Abstract
Among people at genetic risk of schizophrenia, those who use cannabis show smaller thalamic and hippocampal volumes. We evaluated this relationship in people at clinical high risk (CHR) of psychosis. The Alcohol and Drug Use Scale was used to identify 132 CHR cannabis users, the majority of whom were non-dependent cannabis users, 387 CHR non-users, and 204 healthy control non-users, and all participants completed magnetic resonance imaging scans. Volumes of the thalamus, hippocampus and amygdala were extracted with FreeSurfer, and compared across groups. Comparing all CHR participants with healthy control participants revealed no significant differences in volumes of any ROI. However, when comparing CHR users to CHR non-users, a significant ROI × Cannabis group effect emerged: CHR users showed significantly smaller amygdala compared to CHR non-users. However, when limiting analysis to CHR subjects who reported using alcohol at a ‘use without impairment’ severity level, the amygdala effect was non-significant; rather, smaller hippocampal volumes were seen in CHR cannabis users compared to non-users. Controlling statistically for effects of alcohol and tobacco use rendered all results non-significant. These results highlight the importance of controlling for residual confounding effects of other substance use when examining the relationship between cannabis use and neural structure.
Keywords: Amygdala, hippocampus, marijuana, magnetic resonance imaging, neuroanatomy, schizophrenia, thalamus
1. Introduction
Cannabis is the most extensively used illegal substance in people with schizophrenia. A recent review of the literature established that this is also true in people at clinical high risk (CHR) of developing psychosis (Addington et al., 2014), that is, individuals who present with attenuated or brief intermittent psychotic symptoms, or have a genetic risk for psychosis and decline in functioning. There is also some research implicating cannabis as one important factor in the onset of psychosis (Caspi et al., 2005; Fusar-Poli et al., 2012a; Kuepper et al., 2011; Moore et al., 2007). Prospective data suggests that among people at CHR of psychosis who use cannabis, those with higher baseline use severity (Buchy et al., 2015a) and frequency (Valmaggia et al., 2014), and a first use prior to age of 15 (Arseneault et al., 2002; Valmaggia et al., 2014) all confer a greater risk of transition to psychosis.
Recent work has established a link between cannabis use and subcortical volumes in people with schizophrenia. The thalamus, hippocampus and amygdala have been of particular interest as people with schizophrenia show volumetric reductions in these areas relative to healthy people (Bora et al., 2011; Chan et al., 2011; Ellison-Wright et al., 2008) and these regions are rich in cannabinoid 1 (CB1) receptors in the human brain (Glass et al., 1997). For example, chronic, heavy cannabis use has been associated with volumetric reductions in the hippocampus and amygdala (Lorenzetti et al., 2015). People with schizophrenia and a cannabis use disorder show cannabis-related shape differences in thalamus, striatum and globus pallidus, compared to patients without a cannabis use disorder (Smith et al., 2014), and cannabis users with schizophrenia show marked hippocampal shape deflation compared to healthy controls (Solowij et al., 2013). Several studies have established a link between subcortical volumes and cannabis use in people at familial risk of schizophrenia. One study reported that those who used cannabis frequently (i.e. at least once a month but not more than three times per week) had an enlarged third ventricle relative to other use frequencies, which could reflect gray matter loss in the adjacent anterior medial thalamus (Welch et al., 2011a). A second study used manual tracing technique and showed that people at familial risk of schizophrenia who consumed cannabis over a 2-year period showed bilateral volume loss in the thalamus, but not in the hippocampus or amygdala, compared to a non-exposed group (Welch et al., 2011b). A third study used an automated tensor-based morphometry analysis to detect gray matter loss in right anterior hippocampus (Welch et al., 2013). Together these finding suggest that people at familial risk of schizophrenia are particularly sensitive to the risk-modifying effects of cannabis on thalamic and perhaps hippocampal structure, but not on amygdala volumes. Very recent evidence suggests that thalamic functional connectivity may be impacted by cannabis use patterns in youth at clinical high risk of psychosis (Buchy et al., 2015b); however, it is unknown whether thalamic and other subcortical volumes are associated with cannabis use in this population. The CHR population offers a unique opportunity to study the relationship between cannabis use and subcortical volumes in people who are more likely to transition to psychosis than people at familial risk of psychosis or healthy people.
