Abstract
A global data set including 5,087 observations of leaf nitrogen (N) and phosphorus (P) for 1,280 plant species at 452 sites and of associated mean climate indices demonstrates broad biogeographic patterns. In general, leaf N and P decline and the N/P ratio increases toward the equator as average temperature and growing season length increase. These patterns are similar for five dominant plant groups, coniferous trees and four angiosperm groups (grasses, herbs, shrubs, and trees). These results support the hypotheses that (i) leaf N and P increase from the tropics to the cooler and drier midlatitudes because of temperature-related plant physiological stoichiometry and biogeographical gradients in soil substrate age and then plateau or decrease at high latitudes because of cold temperature effects on biogeochemistry and (ii) the N/P ratio increases with mean temperature and toward the equator, because P is a major limiting nutrient in older tropical soils and N is the major limiting nutrient in younger temperate and high-latitude soils.
Nitrogen (N) and phosphorus (P) are generally considered the two most limiting elements to terrestrial vegetation, but global patterns in soil N and P limitation or plant N and P status have not been well characterized (1–10). Here we use a large data set, consisting of 5,087 observations of leaf N and P for 1,280 plant species at 452 sites, to explore global patterns of leaf N and P (expressed herein per unit of dry biomass, mg/g) and their ratio (N/P) in relation to broad-scale variability in geography, temperature, and other climatic factors. Such patterns may provide insights for fields as diverse as ecological stoichiometry, global carbon modeling, and macroecology (1, 11, 12). Given the specific functions of N and P in leaves, N in proteins important in all enzymatic activity, and P in protein synthesis, it would be surprising if temperature did not influence N and P, but the potential ways such influence could occur are complex. Biogeographic gradients in N and P could occur due to temperature effects on plant physiology or soil biogeochemistry, as well as due to geographic patterns of soil substrate age, all of which could be further influenced by correlated variation in plant species composition or trait variation (1–10), disease incidence, herbivory, and other variables. Growing season temperature and length are likely important aspects of the thermal environment that influence geographic patterns in leaf N and P. Other climatic factors, such as precipitation, could also influence patterns of leaf N and P.
Existing publications (7, 10, 13–15) provide limited insight into broad biogeographic leaf N and P patterns. For example, alpine and arctic herbaceous plant species had higher leaf N and P when examined in colder, rather than warmer, habitats (9, 16, 17), but leaf N and P were either similar (17, 18) or lower (19) for given tree species in colder than warmer sites, and forest communities can have regionally lower (15) or higher (20) leaf N with increasing temperatures. In these examples, leaf N and P variation likely results from a variety of potential drivers, described below, not only from direct physiological effects of temperature. We are unaware of any comprehensive survey that attempts to reconcile such differences in relation to climate or plant growth form across global geographic gradients.
The stoichiometry of carbon (C), N, and P is a useful means of assessing mechanisms for N and P variation (1). Gradients of leaf C/N, C/P, and N/P along geographic and temperature gradients could be driven by variation in plant physiology, soil biogeochemistry, and plant community composition. There are other potential drivers of biogeography of leaf N and P (including gradients in precipitation), but we focused on the ones above to develop the following series of hypotheses.
Temperature–Plant Physiological Hypotheses Regarding C/N and C/P
The biochemistry of physiology apparently plays a major role in constraining element concentrations and ratios in most organisms (1). Temperature directly influences virtually all physiological rate processes, the integration of which at the plant scale controls the rate of accumulation and removal of C, N, and P from leaves. If cold temperatures limited photosynthetic C gain more (or less) than root N or P uptake, whole plant C/NorC/P would shift, all else being equal. In contrast to the variable patterns reported above for plants in the field across ecological gradients associated with temperature, plants grown in laboratory conditions typically contain greater leaf N and P when grown at low, rather than high, temperatures (2, 21). This fact is frequently interpreted as evidence for mechanisms that offset reduced rates of biochemical reactions caused by the diminished efficiency of N-rich enzymes and P-rich RNA at low temperatures (2, 9, 21, 22). In essence, because leaf N and P regulate rates of C acquisition and use and because the kinetics of N- and P-regulated processes are temperature-sensitive, changes in N and P can compensate for altered temperature. Regardless of the mechanisms involved, such physiological acclimation could lead to higher leaf N and P in situ in colder, rather than warmer, climates.
