Abstract
To our knowledge, effects of age, placental malaria infection, infections during follow-up, nutritional habits, sickle-cell trait and individual exposure to Anopheles bites were never explored together in a study focusing on the acquisition of malaria antibody responses among infants living in endemic areas.Five hundred and sixty-seven Beninese infants were weekly followed-up from birth to 18 months of age. Immunoglobulin G (IgG), IgG1 and IgG3 specific for 5 malaria antigens were measured every 3 months. A linear mixed model was used to analyze the effect of each variable on the acquisition of antimalarial antibodies in 6-to18-month old infants in univariate and multivariate analyses. Placental malaria, nutrition intakes and sickle-cell trait did not influence the infant antibody levels to P. falciparum antigens. In contrary, age, malaria antibody levels at birth, previous and present malaria infections as well as exposure to Anopheles bites were significantly associated with the natural acquisition of malaria antibodies in 6-to18-month old Beninese infants. This study highlighted inescapable factors to consider simultaneously in an immuno-epidemiological study or a vaccine trial in early life.
Naturally-acquired immunity to malaria develops slowly following repeated exposures to Plasmodium falciparum. Parasite, host, environmental and behavioral factors are essential to be examined for a better understanding of the acquisition of clinical immunity and thus clinical outcomes of malaria infections. To our knowledge, this was not exhaustively investigated in previous studies. Indeed, Dobaño et al. evaluated the age dependence of naturally acquired antibodies to Merozoite Surface Protein (MSP) 1-19, Apical Membrane Antigen 1 (AMA1), and Erythrocyte Binding Antigen 175 only on the basis of past and present parasite exposure, occurrence of previous episodes and neighborhood of residence1. Moreover, Sarr et al. showed that malaria specific antibody responses increased with age in children under 5 years old2. Baird et al. clearly showed that host age, independent of cumulative exposure, represents a determinant key of the quantitative and qualitative immunoglobulin G (IgG) response to P. falciparum, certainly through an immune system maturation process3. In fact, the role of age in this process is complex and cannot be only related to protection increasing with age as a cumulative product of exposure to antigen4.
Environmental factors also influence malaria specific antibody levels. Sarr et al., concluded that malaria antibody responses differed according to the areas where distinct Anopheles species evolve2. Drakeley et al. have observed significant associations between parasite prevalence, altitude, as well as recent rainfalls and the prevalence of antibodies specific for MSP1-19 in individuals between 3 to 45 years old5. Khaireh et al. showed a lower risk to be seropositive to P. falciparum for people living at more than 1.5 km of lakes and rivers6. Brought together, these examples illustrate the necessity to circumscribe the local environmental characteristics that may influence the acquisition of antimalarial antibodies.
Other factors may be incriminated such as malnutrition. Indeed, low levels of IgG directed to SPf66 and the Ring-infected Erythrocyte Surface Antigen have been shown in wasted children7,8.
The genotype AS of hemoglobin (Hb) is the main host genetic factor that confers a resistance to severe malaria9. Williams et al. showed that the HbAS protection occurred during the first 10 years of life. The authors proposed that this innate protection would allow the host to be exposed safely to malaria antigens capable of inducing malaria specific immunity10.
Finally, placental malaria could influence the development of immunity in newborns since studies suggested that offspring of women with placental malaria are more susceptible to malaria during infancy11,12. Moreover, Bonner et al. showed that the development of malaria specific antibody responses was impaired during the first year of an infant born to a mother with placental malaria at delivery13. Dent et al., showed that infants born to mothers with pregnancy-associated malaria without MSP1-driven cord blood lymphocyte responses had a lower functional activity of anti-MSP1 antibodies from 18 to 30 months in comparison to the other infants14. However, these results are inconsistent in the literature. Malhotra et al, did not found any difference in anti-MSP1 and anti-AMA1 IgG levels between infants born to mothers with or without placental malaria11.
The present work is based on an epidemiological study already described12 in which all these concurrent determinants were collected in order to be explored as a whole in relation to malaria immunity. It focused on the IgG, IgG1 and IgG3 – that seem to be main actors in malaria antibody responses15 – specific for 5 malaria antigens selected i) for their abundance on the merozoite surface and thus their accessibility to host antibodies, and ii) because they are promising vaccine candidates that could induce protective immune responses16,17. The aims were i) to explore the development of IgG, IgG1 and IgG3 responses to P. falciparum blood-stage antigens from birth to 18 months and ii) to understand what are the key factors that could influence the emergence of antibody responses in infants.
Results
Descriptive results
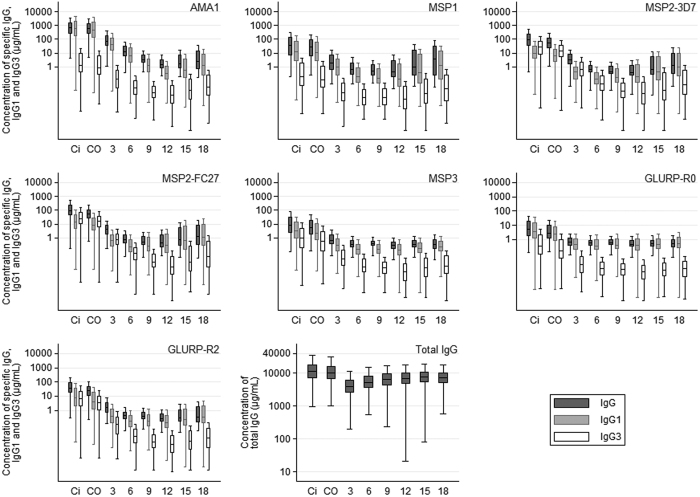
The results presented in the Figs 1, 2 and 3 showed the raw data of IgG, IgG1 and IgG3 acquired in concentration (μg/mL) without statistical test associated.
