I. scapularis have been reported in the northern and central states of Mexico since 1962 [21]. D. andersoni ticks were collected from dogs in the State of Sinaloa, in northwest Mexico. These ticks have been described not only in new regions, but also in new biotopes. Various proposed explanations include global warming, the impact of land use, and a role of wild fauna in tick dispersion [5,13]. Until recently, D. andersoni and D. variabilis in North America, and Amblyomma cajennense in South America were the only species of ticks associated with spotted fever rickettsiae on the American continent [29]. However, other species have recently been reported to be associated with this disease, including Amblyomma triste [38] in Uruguay, Argentina and Brazil, Amblyomma maculatum in the United States and Rhipicephalus sanguineus in Brazil and the United States [25,40].
The geographic distribution of TBD usually follows the distribution of tick vectors [16,39]. In the present study, we documented the presence of tick-borne pathogens from 67 different locations in Mexico, demonstrating that infected vectors are distributed across the country. We found that A. phagocytophilum was distributed in most of the regions studied, R. rickettsii only in coastal Mexico, E. chaffeensis in the central states and E. canis mostly in northern states of Mexico.
These findings extend previous studies in Mexico, which were limited to the search for a few TBD and tick species, and suggest that most of the tick-borne rickettsial (TBR) pathogens are probably endemic in the country. However, more studies are needed to determine which infections these ticks are able to transmit and cause disease [34].
Overall, the results presented herein provide information about potentially pathogenic TBR organisms, and their tick hosts in Mexico, suggesting the presence of previously unrecognized endemic diseases [18]. It is necessary to determine which associations between ticks and pathogens could result in transmission of the agent, or represent a silent zoonotic cycle of the agent. Knowledge of tick distributions may be useful in predicting the epidemiology of diseases associated with particular tick species [18,20,36], and may also provide an opportunity to examine the ecology of emerging or previously undescribed zoonoses for which different ecological determinants of disease transmission may exist [20,33,34].
The distribution of tick-borne pathogens is changing throughout the world, and some of these agents in Mexico may pose an unrecognized health threat. Our results highlight the need for continued survey studies to monitor the appearance of new enzootic diseases, which may represent unknown threats to public health of the populations exposed.
Acknowledgments
The author thanks Dr. David H. Walker at University of Texas Medical Branch, for providing grammatical and critical review.
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, Dail K, Johnson J, Watson DW. Tick-borne disease in North Carolina: is "Rickettsia amblyommii" a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 2008;8:597–606. doi: 10.1089/vbz.2007.0271. [DOI] [PubMed] [Google Scholar]
- 2.Burlini L, Texeira KRS, Szabó MPJ, Famadas KM. Molecular dissimilarities of Rhipicephalus sanguineus (Acari: Ixodidae) in Brazil and its relation with samples throughout the world: is there a geographical pattern? Exp Appl Acarol. 2010;50:361–374. doi: 10.1007/s10493-009-9321-8. [DOI] [PubMed] [Google Scholar]
- 3.Bustamante-Calvillo ME, Varela G, Mariotte CO. Studies of spotted fever in Mexico: spotted fever in the Laguna. Rev Inst Salubr Enferm Trop. 1946;7:39–49. [Google Scholar]
- 4.Bustamante ME, Varela G. Studies of spotted fever in Mexico: the role of Rhipicephalus sanguineus in the transmission of spotted fever in Mexico. Rev Inst Salubr Enferm Trop. 1947;8:139–141. [Google Scholar]
- 5.Brouqui P. Ehrlichiosis in Europe. In: Raoult D, Brouqui P, editors. Rickettsiae and Rickettsial Diseases at the Turn of the Third Millennium. Paris: Elsevier; 2002. pp. 220–232. [Google Scholar]
- 6.Bröker M. Following a tick bite: double infections by tick-borne encephalitis virus and the spirochete Borrelia and other potential multiple infections. Zoonoses Public Health. 2012;59:176–180. doi: 10.1111/j.1863-2378.2011.01435.x. [DOI] [PubMed] [Google Scholar]
- 7.Chmielewska-Badora J, Moniuszko A, Żuliewicz-Sobczak W, Zwoliński J, Piatek J, Pancewicz S. Serological survey in persons occupationally exposed to tick-borne pathogens in cases of co-infections with Borrelia burgdorferi, Anaplasma phagocytophilum, Bartonella spp. and Babesia microti. Ann Agric Environ Med. 2012;19:271–274. [PubMed] [Google Scholar]