Based on the literature described above, the aim of the current report was to evaluate thalamic, hippocampal and amygdala volumes in CHR participants who used cannabis at baseline compared to CHR non-users. We hypothesized that CHR cannabis users would show significantly smaller thalamic and hippocampal volumes compared to CHR participants who did not use cannabis.
2. Methods
2.1 Participants
Participants were recruited for the second phase of the multi-site North American Prodrome Longitudinal Study (NAPLS-2) (Addington et al., 2012), a 2-year longitudinal study which was established to investigate predictors and mechanisms of transition to psychosis. The final NAPLS sample consists of 764 CHR participants and 280 healthy controls (HC). The present paper reports on the 519 CHR participants in NAPLS-2 who provided baseline magnetic resonance (MR) scans and also completed a baseline assessment on cannabis use, as well as 204 HC participants who were not using cannabis at a baseline and provided MR scans. All CHR participants were required to meet the Criteria of Prodromal Syndromes (COPS) using the Structured Interview for Prodromal-Risk Syndromes (SIPS) (McGlashan et al., 2010).
Participants were excluded if they met criteria for any current or lifetime axis I psychotic disorder, IQ<70, past or current history of central nervous system disorder or DSM-IV criteria for current substance dependence disorder. HC participants were also excluded if they had a first-degree relative with a current or past psychotic disorder. A more detailed description of ascertainment, inclusion and exclusion criteria, and participant details is provided elsewhere (Addington et al., 2012).
2.2 Measures
The SIPS and the Scale of Prodromal Symptoms (SOPS) (McGlashan et al., 2010) were used to assess criteria for a prodromal syndrome and severity of attenuated positive symptoms. Post-training agreement on determining the prodromal diagnoses was excellent (kappa=0.90) (Addington et al., 2012).
Cannabis use in the last month was rated using the Alcohol and Drug Use Scale (AUS/DUS) (Drake et al., 1996) which records severity (1=abstinent, 2=use without impairment, 3=abuse, 4=dependence) and frequency of use (0=no use, 1=once or twice per month, 2=3–4 times per month, 3=1–2 times per week, 4=3–4 times per week, 5=almost daily) in the last month. Alcohol and tobacco use were also recorded.
2.3 MRI scans
Scanning was performed at eight sites. Five sites (UCLA, Emory, Harvard, UNC, and Yale) used Siemens-Trio 3T scanners, two sites (Zucker-Hillside Hospital and UCSD) used GE HDx scanners, and one site (Calgary) used a GE Discovery scanner. All Siemens sites used a 12-channel head coil and all GE sites used an 8-channel head coil. Sequence parameters were optimized for each scanner manufacturer, software version and coil configuration according to the ADNI protocol (http://adni.loni.usc.edu/methods/documents/mri-protocols/). Scans were acquired in the sagittal plane with a 1 mm * 1 mm in-plane resolution and 1.2 mm slice thickness. Siemens scanners used an MPRAGE sequence with a 256 (axial) × 240 (sagittal) × 176 (coronal) mm field of view, TR/TE/TI=2300/2.91/900 ms and a 9 degree flip angle, while GE scanners used an IR-SPGR sequence with a 26 cm field of view, TR/TE/TI=7.0/minimum full/400 ms and an 8 degree flip angle.
2.4 Image Processing
Subcortical volumetric segmentation of the thalamus was processed using FreeSurfer version 5.2 (http://surfer.nmr.mgh.harvard.edu/) at Yale University by investigators who had participated in the FreeSurfer training course at the Martinos Center for Biomedical Imaging. The subcortical segmentation procedure assigns a neuroanatomical label to each voxel of the MRI volume using a probabilistic atlas and a Bayesian classification rule (Fischl et al., 2002). See Cannon et al. (2014) for details on the quality assurance procedure.