Figure 1. Total IgG and IgG, IgG1 and IgG3 concentrations specific to blood stage antigens in mothers and infants up to 18 months of age Antibody levels determined in: Ci: peripheral maternal blood; CO: cord blood; 3, 6, 9, 12, 15 and 18 months of infants’ age.
The full line in the box represents median values. Also shown are 10, 25, 75 and 90% percentiles of the IgG, IgG1 and IgG3 concentrations specific for each malaria antigen. For each time point, the group size is: Ci: n = 525, CO: n = 525, 3: n = 374, 6: n = 384, 9: n = 409, 12: n = 418, 15: n = 431, 18: n = 442.
Figure 2.
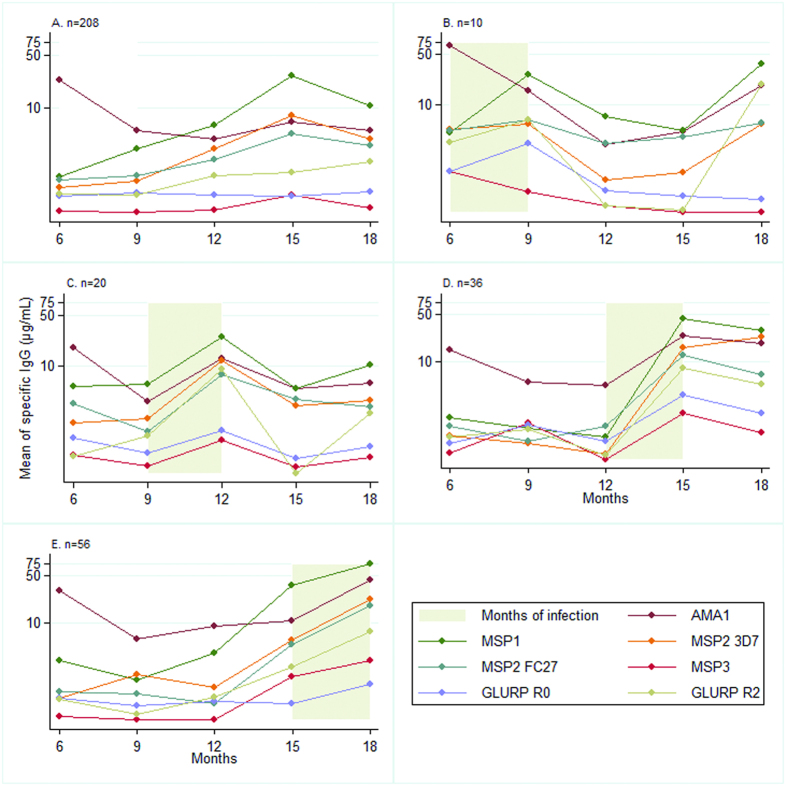
Acquisition of IgG to malaria blood stage antigens for infant with one malaria infection during the follow-up: effect of age (A): infants with no malaria infection. Each B-to-E panel concerns distinct children selected on the basis of their first (and only one) malaria infection that occurred either between (B) 6–9 months of age or (C) 9–12 months of age or (D) 12–15 months of age or (E) 15–18 months of age; n: number of infants in each group.
Figure 3.
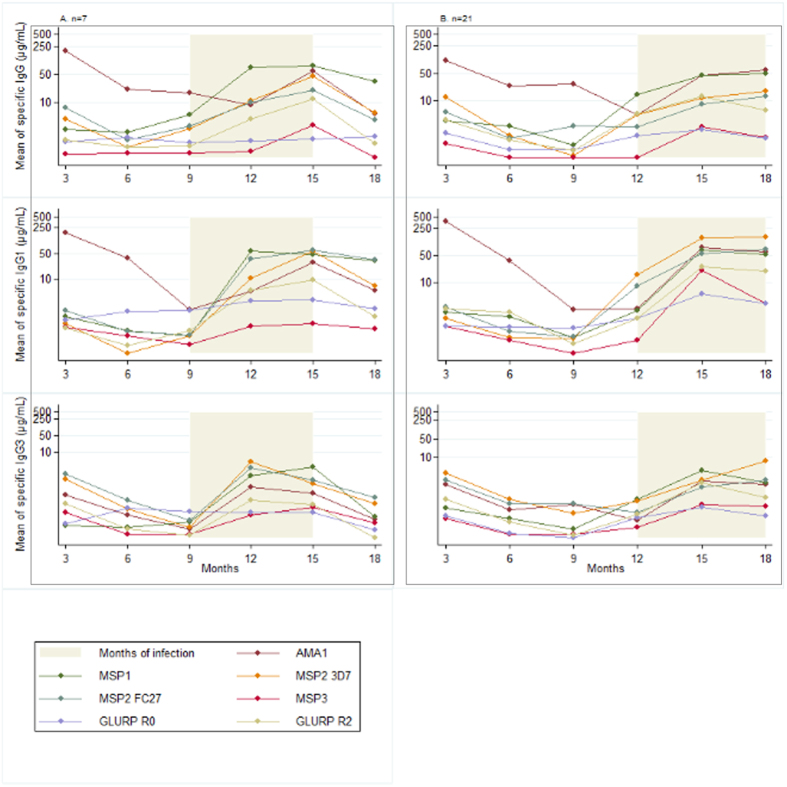
Effect of two successive 3-month periods with malaria infections on the acquisition of IgG, IgG1 and IgG3 to malaria blood stage antigens The panel (A) represents the mean concentration of IgG, IgG1 and IgG3 specific for malaria antigens in the 7 infants with a first malaria infection between 9–12 months and a second infection between 12–15 months. The panel (B) represents the mean concentration of IgG, IgG1 and IgG3 specific for malaria antigens in the 21 infants with a first malaria infection between 12–15 months and a second infection between 15–18 months; n: number of infants in each group.
