Abstract
AIM
To identify the current indications and outcomes of total pancreatectomy at a high-volume center.
METHODS
A single institutional retrospective study of patients undergoing total pancreatectomy from 1995 to 2014 was performed.
RESULTS
One hundred and three patients underwent total pancreatectomy for indications including: Pancreatic ductal adenocarcinoma (n = 42, 40.8%), intraductal papillary mucinous neoplasms (n = 40, 38.8%), chronic pancreatitis (n = 8, 7.8%), pancreatic neuroendocrine tumors (n = 7, 6.8%), and miscellaneous (n = 6, 5.8%). The mean age was 66.2 years, and 59 (57.3%) were female. Twenty-four patients (23.3%) underwent a laparoscopic total pancreatectomy. Splenic preservation and portal vein resection and reconstruction were performed in 24 (23.3%) and 18 patients (17.5%), respectively. The 90 d major complications, readmission, and mortality rates were 32%, 17.5%, and 6.8% respectively. The 1-, 3-, 5-, and 7-year survival for patients with benign indications were 84%, 82%, 79.5%, and 75.9%, and for malignant indications were 64%, 40.4%, 34.7% and 30.9%, respectively.
CONCLUSION
Total pancreatectomy, including laparoscopic total pancreatectomy, appears to be an appropriate option for selected patients when treated at a high-volume pancreatic center and through a multispecialty approach.
Keywords: Intraductal papillary mucinous neoplasms, Laparoscopic total pancreatectomy, Pancreatic ductal adenocarcinoma, Laparoscopy, Pancreas cyst, Pancreas cancer
Core tip: Treatment by total pancreatectomy for diseases of the pancreas has been gained acceptance and used more frequently by pancreatic surgeons. This review highlights a large volume single institutional experience with this operation demonstrating acceptable short-term and long-term outcomes.
INTRODUCTION
In selected patients, total pancreatectomy (TP) has been established as a potential option in the treatment of pancreatic ductal adenocarcinoma (PDAC), invasive or diffuse intraductal papillary mucinous neoplasms (IPMN), multiple pancreatic neuroendocrine tumors (PNET)[1-4]. TP is still also one of the treatment modalities in chronic pancreatitis with severe pain, pancreatic fistula or hemorrhage after pancreaticoduodenectomy (PD)[3,5-7]. Improvement in postoperative management, including better pancreatic enzyme formula, long-acting insulin, and autologous islet cell transplantation, has made TP a viable choice in the treatment of different pancreatic diseases[8,9].
Although TP is performed at an increasing rate at major pancreatic centers, there is still debate regarding its indications and outcomes[2-4]. This study aimed to analyze the indications and short- and long-term outcomes of TP in the spectrum of pancreatic resections in our high-volume center.
MATERIALS AND METHODS
We conducted a retrospective study of 103 patients who underwent TP between March 1995 and December 2014 at Mayo Clinic in Jacksonville, Florida using data collected from an institutional review board-approved prospective database.
The preoperative data, including demographic data and clinical picture, operative details, and postoperative data were collected and analyzed.
Operative strategy
The American Society of Anesthesiologists classification[10] and Eastern Cooperative Oncology Group performance status[11] were used for evaluation of the preoperative risk. All patients were optimized medically prior to surgery.
TP was performed either open or laparoscopic. It was done as pylorus-preserving, standard, or completion TP (of previous distal pancreatectomy or PD). The operation was done with and without splenectomy.
In cases with partial resection in pancreatic tumors, the specimen margin was analyzed by frozen section. The procedure was converted to TP if the margins showed carcinoma in situ or invasive carcinoma. TP was not performed for frozen section findings of moderate dysplasia or adenomatous changes at the margin. International consensus guidelines for the management of patients diagnosed with IPMN was used throughout the study time period, as appropriate. Guidelines from 2006[12] were followed up until these were updated in 2012[13].
The International Study Group of Pancreatic Surgery (ISGPS) classification of venous resection was used as follows[14]: Type I: Partial venous resection with direct closure (venorraphy); Type II: Partial venous excision with closure by patch graft; Type III: Excision with primary end to end venous anastomosis; Type IV: Venous resection with interposition venous graft.
Histopathological data on pancreatic tumor staging were collected according to the tumor, node and metastases staging system. IPMN pathology was defined as per World Health Organization criteria into 4 categories based upon the degree of dysplasia: Adenoma, border line (low-moderate dysplasia), carcinoma in situ (CIS) high-grade dysplasia), or invasive carcinoma[2]. PDAC without IPMN was considered to be de novo, but PDAC associated with IPMN was considered to be arisen from these IPMN.
Postoperative complications occurring in the first 90 d after surgery were graded from (0 to 5) according to the Clavien system[15]. Grade I and II complications were considered minor and Grade III, IV and V were considered major complications. International consensus guidelines were used to evaluate specific complications[16,17]. Major glycemic events included complications or readmissions related to severe hyperglycemia or hypoglycemia. Cardiac complications, pulmonary complications, renal insufficiency, or hepatic insufficiency were defined as temporary organ system dysfunction requiring supportive care over the usual standard postoperative measures.
