Abstract
Clonally expanded populations of B cells carrying somatic mutations of Ig variable (V) region genes have been detected in the CNS of subjects with multiple sclerosis (MS), suggesting that a process of B cell affinity maturation with ensuing production of potentially pathogenic autoantibodies may occur inside the CNS. Here, we have characterized the B cell subsets present in the cerebrospinal fluid (CSF) of MS patients and of individuals with other inflammatory neurological disorders by flow cytometry. CD19+CD38high+CD77+, Ki67+, Bcl-2– centroblasts, i.e., a B cell subset found exclusively in secondary lymphoid organs, were detected in the CSF but not in paired peripheral blood from both patient groups. CD27+IgD– memory B cells, i.e., cells with hyper-mutated IgV genes, were significantly increased in the CSF vs. paired peripheral blood and displayed up-regulation of the CD80 and CD86 costimulatory molecules and of CC chemokine receptor (CCR) 1, CCR2, and CCR4 in both patient groups. Lymphotoxin-α, CXC ligand (CXCL) 12, and CXCL13, key mediators of lymphoid neogenesis, were present in the CSF from patients with MS and other inflammatory neurological disorders and were expressed in MS brain tissue, with selective localization in the outer layer of the capillary vessel wall. In conclusion, this study suggests that a compartmentalized B cell response occurs within the CNS during an ongoing inflammatory reaction, through a recapitulation of all stages of B cell differentiation observed in secondary lymphoid organs. The presence of lymphotoxin-α, CXCL12, and CXCL13 in the CNS may provide favorable microenvironmental conditions for these events.
A rich mononuclear cell infiltrate, comprised of macrophages, T cells, and, to a lesser extent, B cells, is typical of the multiple sclerosis (MS) lesion (1–3). So far, the majority of studies have addressed the characterization of T cells involved in the autoimmune attack to the CNS (1).
A role of B cells in MS pathogenesis is suggested by the presence of oligoclonal Ig bands within the cerebrospinal fluid (CSF) that represent a hallmark of the disease (4). In addition, clonally expanded B cells accumulate in chronic MS lesions (5, 6) and in the CSF (7, 8) from MS patients. Finally, antimyelin Abs may be involved in demyelination (9) and may have prognostic value (10).
Ectopic structures resembling classical lymphoid follicles (11, 12) may develop in the parenchyma of nonlymphoid tissues during the course of both autoimmune (13) and chronic infectious (14) diseases. Newly formed lymphoid follicles recently have been detected in the CNS from MS patients, indicating occurrence of lymphoid neogenesis supported by the CXL13 and CC chemokine ligand 21 chemokines (15).
In this study, we characterize the B cell compartment within the CSF of subjects with MS and other inflammatory neurological disorders (OIND) and demonstrate that a complete recapitulation of B cell differentiation occurs in the CSF, where favorable microenvironmental conditions have been identified.
Materials and Methods
Patients and Sample Preparation. CSF and peripheral blood (PB) samples were obtained with informed consent from 26 patients with MS and 22 patients with OIND, including viral meningitis (n = 6), myeloradiculitis (n = 7), encephalomyelitis (n = 4), and vasculitis (n = 5), free of immunosuppressive or glucocorticoid treatment in the 3 months preceding lumbar puncture (Table 1). MS individuals were classified according to the recommended guidelines for the diagnosis of MS (16). PB samples from 20 age-matched normal individuals were tested as controls. PB mononuclear cells were isolated on Ficoll–Hypaque density gradients (Sigma). CSF cells were collected after centrifugation. Cell-free supernatants were stored at –80°C until analysis. The absolute cell counts in the CSF samples tested ranged from 2 × 105 to 5 × 105.
Table 1. Demographic and laboratory features of MS and OIND patients.
| Number | Mean age | IgG, mg% | Link index | Cells, mm3 | Oligoclonal bands | |
|---|---|---|---|---|---|---|
| OIND | 22 (10 F, 12 M) | 50 (20-80) | 7.88 (2.52-55.9) | 0.67 (0.445-1.54) | 73.09 (4-465) | 13/22 |
| MS | 26 (18 F, 8 M) | 36.8 (17-70) | 6.6 (1.45-31.9) | 1.13 (0.464-3.089) | 14.3 (3-38) | 26/26 |
Normal values: IgG, 0.741-4.19; link index, 0.236-0.699; cell number, 0-4. F, female; M, male. Ranges appear in parentheses.