From birth to 6 months of age, maternal IgG, IgG1 and IgG3 are decreasing in infant blood and after 6 months of age the infant antibodies are produced. This production is increasing with age as we can visualize that at 6 months the level of specific IgG, IgG1 or IgG3 and total IgG are lower than at 18 months of age. For AMA1, the concentration of specific IgG/IgG1/IgG3 is stronger than for all the other antigens. The same effect is still observed but instead to see a decrease/increase at 6 months of age, the phenomenon is observed at 12 months of age (raw data in Fig. 1). For all antigens, including MSP2 from the age of 6 months, IgG1 represented the major part of the IgG response. Concerning MSP2 isoforms, IgG3 levels in mother, cord and 3-month blood were i) higher or equal to IgG1 levels and ii) higher than IgG3 levels directed to the other antigens.
Infants with no detected malaria infection during the follow-up were selected in the Fig. 2A. Antibody levels are never null (consistent with undetected infections) but still lower than in infants with at least one infection. In graphs B to E, infants infected only once during the follow-up were selected. Antibody responses following a malaria infection were represented by a positive slope. Antibody levels increased with age and this effect was more marked for infections occurring after 1 year of age.
Figure 3 illustrates the boosting effect on the IgG, IgG1 and IgG3 levels of two consecutive infections during a 6-month observation window. For most antigens, the second infection seems associated with an increasing specific IgG response whereas this trend is less clear for IgG1 and IgG3. The multivariate analysis will examine these results in more depth.
Univariate analyses
The nutritional status was not associated with antibody levels (P > 0.20). Although the sickle-cell trait was negatively associated with the acquisition of IgG3 to MSP2-FC27, this effect was not significant (P = 0.26). IgG levels to P. falciparum in infants aged more than 6 months were positively associated with maternal (all P < 0.001 except IgG3 to MSP2-FC27 (P = 0.021) and to MSP3 (P = 0.004)), cord (all P < 0.001 except IgG3 to GLURP-R2 (P = 0.017)) and 3-month-infant (all P < 0.001) antibody levels with the strongest effect observed in cord blood. Placental malaria infection was not associated with specific IgG levels.
Multivariate final model
Table 1 shows the final model for each antibody response in infants aged more than six months. Overall, age, malaria specific antibody levels in cord blood and at 3 months, an infection having occurred either at the 3-month period or at the 3-to-6-month period before antibody measurement and environmental factors were significantly associated with the acquisition of antigen-specific IgG (data not shown), IgG1 and IgG3 in 6-to-18-month old infants. Age has a polynomial effect and, after a decrease from 6 to 9 or 12 months (depending on antibody), all the levels increased regularly until the age of 18 months (data not shown) as illustrated by the raw data in Fig. 1. Infections at 3–6 months of age were not significant, probably due to the immaturity of the newborn immune system and to the amount of maternal antibodies that could hide the slow acquisition. Contrarily, infections occurring either at the 3-month period or at the 3-to-6-month period before antibody measurement were positively significantly associated with the levels of antibodies. However, the effects of age and of infection on the evolution of antibody levels are complex, and a strong interaction (p < 10−3 for each antibody response) was detected between age and infections. This result is consistent with the fact that an infection occurring later in the follow-up had a stronger effect on the antibody response than an infection occurring early after birth when the immune system can be considered as less mature (Fig. 4). This effect of age has been already shown by Baird et al., who hypothesized that heavy exposure to infection among children, whether recent or lifelong, does not induce adult-like protection due to the maturation of adult immune system compared with infants3. However, we also showed that infection having occurred at the 3-to-6-month period before antibody measurement significantly increased the antibody response showing that the repetitions of infections and, indirectly the delay from the last infection, is an important factor in the acquisition of immunity. The environmental variable was very often significantly associated with high antibody levels (Table 1). Placental malaria and sickle-cell trait were not significant in the multivariate analyses.
Table 1. Multivariate analysis: final model indicating factors associated with the acquisition of malaria specific cytophilic IgG in infants from 6 to 18 months of age.