Genotypic variation related to temperature could also influence leaf N and P patterns. Within species, when populations native to sites differing in temperature are grown under common garden conditions, populations from colder habitats often have greater leaf N and P (16, 23–25). This trait is typically considered an adaptation that enhances metabolic activity and growth rates under the low temperatures of native habitats (2, 9, 23, 24). Adaptation of this kind could also generally lead to higher leaf N and P in situ in colder, rather than warmer, climates. Because of both physiological acclimation and adaptation to temperature, N and P should decline monotonically with increasing temperature, which we label the °T–Plant Physiology hypothesis.
Biogeochemical Hypotheses Regarding C/N and C/P
Because leaf N and P typically reflect soil N and P availability (7, 10, 26, 27), temperature effects on biogeochemistry could also drive the biogeography of leaf N and P, but in the opposite direction as the temperature effects on plants. Cold temperatures influence physical properties such as water viscosity and membrane permeability, which, coupled with influences on metabolic processes, typically limit microbial activity (3, 9, 28). Hence, low temperatures have well known depressing effects on decomposition and mineralization of organic matter, which reduce the availability of N and P (3, 9, 10, 28, 29) and, therefore, likely of leaf N and P. Low-temperature suppression of nutrient movement in soils and nutrient uptake by roots are also well known phenomena (9, 16). Moreover, differences in leaf N and P generally correspond with differences in litter N and P (7, 10, 30), which could lead to temperature-related positive feedback on nutrient cycling (3, 10, 31); nutrient availability may thus be further raised to some degree at warmer, rather than colder, sites. All of these temperature-related effects on biogeochemistry would lead to leaf N and P's increasing monotonically with temperature across the globe (the °T–Biogeochemistry hypothesis), which is the reverse prediction of the °T–Plant Physiology hypothesis.
However, this simple temperature–biogeochemistry relationship is made more complex by earth history, including major geological disturbances such as glaciation that have led, on average, to older and more highly leached soils in the tropics than elsewhere (4–8, 32–34), and by precipitation gradients, because annual rainfall and associated leaching are, on average, greater in the tropics as well. Soil substrate age has been shown to influence soil N and P availability and leaf N and P, with lower levels in very young and old soils than in young to intermediate-age soils (6–8, 34). Although tropical and temperate soils vary widely in soil age, tropical soils are considered on average to be older, to be more leached, and to have lower fertility (4–8, 32, 33), which would raise leaf C/N and C/P and lower N and P with increasing temperature (the Soil Substrate Age hypothesis).
Species Composition and Plant Trait Effects on C/N and C/P
If species or life-forms with intrinsically high P and N are successful where N and P availability are high (7, 10, 27) and vice versa, species compositional shifts could exacerbate biogeographic patterns in N and P, leading to the Species Composition hypothesis: the overall pattern for all species pooled will be greater [i.e., response functions will have steeper changes per unit variation in mean annual temperature (MAT) or growing season temperature] than for major groups or individual genera. Furthermore, the major plant groups will differ on average in N, P, and N/P because of intrinsic anatomical differences that lead to consistent differences in stoichiometry of C/N, C/P, and N/P (1).
Variation in community-scale leaf traits could also influence geographic patterns in N and P. If climate variation alters leaf lifespan or leaf mass per area, these changes could affect leaf N and P, given the generally close association of these leaf attributes (35, 36). For example, if evergreen leaves with a lifespan of >1 year are favored in the wet tropics and in short growing seasons at high latitudes, but deciduous species are favored at midlatitudes (37, 38), this variation would decrease N and P at the highest and lowest temperatures, because species with longer-lived foliage have lower N and P (35, 36) (Evergreen–Deciduous hypothesis). However, for evergreen species, leaf lifespan is greater in colder environments (39–41); given that leaf N and P increase with decreasing leaf lifespan, this trait should lead to lower leaf N and P in evergreen species in increasingly cold climates. Hence, variation in leaf lifespan of evergreen species and the relative balance of evergreen vs. deciduous species could counteract one another in the tropics but act in concert at high latitudes.