Flow Cytometric Analysis. CD19-phycoerythrin (PE)-cyanin 5 was from Caltag (South San Francisco, CA); CD38-PE was from Serotec; CD27-PE, CD27-FITC, CD80-FITC, CD86-PE, anti-Ig κ light chain-FITC, and anti-Ig λ light chain-FITC mAbs were from Pharmingen. FITC-conjugated anti-human IgD mAb was from DAKO. Anti-CC chemokine receptor (CCR) 1-PE, CCR2-PE, CCR4-PE, CCR6-PE, and CCR7-PE mAbs were from R & D Systems. Unconjugated CD77 (Immunotech, Luminy, France), CD138 (Beckman Coulter), and anti-CXC chemokine receptor (CXCR) 3, CXCR4, and CXCR5 mAbs (Serotec) also were used. Equal numbers of CSF cells and PB mononuclear cells were stained with fluorochrome-conjugated mAbs, with unconjugated mAbs followed by fluorochrome-conjugated secondary reagents, or with isotype- and fluorochrome-matched control Abs. Cells were then run on a fluorescence-activated cell sorter (FAC-Scan, Becton-Dickinson). On average, 104 events were acquired and analyzed by using cellquest software (17). For intracellular staining, cells were fixed, permeabilized, stained with FITC-labeled anti-Ig κ and λ light chain, anti-Bcl-2 mAb (DAKO), anti-Ki-67 mAb (DAKO), or isotype-control mAbs, as described in ref. 18, and analyzed as above.
CSF CD19+ cells ranged from 1% to 12% and 1% to 10% in MS and OIND patients, respectively. Thus, the mean absolute numbers of CSF CD19+ cells were 35,000 and 34,000 for MS and OIND patients, respectively. PB mononuclear CD19+ cells ranged from 3% to 10% in all groups tested.
ELISA. CXCL12, CXCL13, and lymphotoxin (LT) α in cell-free CSF samples from 22 MS individuals and 21 OIND subjects were detected by ELISA (Quantikine, R & D Systems).
Immunohistochemistry. Tissue sections 5–7 μm thick from chronic active lesions of two MS brains were fixed in formalin, paraffin-embedded, and stained by hematoxylin/eosin, luxol fast blue, or trichromic standard. CD3 (1:100 dilution), CD68 (1:100 dilution), and CD31 from DakoCytomation (1:50 dilution), anti-α-actin mAb from BioGenex Laboratories (San Ramon, CA, 1:50 dilution), and anti-LT-α, anti-CXCL12, and anti-CXCL13 goat polyclonal Abs from R & D Systems (1:500 dilution) were used for immunohistochemical studies. Secondary reagents were an anti-mouse Ig Ab conjugated to peroxidase and streptavidin (Supersensitive Detection Kit, BioGenex) for CD3, CD68, and CD31, and an anti-goat IgG biotinilated Ab for LT-α, CXCL12, and CXCL13 (DakoCytomation, 1:200 dilution). Sections from an autoptic normal brain (from a person killed in a car accident) also were analyzed. Control reactions were performed in the absence of primary Ab and with isotype-matched irrelevant Abs. Staining was performed by using the peroxidase technique (DakoCytomation).
Statistical Analysis. Descriptive statistics were reported as absolute frequencies for qualitative data and as means and SD, minimum and maximum value, for quantitative data. The Mann–Whitney U test was used to compare parameters in the two groups of data (CSF vs. PB samples). One-way nonparametric ANOVA (Kruskal–Wallis test) was used to compare parameters in more than two groups of data, and the Bonferroni correction was applied for multiple comparisons to explore post hoc differences between pairs of groups. All statistical tests were two-sided; P < 0.05 was considered statistically significant. The statistical package statistica (Version 6, StatSoft, Tulsa, OK) was used to perform all of the analyses.
Results
B Cell Subset Characterization. Cells obtained simultaneously from the CSF and PB of 16 MS and 16 OIND patients were stained with CD19 mAb in combination with anti-IgD, CD38, or CD27 mAbs, which broadly detect naïve, germinal center (GC), and memory B lymphocytes, respectively (19, 20).
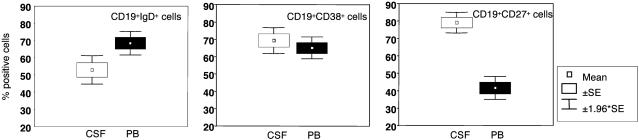
IgD+ B cells were significantly enriched in PB vs. CSF from MS patients (P = 0.006), whereas the percentages of CD38+ B cells in CSF and PB were similar (Fig. 1 Left and Center, respectively). The proportion of CD27+ B cells was significantly higher in CSF than PB from MS patients (P < 0.0001) (Fig. 1 Right). Similar results were obtained when CSF and PB from OIND patients were tested (data not shown).
Fig. 1.
Detection of B cell subsets in patients with MS. Cells from CSF and PB of 16 MS patients were stained with CD19 mAb in combination with anti-IgD (Left), CD38 (Center), or CD27 (Right) mAbs that broadly detect naïve, GC, and memory B lymphocytes, respectively, and analyzed by flow cytometry, setting the gate on CD19+ cells. Results are expressed as mean percent positive cells ±SE.
The sum of the percentages of IgD+, CD38+, and CD27+ B cells largely exceeded 100% in all samples because of the partial overlap of these surface markers (21, 22).