| Variables | AMA1 | MSP1 | MSP2-3D7 | MSP2-FC27 | MSP3 | GLURP-R0 | GLURP-R2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG1 | IgG3 | IgG1 | IgG3 | IgG1 | IgG3 | IgG1 | IgG3 | IgG1 | IgG3 | IgG1 | IgG3 | IgG1 | IgG3 | |
| Constant | 3.819 | −1.180 | −1.487 | −4.182 | 3.784 | −0.961 | −2.805 | −0.322 | −2.350 | −2.834 | −2.171 | 3.603 | −1.685 | −2.410 |
| Age | −1.018a | −0.581 | −0.325 | −0.181 | 0.090 | −0.781 | 0.068 | −0.862 | −0.078* | −0.419 | 0.050 | −0.224 | −0.189 | −0.552 |
| Age squared | 0.037 | 0.023 | 0.015 | 0.008 | NS | 0.031 | NS | 0.033 | 0.005** | 0.017 | NS | 0.009 | 0.010 | 0.022 |
| Antibody levels in cord blood | 0.131 | 0.097** | 0.132 | 0.060* | 0.148 | 0.209 | 0.072 | 0.114** | 0.085** | NS | 0.073** | 0.126* | NS | NS |
| Antibody levels at 3 months | 0.063** | 0.135 | 0.113 | 0.206 | 0.129 | 0.173 | 0.147 | 0.140 | 0.183 | 0.206 | 0.147 | 0.196 | 0.164 | 0.169 |
| Infection at 3–6 months of age | NS | NS | NS | −0.345** | 0.244* | NS | NS | NS | NS | NS | NS | −0.406 | NS | NS |
| Infection from 6 months of age: | ||||||||||||||
| in the 3-month period preceding antibody measurement | 1.335 | 1.445 | 2.210 | 2.222 | 1.716 | 1.826 | 1.696 | 1.778 | 0.633 | 0.945 | 0.588 | 0.834 | 1.436 | 1.362 |
| in the 3-to-6-month period preceding antibody measurement | 1.443 | 1.114 | 1.909 | 1.277 | 1.519 | 1.343 | 1,494 | 1.439 | 0,380 | 0.51 | 0.221* | 0.240** | 0.697 | 0.499 |
| Environmental variable | 0.032 | 0.024** | 0,027* | 0,040** | 0.039 | 0.029 | NS | 0,038** | NS | NS | 0,039 | 0,019* | NS | 0,024* |
a: The numbers of the entire Table 1 are coefficient of mixed linear regression: a positive (negative) coefficient indicates a positive (negative) effect of the variable on antibody levels from 6 until 18 months of life. All associations are significant at a P value of ≤0.001 unless otherwise indicated: NS non-significant, *P < 0.05, **P ≤ 0.01, with quarterly febrile infection cases, with quarterly non febrile infection cases. Malaria infections in the previous 3-month period are defined as the presence of at least one malaria attack between two successive antibody measurements and could be feverish or not. The infections in the previous 3-to-6-month period allowed the creation a variable for testing the effect of 2 consecutive infections (separated by at least 3 months) on malaria specific antibody responses. Age is in months; Infections are coded as: at least one infection = 1, no infection = 0; antibody levels are represented by quantitative residual values; the environmental variable is a quantitative index related to the malaria exposure of the infant in the area (described elsewhere18).
Figure 4. Level of IgG1 specific to MSP3 predicted by the mixed linear model IgG1 specific to MSP3 levels are the residuals of adjustments (cf Materials and Methods -Outcome variable).
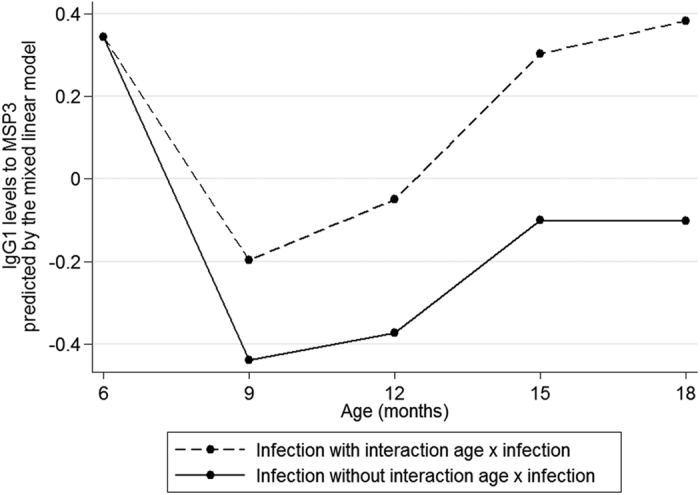
The effect of an infection at each 3-month period is estimated in presence or not of an interaction between age and infection. The best fitted model includes an interaction between age and infection: the difference between the 2 curves increases with age illustrating that more an infant is old and stronger is his response to a first malaria infection.
Discussion
The purposes of this work were i) to explore the acquisition of IgG, IgG1 and IgG3 responses to P. falciparum blood-stage antigens from birth to 18 months and ii) to highlight the factors that could influence the acquisition of these antibody responses in infant.
Infants with no detected malaria infection presented low but nonzero P. falciparum antibody responses allowing 2 complementary interpretations: i) even with a close survey, an infant can develop unperceived infections and ii) malaria specific immune response would subtly develop in infant following submicroscopic infections. The strong effect of the environmental variable corroborates both explanations: P. falciparum exposure was related to the specific antibody response in 6–18 months old infants. This variable was inseparable from the presence of anopheles18 and furthermore was found strongly associated with the occurrence of the first malaria infection in the same cohort19. Pombo et al. showed that protection could be induced by sub-clinical infections with very low circulating parasites20 allowing to conclude that residing in a malaria endemic area is sufficient to induce a subtle development of anti-plasmodial immune responses. The present results underline the importance to take into account as precisely as possible the exposure to P. falciparum during sero-epidemiological studies.