Hypotheses Regarding N/P
Given differences in the biochemistry and function of N-rich proteins and P-rich RNA, reductions in leaf N/P with increasing temperatures could occur due to stoichiometric effects of physiological acclimation and adaptation to temperature (the °T–Physiology N/P hypothesis), as occurs generally in heterotrophs (2, 42, 43) and in relation to growth rate (1, 42, 43). However, soil age may also influence the balance between N and P, because young soils tend to be more N- than P-limited, with the reverse true of older soils (6–8, 34, 44), and the relative limitation by soil P is likely greater in tropical than in younger soils in cooler regions of the Earth (10, 32, 33). Thus, geographic variation in soil age should result in leaf N/P's increasing globally with increasing temperature (the Soil Substrate Age N/P hypothesis), because P is equally or more limiting than N in the older and more highly leached soils in warm regions, whereas N is the dominant limitation in younger soils at mid- and high latitudes.
Methods
In this report we use a data set (273 sources) including 5,087 observations of higher plant leaf N and P concentrations from 1,280 species and 704 genera from 452 sites for which we could identify MAT (°C) and other climate variables (see Table 1, which is published as supporting information on the PNAS web site). We did not use data for fertilized plants or planted, urban, or polluted sites. When not supplied by authors, we used data from the WorldClimate web site (www.worldclimate.com) and a global data set (45) to generate data for MAT, rainfall, vapor pressure, and irradiance. For missing latitude, longitude, or altitude of plant origin we used data obtained from the Global Gazetteer web site (www.calle.com/world/index.html).
Sites were located on six continents and ranged from –12.8°C to 28.0°C MAT and from 43°S to 70°N latitude. Despite variability in MAT due to continentality, ocean currents, and elevation, in this data set MAT was highly negatively correlated with the absolute value of latitude [coefficient of determination (r2) = 0.87, n = 1,264, for all species]. MAT was also closely correlated (0.66 < r2 < 0.89) with growing season length, growing season mean temperature, irradiance, and vapor pressure and modestly correlated (r2 = 0.40) with annual rainfall. Sites with higher MAT had longer growing seasons and higher mean growing season temperatures. As is typical in cross-species analyses, we used log10 data to normalize the distributions and minimize patterns in residuals (1, 36). We also compared species in five major plant groups for which data were most abundant, coniferous trees and four angiosperm groups (trees, shrubs, grasses, and herbs).
We analyzed data in several ways: (i) using all data (i.e., treating all observations equally), (ii) averaging by species, and (iii) averaging by species within temperature ranges (hereafter called bins). Each approach has its own bias, but trends were generally similar regardless. In this data set, using all data excessively weights frequently sampled species, whereas averaging by species gives more weight to species sampled rarely and eliminates and thus obscures MAT variation among sites for frequently sampled species. Averaging by bins eliminates and thus obscures sources of variation, such as local site, species, and climate variability, but balances the influence of unevenly sampled climate zones. Temperature bins were developed to balance as much as possible the sample size (total and by species) for all data and within each of the five major groups (n = 1,679 data, when each species was counted once per bin).
We present data averaged by species (Figs. 1, 2, 3) and by temperature bins (Fig. 1 and Figs. 5 and 6, which are published as supporting information on the PNAS web site), although within genera (Fig. 4) we used all observations. We fit the relationships of N, P, and N/P to climate indices using regression. Regressions were linear unless nonlinear fits resulted in improvements in homoscedasticity or fit. In that case, the significant nonlinear equation that had the best fit and residual distribution was used. Given multiple and diverse potential drivers of the response of N and P to temperature, we do not imply that these represent specific biologically relevant functions. The full equations are provided in Table 2, which is published as supporting information on the PNAS web site.
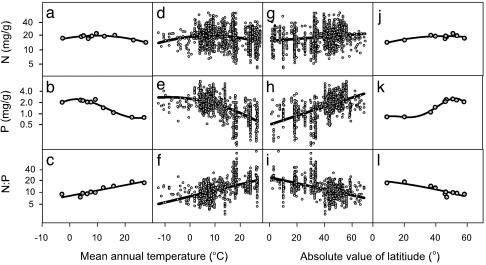
Fig. 1.