The follow-up period (1-18 years) was from date of surgery. Any death during the hospital stay or within the first 90 d after surgery was defined as perioperative mortality. Readmissions to any facility were recorded for 90 d after surgery.
After analysis of results and outcomes from our previous publication in 2009[4], patients undergoing consideration for total pancreatectomy were sent for preoperative evaluation and counseling by a nutrition and endocrine team for the anticipated exocrine and endocrine insufficiency caused by surgical intervention. Postoperatively, patients were seen and followed in the hospital setting by the inpatient nutrition and endocrine team for ongoing education and management regarding the subsequent pancreatic insufficiency. Insulin and enzyme replacement were determined according to individual patient needs. In addition, percutaneous jejunostomy feeding tube placement became a standard procedure during TP after 2009, and many patients were started on enteral tube feeds in the hospital setting and continued on after discharge to aid in avoiding readmissions for malnutrition.
Postoperatively, patients were treated on the medical-surgical floor and intensive care use was not routine unless indicated. Perioperative use of parenteral nutrition and blood product transfusion was also limited unless indicated. Based on final pathology, adjuvant treatments including chemotherapy or radiotherapy were recommended to patients undergoing TP for periampullary adenocarcinoma.
Statistical analysis
Data were collected and analyzed by a biomedical statistician using SPSS version 21.0 (SPSS Inc., Chicago, IL, United States). A Kaplan-Meier curve was used for the analysis of survival.
RESULTS
From March 1995 to December 2014, 983 pancreatic resections were performed for benign and malignant pancreatic diseases; TP was performed in 103 patients (10.5%). The demographic and preoperative clinicopathological data are listed in Table 1. Subgroup analysis for those undergoing TP for PDAC and IPMN is shown. Indications for TP (rather than partial pancreatectomy) was multifocal disease (55 patients, 53.4%), positive margins (23 patients, 22.3%), elective completion TP for recurrence of the primary pathology (5 patients, 4.9%), or other (20 patients, 19.4%). There were no cases with emergent TP as treatment for postoperative pancreatic fistula (POPF) or hemorrhage from patients undergoing partial pancreatectomy in this study. POPF was treated with interventional radiological procedures, and in this study, all completion TP was performed in an elective fashion for pancreatic pathology.
Table 1.
Demographics for 103 patients undergoing total pancreatectomy with subgroup analysis
| Variable | Overall (n = 103) | PDAC (n = 42) | IPMN (n = 40) | LTP (n = 24) |
| Age, yr1 | 70.2 (32-84.5) | 70.3 (33.3-84.5) | 71.3 (42.9-81.1) | 70.3 (37.3-84.5) |
| Body mass index1 | 25.1 (17.7-42.1) | 24.2 (17.7-39.8) | 25.8 (18.5-42.1) | 26.7 (17.7-36.3) |
| Male | 44 (42.7%) | 18 (42.9%) | 19 (47.5%) | 13 (54.2%) |
| Hypertension | 70 (68%) | 29 (69%) | 31 (77.5%) | 14 (58.3%) |
| Diabetes | 46 (44.7%) | 19 (45.2%) | 16 (40%) | 13 (54.2%) |
| Cardiac disease | 31 (30.1%) | 8 (19%) | 14 (35%) | 7 (29.2%) |
| Pulmonary disease | 19 (18.4%) | 7 (16.7%) | 7 (17.5%) | 5 (20.8%) |
| ASA | ||||
| II | 24 (23.3%) | 11 (26.2%) | 9 (22.5%) | 2 (8.3%) |
| III | 72 (69.9%) | 26 (61.9%) | 29 (72.5%) | 22 (91.7%) |
| IV | 7 (6.8%) | 5 (11.9%) | 2 (5%) | 0 |
| Type of resection | ||||
| Standard | 35 (34%) | 17 (40.5%) | 10 (25%) | 5 (20.8%) |
| Pylorus preserving | 56 (54.4%) | 20 (47.6%) | 27 (67.5%) | 16 (66.7%) |
| Completion | 12 (11.6%) | 5 (11.9%) | 3 (7.5%) | 3 (12.5%) |
Values are median (range), values in parenthesis are percentages unless otherwise indicated. ASA: American Society of Anesthesiologists score; IPMN: Intraductal papillary mucinous neoplasm; LTP: Laparoscopic total pancreatectomy; PDAC: Pancreatic ductal adenocarcinoma.
Overall, 79 patients (76.7%) underwent an open TP. Laparoscopic TP (LTP) was attempted in 31 patients (30.1%), and conversion to open occurred in 7 patients (22.6%) due to adhesions from chronic pancreatitis in 5 patients, bleeding in 1 patient, and portal vein involvement by the tumor in 1 patient. Of the 24 patients who did not require conversion from LTP, a hand-assisted approach was used for 2 patients, and subgroup analysis is given in the tables. LTP was introduced in November 2008, and a total of 52 patients (50.5%) had TP after this date. Of these, 28 (53.8%) underwent open surgery and 24 (46.2%) underwent LTP. Spleen-preserving TP was done in 29 patients (28.2%), four of whom underwent spleen-preserving LTP.