An infection occurring in the 3-to-6-month period before the antibody measurement could be followed or not by an infection in the 3-month period. In case of no infection occurring during the 3-month period before the antibody measurement, there was a positive “residual effect” on the level of total and cytophilic IgG, of the infection having occurred in the last 3-to-6 month period. In case of an additional infection during the 3-month period before the antibody measurement, the malaria specific antibody levels were boosted (2 consecutive infections). This is consistent with the development of an immune memory for infants living in an endemic area. Additionally, the quantification of memory B cells (CD19+ CD21+ CD27+) would represent a substantial information especially as Ngundu et al., did not show any correlation between malaria antibody levels and memory B cell frequencies21.
In the present study, the 6–18 months infants’ levels of antimalarial antibodies were not related to placental malaria. These results are consistent with those of Malhotra et al. who followed-up a birth cohort in Kenya and found no age-linked differences in anti-malaria IgG levels between not exposed, exposed-and-not-sensitized and sensitized groups of children11. Contrarily, Bonner et al. showed that antimalarial antibody responses decreased during the first year of an infant born to a mother with placental malaria at delivery13. However, these authors compared time-pooled measures (0–4 months and 4–12 months) that could amplify the differences between infants born to mothers with and without placental malaria at delivery. In the birth cohort under study, infants born to mothers with placental malaria developed a malaria infection earlier compared to others12. According to the present results, the evolution of the infants’ malaria antibody responses seems not to be involved in this phenomenon. Indeed, Malhotra et al. also observed that tolerant (exposed-and-not-sensitized) children had impaired functional antibody responses to MSP1-1914. An investigation of the impact of antimalarial antibodies on protection to clinical malaria would lead to conclude on the role of these antibodies in infants born to mothers with placental malaria.
Cord blood malaria antibody levels were positively associated with P. falciparum IgG, IgG1 and IgG3 levels during the follow-up were not associated with placental malaria. This result means that the infants born with high levels of transplacental transferred maternal antibodies to malaria produced high levels of IgG specific for malaria. If we consider the hypothesis according to which antibodies are markers of exposure, the higher malaria infections would occur in infants the higher the malaria antibody levels would be. It was previously demonstrated in this cohort that infants born to mothers with placental malaria had a higher risk of malaria infection in their 18 first months of age19. Antibodies as markers of exposure could thus potentially explain this result. The literature provides contradictory conclusions regarding maternal antimalarial antibodies transferred to the fetus and protection of infant from early malaria infections22. Regarding the present study, the number of malaria infections up to the age of 6 months was not associated with the transfer of malaria-specific IgG from the mother to the fetus, leading to the conclusion that malaria-specific IgG represented a marker of maternal exposure rather than a marker of infant protection from malaria23.
Nutritional intakes had no effect on malaria specific antibody levels. Genton et al. found no association between antibody levels to MSP1 and MSP2 and nutritional status7. However, in the present study nutritional intakes were assessed by means of 24-Hour Dietary Recall questionnaire and not by nutrients measurements whereas nutrients are constitutional components of the immune response24. Among them, zinc deficiency has been shown to compromise the B lymphocyte development and therefore the antibody production25. Further investigations on the nutritional status at the nutrient level and its impact on the acquisition of malaria specific immunity are necessary.
Sickle-cell trait was not significantly associated with antibody evolution in the univariate or multivariate analyses.
In the present work, antibody responses were analyzed independently. The profiles of malaria antibody concentrations in infant are similar over the time as those expressed in arbitrary units or in OD values in other studies26,27,28. Antibody response curves vary depending on merozoite antigens and IgG subclasses - as illustrated by the higher level of IgG3 specific for MSP2 in women (as described elsewhere29) - and they result from the sum of individual responses, each being characterized by defined subpopulations of IgG antibody-secreting cells28. The next step will consist to take into account the synergy of the antibody responses directed to the five studied antigens, in order to obtain for each infant a fine picture of the acquisition process of malaria humoral immunity30.
At last, this study demonstrates the huge importance to give to key factors conditioning the antibody response in early life (age, malaria antibody levels at birth, previous and present malaria infections as well as exposure to Anopheles bites). Such factors have to be considered simultaneously in follow-ups of cohorts aimed at elucidating the specific humoral response mainly in the context of vaccine trials.
Methods
Study design and sample collection
The study took place in Southern Benin where P. falciparum is the commonest species (95%)31 and where the entomological inoculation rate is 15.48 infected anopheles/person per year12. Between June 2007 and July 2008, 656 newborns were enrolled and 567 infants remained for this study (10 stillbirths, 16 infants deceased before Day 28, 10 infants lost of sight, 25 twin pairs and one set of triplets)12.
Briefly, at delivery, (i) a questionnaire collecting information on maternal age, parity, use of Intermittent Preventive Treatment during pregnancy (IPTp) and bed net possession was administered and (ii) placental blood smears were performed and maternal peripheral as well as cord bloods were collected into Vacutainer® EDTA (Ethylene diaminetetraacetic acid) tubes.
Every child was visited weekly. In case of fever (axillary temperature ≥37.5 °C) and/or a reported 48-hours-fever episode, a questionnaire and both a rapid diagnostic test (RDT) and a thick blood smear (TBS) were performed. Symptomatic malaria infection (fever and positive TBS and/or RDT) was treated with artemether and lumefantrine combination therapy as recommended by the National Malaria Control Program. Every month, a systematic TBS to detect asymptomatic malaria infection and a qualitative dietary questionnaire32 were performed. Every 3 months (at 3, 6, 9, 12, 15, 18 months of age), infant blood samples were collected (in EDTA). Plasmas were conserved at −80 °C.