2.5 Statistical Analyses
A regression analysis adjusted “raw” ROI volumes for normal variation in age based on observed relationships of the ROIs with age in the HC sample (Mathalon et al., 2003; Pfefferbaum et al., 1995). Specifically, for each ROI, volumetric data were linearly regressed on age in the HC group, and the resulting regression equation was used to derive predicted ROI volumes based on age for all subjects. These age-specific predicted volumes were then subtracted from observed volumes, and the difference was divided by the standard error of regression from the HC age-regression model. This resulted in an age-adjusted z-score for each participant based on the normative data provided by the HC group. By definition, the mean ± standard deviation for age-adjusted z-scores in the HC group equals 0 ± 1. For CHR participants, z-scores provide volume estimates relative to that which would be expected from healthy individuals of a particular age. For all groups, z-scores express deviations of ROI volumes from the age-specific normative ROI volume estimates in standard units. Accordingly, the profile of ROI z-score means for the HC’s used herein in group comparisons was nearly flat (i.e., all ROI means equal to zero), and the profile of the CHR group reflects regional variation in the extent of volumetric abnormalities. Use of z-scores enabled comparison of MRI data across groups with different mean ages and across brain regions with fundamentally different sizes, rendering the ROI measures commensurable for comparison of ROI profiles between groups.
Differences in profiles of ROI volume age-adjusted z-scores (thalamus, hippocampus, amygdala) were evaluated in 1) all CHR and HC participants, and 2) CHR cannabis users vs. CHR non-users. The Subject Type effect was defined as CHR vs. HC participants, the Cannabis effect was defined as CHR cannabis user vs. CHR non-user, and ROI was defined as thalamus, hippocampus and amygdala. Difference in ROIs for CHR cannabis users and CHR non-users was assessed with the interaction effect (ROI × Cannabis) of a mixed model analysis of variance (ANOVA) with scanner site entered as an additional between-subjects factor to control for any potential site variation in MRI volumes. All imaging analyses included intracranial volume as a covariate. Chi-square for categorical variables and t-tests for continuous variables were used to compare CHR and HC groups on demographics and cannabis use. Chi-squares and ANOVAs were used to compare cannabis users and non-users on demographic and substance use variables. The critical p-value was set to p=0.05.
2.6 Procedures
All eight NAPLS sites (Emory University, Harvard University, University of Calgary, University of California at Los Angeles, University of California at San Diego, University of North Carolina at Chapel Hill, Yale University, and Zucker Hillside Hospital) recruited CHR and HC individuals. Raters were experienced research clinicians who demonstrated adequate reliability at routine reliability checks. Gold standard post-training agreement on the critical threshold for determining initial eligibility and subsequent conversion status based on the SIPS was excellent (kappa=.90). The Principal Investigator/psychiatrist/psychologist at each site conducted a comprehensive clinical assessment to determine if entry criteria were met. JA chaired weekly conference calls to review criteria for all individuals admitted to the study. Clinical assessments that included the AUS/DUS were conducted at baseline. The study protocols and informed consents were reviewed and approved by the ethical review boards of all eight NAPLS study sites.
3. Results
3.1 Demographics and cannabis use patterns
Demographic and clinical characteristics, as well as mean substance use ratings of CHR and HC participants are summarized in Table 1. Compared to the HC group, the CHR group was significantly younger, had fewer years of education, and had relatively more males. Groups did not differ on racial background. CHR participants had significantly higher cannabis use severity and frequency, lower alcohol use and higher tobacco use than HC participants.
Table 1.
Demographic and clinical characteristics, and substance use ratings, in CHR and HC groups.