IgD+, CD38+, and CD27+ B cells were similarly represented in PB from MS and OIND patients and from controls (data not shown).
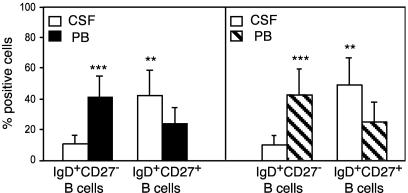
Heterogeneity of CD19+, IgD+ Cells. IgD+ B lymphocytes represent a heterogeneous cell fraction including naïve B cells together with GC founder and memory B cells (19–23). Cells isolated simultaneously from CSF and PB samples of 15 MS and 15 OIND patients, as well as from PB of 15 normal controls, were stained with CD19 mAb in combination with anti-IgD and CD27 mAbs. IgD+, CD27– cells are bona fide naïve B cells (19), whereas IgD+, CD27+ cells represent a minor but sizeable subset of memory B cells (19, 21, 23, 24).
IgD+, CD27+ B cells were significantly enriched in the CSF vs. PB, as opposed to IgD+, CD27– naïve B cells, which were significantly more abundant in PB than in CSF (Fig. 2). No difference in the percentages of PB IgD+, CD27+ and IgD+, CD27– B cells was observed among MS and OIND patients and healthy donors (data not shown).
Fig. 2.
Immunophenotypic dissection of CD19+, IgD+ cells in patients with MS or OIND. Cells from CSF and PB of 15 MS (Left) and 15 OIND (Right) patients were stained with CD19 mAb in combination with anti-IgD and CD27 mAbs and analyzed by flow cytometry setting the gate on CD19+ cells. Results are mean percent positive cells ±SD. ***, P < 0.0001; **, P < 0.001.
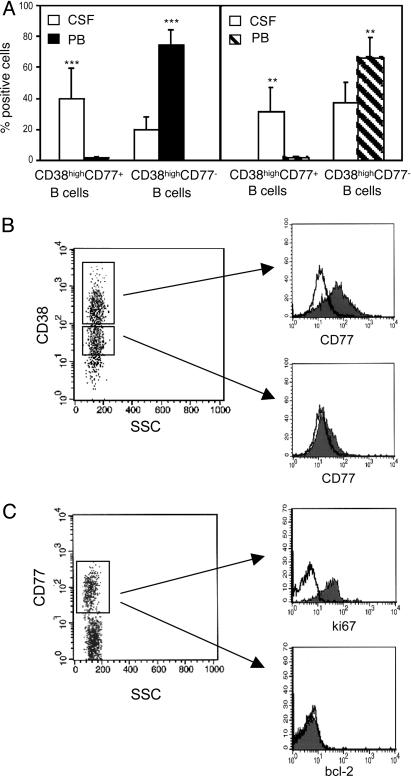
Detection of Centroblasts in the CSF. CSF and PB samples obtained simultaneously from 10 MS and 10 OIND patients and PB from 10 normal controls were stained with CD19, CD38, and CD77 mAbs. High-intensity CD38 (CD38high), CD77+ centroblasts (20, 25) were detected in CSF but not in PB of all patients (Fig. 3A) or healthy donors (data not shown). In contrast, CD38high, CD77– centrocytes were significantly enriched in PB vs. CSF from both patient groups (Fig. 3A). CD38high, CD77– B cells were similarly represented in the PB from MS and OIND patients and healthy donors (data not shown).
Fig. 3.
Identification of CD19+, CD38high, CD77+, Ki67+, Bcl-2– centroblasts in the CSF of patients with MS or OIND. (A) Cells from CSF and PB of 10 MS (Left) and 10 OIND (Right) patients were stained with CD19 and CD38 mAbs in combination with CD77 and analyzed by flow cytometry setting the gate on CD19+ cells. Coexpression of CD77 and CD38 was determined by setting the marker on CD38high cells, which represent bona fide GC B cells (B). Results are mean percent positive cells ±SD. ***, P = 0.0005; **, P = 0.001. (B) Cells from the CSF of a MS patient were stained with CD19, CD38, and CD77 mAbs and analyzed by gating first on CD19+ (data not shown) and then on CD38high or CD38low cells (Left). CD77+ cells were detected in the CD38high (Upper Right) but not in the CD38low (Lower Right) B cell fraction. A representative experiment is shown. (C) Cells from the CSF of a MS patient were stained for surface CD19 and CD77, as well as intracellular Ki67 or Bcl-2, and analyzed by gating first for CD19 (data not shown) and then for CD77 (Left). CD77+ B cells expressed Ki67 (Upper Right) but not Bcl-2 (Lower Right). A representative experiment is shown.
CD77+ cells were detected in the CD38high, but not in the low-intensity CD38 (CD38low) B cell fraction, which comprises non-GC B cells (25) (Fig. 3B).