Antibody measurements
Antibody measurement by Enzyme-Linked ImmunoSorbent Assay (ELISA)
ELISA was performed as described previously17 and recalled in the Supplementary material33,34,35,36,37,38. The Afro Immuno Assay protocols - standard methods developed for evaluating malaria vaccines sponsored by the African Malaria Network Trust (AMANET [www.amanet-trust.org]) - were performed to assess malaria antibody concentrations by ELISA39,40.
Standard curves were established using human IgG, IgG1 and IgG3 purified proteins (The Binding Site, France) to determine the concentration of specific antibodies. Each point was tested in duplicate.
Data Management for ELISA data
The ADAMSEL FLP b039 software (http://www.malariaresearch.eu/content/software) was used to analyze automatically the ELISA optical density (OD) leading to antibody concentrations. Discordant duplicates (with a variation coefficient >15%) were technically repeated.
OD that were below detection threshold or over saturation were referred as “Low” and “High” concentrations (μg/mL) respectively. These thresholds depend on standard concentrations, normalization procedures and are therefore specific to each ELISA plate. These censored values were imputed using a (log) linear regression model taking into account confounding variables. The model was fitted using a stochastic expectation maximization algorithm41 already applied to ELISA analyses42, and estimated values were attributed to the “Low” and “High” censored values. The quality of imputation, assessed by the R2 between imputed and measured values (both in log-scale), was very good for both low (R2 = 0.9082 for the 5156 concerned measurements) and high (R2 = 0.9498 for the 192 concerned measures). The residuals were classically standardized to obtain a Normal distribution. The R2 between the imputed values and adjusted residuals (both in log-scale) was 0.9906.
Outcome variable: adjusted antibody level
The outcome variable was time-dependent (at 6, 9, 12, 15 and 18 months). Antibody levels were firstly adjusted on the nuisance variables using a linear regression for: health center, prematurity, parity, use of IPTp, bed net possession and maternal age in order to focus on the effects of variables of interest (detailed in “Independent variables” section). The residuals of these adjustments - referred as “adjusted antibody level” - were then used as outcome variable.
Independent variables
Maternal, cord and 3-month infant blood samples
Birth and 3-month infant samples are strongly represented by maternal antibodies that could interfere with the evaluation of acquired IgG of neonates43. Antibody measures in these samples were thus used as quantitative variables in the analyses.
Infections
Placental malaria infection was defined by the presence of P. falciparum in thick placental smears.
The number of malaria attacks between two successive antibody measurements was used either as a quantitative or a qualitative variable. Malaria infections could be feverish or not.
The effect of an infection on the antibody levels was tested by two ways: a prospective effect and a boosting effect of a second infection. To evaluate the prospective effect, infections occurring in the 3-month period before the antibody measurements were considered to be associated with antibody levels. To evaluate the boosting of the antibody response in children being infected in the 3-month period before the antibody measurement, we considered whether they were also infected in the 3-to-6 month period (2 consecutive infections: one occurring in the 3-to-6 month period and one in the 3-month period before the antibody measurements).
Infant’s feeding
Individual 24-Hour Dietary Recall questionnaires - based on child feeding practices32 - were administered monthly from birth. A time-dependent quantitative variable accounting for the past 3-month feeding practices was created12.
Sickle-cell trait
Genomic DNA was extracted from buffy coat using QIAamp DNA blood midi kit (Qiagen, France). The amplified beta globin DNA was then cleaved by the DdeI enzyme into 3 fragments when normal and into 2 fragments when mutated44.
Environmental factors
The environmental risk of exposure to malaria was modeled for each child based on climatic, entomological, and characteristics of the children immediate surroundings18. Using this model an individual time-dependent risk of exposure was attributed to each child, depending on his birthday and on his house’s location in the area.
Statistical analyses
During the study period, antibodies levels were determined quarterly, and the child’s measurements cannot be considered independent. Therefore, child’s antibody levels presented a two-level hierarchy: antibody measurements (level 1) were clustered within children (level 2)45. A linear mixed model with assumed independent random-coefficients was used for both univariate and multivariate analyses. Hierarchical mixed models are well suited to this type of data, allowing analyzing the time dependent evolution of antibodies, and were used in this study45 for both univariate and multivariate analyses. Variance components and fixed effects parameters were estimated using the restricted and the not restricted maximum likelihood methods, respectively45.
The effect of age on the acquisition of natural antibodies was tested using polynomial models. The best fitted model (Bayesian Information Criterion) was used for further analyses.
Multivariate analysis was performed including the variables with P ≤ 0.20 in the univariate step. Placental malaria was systematically included in multivariate analyses. An interaction between age and infection was tested in the model. All statistical analyses were performed using Stata, version 11.0 (StatCorp LP, TX, USA). Statistical significance was set at P < 0.05.
Ethics
The study protocol was approved by the University of Abomey-Calavi’s institutional review board and the IRD’s Consultative Ethics Committee. All women in this study signed an informed consent before enrollment (which also included their children) with the possibility to withdraw at any time. All the methods were carried out in accordance with the approved guidelines.
Additional Information
How to cite this article: Dechavanne, C. et al. Acquisition of natural humoral immunity to P. falciparum in early life in Benin: impact of clinical, environmental and host factors. Sci. Rep. 6, 33961; doi: 10.1038/srep33961 (2016).