Leaf N, P, and the leaf N/P ratio in relation to MAT and absolute latitude for all species averaged by species (d–i) and pooled within temperature bins (a–c and j–l). For all species, the fits for MAT were for leaf N (r2 = 0.05, P < 0.0001, n = 1,251) (d), leaf P (r2 = 0.39, P < 0.0001, n = 907) (e), and leaf N/P(r2 = 0.31, P < 0.0001, n = 894) (f). The fits for absolute value of latitude were for leaf N (r2 = 0.04, P < 0.0001, n = 1,235) (g), leaf P (r2 = 0.34, P < 0.0001, n = 908) (h), and leaf N/P(r2 = 0.24, P < 0.0001, n = 878) (i). For species in temperature bins (n = 10), the fits for MAT were for leaf N (r2 = 0.75, P < 0.008) (a), leaf P (r2 = 0.96, P < 0.001) (b), and leaf N/P(r2 = 0.85, P < 0.0001) (c). The fits for absolute value of latitude were for leaf N (r2 = 0.69, P = 0.02) (j), leaf P (r2 = 0.98, P < 0.001) (k), and leaf N/P(r2 = 0.77, P < 0.0008) (l). The full equations (for all figures) are provided in Table 1. The number of species in each MAT bin averaged 167, with a range from 72 to 292.
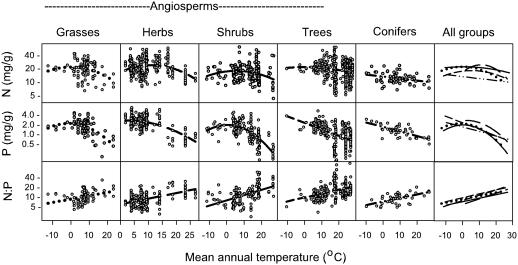
Fig. 2.
Leaf N, P, and N/P in relation to MAT for all species in five major plant groups. Among the angiosperm groups, for grasses the fits were for N (r2 = 0.10, P < 0.0001, n = 113), P (r2 = 0.11, P < 0.0001, n = 96), and N/P(r2 = 0.31, P < 0.0001, n = 95); for herbs, N (r2 = 0.08, P < 0.0001, n = 312), P (r2 = 0.35, P < 0.0001, n = 250), and N/P(r2 = 0.24, P < 0.0001, n = 244); for shrubs, N (r2 = 0.07, P < 0.0001, n = 212), P (r2 = 0.48, P < 0.0001, n = 173), and N/P(r2 = 0.25, P < 0.0001, n = 168); and for trees, N (r2 = 0.05, P < 0.0001, n = 491), P (r2 = 0.25, P < 0.0001, n = 302), and N/P(r2 = 0.20, P < 0.0001, n = 287). For the coniferous trees, the fits were for N (r2 = 0.10, P < 0.0001, n = 69), P (r2 = 0.49, P < 0.0001, n = 45), and N/P(r2 = 0.36, P < 0.0001, n = 45).
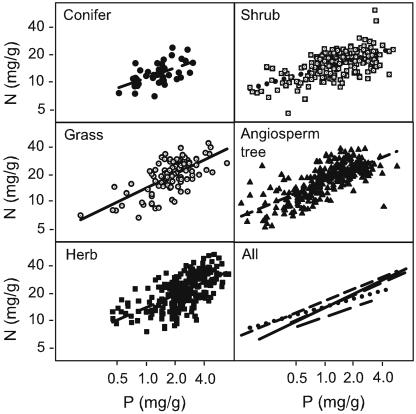
Fig. 3.
Leaf N in relation to leaf P for five plant groups, arrived at by using data averaged by species as in Fig. 2, is shown. For all groups, the following fits (all P < 0.0001) were linear: grass (r2 = 0.46, n = 96), herb (r2 = 0.38, n = 246), shrub (r2 = 0.37, n = 169), angiosperm tree (r2 = 0.58, n = 286), and conifer (r2 = 0.31, n = 45).
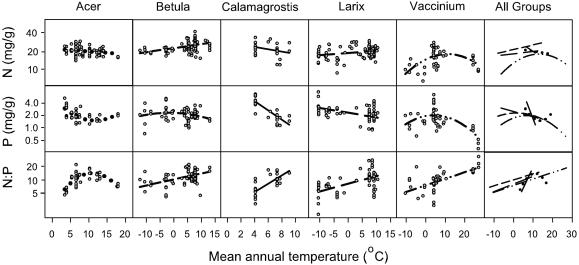
Fig. 4.