CSF cells from five additional MS and OIND cases were stained for surface CD19, CD77, and intracellular Ki67 or Bcl-2 (Fig. 3C). CD77+ B cells were found to express the Ki67 proliferation marker (Fig. 3C Upper Right) but not the Bcl-2 antiapoptotic protein (Fig. 3C Lower Right).
The CD19+CD38highCD77+, Ki67+, Bcl-2– immunophenotype unambiguously detects centroblasts in the CSF from MS and OIND patients.
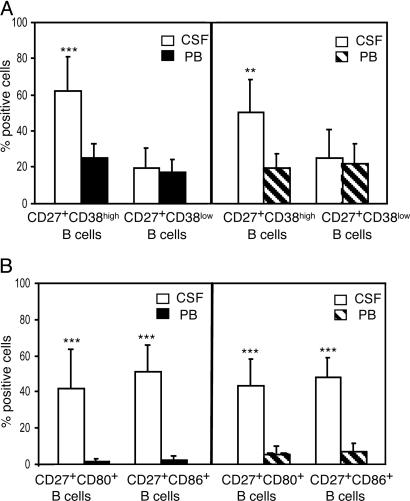
Heterogeneity of CD19+, CD27+ Cells. CSF and PB samples obtained simultaneously from 10 MS and 10 OIND patients were stained with CD19 and CD27 mAbs in conjunction with CD38, CD80, or CD86 mAbs. PB samples from 10 healthy donors also were tested. CD27+, CD38high B cells were significantly enriched in the CSF vs. PB from both patient groups, whereas CD27+, CD38low B cells were detected in similar proportions in the two compartments (Fig. 4A). The latter cells represent fully differentiated memory cells, whereas the former are GC cells undergoing differentiation to memory cells (21, 26).
Fig. 4.
Characterization of CD19+, CD27+ memory cells in patients with MS or OIND. Cells from CSF and PB of 10 MS (Left) and 10 OIND (Right) patients were stained with CD19 and CD27 mAbs in conjunction with CD38 (A) or with CD80 or CD86 (B) mAbs and analyzed by flow cytometry gating on CD19+ cells. Results are mean percent positive cells ±SD. ***, P = 0.0005; **, P = 0.001.
CD27+ B cells coexpressing the CD80 or CD86 costimulatory molecules were significantly more abundant in CSF than in PB from both MS and OIND patients (Fig. 4B).
PB from MS and OIND patients and healthy donors contained similar proportions of all of the above-mentioned memory B cell subsets (data not shown).
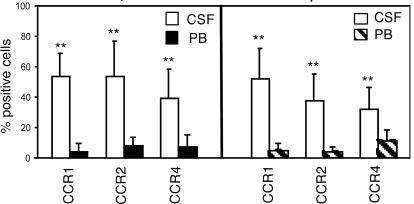
CD19+, CD27+ cells also were characterized for the expression of CCR1, CCR2 CCR4, CCR6, CCR7, CXCR3, CXCR4, and CXCR5, selected upon their involvement in B cell chemotaxis (27, 28). CCR1, CCR2, and CCR4 expression was significantly higher in CSF than in PB CD27+ B cells (Fig. 5) but was comparable in PB from MS and OIND patients and normal controls. The other chemokine receptors were similarly expressed in CD27+ B cells from CSF and PB of both patient groups and from normal PB.
Fig. 5.
Expression of CCR1, CCR2, and CCR4 on CD19+, CD27+ memory cells in MS and OIND patients. Cells from CSF and PB of 10 MS (Left) and 10 OIND (Right) patients were stained with CD19, CD27, and anti-CCR1, CCR2, or CCR4 mAbs. CD19+ cells were gated first, and then CD27+ cells. Results are mean percentage of positive cells. **, P = 0.002 for CCR1 and CCR2 expression in both patient groups. **, P = 0.003 and 0.019 for CCR4 expression in MS and OIND patients, respectively.
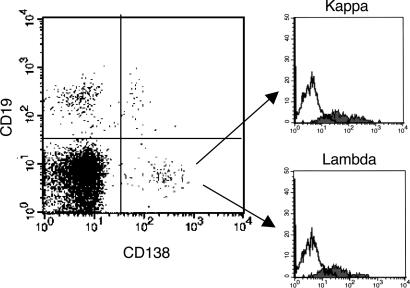
Plasma Cell Detection in Patient CSF. CSF and PB samples obtained simultaneously from five MS and five OIND patients with oligoclonal bands, as well as PB samples from five healthy controls, were stained with CD19 and CD138, a plasma cell-specific marker (29) (Fig. 6). Most CD138+ cells were CD19–, i.e., bona fide plasma cells (29), whereas only a minor proportion were CD19+ (Fig. 6 Left). The mean percentage ±SD of CD138+, CD19– cells in the CSF from MS patients was 7.1 ± 1.1, whereas that in the CSF from OIND patients was 1.5 ± 0.9. The mean percentage ±SD of CD138+, CD19+ cells in the CSF from MS patients was 1.8 ± 0.8, whereas that in the CSF from OIND patients was 1.0 ± 0.6.