Supplementary Material
Acknowledgments
We thank the mothers of Tori Bossito and their infants for their diligently participation to this project; Edgard Godonou, Stéphane Gehou, Sylvestre Zehounkpe, Pierre Adanchoedo, Patrick Pomalegni, and Agnès Le Port for their supervision of the field study; the field medical staff for their precious contribution to the project: the 18 community health workers of villages, the midwives, nurses health centres and in particular Franck Loumedjinon and Messanh Daoudou and to the Faculté des Sciences de la Santé (FSS), the Institut des Sciences Biomédicales Appliquées de Cotonou (ISBA), the Programme National de Lutte contre le Paludisme (PNLP) for their institutional support. We are grateful to collaborators and in particular S. Longacre (Pasteur Institute, Paris, France), R. Anders (La Trobe University, Melbourne, Australia) for their gift of recombinant proteins. This work was supported by the Agence Nationale de la Recherche (ANR) Santé Environnement Santé Travail (SEST 2006 ; 040 01). A PhD prize was awarded by The Treilles Foundation to CD and a PhD scholarship was awarded by IRD and Ambassade de France à Cotonou to IS.
Footnotes
Author Contributions Conceived and designed the project: F.M.-N. and A.G. Collected samples on the field: C.D., A.B., C.A., F.M.-N. and A.G. Prepared antigen: M.T. Performed the experiments: C.D., I.S., A.B., D.C. and F.M.N. Established the data files: C.D., I.S., R.A. and E.J.R. Supervised data collection, experiments or analysis: K.M., A.M., D.C., G.N., F.M.-N and A.G. Performed statistical analyses: C.D., J.M., G.N. and A.G. Wrote the paper: C.D. and A.G. All authors reviewed the manuscript before submission.
References
- Dobaño C. et al. Age-dependent IgG subclass responses to Plasmodium falciparum EBA-175 are differentially associated with incidence of malaria in Mozambican children. Clin. Vaccine Immunol. CVI 19, 157–166 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarr J. B. et al. Evaluation of antibody response to Plasmodium falciparum in children according to exposure of Anopheles gambiae s.l or Anopheles funestus vectors. Malar. J. 6, 117 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird J. K. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol. Today Pers. Ed 11, 105–111 (1995). [DOI] [PubMed] [Google Scholar]
- Reeder J. C., Davern K. M., Baird J. K., Rogerson S. J. & Brown G. V. The age-specific prevalence of Plasmodium falciparum in migrants to Irian Jaya is not attributable to agglutinating antibody repertoire. Acta Trop. 65, 163–173 (1997). [DOI] [PubMed] [Google Scholar]
- Drakeley C. J. et al. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc. Natl. Acad. Sci. USA. 102, 5108–5113 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaireh B. A. et al. Plasmodium vivax and Plasmodium falciparum infections in the Republic of Djibouti: evaluation of their prevalence and potential determinants. Malar. J. 11, 395 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genton B., Al-Yaman F., Ginny M., Taraika J. & Alpers M. P. Relation of anthropometry to malaria morbidity and immunity in Papua New Guinean children. Am. J. Clin. Nutr. 68, 734–741 (1998). [DOI] [PubMed] [Google Scholar]
- Fillol F. et al. Impact of child malnutrition on the specific anti-Plasmodium falciparum antibody response. Malar. J. 8, 116 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aidoo M. et al. Protective effects of the sickle cell gene against malaria morbidity and mortality. The Lancet 359, 1311–1312 (2002). [DOI] [PubMed] [Google Scholar]
- Williams T. N. et al. An immune basis for malaria protection by the sickle cell trait. PLoS Med. 2, e128 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra I. et al. Can prenatal malaria exposure produce an immune tolerant phenotype ? A prospective birth cohort study in Kenya. PLoS Med. 6, e1000116 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Port A. et al. First malaria infections in a cohort of infants in Benin: biological, environmental and genetic determinants. Description of the study site, population methods and preliminary results. BMJ Open 2, e000342 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner P. C. et al. Placental malaria diminishes development of antibody responses to Plasmodium falciparum epitopes in infants residing in an area of western Kenya where P. falciparum is endemic. Clin. Diagn. Lab. Immunol. 12, 375–379 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent A. et al. Prenatal malaria immune experience affects acquisition of Plasmodium falciparum merozoite surface protein-1 invasion inhibitory antibodies during infancy. J. Immunol. Baltim. Md 1950 177, 7139–7145 ( 2006). [DOI] [PubMed] [Google Scholar]
- Stanisic D. I. et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect. Immun. 77, 1165–1174 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodoo D. et al. Cohort study of the association of antibody levels to AMA1, MSP119, MSP3 and GLURP with protection from clinical malaria in Ghanaian children. Malar. J. 7, 142 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtin D. et al. The quantity and quality of African children’s IgG responses to merozoite surface antigens reflect protection against Plasmodium falciparum malaria. PloS One 4, e7590 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell G. et al. Modeling the influence of local environmental factors on malaria transmission in Benin and its implications for cohort study. PloS One 7, e28812 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Port A. et al. Importance of adequate local spatiotemporal transmission measures in malaria cohort studies: application to the relation between placental malaria and first malaria infection in infants. Am. J. Epidemiol. 178, 136–143 (2013). [DOI] [PubMed] [Google Scholar]