Operative variables are found in Table 2. LTP was found to have longer operative times, but less blood loss. Vein resection was performed in 18 patients (17.5%). The resections were conducted according to the ISGPS classification of vein resection, which included type I (lateral venorraphy) in 4 patients (22.22%), type II (patch graft) in 2 (11.11%; 1 from gonadal vein and 1 from bovine graft), type III (primary anastomosis) in 6 (33.33%), and type IV (interposition venous graft) in 6 (33.33%; 4 by 14 mm polytetrafluoroethylene synthetic graft, 1 from gonadal vein, and 1 from splenic vein). One patient of LTP had venous resection and laparoscopic lateral venorraphy.
Table 2.
Operative variables for 103 patients undergoing total pancreatectomy with subgroup analysis
| Variable | Overall (n = 103) | PDAC (n = 42) | IPMN (n = 40) | LTP (n = 24) |
| Operative time (min)1 | 426 (165-930) | 390 (165-636) | 435 (240-930) | 534 (234-770) |
| Estimated blood loss (mL)1 | 500 (50-18000) | 500 (50-7800) | 525 (50-18000) | 200 (50-600) |
| Intraoperative pRBC transfusion (unit)1 | 1 (0%-40%) | 2 (0%-30%) | 1 (0%-40%) | 0 (0%-2%) |
| Vein resection | 18 (17.5%) | 13 (40%) | 4 (10%) | 1 (4.2%) |
Values are median (range), values in parenthesis are percentages unless otherwise indicated. IPMN: Intraductal papillary mucinous neoplasm; LTP: Laparoscopic total pancreatectomy; PDAC: Pancreatic ductal adenocarcinoma; pRBC: Intraoperative packed red blood cell.
Table 3 gives the 90-d complications and postoperative outcomes, including length of stay and readmission rate, for those undergoing TP overall and by subgroup. Major postoperative complications were found in 33 (32%) patients, and reoperation was done for 7 patients (6.8%) due to abdominal collections (4 patients), post-pancreatectomy hemorrhage (2 patients), and intestinal fistula not responding to conservative or radiological interventions (1 patient).
Table 3.
Postoperative outcomes and complications (90 d) for 103 patients undergoing total pancreatectomy
| Variable | Overall (n = 103) | PDAC (n = 42) | IPMN (n = 40) | LTP (n = 24) |
| Cardiac | 10 (9.7%) | 5 (11.9%) | 3 (7.5%) | 1 (4.2%) |
| Pulmonary | 15 (14.6%) | 7 (16.7%) | 6 (15%) | 5 (20.8%) |
| Renal insufficiency | 8 (7.8%) | 3 (7.1%) | 6 (15%) | 4 (16.7%) |
| Hepatic insufficiency | 3 (2.4%) | 1 (2.4%) | 3 (7.5%) | 2 (8.3%) |
| Major glycemic event | 6 (5.8%) | 3 (7.1%) | 2 (5%) | 1 (4.2%) |
| Post-pancreatectomy hemorrhage | 5 (4.9%) | 0 (0.0%) | 4 (10%) | 4 (16.7%) |
| A | 2 (1.9%) | 0 | 2 (5%) | 2 (8.3%) |
| B | 1 (0.8%) | 0 | 0 | 1 (4.2%) |
| C | 2 (1.9%) | 0 | 2 (5%) | 1 (4.2%) |
| Delayed gastric emptying | 14 (13.6%) | 5 (11.9%) | 5 (12.5%) | 2 (8.3%) |
| A | 4 (4.9%) | 2 (4.8%) | 2 (5%) | 0 |
| B | 5 (4.9%) | 2 (4.8%) | 1 (2.5%) | 1 (4.2%) |
| C | 5 (4.9%) | 1 (2.4%) | 2 (5%) | 1 (4.2%) |
| Wound infection | 11 (10.7%) | 5 (11.9%) | 2 (5%) | 2 (8.3%) |
| Intra-abdominal abscess | 14 (13.6%) | 2 (4.8%) | 8 (20%) | 4 (16.7%) |
| Biliary fistula | 2 (1.9%) | 0 | 1 (2.5%) | 0 |
| Mesenteric/portal vein thrombosis | 5 (4.9%) | 2 (4.8%) | 2 (5%) | 1 (4.2%) |
| Reoperation | 7 (6.8%) | 2 (4.8%) | 2 (5%) | 2 (8.3%) |
| Patients intensive care stay | 64 (62.1%) | 24 (57.1%) | 30 (75%) | 10 (41.7%) |
| Median intensive care stay, d1 (range) | 2 (1-59) | 2 (1-12) | 2 (1-59) | 2 (1-33) |
| Overall morbidity | 66 (64.1%) | 27 (64.3%) | 25 (62.5%) | 10 (41.7%) |
| Major (III-V) | 33 (32%) | 13 (31%) | 13 (32.5%) | 5 (20.8%) |
| IIIa | 14 (13.6%) | 5 (11.9%) | 6 (15%) | 0 |
| IIIb | 3 (2.9%) | 2 (4.8%) | 0 | 0 |
| IVa | 4 (4.9%) | 1 (2.4%) | 2 (5%) | 2 (8.3%) |
| IVb | 5 (4.9%) | 2 (4.8%) | 1 (2.5%) | 2 (8.3%) |
| V | 7 (6.8%) | 3 (7.1%) | 4 (10%) | 1 (4.2%) |
| Length of stay, d1 (range) | 9 (3%-71%) | 9 (3%-71%) | 10 (4%-67%) | 8 (4%-52%) |
| Readmission | 26 (25.2%) | 12 (28.6%) | 8 (20%) | 3 (12.5%) |
Values are median (range), values in parenthesis are percentages unless otherwise indicated. IPMN: Intraductal papillary mucinous neoplasm; LTP: Laparoscopic total pancreatectomy; PDAC: Pancreatic ductal adenocarcinoma.