| CHRA n=519 | HC n=204 | ||||
|---|---|---|---|---|---|
|
| |||||
| n (%) | n (%) | χ2 | p-value | ||
| Sex | |||||
| Male | 314 (60.5) | 107 (52.5) | 3.90 | 0.05 | |
| Female | 205 (39.5) | 97 (47.5) | |||
| Race | |||||
| First NationsAsian Black Latin America/Middle East/White Interracial |
6 (1.2) 40 (8.7) 88 (17.0) 332 (64.1) 52 (10.0) |
4 (2.0) 23 (11.2) 36 (17.6) 120 (58.9) 21 (10.3) |
5.81 | 0.76 | |
| AUS/DUS cannabis use severity | |||||
| 387 (74.6) | 204 (100.0) | 63.47 | <0.001 | ||
| Abstinent Use without impairment Abuse Dependence |
109 (21.0) 21 (4.0) 2 (0.4) |
0 (0.0) 0 (0.0) 0 (0.0) |
|||
| AUS/DUS cannabis use frequency | |||||
| Abstinent 1–4 times per month 1–4 times per week Almost daily Missing | 387 (74.7) 67 (12.9) 49 (9.5) 15 (2.9) 0 (0.0) |
204 (100.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) |
60.39 | <0.001 | |
| AUS/DUS tobacco use severity | |||||
| Abstinent Use without impairment Abuse Dependence Missing |
387 (74.6) 120 (23.1) 5 (1.0) 7 (1.3) 0 (0.0) |
187 (91.7) 15 (7.4) 0 (0.0) 0.(0.0) 2 (1.0) |
29.73 | <0.001 | |
| AUS/DUS alcohol use severity | |||||
| 296 (57.0) | 101 (49.5) | 9.72 | 0.02 | ||
| Abstinent Use without impairment Abuse Dependence Missing |
210 (40.5) 9 (1.7) 6 (1.2) 0 (0.0) |
102 (50.0) 0 (0.0) 0 (0.0) 1 (0.5) |
|||
| Mean (SD) | Mean (SD) | t | p-value | ||
| Age (years) | 18.6 (4.2) | 20.1 (4.8) | 4.00 | <0.001 | |
| Education (years) | 11.4 (2.8) | 12.9 (3.6) | 5.95 | <0.001 | |
Note. CHR, Clinical High Risk; HC, Healthy Controls; SD, Standard Deviation.
Includes CHR cannabis users and non-users.
One-hundred thirty-two CHR participants used cannabis (i.e. scored ‘2’ or higher on the AUS/DUS severity scale) at baseline. Demographic and clinical characteristics, as well as mean substance use ratings of CHR cannabis users vs. non-users and HC non-users are summarized in Supplementary Table 1. CHR cannabis users and CHR non-users significantly differed on education, and alcohol and tobacco use.
3.2 Regional brain volume differences
Volumetric analysis was conducted on age-adjusted z-scores. Group means for the cortical gray matter ROI raw volumes, although not analyzed statistically, are presented in Table 2.
Table 2.
Volumes of 3 subcortical regions of interest.
| CHR | HC | ||
|---|---|---|---|
| User n=132 | Non-user n=387 | Non-user n=204 | |
| Thalamus | 15735.7 ± 1660.9 | 15362.7 ± 1783.6 | 15432.6 ± 1588.7 |
| Hippocampus | 8442.7 ± 893.0 | 8454.8 ± 904.5 | 8536.8 ± 876.1 |
| Amygdala | 3454.5 ± 435.4 | 3486.0 ± 474.4 | 3469.4 ± 421.4 |
Note. Data are shown as mean ± standard deviation. HC = Healthy Control; CHR = Clinical High Risk.
First, we examined volumetric differences in our 3 a priori ROIs in all CHR participants compared to HC participants, to evaluate baseline differences across groups. As shown in Table 3, the main effect of ROI was significant, indicating significantly larger thalamus compared to both hippocampus and amygdala, and larger hippocampus than amygdala, Main effects of Subject Type and Site were non-significant. The ROI × Site effect was significant, which may have been related to scanner differences or subject cohort differences between sites. All other effects in the model controlled for these Site effects. The ROI × Subject Type effect was non-significant. Thus CHR and HC participants did not differ on volumes of thalamus, hippocampus or amygdala.
Table 3.
Analysis of variance (ANOVA) of thalamus, hippocampus and amygdala in CHR and HC participants.
| Effect | df | F | p-value | |
|---|---|---|---|---|
| ROI | 2 | 16.13 | <0.001 |
Thalamus > Hippocampus Thalamus > Amygdala Hippocampus > Amygdala |
| Subject type | 1 | 2.29 | 0.13 | |
| Site | 7 | 1.89 | 0.07 | |
| ROI × Site | 14 | 8.74 | <0.001 | |
| ROI × Subject Type | 2 | 1.23 | 0.29 |
Note. Subject type = healthy control or clinical high risk, collapsed across cannabis users and non-users. ANOVA results are based on multivariate assumptions for repeated measures, and all F-tests are based on Wilks’ Lambda. Significant p-values are bolded. Follow-up tests are shown in italics.