Fig. 6.
Detection of CD138+, CD19– plasma cells in the CSF from patients with MS. Cells from CSF and PB of a representative MS patient were stained for surface CD19 and CD138 and for intracellular Ig light chains. CD138+, CD19– plasma cells, as shown in the right lower quadrant of the histogram, expressed both κ (Upper Right) and λ (Lower Right) Ig light chains.
CD138+ cells, either CD19– or CD19+, were not detected in PB samples from MS and OIND patients or healthy controls (data not shown).
CD138+, CD19– plasma cells were subsequently shown by flow cytometry to contain κ and λ Ig light chains (Fig. 6 Right). Most CD138+ cells, either CD19– or CD19+, coexpressed CD38 and CD27 (data not shown).
LT-α, CXCL13, and CC Chemokine Ligand 19 Detection in Patient CSF. Because LT-α has been shown to play a pivotal role in lymphoid organogenesis (30–33), we determined its CSF levels. LT-α was detected in all CSF samples at lower levels in MS than in OIND patients (10.8 ± 4.9 pg/ml and 17.8 ± 5.9 pg/ml, respectively; P < 0.001).
Because LT-mediated events result in the production of lymphoid chemokines (31–33), we next tested CXCL12 and CXCL13 levels in the CSF from both patient groups. CXCL12 and CXCL13 concentrations were higher in OIND patients, but the difference did not reach statistical significance (CXCL12: MS 971.7 ± 882.9 pg/ml, OIND 1,201.9 ± 1,116.3 pg/ml; CXCL13: MS 73.1 ± 63.7 pg/ml, OIND 176.6 ± 305.8 pg/ml).
CXCL12, CXCL13, and LT-α in MS Brain Lesions. Next, we investigated the expression of LT-α, CXCL12, and CXCL13 in MS brain lesions. These molecules were detected exclusively in a relevant number (≈50%) of vessel walls within MS plaques. Such expression was often but not always associated with inflammatory infiltrates and demyelination, as assessed by luxol fast blue staining (data not shown). Infiltrating mononuclear cells and neural cells within the affected CNS parenchyma did not express LT-α, CXCL12, or CXCL13 (data not shown). Moreover, no immunoreactivity was observed in adjacent, normal-appearing white matter from the same subjects, including vessels, and from a normal control brain (data not shown).
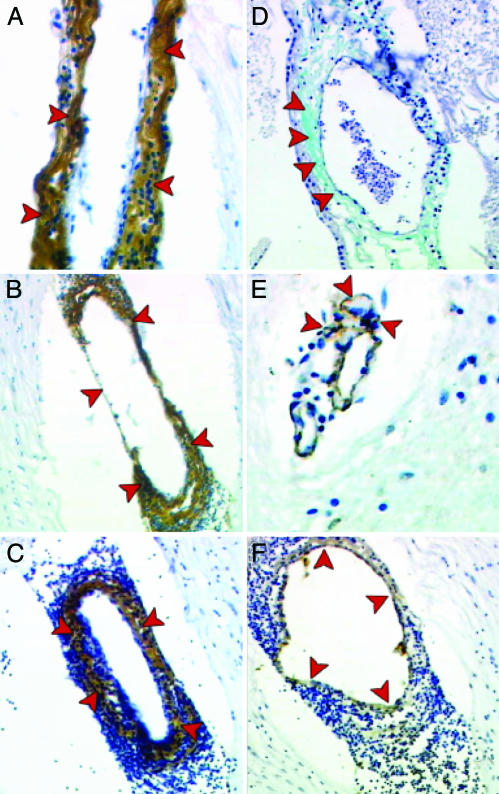
In particular, LT-α, CXCL12, and CXCL13 (Fig. 7 A–C, respectively) were detected in the outer layers of the capillary wall, with a pattern similar to that obtained for the extracellular matrix with the trichromic staining (Fig. 7D) and for pericytes, which are detected with the anti-α actin mAb (Fig. 7E). In contrast, endothelial cells forming the inner layer of the vessel walls, detected by CD31 (Fig. 7F), did not stain for LT-α, CXCL12, or CXCL13 (Fig. 7 A–C).
Fig. 7.
Immunohistochemical detection of LT-α, CXCL12, and CXCL13 in the inflamed brain vessels of an MS patient. Anti-LT-α (A), CXCL12 (B), and CXCL13 (C) Abs intensely stain (brown) the outer layer of the vessel walls (A–C, red arrows). Such immunoreactivity mimics that obtained with trichromic staining for the extracellular matrix (D and A, red arrows, respectively) and, to a lesser extent, with the anti-α actin mAb, which stains pericytes (E, red arrows). In contrast, endothelial cells, detected by CD31 staining (F, red arrows), do not show any immunoreactivity for LT-α, CXCL12, or CXCL13 (A–C, respectively).