- 8.Dantas-Torres F, Chomel BB, Otranto D. Ticks and tick-borne diseases: a One Health perspective. Trends Parasitol. 2012;28:437–446. doi: 10.1016/j.pt.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Dantas-Torres F, Giannelli A, Otranto D. Starvation and overwinter do not affect the reproductive fitness of Rhipicephalus sanguineus. Vet Parasitol. 2012;185:260–264. doi: 10.1016/j.vetpar.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Demma LJ, Traeger MS, Nicholson WL, Paddock CD, Blau DM, Eremeeva ME, Dasch GA, Levin ML, Singleton J, Jr, Zaki SR, Cheek JE, Swerdlow DL, McQuiston JH. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med. 2005;353:587–594. doi: 10.1056/NEJMoa050043. [DOI] [PubMed] [Google Scholar]
- 11.Dong J, Olano JP, McBride JW, Walker DH. Emerging pathogens: challenges and successes of molecular diagnostics. J Mol Diagn. 2008;10:185–197. doi: 10.2353/jmoldx.2008.070063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durden LA. Taxonomy, host associations, life cycles and vectorial importance of ticks parasitizing small mammals. In: Morand S, Krasnov BR, Poulin R, editors. Micromammals and Macroparasites: From Evolutionary Ecology to Management. Tokyo: Springer; 2006. pp. 91–102. [Google Scholar]
- 13.Feria-Arroyo TP, Castro-Arellano I, Gordillo-Perez G, Cavazos AL, Vargas-Sandoval M, Grover A, Torres J, Medina RF, de León AAP, Esteve-Gassent MD. Implications of climate change on the distribution of the tick vector Ixodes scapularis and risk for Lyme disease in the Texas-Mexico transboundary region. Parasit Vectors. 2014;7:199. doi: 10.1186/1756-3305-7-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gongóra-Biachi RA, Zavala-Velázquez J, Castro-Sansores CJ, González-Martínez P. First case of human ehrlichiosis in Mexico. Emerg Infect Dis. 1999;5:481. doi: 10.3201/eid0503.990327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordillo-Pérez G, Vargas M, Solórzano-Santos F, Rivera A, Polaco OJ, Alvarado L, Muñóz O, Torres J. Demonstration of Borrelia burgdorferi sensu stricto infection in ticks from the northeast of Mexico. Clin Microbiol Infect. 2009;15:496–498. doi: 10.1111/j.1469-0691.2009.02776.x. [DOI] [PubMed] [Google Scholar]
- 16.Gubler DJ, Reiter P, Ebi KL, Yap W, Nasci R, Patz JA. Climate variability and change in the United States: potential impacts on vector- and rodent-borne diseases. Environ Health Perspect. 2001;102:223–233. doi: 10.1289/ehp.109-1240669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guzmán-Cornejo C, Robbins RG. The genus Ixodes (Acari: Ixodidae) in Mexico: adult identification keys, diagnoses, host, and distribution. Rev Mex Biodivers. 2010;81:289–298. [Google Scholar]
- 18.Hai VV, Almeras L, Socolovschi C, Raoult D, Parola P, Pagès F. Monitoring human tick-borne disease risk and tick bite exposure in Europe: available tools and promising future methods. Ticks Tick Borne Dis. 2014;5:607–619. doi: 10.1016/j.ttbdis.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Hamer SA, Tsao JI, Walker ED, Mansfield LS, Foster ES, Hickling GJ. Use of tick surveys and serosurveys to evaluate pet dogs as a sentinel species for emerging Lyme disease. Am J Vet Res. 2009;70:49–56. doi: 10.2460/ajvr.70.1.49. [DOI] [PubMed] [Google Scholar]
- 20.Hinrichsen VL, Whitworth UG, Breitschwerdt EB, Hegarty BC, Mather TN. Assessing the association between the geographic distribution of deer ticks and seropositivity rates to various tick-transmitted disease organisms in dogs. J Am Vet Med Assoc. 2001;218:1092–1097. doi: 10.2460/javma.2001.218.1092. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman A. Monograph of Ixodoiea in Mexico. Part 1. Rev Soc Mex Hist Nat. 1962;23:191–307. [Google Scholar]
- 22.Illoldi-Rangel P, Rivaldi CL, Sissel B, Trout Fryxell R, Gordillo-Pérez G, Rodríguez-Moreno A, Williamson P, Montiel-Parra G, Sánchez-Cordero V, Sarkar S. Species distribution models and ecological suitability analysis for potential tick vectors of Lyme disease in Mexico. J Trop Med. 2012;2012:959101. doi: 10.1155/2012/959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2009;30:261–292. doi: 10.1016/j.cll.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones EK, Clifford CM, Keirans JE, Kohls GM. The ticks of Venezuela (Acarina: Ixodidae), with a key to the species of Amblyomma in the western hemisphere. Brigham Young Univ Sci Bull Biol Ser. 1972;17:1–46. [Google Scholar]