Secondly, to evaluate our hypothesis that CHR cannabis users would show significantly smaller thalamic volumes compared to CHR non-users, we evaluated volumetric differences in the 3 ROIs amongst these two CHR groups. These results are displayed in Table 4. Main effects of ROI and Site were non-significant. A significant main effect of Cannabis was seen. The ROI × Site interaction was significant; all other effects in the model controlled for Site effects. A significant ROI × Cannabis effect can be seen. Follow-up tests to parse this interaction showed that the Cannabis effect was significant for the amygdala, but not for the other ROIs, with cannabis users having significantly smaller amygdala than non-users (see Figure 1). Further follow-up tests indicated a significant ROI effect in CHR non-users, reflecting significantly larger hippocampus than amygdala. The ROI effect was non-significant among CHR cannabis users.
Table 4.
Analysis of variance (ANOVA) of thalamus, hippocampus and amygdala in CHR cannabis users (n=132) vs. CHR non-users (n=387).
| Effect | df | F | p-value | Direction of effect |
|---|---|---|---|---|
| ROI | 2 | 9.86 | <0.001 | |
| Cannabis | 1 | 4.40 | 0.04 | |
| Site | 7 | 0.92 | 0.49 | |
| ROI × Site | 14 | 6.49 | <0.001 | |
| ROI × Cannabis | 2 | 3.46 | 0.03 | |
| Cannabis effect for thalamus | 1 | 0.21 | 0.65 | |
| Cannabis effect for hippocampus | 1 | 1.44 | 0.23 | |
| Cannabis effect for amygdala | 1 | 4.78 | 0.03 | Users < Non-users |
| ROI effect in users | 2 | 0.09 | 0.91 | |
| ROI effect in non-users | 2 | 11.4 | <0.001 | Hippocampus > Amygdala |
Note. HC = Healthy Control; CHR = Clinical High Risk; Cannabis = cannabis user or non-user. ANOVA results are based on multivariate assumptions for repeated measures, and all F-tests are based on Wilks’ Lambda. Follow-up ANOVAs are shown in italics. Significant p-values are bolded.
Figure 1.
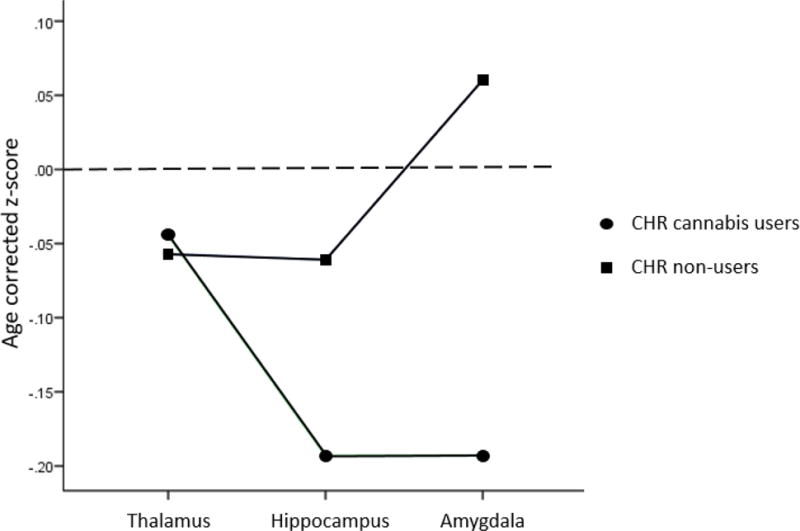
Estimated marginal means of age-corrected z-scores for clinical high risk cannabis users (n=132) and cannabis non-users (n=387) for thalamus, hippocampus and amygdala, adjusted for intracranial volume. Significantly smaller amygdala volumes were seen in CHR cannabis users compared to CHR non-users. Dotted line represents the approximate mean value of healthy controls.
We re-ran this latter analysis after removing 15 CHR cannabis users and 88 non-users who were taking antipsychotic medication. The results changed such that the ROI X Cannabis interaction went from p=0.03 to 0.10. Follow-up tests indicated that the cannabis effect for amygdala went from 0.03 to 0.01, reflecting significantly smaller amygdala volumes but not hippocampal or thalamic volumes (p=0.13 and p=0.66, respectively) in CHR cannabis users compared to CHR non-users.
When entering tobacco use as a statistical covariate in the repeated measures ANOVA, the ROI X Cannabis interaction was reduced to a trend, F(2,507)=2.72, p=0.07. Follow-up tests indicated significantly smaller amygdala in CHR cannabis users compared to non-users (p=0.04), but no difference in hippocampal or thalamic volumes (p=0.08 and p=0.98, respectively).