Discussion
This study provides previously uncharacterized evidence for a recapitulation of B cell differentiation in the CNS from MS and OIND patients and complements the previous demonstration of CNS-restricted somatic IgV gene mutations in brain lesions (5, 6) and in the CSF from MS patients (7, 8).
CD38high, CD77+, Ki67+, Bcl-2– centroblasts were detected in CSF but not PB from both patient groups. Centroblasts may derive from intrathecal differentiation of naïve B cells and/or from recruitment to the CNS of circulating memory B cells undergoing further cycles of IgV gene mutations (34). The finding that naïve B cells were less numerous in the CSF than in PB from both patient groups may suggest that memory B cells recruited to the CNS represent a major source of centroblasts.
The recent identification of newly formed lymphoid follicles in the CNS from MS patients (15) suggests that ectopic GCs provide the appropriate microenvironment for both pathways of B cell differentiation.
Alternatively, but less likely, somatic hypermutation of IgV genes may take place outside GC (35), or centroblasts may be generated in peripheral lymphoid tissues and subsequently migrate to the CNS. However, the latter cells are highly susceptible to spontaneous apoptosis and do not recirculate (21).
Physiological organogenesis of lymphoid tissues is sustained by the coordinated action of LT-α1β2 and LT-α on stromal cells and the consequent production of lymphoid chemokines, including CXCL12 and CXCL13 (30–33). Similar mechanisms operate in the de novo formation of ectopic lymphoid structures (36).
Here, CXCL12, CXCL13, and LT-α were detected in the CSF of subjects with inflammatory neurological disorders, supporting the possibility that these molecules play a role in the organization of the immune response in the CNS. Moreover, CXCL12, CXCL13, and LT-α were detected by immunohistochemistry in the outer layer of vessel walls. This layer is composed of pericytes and extracellular matrix forming the leptomeningeal coating, which reflects from the surface of the brain on the veins leaving the inflamed parenchyma. These structures delimitate a perivascular space that is not connected with the subarachnoid space (37). Such perivascular space surrounds the veins leaving the brain parenchyma and represents a route of trafficking from the blood into the CNS (38). Indeed, cytokines and chemokines attached to the extracellular matrix provide regulatory stimuli for migrating immune cells (39, 40). Thus, the presence of follicular aggregates in the meninges and in the CNS perivascular spaces (15) suggests that these sites may indeed promote immune interactions similar to those occurring in secondary lymphoid follicles. Such findings are in accordance with the detection of lymphoid-like structures in the subarachnoid spaces in some experimental models of autoimmune encephalomyelitis (41).
In secondary lymphoid tissues, centrocytes are committed to differentiate to plasma or memory cells. In this study, we demonstrated that both pathways operate in the CNS of MS and OIND patients. Thus, Ig-secreting plasma cells were found in the CSF from both groups consistently with the presence of oligoclonal Ig bands in the patients studied. The higher percentage of plasma cells in CSF from MS individuals may be related to the chronic course of this disease as opposed to the acute one typical of the OIND cases studied. Furthermore, CD27+ memory B cells were significantly enriched in the CSF vs. PB from all patients.
Up-regulation of the CD80 and CD86 costimulatory molecules on memory B cells suggests that the latter may serve as local antigen-presenting cells and sustain T cell activation (42). In addition, CSF memory B cells were found to up-regulate CCR1, CCR2, and CCR4, which trigger chemotaxis of tonsil naïve and memory B cells (27, 28) and are quickly up-modulated by inflammatory stimuli (43). These receptors may drive migration of memory B cells to the CNS, where their ligands are available (44–47), or help retain memory B cells in the CNS.
Altogether, the similarities in B cell subset distribution in the CSF from MS and OIND patients suggest that common pathways sustain the immune response within the CNS in these neurological disorders. Persistence of antigenic stimulation and defective immune regulation may account for the chronic course of MS as opposed to the self-limiting course of most OIND.
Acknowledgments
We thank Drs. Francesca Aloisi, Federico Caligaris-Cappio, and Roy Weller for critical revision of the manuscript; Mrs. Chiara Bernardini for excellent secretarial assistance; and Mr. Luca Tedesco for photographical assistance. This work was supported by Fondazione Italiana per la Sclerosi Multipla, Ministero dell'Istruzione, dell'Università e della Ricerca, and Ministero della Salute (Progetti Finalizzati and Ricerca Corrente).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CCR, CC chemokine receptor; CSF, cerebrospinal fluid; CXCL, CXC chemokine ligand; CXCR, CXC chemokine receptor; GC, germinal center; LT, lymphotoxin; MS, multiple sclerosis; OIND, other inflammatory neurological disorders; PB, peripheral blood; PE, phycoerythrin; V, variable.