- Pombo D. J. et al. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet 360, 610–617 (2002). [DOI] [PubMed] [Google Scholar]
- Ndungu F. M. et al. Memory B cells are a more reliable archive for historical antimalarial responses than plasma antibodies in no-longer exposed children. Proc. Natl. Acad. Sci. USA. 109, 8247–8252 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs K. R. & Dent A. Plasmodium malaria and antimalarial antibodies in the first year of life. Parasitology 143, 129–138 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechavanne C., Cottrell G., Garcia A. & Migot-Nabias F. Placental Malaria: Decreased Transfer of Maternal Antibodies Directed to Plasmodium falciparum and Impact on the Incidence of Febrile Infections in Infants. PloS One 10, e0145464 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field C. J., Johnson I. R. & Schley P. D. Nutrients and their role in host resistance to infection. J. Leukoc. Biol. 71, 16–32 (2002). [PubMed] [Google Scholar]
- Shankar A. H. & Prasad A. S. Zinc and immune function: the biological basis of altered resistance to infection. Am. J. Clin. Nutr. 68, 447S–463S (1998). [DOI] [PubMed] [Google Scholar]
- Kangoye D. T. et al. Plasmodium falciparum malaria in children aged 0-2 years: the role of foetal haemoglobin and maternal antibodies to two asexual malaria vaccine candidates (MSP3 and GLURP). PloS One 9, e107965 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duah N. O., Miles D. J. C., Whittle H. C. & Conway D. J. Acquisition of antibody isotypes against Plasmodium falciparum blood stage antigens in a birth cohort. Parasite Immunol. 32, 125–134 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. T. et al. Dynamics of the antibody response to Plasmodium falciparum infection in African children. J. Infect. Dis. 210, 1115–1122 (2014). [DOI] [PubMed] [Google Scholar]
- Metzger W. G. et al. Serum IgG3 to the Plasmodium falciparum merozoite surface protein 2 is strongly associated with a reduced prospective risk of malaria. Parasite Immunol. 25, 307–312 (2003). [DOI] [PubMed] [Google Scholar]
- Gray J. C. et al. Profiling the antibody immune response against blood stage malaria vaccine candidates. Clin. Chem. 53, 1244–1253 (2007). [DOI] [PubMed] [Google Scholar]
- Djènontin A. et al. Culicidae diversity, malaria transmission and insecticide resistance alleles in malaria vectors in Ouidah-Kpomasse-Tori district from Benin (West Africa): A pre-intervention study. Parasit. Vectors 3, 83 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Indicators for assessing infant and young child feeding practices: part 2: measurement. (2010).
- Faber B. W. et al. Production, quality control, stability and pharmacotoxicity of cGMP-produced Plasmodium falciparum AMA1 FVO strain ectodomain expressed in Pichia pastoris. Vaccine 26, 6143–6150 (2008). [DOI] [PubMed] [Google Scholar]
- Bonnet S. et al. Soluble and glyco-lipid modified baculovirus Plasmodium falciparum C-terminal merozoite surface protein 1, two forms of a leading malaria vaccine candidate. Vaccine 24, 5997–6008 (2006). [DOI] [PubMed] [Google Scholar]
- Anders R. F., Adda C. G., Foley M. & Norton R. S. Recombinant protein vaccines against the asexual blood stages of Plasmodium falciparum. Hum. Vaccin. 6, 39–53 (2010). [DOI] [PubMed] [Google Scholar]
- McCarthy J. S. et al. A phase 1 trial of MSP2-C1, a blood-stage malaria vaccine containing 2 isoforms of MSP2 formulated with Montanide® ISA 720. PloS One 6, e24413 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho L. J. M. et al. Immunization of Saimiri sciureus monkeys with a recombinant hybrid protein derived from the Plasmodium falciparum antigen glutamate-rich protein and merozoite surface protein 3 can induce partial protection with Freund and Montanide ISA720 adjuvants. Clin. Diagn. Lab. Immunol. 12, 242–248 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen M., Vuust J., Gottschau A., Jepsen S. & Høgh B. Antigenicity and immunogenicity of recombinant glutamate-rich protein of Plasmodium falciparum expressed in Escherichia coli. Clin. Diagn. Lab. Immunol. 2, 30–34 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebie I. et al. Humoral responses to Plasmodium falciparum blood-stage antigens and association with incidence of clinical malaria in children living in an area of seasonal malaria transmission in Burkina Faso, West Africa. Infect. Immun. 76, 759–766 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusingu J. P. A. et al. Cytophilic antibodies to Plasmodium falciparum glutamate rich protein are associated with malaria protection in an area of holoendemic transmission. Malar. J. 4, 48 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeux G. & Diebolt J. SEM algorithm: a probabilistic learning algorithm for recognition of density mixtures. Rev. Stat. Appliquée 34, 35–52 (1986). [Google Scholar]
- Moulton L. H. & Halsey N. A. A mixture model with detection limits for regression analyses of antibody response to vaccine. Biometrics 51, 1570–1578 (1995). [PubMed] [Google Scholar]
- Rasheed F. N. et al. Relationships between maternal malaria and malarial immune responses in mothers and neonates. Parasite Immunol. 17, 1–10 (1995). [DOI] [PubMed] [Google Scholar]
- Migot-Nabias F. et al. Red blood cell polymorphisms in relation to Plasmodium falciparum asymptomatic parasite densities and morbidity in Senegal. Microbes Infect. Inst. Pasteur 8, 2352–2358 (2006). [DOI] [PubMed] [Google Scholar]
- McCulloch C. E., Searle S. R. & Neuhaus John M. Generalized, linear, and mixed models. (Wiley, 2008). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.