Finally, we re-ran the analysis presented in Table 4, but this time included only CHR participants who endorsed using alcohol at the ‘use without impairment’ severity level, to account for potential effect of alcohol use on observed effects reported above. This subgroup was selected as there were large and relatively similar sample sizes of people who endorsed alcohol use without impairment amongst CHR cannabis users (n=100) and CHR cannabis nonusers (n=110, see Supplementary Table 1); in comparison, number of CHR cannabis users and CHR non-users were quite diverse in proportion of individuals who were abstinent from alcohol use or used with abuse or dependence. The results indicated that the ROI X Cannabis interaction was statistically significant at p=0.03. However, follow-up tests indicated that the cannabis effect for amygdala went from 0.02 to 0.10, reflecting a trend toward smaller amygdala volumes in CHR users compared to CHR non-users. A significant effect was observed for the hippocampus (p=0.007), reflecting significantly smaller hippocampus in CHR cannabis users compared to CHR non-users, and no difference in thalamic volumes (p=0.94). See Figure 2.
Figure 2.
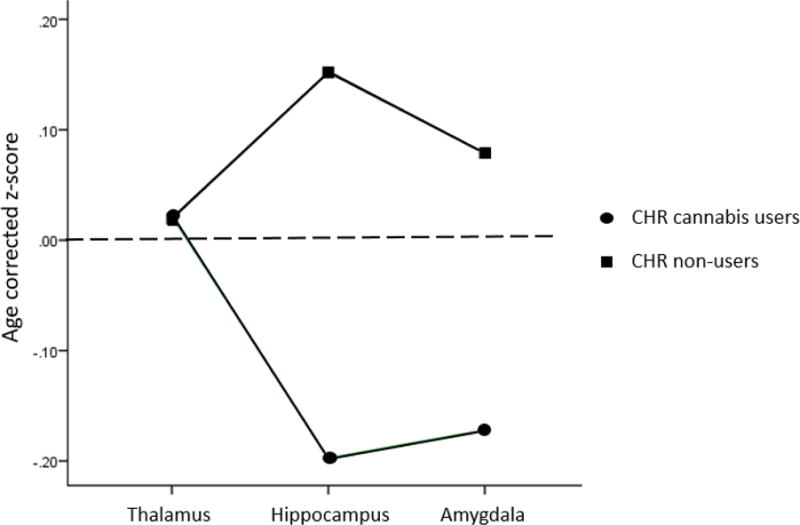
Estimated marginal means of age-corrected z-scores for clinical high risk participants who endorsed using alcohol at a ‘use without impairment’ severity level (cannabis users, n=100, and cannabis non-users, n=110) for thalamus, hippocampus and amygdala, adjusted for intracranial volume. Significantly smaller hippocampi were seen in CHR cannabis users compared to CHR non-users. Dotted line represents the approximate mean value of healthy controls.
In a final analysis, we re-ran this same analysis in all CHR cannabis users (n=132) and all CHR non-users (n=387), entering alcohol use and tobacco use as statistical covariates. The results changed such that the ROI X Cannabis interaction was non-significant, F(2,506)=2.16, p=0.12, indicating that CHR cannabis users and non-users did not significantly differ on volumes of any subcortical structure.
4. Discussion
The current study evaluated thalamic, hippocampal and amygdala volumes in a large sample of people at CHR of psychosis who used cannabis compared to CHR non-users. Our first analysis showed that CHR and HC participants did not significantly differ on volumes of any ROI. Our second analysis showed that CHR cannabis users had significantly smaller amygdala volumes, but not hippocampal or amygdala volumes, compared to CHR non-users. Covarying for tobacco use reduced this effect to a trend. When results were limited to CHR participants who endorsed using alcohol without impairment, the smaller amygdala in CHR cannabis users was no longer seen, and a significant effect emerged such that CHR cannabis users showed significantly smaller hippocampal volumes compared to CHR non-users. This suggests that alcohol use may have confounded the observed effect between cannabis use and smaller amygdala volumes in the CHR group, and that CHR cannabis users show smaller hippocampi compared to CHR non-users when restricting analyses to CHR subjects who use alcohol at a ‘use without impairment’ severity level.