Leaf N, P, and N/P in relation to MAT for five genera are shown. The regression fits were as follows: Acer, for N (r2 = 0.06, P = 0.05, n = 70), P (r2 = 0.39, P < 0.0001, n = 43), and N/P(r2 = 0.54, P < 0.0001, n = 40); Betula, for N (r2 = 0.16, P < 0.0001, n = 86), P (r2 = 0.04, P = 0.09, n = 74), and N/P(r2 = 0.21, P < 0.0001, n = 71); Calamagrostis, for N (r2 = 0.10, P = 0.07, n = 33), P (r2 = 0.69, P < 0.0001, n = 33), and N/P(r2 = 0.57, P < 0.0001, n = 33); Larix, for N (r2 = 0.07, P = 0.005, n = 109), P (r2 = 0.22, P < 0.0001, n = 81), and N/P(r2 = 0.34, P < 0.0001, n = 80); and Vaccinium, for N (r2 = 0.23, P = 0.002, n = 50), P (r2 = 0.44, P < 0.0001, n = 47), and for N/P(r2 = 0.42, P < 0.0001, n = 45).
Results
There are significant relations of leaf N, P, and N/P to MAT and latitude (Figs. 1, 2, 3, 4, 5, 6 and Table 2) found by using data for all species, by plant types, or within genera. Among all species, MAT was more strongly correlated with leaf N, P, and N/P than were growing season length, growing season temperature, annual rainfall, or any other climate variable. However, given the strong correlations between MAT and growing season temperature and length, we cannot effectively distinguish among these and hence use MAT as a surrogate for their collective effects.
For all species (Fig. 1), leaf N and P are lowest at warmer temperatures (>15°C MAT) and increasingly equatorial latitudes. There is also a tendency for leaf N to be lower at the coldest MAT. When using data for all species, MAT explains very little of the total variation in leaf N but a substantial proportion of the total variability in P. The N/P ratio increases markedly with MAT and decreases with latitude. Given the strong correlation between MAT and latitude, all subsequent results are presented in relation only to MAT.
These patterns are generally similar when the major plant groups are examined individually (Figs. 2 and 5). In all five groups, leaf N decreases with MAT (Figs. 2 and 5) from the 5–10°C range to the warmest MAT. Groups appear to differ in whether leaf N declines or plateaus at the coldest MAT, but there are insufficient data at very low MATs to definitively characterize such differences. In all five groups, leaf P generally decreased with MAT and N/P increased with MAT (Figs. 2 and 5 and Table 2).
However, the stoichiometry of C/N, C/P, and N/P varies among groups (Figs. 2 and 3). For both N and P, at any MAT < 18°C, the ranking of N and P from high to low was herbs > grasses ≈ angiosperm trees or shrubs > conifers. In all groups N and P were strongly correlated (P ≤ 0.0001, r2 = 0.30–0.58) (Fig. 3). Conifers and angiosperm shrubs had lower slopes of the N vs. P relationship than angiosperms or the two herbaceous groups (slope differences among groups, P = 0.0004). At any level of leaf P, N was least in coniferous trees.
Individual genera with sufficient data had relationships (of leaf N, P, and/or N/P in relation to MAT) (Figs. 4 and 6) that fit roughly within the overall relationship data space representing the broadest patterns observed among all groups (Figs. 1 and 2), but the genera varied in their patterns. Vaccinium occupied most of the total MAT range, and leaf N and P both had a rise-and-fall shape in relation to MAT (Fig. 4). However, the other four genera shown, each of which occupied roughly half the MAT range or less, differed in their patterns. In Betula and Larix leaf N increased with MAT, whereas in Acer and Calamagrostis leaf N decreased with MAT (Fig. 4). These five genera also had different shaped leaf P–MAT and N/P–MAT patterns.
Discussion
Our results demonstrate that leaf N and P decrease and the N/P ratio increases with increasing environmental temperature and with nearness to the equator (see also ref. 46). These trends were true in analyses using all data as well as in those using data pooled by temperature bins and were similar in diverse life-forms and taxonomic groupings. Climate was an important correlate of leaf P and N/P, with indices such as MAT explaining a substantial fraction of their total variation, which is impressive given that the total variation in the data set includes much local variation (35) that is unrelated to broad biogeography. Leaf N was always significantly related to MAT, but the patterns were less consistent, and less of its variance was explained. The question remains open as to whether the observed biogeographical patterns in leaf N, P, and N/P involve adaptation or acclimation responses of plants to their thermal environments, variability in N and P availability, changes in plant communities and traits, or a combination thereof.