References
- 1.Steinman, L. (1991) Adv. Immunol. 49, 357–379. [DOI] [PubMed] [Google Scholar]
- 2.Esiri, M. M. (1977) Lancet ii, 478–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prineas, J. W. & Wright, R. G. (1978) Lab. Invest. 38, 409–421. [PubMed] [Google Scholar]
- 4.Archelos, J. J., Storch, M. K. & Hartung, H. P. (2000) Ann. Neurol. 47, 694–706. [PubMed] [Google Scholar]
- 5.Baranzini, S. E., Jeong, M. C., Butunoi, C., Murray, R. S., Bernard, C. C. & Oksenberg, J. R. (1999) J. Immunol. 163, 5133–5144. [PubMed] [Google Scholar]
- 6.Owens, G. P., Kraus, H., Burgoon, M. P., Smith-Jensen, T., Devlin, M. E. & Gilden, D. H. (1998) Ann. Neurol. 43, 236–243. [DOI] [PubMed] [Google Scholar]
- 7.Colombo, M., Dono, M., Gazzola, P., Roncella, S., Valetto, A., Chiorazzi, N., Mancardi, G. L. & Ferrarini, M. (2000) J. Immunol. 164, 2782–2789. [DOI] [PubMed] [Google Scholar]
- 8.Qin, Y., Duquette, P., Zhang, Y., Talbot, P., Poole, R. & Antel, J. (1998) J. Clin. Invest. 102, 1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genain, C. P., Cannella, B., Hauser, S. L. & Raine, C. S. (1999) Nat. Med. 5, 170–175. [DOI] [PubMed] [Google Scholar]
- 10.Berger, T., Rubner, P., Schautzer, F., Egg, R., Ulmer, H., Mayringer, I., Dilitz, E., Deisenhammer, F. & Reindl, M. (2003) N. Engl. J. Med. 349, 139–145. [DOI] [PubMed] [Google Scholar]
- 11.Liu, Y. J. & Arpin, C. (1997) Immunol. Rev. 156, 111–126. [DOI] [PubMed] [Google Scholar]
- 12.MacLennan, I. C. (1994) Annu. Rev. Immunol. 12, 117–139. [DOI] [PubMed] [Google Scholar]
- 13.Hjelmstrom, P. (2001) J. Leukocyte Biol. 69, 331–339. [PubMed] [Google Scholar]
- 14.Murakami, J., Shimizu, Y., Kashii, Y., Kato, T., Minemura, M., Okada, K., Nambu, S., Takahara, T., Higuchi, K., Maeda, Y., et al. (1999) Hepatology 30, 143–150. [DOI] [PubMed] [Google Scholar]
- 15.Serafini, B., Rosicarelli, B., Magliozzi, R., Stigliano, E. & Aloisi, F. (2004) Brain Pathol. 14, 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald, W. I., Compston, A., Edan, G., Goodkin, D., Hartung, H. P., Lublin, F. D., McFarland, H. F., Paty, D. W., Polman, C. H., Reingold, S. C., et al. (2001) Ann. Neurol. 50, 121–127. [DOI] [PubMed] [Google Scholar]
- 17.Corcione, A., Arduino, N., Ferretti, E., Raffaghello, L., Roncella, S., Rossi, D., Fedeli, F., Ottonello, L., Trentin, L., Dallegri, F., et al. (2004) Clin. Cancer Res. 10, 964–971. [DOI] [PubMed] [Google Scholar]
- 18.Dufour, C., Corcione, A., Svahn, J., Haupt, R., Poggi, V., Beka'ssy, A. N., Scime, R., Pistorio, A. & Pistoia, V. (2003) Blood 102, 2053–2059. [DOI] [PubMed] [Google Scholar]
- 19.Klein, U., Rajewsky, K. & Kuppers, R. (1998) J. Exp. Med. 188, 1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pascual, V., Liu, Y. J., Magalski, A., de Bouteiller, O., Banchereau, J. & Capra, J. D. (1994) J. Exp. Med. 180, 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bohnhorst, J. O., Bjorgan, M. B., Thoen, J. E., Natvig, J. B. & Thompson, K. M. (2001) J. Immunol. 167, 3610–3618. [DOI] [PubMed] [Google Scholar]
- 22.Lebecque, S., de Bouteiller, O., Arpin, C., Banchereau, J. & Liu, Y. J. (1997) J. Exp. Med. 185, 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dono, M., Zupo, S., Leanza, N., Melioli, G., Fogli, M., Melagrana, A., Chiorazzi, N. & Ferrarini, M. (2000) J. Immunol. 164, 5596–5604. [DOI] [PubMed] [Google Scholar]