- 25.Medina-Sanchez A, Bouyer DH, Alcantara-Rodriguez V, Mafra C, Zavala-Castro J, Whitworth T, Popov VL, Fernandez-Salas I, Walker DH. Detection of typhus group Rickettsia in Amblyomma ticks in the State of Nuevo Leon, Mexico. Ann N Y Acad Sci. 2005;1063:327–332. doi: 10.1196/annals.1355.052. [DOI] [PubMed] [Google Scholar]
- 26.Labruna MB, Pacheco RC, Nava S, Brandão PE, Richtzenhain LJ, Guglielmone AA. Infection by Rickettsia belli and Candidatus "Rickettsia amblyommii" in Amblyomma neumanni ticks from Argentina. Microb Ecol. 2007;54:126–133. doi: 10.1007/s00248-006-9180-3. [DOI] [PubMed] [Google Scholar]
- 27.Labruna MB, Whitworth T, Bouyer DH, McBride J, Camargo LMA, Camargo EP, Popov V, Walker DH. Rickettsia bellii and Rickettsia amblyommii in Amblyomma ticks from the State of Rondônia, western Amazon, Brazil. J Med Entomol. 2004;41:1073–1081. doi: 10.1603/0022-2585-41.6.1073. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira KA, Pinter A, Medina-Sanchez A, Boppana VD, Wikel SK, Saito TB, Shelite T, Blanton L, Popov V, Teel PD, Walker DH, Galvao MAM, Mafra C, Bouyer DH. Amblyomma imitator ticks as vectors of Rickettsia rickettsii, Mexico. Emerg Infect Dis. 2010;16:1282–1284. doi: 10.3201/eid1608.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- 30.Pfäffle M, Littwin N, Muders SV, Petney TN. The ecology of tick-borne diseases. Int J Parasitol. 2013;43:1059–1077. doi: 10.1016/j.ijpara.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Sanogo YO, Parola P, Shpynov S, Camicas JL, Brouqui P, Caruso G, Raoult D. Genetic diversity of bacterial agents detected in ticks removed from asymptomatic patients in norrtheastern Italy. Ann N Y Acad Sci. 2003;990:182–190. doi: 10.1111/j.1749-6632.2003.tb07360.x. [DOI] [PubMed] [Google Scholar]
- 32.Solano-Gallego L, Rossi L, Scroccaro AN, Montarsi F, Caldin M, Furlanello T, Trotta M. Detection of Leishmania infantum DNA mainly in Rhipicephalus sanguineus male removed from dogs living in endemic areas of canine leishmaniosis. Parasit Vectors. 2012;5:98. doi: 10.1186/1756-3305-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sosa-Gutierrez CG, Quintero Martinez MT, Gaxiola Camacho SM, Cota Guajardo S, Esteve-Gassent MD, Gordillo-Pérez MG. Frequency and clinical epidemiology of canine monocytic ehrlichiosis in dogs infested with ticks from Sinaloa, Mexico. J Vet Med. 2013;2013:797019. doi: 10.1155/2013/797019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sosa-Gutiérrez CG, Vargas M, Torres J, Gordillo-Pérez MG. Tick-borne rickettsial pathogens in rodents from Mexico. J Biomed Sci Eng. 2014;7:884–889. [Google Scholar]
- 35.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetic Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tinoco-Gracia L, Quiroz-Romero H, Quintero-Martínez MT, Rentería-Evangelista TB, Barreras-Serrano A, Hori-Oshima S, Medina-Basulto G, Vinasco J, Moro MH. Prevalence and risk factors for Borrelia burgdorferi infection in Mexicali, Baja California, a Mexico-US border city. Int J Appl Res Vet Med. 2008;6:161–165. [Google Scholar]
- 37.Vargas-Sandoval M, Priego-Santander AG, Larrazábal A, Sosa-Gutierrez CG, Lara-Chávez MB, Ávila-Val TC. Potential species distribution and richness of Ixodidae ticks associated with wild vertebrates from Michoacán, Mexico. J Geogr Inf Syst. 2014;6:467–477. [Google Scholar]
- 38.Venzal JM, Estrada-Peña A, Portillo A, Mangold AJ, Castro O, De Souza CG, Félix ML, Pérez-Martínez L, Santibánez S, Oteo JA. Rickettsia parkeri: a rickettsial pathogen transmitted by ticks in endemic areas for spotted fever rickettsiosis in southern Uruguay. Rev Inst Med Trop Sao Paulo. 2012;54:131–134. doi: 10.1590/s0036-46652012000300003. [DOI] [PubMed] [Google Scholar]
- 39.Walker DH, Paddock CD, Dumler JS. Emerging and re-emerging tick-transmitted rickettsial and ehrlichial Infections. Med Clin North Am. 2008;92:1345–1361. doi: 10.1016/j.mcna.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Wikswo ME, Hu R, Metzger ME, Eremeeva ME. Detection of Rickettsia rickettsii and Bartonella henselae in Rhipicephalus sanguineus ticks from California. J Med Entomol. 2007;44:158–162. doi: 10.1603/0022-2585(2007)44[158:dorrab]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 41.Williamson P, Billingsley P, Teltow GJ, Seals JP, Turnbough MA, Atkinson SE. Borrelia, Ehrlichia, and Rickettsia spp. in ticks removed from persons, Texas, USA. Emerg Infect Dis. 2010;16:441–446. doi: 10.3201/eid1603.091333. [DOI] [PMC free article] [PubMed] [Google Scholar]