C/N and C/P Discussion. The results are consistent with both the °T–Plant Physiology and Soil Substrate Age hypotheses (see above) that suggest that both C/N and C/P should increase (and N and P decrease) with increasing temperatures. Decreasing N and P with increasing MAT is consistent with both phenotypic and genotypic responses to temperature identified for specific taxa (2, 9, 16, 21, 23–25). Such responses are generally considered to be of acclimative and adaptive value (9, 16, 21, 23–25) and may also reflect stoichiometric constraints (1, 2, 21). The trends also are consistent with substrate age and rainfall effects: the tendency of old soils in warm (i.e., tropical), higher-rainfall habitats to have low availability of both N and P, compared with temperate soils that are typically younger and less leached (4–8, 32, 33), would also drive down leaf N and P with increasing MAT across temperate to tropical latitudinal gradients.
However, for temperatures >5°C, there was no support (Figs. 1 and 2) for the °T–Biogeochemistry hypothesis that N and P would increase with MAT as cold temperature suppression of biogeochemical processes was removed. This lack of support may be because this driving force was swamped in magnitude by the physiological and soil age effects, or perhaps interactions with many other processes limit the degree to which this increase occurs, especially at all but the lowest temperatures (28).
Overall, the data support the idea that the combination of temperature-related physiology and rainfall- and substrate-related biogeochemical constraints collectively result in the observed N and P patterns that were consistent among all plant groups. Data are insufficient, however, to differentiate between aspects of the thermal environment, such as the growing season mean temperature or length, and annual indices, such as MAT, and how they might influence nutrient availability and leaf chemistry. Moreover, the tendency of N or P to plateau or even decline at the coldest temperatures is not consistent with the °T–Plant Physiology hypothesis and perhaps reflects direct and indirect biogeochemical effects of low temperature, including permafrost effects, paludification, or high accumulation of surface organic matter. The overall patterns across the full terrestrial MAT spectrum could arise because of the dominance at high MAT (i.e., >15°C) of old, highly leached soils with low N and P availability and of cold soil temperatures suppressing biogeochemical processes at low MAT (i.e., <5°C).
Although it could theoretically influence patterns for all species pooled, in this data set composition per se doesn't explain low N and P at the highest MAT. Although herbs (the group with the highest N and P at MAT of <18°C) make up a smaller proportion of the data pool at higher MAT (data not shown), herb N and P decline markedly with increasing MAT in any case (Figs. 2 and 5). Hence, biogeographic variation in communities (if data are representative) or in sampling bias has a modest effect on the total patterns reported here.
Additionally, the geographic distribution of evergreen vs. deciduous species also contributes to the geographic gradients shown in Figs. 1 and 2. Deciduous species, known to have higher leaf N and P than evergreens with leaf lifespan of >1 year (35), make up a greater fraction of all species in temperate than in tropical zones (data not shown), thus contributing to higher leaf N and P in temperate than in tropical zones. However, deciduous species dominated both the angiosperm tree and shrub groups at temperatures of <10°C(>90% of species on average), and, with shorter growing seasons at coldest temperatures, leaf lifespan should generally be briefest. Given the inverse relationship between leaf N and P vis-a-vis leaf lifespan (35, 36), this trend should lead to higher N and P at the coldest temperatures in both angiosperm woody groups, which was not observed. These contrasts suggest that ecological sorting of species types and of species traits such as leaf lifespan, although real, likely play a modest role in the broadest biogeographic structuring of leaf N and P.
N/P Discussion. In all groups and for all species pooled, leaf N/P increases with MAT. These results refute the °T–Physiology N/P hypothesis that predicts decreasing N/P with increasing temperature. The results do support the Soil Substrate Age N/P hypothesis, which suggests strong control on leaf N and P due to soils: plants in warm (i.e., tropical) habitats are more P- than N-limited, likely because of substrate age and higher rates of leaching associated with higher rainfall (6, 8, 44), whereas plants in temperate soils, which are typically younger and less leached, are by far N-limited (4–8, 32, 33). The hypothesized leaf N/P breakpoint (10, 47) between N-limitation (N/P < 14) and P-limitation (N/P > 16) falls at roughly 18–22°C MAT for all data pooled and, if it applied equally to all species types, would suggest a potential transition from P- to N-limitation at < 25°C, 20°C, 15°C, and 12°C for coniferous trees, herbs, grasses, and angiosperm woody plants, respectively. However, it is likely that because of anatomical differences (1), critical N/P values for this threshold vary among groups. If the relative N- vs. P-limitation is similar for all five groups in our study at any given MAT (e.g., all are P-limited at the highest MAT and N-limited at <10°C), differences in the elevation of the N-to-P line would suggest that angiosperm trees operate without P-limitation at higher N/P than conifers.