Pathological indications for TP are listed in Table 4. PDAC and IPMN were the most common indications for surgery. Sixty-two patients (60.2%) were found to have IPMN upon final pathology. Forty patients (38.8%) had this as the only pathologic process, while IPMN was associated with other pancreatic pathology in 22 patients (21.4%).
Table 4.
Pathologic findings for 103 patients undergoing total pancreatectomy
| Variable | Overall (n = 103) | LTP (n = 24) |
| Malignant | n = 53 | n = 12 |
| Pancreatic ductal adenocarcinoma | 42 (40.8%) | 5 (20.8%) |
| De novo | 23 (22.3%) | 3 (12.5%) |
| Arising from IPMN | 19 (18.5%) | 2 (8.3%) |
| Neuroendocrine | 7 (6.8%) | 6 (25%) |
| Cholangiocarcinoma with IPMN | 1 (0.97%) | 0 |
| Ampullary adenocarcinoma with IPMN | 1 (0.97%) | 1 (4.2%) |
| Renal cell carcinoma | 1 (0.97%) | 0 |
| Sarcoma | 1 (0.97%) | 0 |
| Tumor size, cm1 | 3.5 ± 2.4 (0.5%-14%) | 3.2 ± 2.5 (1.3%-10%) |
| Margin negative (R0) | 41 (77.4%) | 11 (91.7%) |
| Number of lymph nodes harvested1 | 23 ± 14 (1%-61%) | 28 ± 11 (11%-41%) |
| Non malignant | n = 50 | n = 12 |
| IPMN | 40 (38.8%) | 11 (45.8%) |
| Chronic pancreatitis | 8 (7.8%) | 1 (4.2%) |
| Ampullary adenoma with IPMN | 1 (0.97%) | 0 |
| Trauma | 1 (0.97%) | 0 |
Values are mean (range), values in parenthesis are percentages unless otherwise indicated. IPMN: Intraductal papillary mucinous neoplasm; LTP: Laparoscopic total pancreatectomy.
Twenty of 42 patients with PDAC (47.6%), had tumor recurrence; 10 (50%) had distant metastasis (mainly to the liver and lung), 3 (15%) local recurrence, and 7 (35%) had both distant and local recurrence. The mean time of recurrence was 9.5 mo (range: 2.5-27 mo).
Overall, the 90-d perioperative mortality was 7 patients (6.8%). The 1-, 3-, 5-, and 7-year total survival rate for all 103 patients were 73.7%, 61.3%, 57.5%, and 53.8%, respectively (Figure 1A). The 1-, 3-, 5-, and 7-year survival for patients without malignant tumors (50 patients) were 84%, 82%, 79.5%, and 75.9%, respectively, while in patients with malignant findings (53 patients) the survival rates were 64%, 40.4%, 34.7%, and 30.9%, respectively. The 1-, 3-, 5-, and 7-year survival rates in patients who had PDAC (42 patients) were 59.5%, 29.2%, 21.9%, and 18.3%, respectively (Figure 1B).
Figure 1.