- 24.Paramithiotis, E. & Cooper, M. D. (1997) Proc. Natl. Acad. Sci. USA 94, 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein, U., Tu, Y., Stolovitzky, G. A., Keller, J. L., Haddad, J., Jr., Miljkovic, V., Cattoretti, G., Califano, A. & Dalla-Favera, R. (2003) Proc. Natl. Acad. Sci. USA 100, 2639–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung, J., Choe, J., Li, L. & Choi, Y. S. (2000) Eur. J. Immunol. 30, 2437–2443. [DOI] [PubMed] [Google Scholar]
- 27.Corcione, A., Tortolina, G., Bonecchi, R., Battilana, N., Taborelli, G., Malavasi, F., Sozzani, S., Ottonello, L., Dallegri, F. & Pistoia, V. (2002) Int. Immunol. 14, 883–892. [DOI] [PubMed] [Google Scholar]
- 28.Brandes, M., Legler, D. F., Spoerri, B., Schaerli, P. & Moser, B. (2000) Int. Immunol. 12, 1285–1292. [DOI] [PubMed] [Google Scholar]
- 29.Luque, R., Brieva, J. A., Moreno, A., Manzanal, A., Escribano, L., Villarrubia, J., Velasco, J. L., Lopez-Jimenez, J., Cervero, C., Otero, M. J., et al. (1998) Clin. Exp. Immunol. 112, 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu, Y. X., Huang, G., Wang, Y. & Chaplin, D. D. (1998) J. Exp. Med. 187, 1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ngo, V. N., Korner, H., Gunn, M. D., Schmidt, K. N., Riminton, D. S., Cooper, M. D., Browning, J. L., Sedgwick, J. D. & Cyster, J. G. (1999) J. Exp. Med. 189, 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuff, C. A., Schwartz, J., Bergman, C. M., Russell, K. S., Bender, J. R. & Ruddle, N. H. (1998) J. Immunol. 161, 6853–6860. [PubMed] [Google Scholar]
- 33.Drayton, D. L., Ying, X., Lee, J., Lesslauer, W. & Ruddle, N. H. (2003) J. Exp. Med. 197, 1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, Y. J. & Banchereau, J. (1996) Immunologist 4, 55–66. [Google Scholar]
- 35.William, J., Euler, C., Christensen, S. & Shlomchik, M. J. (2002) Science 297, 2066–2070. [DOI] [PubMed] [Google Scholar]
- 36.Weyand, C. M., Kurtin, P. J. & Goronzy, J. J. (2001) Am. J. Pathol. 159, 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weller, R. O., Kida, S. & Zhang, E. T. (1992) Brain Pathol. 2, 277–284. [DOI] [PubMed] [Google Scholar]
- 38.Ransohoff, R. M., Kivisakk, P. & Kidd, G. (2003) Nat. Rev. Immunol. 3, 569–581. [DOI] [PubMed] [Google Scholar]
- 39.Vaday, G. G., Franitza, S., Schor, H., Hecht, I., Brill, A., Cahalon, L., Hershkoviz, R. & Lider, O. (2001) J. Leukocyte Biol. 69, 885–892. [PubMed] [Google Scholar]
- 40.Zhao, X., Sato, A., Dela Cruz, C. S., Linehan, M., Luegering, A., Kucharzik, T., Shirakawa, A. K., Marquez, G., Farber, J. M., Williams, I. & Iwasaki, A. (2003) J. Immunol. 171, 2797–2803. [DOI] [PubMed] [Google Scholar]
- 41.Magliozzi, R., Columba-Cabezas, S., Serafini, B. & Aloisi, F. (2004) J Neuroimmunol. 148, 11–23. [DOI] [PubMed] [Google Scholar]
- 42.Liu, Y. J., Barthelemy, C., de Bouteiller, O., Arpin, C., Durand, I. & Banchereau, J. (1995) Immunity 2, 239–248. [DOI] [PubMed] [Google Scholar]
- 43.Baggiolini, M. & Loetscher, P. (2000) Immunol. Today 21, 418–420. [DOI] [PubMed] [Google Scholar]
- 44.Giunti, D., Borsellino, G., Benelli, R., Marchese, M., Capello, E., Valle, M. T., Pedemonte, E., Noonan, D., Albini, A., Bernardi, G., et al. (2003) J. Leukocyte Biol. 73, 584–590. [DOI] [PubMed] [Google Scholar]
- 45.Simpson, J., Rezaie, P., Newcombe, J., Cuzner, M. L., Male, D. & Woodroofe, M. N. (2000) J. Neuroimmunol. 108, 192–200. [DOI] [PubMed] [Google Scholar]
- 46.Boven, L. A., Montagne, L., Nottet, H. S. & De Groot, C. J. (2000) Clin. Exp. Immunol. 122, 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McManus, C., Berman, J. W., Brett, F. M., Staunton, H., Farrell, M. & Brosnan, C. F. (1998) J. Neuroimmunol. 86, 20–29. [DOI] [PubMed] [Google Scholar]