In summary, our analyses indicate the existence of global patterns in higher plant leaf N, P, and N/P in relation to broad latitudinal and temperature gradients. These biogeographic gradients exist likely as a result of the collective forcing of several drivers. These patterns have important implications for our understanding of the biogeographic scaling of vegetation chemistry and ecosystem function and offer promise for the development of modeling tools useful at regional to global scales.
Supplementary Material
Acknowledgments
We thank I. Wright for helpful suggestions on the manuscript. This work was supported by National Science Foundation Long-Term Ecological Research Grant DEB-0080382 and International Programs Grant INT-9814047.
Abbreviations: MAT, mean annual temperature; r2, coefficient of determination.
See Commentary on page 10849.
References
- 1.Sterner, R.W. & Elser, J. J. (2002) Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere (Princeton Univ. Press, Princeton), pp. 1–439.
- 2.Woods, H. A., Makino, W., Cotner, J. B., Hobbie, S. E., Harrison, J. F., Acharya, K. & Elser, J. J. (2003) Funct. Ecol. 17, 237–245. [Google Scholar]
- 3.Hobbie, S. E., Schimel, J. P., Trumbore, S. E. & Randerson, J. R. (2000) Global Change Biol. 6, 196–210. [DOI] [PubMed] [Google Scholar]
- 4.Food and Agriculture Organization of the United Nations (1998) World Soil Resources Report 84: World Reference Base for Soil Resources (Food and Agric. Org., Rome).
- 5.U.S. Department of Agriculture/Natural Resources Conservation Service (2000) Global Soil Regions Map (U.S. Dept. Agric./Nat. Resources Conservat. Service, Washington, DC).
- 6.Crews, T. E., Kitayama, K., Fownes, J. H., Riley, R. H., Herbert, D. A., Mueller-Dombois, D. & Vitousek, P. M. (1995) Ecology 76, 1407–1424. [Google Scholar]
- 7.Vitousek, P. M., Turner, D. R. & Kitayama, K. (1995) Ecology 76, 712–720. [Google Scholar]
- 8.Chadwick, O. A., Derry, L. A., Vitousek, P. M., Huebert, B. J. & Hedin, L. O. (1999) Nature 397, 491–497. [Google Scholar]
- 9.Körner, C. (1999) Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems (Springer, Berlin), pp. 1–338.
- 10.Aerts, R. & Chapin, F. S. (2000) Adv. Ecol. Res. 30, 1–67. [Google Scholar]
- 11.Moorcroft, P. R., Hurtt, G. C. & Pacala, S. W. (2001) Ecol. Monogr. 71, 557–585. [Google Scholar]
- 12.Brown, J. H. (1999) Oikos 87, 3–14. [Google Scholar]
- 13.Walbridge, M. R. (1991) Ecology 72, 2083–2100. [Google Scholar]
- 14.Thompson, K., Parkinson, J. A., Band, S. R. & Spencer, R. E. (1997) New Phytol. 136, 679–689. [DOI] [PubMed] [Google Scholar]
- 15.Yin, X. W. (1993) Can. J. For. Res. 23, 1587–1602. [Google Scholar]
- 16.Chapin, F. S. & Oechel, W. C. (1983) Ecology 64, 743–751. [Google Scholar]
- 17.Körner, C. (1989) Oecologia 81, 379–391. [DOI] [PubMed] [Google Scholar]
- 18.Cordell, S., Goldstein, G., Mueller-Dombois, D., Webb, D. & Vitousek, P. M. (1998) Oecologia 113, 188–196. [DOI] [PubMed] [Google Scholar]