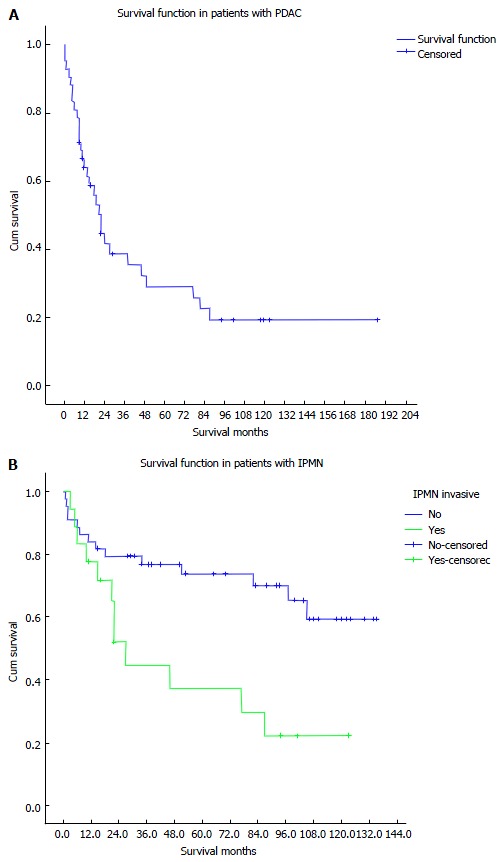
Kaplan Meier curve for survival. A: Survival in patients with pancreatic ductal adenocarcinoma; B: Survival in patients with intraductal papillary mucinous neoplasms. PDAC: Pancreatic ductal adenocarcinoma; IPMN: Intraductal papillary mucinous neoplasms.
The 1-, 3-, 5-, and 7-year survival rates in patients with non-invasive IPMN (44 patients) were 84.1%, 76.9%, 73.8%, and 70.1%, respectively, while in patients with invasive IPMN (18 patients) the survival rates were 77.8%, 44.8%, 37.3%, and 29.8%, respectively.
DISCUSSION
Enthusiasm for TP has varied with time. This major operation should be assessed carefully for operative risk and postoperative outcomes after the loss of the exocrine and endocrine functions of the pancreas[1,3].
Murphy et al[5] reported that there was an increase in the rate of TP in the United States between 1998 and 2006. In about a 20-year span, 50.5% of the TP on this series were performed during the last 6 years of the study. In this publication, there was increased utilization of elective TP, especially after early diagnosis of multifocal pancreatic pathologies like IPMN and multiple PNET, and the role of TP in the management of chronic pancreatitis was limited only to patients with refractory pain not responding to medical treatment.
On the other hand, there was significant decline in the use of emergency TP as in POPF and hemorrhage. This was mainly due to better use of radiologic drainage and arterial embolization which became available and preferable to relaparotomy[6]. In our study, we had only 1 patient who underwent an emergency TP for abdominal trauma.
The recommendations of the international consensus guidelines in the management of IPMN depended on its site in the main duct or side branches and its clinical and morphological picture in preoperative imaging study. TP should be performed in patients with positive multiple surgical margins for invasive IPMN or high-grade dysplasia on frozen section[13]. In our study, the existence of main duct IPMN as the primary pathology accounted for 38.8% of all TP performed, and IPMN associated with other tumors accounted for 21.4%, but elsewhere IPMN has been reported to encompass 22% of all TP performed[3].
Dallemagne et al[18] demonstrated the feasibility and advantage of TP with the laparoscopic approach. They reported two cases of TP with pylorus and splenic preservation with good postoperative outcomes. Blood loss, intensive care unit length of stay, and overall hospital length of stay were shorter[19-21]. Asbun and Stauffer also reported 11 patients with LTP[19].
Zeh et al[22] and Buchs et al[23] reported that robotic assistance LTP can offer more advantages. Giulianotti documented five cases with laparoscopic robotic surgery, with spleen-preserving technique in two of them (Kimura technique)[24]. Also, Boggi et al[25] showed the feasibility of robot-assisted LTP in a series of 11 patients without conversion to open surgery.
Choi et al[26] and Ferrone et al[27] reported four patients with laparoscopic-assisted pylorus and spleen-preserving TP with segmental excision of the splenic artery and vein (Warshaw’s procedure), but with a small midline opening for completion of the anastomosis.
In our study, 24 patients underwent full LTP, 16 underwent pylorus-preserving LTP, and 5 underwent spleen-preserving LTP. Patients with LTP had a higher negative margins rate and significantly more lymph nodes removed than in open surgery.
There are still high postoperative complications after TP. In our study, 32% of patients had a major complication after TP. This matches with other series that had complication rates of 32%-54%[4,28]. However, the complications after partial resection and TP were not significantly different in one study done on 124 patients with TP[29].
The postoperative outcomes related to exocrine and endocrine pancreatic functions may affect the enthusiasm for TP. Postoperative diabetes may be difficult to control, with reported mortality from hypoglycemia[30,31]. In our study, no such deaths were reported. The improvements in insulin, well-trained nurses, and exposure to a diabetic care team prior to the procedure led to dramatically improved diabetic outcomes post-TP[30,32]. Since 2008, at our institution, we have implemented a preoperative TP pathway in which patients receive glucose management, enzyme replacement, and nutritional education prior to the operation. The indications and management decisions are done through discussion at a multidisciplinary Pancreas Board that is held on a weekly basis.
Wu et al[33] reported that islet autotransplantation (IAT) is a safe modality for patients who had chronic pancreatitis and underwent TP, and a significant number of patients can achieve insulin independence for a long time after receiving enough islet equivalent per kg body weight. None of our patients who underwent TP for chronic pancreatitis were candidates for TP and IAT as all had concerns for neoplasia. Any candidate for TP and IAT are sent to those referral centers. Hence, there is a possible bias towards a small number of patients undergoing TP for chronic pancreatitis at our institution.
The development of enzyme replacement formulations and the use of the duodenum-preserving TP have improved morbidity from exocrine insufficiency. However, in one study, the pylorus-preserving TP was not associated with significantly different nutritional status than the standard TP[34].
Intraoperative frozen section analysis of the resection margins during partial pancreatectomies is important to emphasize R0 resection. There was a significantly better survival in patients undergoing completion TP after positive margins during PD than patients undergoing R1 resection in a study done on 33 patients with PDAC[35]. In our study, 21.4% of the patients who underwent distal pancreatectomies and pancreaticoduodenectomies also had positive resection margins in the pathological study and required TP in the same operative sitting.
Survival of patients undergoing TP varied according to the underlying disease process. Patients with benign disease had a high survival rate whereas those with invasive IPMN or other malignant tumors had poor survival as shown in our study. Some institutions reported high operative mortality rates to be greater than 20% and associated with high morbidity, and this led these centers to stand against the role of TP[36-38]. However, recent institution series have reported lower perioperative mortality rates to be 3%-6.1%[4,5,32], which was near to our 90-d perioperative mortality rate of 6.8%. The improved results in recent years are likely due to improvements in perioperative support, education, and possibly, enhanced surgical technique.
In a recent study, Johnston et al[7] reported that the first month perioperative mortality after TP was 5.5%. The median survival was 15 ms, and the 1-, 3-, and 5-year overall survival rates were 60%, 22% and 13%, respectively. In multivariate analysis, the factors that affected survival were age, positive lymph nodes, positive surgical margin, tumor grade, tumor size, and adjuvant chemotherapy.
Baiocchi et al[2] and Salvia et al[39] reported that IPMN was resectable in 90%-100% of the patients. The survival rates for CIS, invasive carcinoma, and presence of nodal metastasis were 80%-90%, 50%-70% and 40%-50%, respectively.
The role of emergency TP is declining in favor of alternative interventional radiological strategies for the management of POPF and hemorrhage. Venous reconstruction in appropriately selected patients with PDAC and locally advanced tumor can be done safely without negatively affecting recurrence or survival when compared to patients without vein involvement by the tumor. LTP appears to be a feasible and safe procedure when performed by experienced hands at a high-volume center. The significant metabolic derangements after TP may not become immediately apparent in the postoperative inpatient recovery phase, and may lead to a high rate of readmissions later on, so strict follow-up protocol should be available for these patients. A multidisciplinary approach with all-encompassing perioperative education that includes diabetic and nutritional counseling appears to be essential in the successful management of these patients.
ACKNOWLEDGMENTS
The authors would like to acknowledge the assistance provided by Mauricia Buchanan in data collection.
COMMENTS
Background
In selected patients, total pancreatectomy (TP) has been established as a potential option in the treatment of pancreatic ductal adenocarcinoma, invasive or diffuse intraductal papillary mucinous neoplasms, multiple pancreatic neuroendocrine tumors.
Research frontiers
A single institutional retrospective study of patients undergoing total pancreatectomy from 1995 to 2014 was performed.
Innovations and breakthroughs
TP is performed at an increasing rate at major pancreatic centers, there is still debate regarding its indications and outcomes. This study aimed to analyze the indications and short- and long-term outcomes of TP in the spectrum of pancreatic resections in this high-volume center.
Applications
Total pancreatectomy, including laparoscopic total pancreatectomy, appears to be an appropriate option for selected patients when treated at a high-volume pancreatic center and through a multispecialty approach.
Terminology
The authors conducted a retrospective study of 103 patients who underwent TP between March 1995 and December 2014 at Mayo Clinic in Jacksonville, Florida using data collected from an institutional review board-approved prospective database.
Peer-review
The staff and surgeons are very experienced, presenting excellent postoperative outcome and long-term survival.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Institutional review board statement: We conducted a retrospective study of 103 patients who underwent TP between March 1995 and December 2014 at Mayo Clinic in Jacksonville, Florida using data collected from an institutional review board-approved prospective database.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient gave informed consent for institutional data collection and sharing.
Conflict-of-interest statement: Dr. Zakaria, Dr. Stauffer, Dr. Raimondo, Dr. Woodward, Dr. Wallace and Dr. Asbun report no biomedical financial interests or potential conflict of interest.
Data sharing statement: No additional data are available.