- 19.Oleksyn, J., Reich, P. B., Zytkowiak, R., Karolewski, P. & Tjoelker, M. G. (2003) Oecologia 136, 220–235. [DOI] [PubMed] [Google Scholar]
- 20.Tanner, E. V. J., Vitousek, P. M. & Cuevas, E. (1998) Ecology 79, 10–22. [Google Scholar]
- 21.Tjoelker, M. G., Reich, P. B. & Oleksyn, J. (1999) Plant Cell Environ. 22, 767–778. [Google Scholar]
- 22.Weih, M. & Karlsson, P. S. (2001) New Phytol. 150, 147–155. [Google Scholar]
- 23.Reich, P. B., Oleksyn, J. & Tjoelker, M. G. (1996) Funct. Ecol. 10, 768–776. [Google Scholar]
- 24.Oleksyn, J., Modrzynski, J., Tjoelker, M. G., Zytkowiak, R., Reich, P. B. & Karolewski, P. (1998) Funct. Ecol. 12, 573–590. [Google Scholar]
- 25.Oleksyn, J., Reich, P. B., Zytkowiak, R., Karolewski, P. & Tjoelker, M. G. (2002) Ann. For. Sci. 59, 1–18. [Google Scholar]
- 26.Hobbie, S. E. & Gough, L. (2002) Oecologia 131, 453–462. [DOI] [PubMed] [Google Scholar]
- 27.Van den Driessche, R. (1974) Bot. Rev. 40, 347–394. [Google Scholar]
- 28.Hobbie, S. E., Nadelhoffer, K. J. & Hogberg, P. (2002) Plant Soil 242, 163–170. [Google Scholar]
- 29.Kirschbaum, M. U. F. (1995) Soil Biol. Biochem. 27, 753–760. [Google Scholar]
- 30.Aerts, R. (1996) J. Ecol. 84, 597–608. [Google Scholar]
- 31.Treseder, K. K. & Vitousek, P. M. (2001) Ecology 82, 946–954. [Google Scholar]
- 32.Vitousek, P. M. (1982) Am. Nat. 119, 553–572. [Google Scholar]
- 33.Vitousek, P. M. & Sanford, R. L. (1986) Ann. Rev. Ecol. Syst. 17, 137–167. [Google Scholar]
- 34.Hedin, L. O., Vitousek, P. M. & Matson, P. A. (2003) Ecology 84, 2231–2255. [Google Scholar]
- 35.Reich, P. B., Walters, M. B. & Ellsworth, D. S. (1997) Proc. Natl. Acad. Sci. USA 94, 13730–13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reich, P. B., Uhl, C., Walters, M. B. & Ellsworth, D. S. (1991) Oecologia 86, 16–24. [DOI] [PubMed] [Google Scholar]
- 37.Kikuzawa, K. (1996) Vegetatio 122, 61–67. [Google Scholar]
- 38.Givnish, T. J. (2002) Silva Fenn. 36, 703–743. [Google Scholar]
- 39.Schoettle, A. W. (1990) Tree Physiol. 7, 209–214. [DOI] [PubMed] [Google Scholar]
- 40.Reich, P. B., Oleksyn, J., Modrzynski, J. & Tjoelker, M. G. (1996) Tree Physiol. 16, 643–642. [DOI] [PubMed] [Google Scholar]
- 41.Reich, P. B., Wright, I. J., Cavender-Bares, J., Craine, J. M., Oleksyn, J., Westoby, M. & Walters, M. B. (2003) Int. J. Plant Sci. 164(3S), S143–S164. [Google Scholar]
- 42.Elser, J. J., Acharya, K., Kyle, M., Cotner, J., Makino, W., Markow, T., Watts, T., Hobbie, S., Fagan, W., Schade, J., et al. (2003) Ecol. Lett. 6, 936–943. [Google Scholar]
- 43.Makino, W., Cotner, J. B., Sterner, R. W. & Elser, J. J. (2003) Funct. Ecol. 17, 121–130. [Google Scholar]
- 44.Richardson, S. W., Peltzer, D. A., Allen, R. B., McGlone, M. S. & Parfitt, R. L. (2004) Oecologia, in press. [DOI] [PubMed]
- 45.New, M., Hulme, M. & Jones, P. (1999) J. Clim. 12, 829–856. [Google Scholar]
- 46.McGroddy, M. E., Daufresne, T. & Hedin, L. O. (2004) Ecology, in press.
- 47.Koerselman, W. & Meuleman, A. F. M. (1996) J. Appl. Ecol. 33, 1441–1450. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.