Peer-review started: March 20, 2016
First decision: April 19, 2016
Article in press: July 13, 2016
P- Reviewer: Sandblom G, Shelat VG S- Editor: Qiu S L- Editor: A E- Editor: Li D
References
- 1.Kulu Y, Schmied BM, Werner J, Muselli P, Büchler MW, Schmidt J. Total pancreatectomy for pancreatic cancer: indications and operative technique. HPB (Oxford) 2009;11:469–475. doi: 10.1111/j.1477-2574.2009.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baiocchi GL, Portolani N, Missale G, Baronchelli C, Gheza F, Cantù M, Grazioli L, Giulini SM. Intraductal papillary mucinous neoplasm of the pancreas (IPMN): clinico-pathological correlations and surgical indications. World J Surg Oncol. 2010;8:25. doi: 10.1186/1477-7819-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almond M, Roberts KJ, Hodson J, Sutcliffe R, Marudanayagam R, Isaac J, Muiesan P, Mirza D. Changing indications for a total pancreatectomy: perspectives over a quarter of a century. HPB (Oxford) 2015;17:416–421. doi: 10.1111/hpb.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stauffer JA, Nguyen JH, Heckman MG, Grewal MS, Dougherty M, Gill KR, Jamil LH, Scimeca D, Raimondo M, Smith CD, et al. Patient outcomes after total pancreatectomy: a single centre contemporary experience. HPB (Oxford) 2009;11:483–492. doi: 10.1111/j.1477-2574.2009.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy MM, Knaus WJ, Ng SC, Hill JS, McPhee JT, Shah SA, Tseng JF. Total pancreatectomy: a national study. HPB (Oxford) 2009;11:476–482. doi: 10.1111/j.1477-2574.2009.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haddad LB, Scatton O, Randone B, Andraus W, Massault PP, Dousset B, Soubrane O. Pancreatic fistula after pancreaticoduodenectomy: the conservative treatment of choice. HPB (Oxford) 2009;11:203–209. doi: 10.1111/j.1477-2574.2009.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston WC, Hoen HM, Cassera MA, Newell PH, Hammill CW, Hansen PD, Wolf RF. Total pancreatectomy for pancreatic ductal adenocarcinoma: review of the National Cancer Data Base. HPB (Oxford) 2016;18:21–28. doi: 10.1016/j.hpb.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jethwa P, Sodergren M, Lala A, Webber J, Buckels JA, Bramhall SR, Mirza DF. Diabetic control after total pancreatectomy. Dig Liver Dis. 2006;38:415–419. doi: 10.1016/j.dld.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Johnston PC, Lin YK, Walsh RM, Bottino R, Stevens TK, Trucco M, Bena J, Faiman C, Hatipoglu BA. Factors associated with islet yield and insulin independence after total pancreatectomy and islet cell autotransplantation in patients with chronic pancreatitis utilizing off-site islet isolation: Cleveland Clinic experience. J Clin Endocrinol Metab. 2015;100:1765–1770. doi: 10.1210/jc.2014-4298. [DOI] [PubMed] [Google Scholar]
- 10.Haynes SR, Lawler PG. An assessment of the consistency of ASA physical status classification allocation. Anaesthesia. 1995;50:195–199. doi: 10.1111/j.1365-2044.1995.tb04554.x. [DOI] [PubMed] [Google Scholar]
- 11.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 12.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, Asbun HJ, Bassi C, Büchler M, Charnley RM, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2014;155:977–988. doi: 10.1016/j.surg.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 15.DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, Yeo CJ, Clavien PA. Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931–937; discussion 931-937. doi: 10.1097/01.sla.0000246856.03918.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Dallemagne B, de Oliveira AT, Lacerda CF, D’Agostino J, Mercoli H, Marescaux J. Full laparoscopic total pancreatectomy with and without spleen and pylorus preservation: a feasibility report. J Hepatobiliary Pancreat Sci. 2013;20:647–653. doi: 10.1007/s00534-013-0593-3. [DOI] [PubMed] [Google Scholar]
- 19.Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg. 2012;215:810–819. doi: 10.1016/j.jamcollsurg.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Kim SC, Song KB, Jung YS, Kim YH, Park do H, Lee SS, Seo DW, Lee SK, Kim MH, Park KM, et al. Short-term clinical outcomes for 100 consecutive cases of laparoscopic pylorus-preserving pancreatoduodenectomy: improvement with surgical experience. Surg Endosc. 2013;27:95–103. doi: 10.1007/s00464-012-2427-9. [DOI] [PubMed] [Google Scholar]
- 21.Casadei R, Marchegiani G, Laterza M, Ricci C, Marrano N, Margiotta A, Minni F. Total pancreatectomy: doing it with a mini-invasive approach. JOP. 2009;10:328–331. [PubMed] [Google Scholar]
- 22.Zeh HJ, Zureikat AH, Secrest A, Dauoudi M, Bartlett D, Moser AJ. Outcomes after robot-assisted pancreaticoduodenectomy for periampullary lesions. Ann Surg Oncol. 2012;19:864–870. doi: 10.1245/s10434-011-2045-0. [DOI] [PubMed] [Google Scholar]
- 23.Buchs NC, Addeo P, Bianco FM, Ayloo S, Benedetti E, Giulianotti PC. Robotic versus open pancreaticoduodenectomy: a comparative study at a single institution. World J Surg. 2011;35:2739–2746. doi: 10.1007/s00268-011-1276-3. [DOI] [PubMed] [Google Scholar]
- 24.Giulianotti PC, Addeo P, Buchs NC, Bianco FM, Ayloo SM. Early experience with robotic total pancreatectomy. Pancreas. 2011;40:311–313. doi: 10.1097/MPA.0b013e3181f7e303. [DOI] [PubMed] [Google Scholar]
- 25.Boggi U, Palladino S, Massimetti G, Vistoli F, Caniglia F, De Lio N, Perrone V, Barbarello L, Belluomini M, Signori S, et al. Laparoscopic robot-assisted versus open total pancreatectomy: a case-matched study. Surg Endosc. 2015;29:1425–1432. doi: 10.1007/s00464-014-3819-9. [DOI] [PubMed] [Google Scholar]
- 26.Choi SH, Hwang HK, Kang CM, Yoon CI, Lee WJ. Pylorus- and spleen-preserving total pancreatoduodenectomy with resection of both whole splenic vessels: feasibility and laparoscopic application to intraductal papillary mucin-producing tumors of the pancreas. Surg Endosc. 2012;26:2072–2077. doi: 10.1007/s00464-011-2113-3. [DOI] [PubMed] [Google Scholar]
- 27.Ferrone CR, Konstantinidis IT, Sahani DV, Wargo JA, Fernandez-del Castillo C, Warshaw AL. Twenty-three years of the Warshaw operation for distal pancreatectomy with preservation of the spleen. Ann Surg. 2011;253:1136–1139. doi: 10.1097/SLA.0b013e318212c1e2. [DOI] [PubMed] [Google Scholar]
- 28.Crippa S, Tamburrino D, Partelli S, Salvia R, Germenia S, Bassi C, Pederzoli P, Falconi M. Total pancreatectomy: indications, different timing, and perioperative and long-term outcomes. Surgery. 2011;149:79–86. doi: 10.1016/j.surg.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Müller MW, Friess H, Kleeff J, Dahmen R, Wagner M, Hinz U, Breisch-Girbig D, Ceyhan GO, Büchler MW. Is there still a role for total pancreatectomy? Ann Surg. 2007;246:966–974; discussion 974-975. doi: 10.1097/SLA.0b013e31815c2ca3. [DOI] [PubMed] [Google Scholar]
- 30.Barbier L, Jamal W, Dokmak S, Aussilhou B, Corcos O, Ruszniewski P, Belghiti J, Sauvanet A. Impact of total pancreatectomy: short- and long-term assessment. HPB (Oxford) 2013;15:882–892. doi: 10.1111/hpb.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts KJ, Blanco G, Webber J, Marudanayagam R, Sutcliffe RP, Muiesan P, Bramhall SR, Isaac J, Mirza DF. How severe is diabetes after total pancreatectomy? A case-matched analysis. HPB (Oxford) 2014;16:814–821. doi: 10.1111/hpb.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Billings BJ, Christein JD, Harmsen WS, Harrington JR, Chari ST, Que FG, Farnell MB, Nagorney DM, Sarr MG. Quality-of-life after total pancreatectomy: is it really that bad on long-term follow-up? J Gastrointest Surg. 2005;9:1059–1066; discussion 1066-1067. doi: 10.1016/j.gassur.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Wu Q, Zhang M, Qin Y, Jiang R, Chen H, Xu X, Yang T, Jiang K, Miao Y. Systematic review and meta-analysis of islet autotransplantation after total pancreatectomy in chronic pancreatitis patients. Endocr J. 2015;62:227–234. doi: 10.1507/endocrj.EJ14-0510. [DOI] [PubMed] [Google Scholar]
- 34.Sugiyama M, Atomi Y. Pylorus-preserving total pancreatectomy for pancreatic cancer. World J Surg. 2000;24:66–70; discussion 70-71. doi: 10.1007/s002689910013. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt CM, Glant J, Winter JM, Kennard J, Dixon J, Zhao Q, Howard TJ, Madura JA, Nakeeb A, Pitt HA, et al. Total pancreatectomy (R0 resection) improves survival over subtotal pancreatectomy in isolated neck margin positive pancreatic adenocarcinoma. Surgery. 2007;142:572–578; discussion 572-578. doi: 10.1016/j.surg.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 36.Cooperman AM, Herter FP, Marboe CA, Helmreich ZV, Perzin KH. Pancreatoduodenal resection and total pnacreatectomy--an institutional review. Surgery. 1981;90:707–712. [PubMed] [Google Scholar]
- 37.Ihse I, Anderson H. Total pancreatectomy for cancer of the pancreas: is it appropriate? World J Surg. 1996;20:288–293; discussion 294. doi: 10.1007/s002689900046. [DOI] [PubMed] [Google Scholar]
- 38.Brooks JR. Where are we with pancreas transplantation? Surgery. 1989;106:935–945. [PubMed] [Google Scholar]
- 39.Salvia R, Fernández-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, Pederzoli P, Warshaw AL. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–685; discussion 685-687. doi: 10.1097/01.sla.0000124386.54496.15. [DOI] [PMC free article] [PubMed] [Google Scholar]