The finding that CHR cannabis users showed reduction in hippocampal volumes compared to CHR non-users after being matched on alcohol use patterns is consistent with the results of one study that used automated tensor-based morphometry analysis to detect gray matter loss in right anterior hippocampus in individuals at genetic risk of schizophrenia who endorsed using cannabis over a 2-year period (Welch et al., 2013). Individuals with heavy cannabis use and otherwise minimal psychiatric comorbidities show morphologic reductions in the hippocampus (Lorenzetti et al., 2015), and dose-related reductions in hippocampal volume have been seen in otherwise healthy chronic cannabis users (Yucel et al., 2008). The hippocampus is one brain regions reported as abnormal in people with schizophrenia and in those at risk of psychosis (Fusar-Poli et al., 2012b; Olabi et al., 2011). One study has reported that people with schizophrenia who use cannabis show hippocampal shape deflation compared to healthy controls (Solowij et al., 2013), and another documented decreased left hippocampal volumes in cannabis using patients with a first-episode psychosis (Bangalore et al., 2008). Hence these findings are consistent with those of the current study, which suggest that individuals at CHR of psychosis may have an enhanced sensitivity to the effects of cannabis on subcortical brain structure, or that volumetric reductions in hippocampus may confer greater risk of cannabis use in this population. However, here it is critical to note that once the joint effects of alcohol and tobacco were accounted for, CHR cannabis users and CHR non-users did not significantly differ on volumes of hippocampus or amygdala. This finding highlights the importance of controlling for residual confounding effects of other substance use when evaluating the covariation between cannabis use and neuroanatomy.
It should be noted that two previous studies in people at genetic risk for schizophrenia showed marked reductions in thalamic volumes in those who consumed cannabis over a 2-year period (Welch et al., 2011b), and an enlarged third ventricle in frequent users compared to other use frequencies which could reflect gray matter loss in the adjacent anterior medial thalamus (Welch et al., 2011a), and this effect was not observed here. One possibility is that there is something specific about being at familial risk for schizophrenia and its relationship with cannabis use and thalamic volumes. There is some support for this hypothesis in the work of Habets and colleagues (Habets et al., 2011) who reported a significant group X cannabis interaction effect, with robust reductions of cortical thickness for people with schizophrenia and their healthy siblings than controls, suggesting genetic liability and cannabis use interaction effects on cortical thickness. Another possibility is that chronic and heavy cannabis use can lead to progressive structural changes in people at risk of psychosis. In previous studies in individuals at familial risk of schizophrenia, cannabis exposure was reported dichotomously (Yes/No) and the number of exposures was not reported. It can be seen that in our study most participants (74%) were abstinent from cannabis, and the majority of CHR participants who endorsed using cannabis at the baseline assessment had very low use severity (21%) and only 4.4% met criteria for abuse or dependence. Thus it is possible that evaluating samples of people at CHR of psychosis with greater proportions of heavy cannabis users may reveal different effects for volumes of the thalamus, hippocampus and amygdala. Indeed, literature in people with schizophrenia has revealed that heavy cannabis users show thalamic shape differences (Smith et al., 2014) and volumetric reductions in hippocampus and amygdala (Lorenzetti et al., 2015). Future research may consider evaluating CHR heavy cannabis users vs. recreational users vs. non-users on subcortical volumes to further understand the relationship between neuroanatomy and varying levels of cannabis use severity.
Several limitation should be noted. The self-report ascertainment of cannabis use may be less reliable gathering biologically based metrics such as urine toxicology data to verify cannabis use. A recent study evaluating concordance between urine screening and self-reported cannabis use in youth at risk for psychosis has shown inconsistencies between urine results and self-reported use, such that some people reported cannabis usage but urine screens were negative, whereas others did not report cannabis use but urine screens were positive for tetrahydrocannabinol (Carol and Mittal, 2014). Further, details on cannabis dosage or potency were not collected and therefore their potential impact on subcortical structure cannot be determined. Importantly, the present cross-sectional analysis cannot infer causality between cannabis use patterns and morphology of subcortical structures. Rather, our cross-sectional analysis only presents a snapshot of the association between cannabis and brain structure, by suggesting smaller amygdala in CHR cannabis users compared to CHR non-users, and smaller hippocampi amongst CHR cannabis users with minimal alcohol use compared to CHR non-users with equivalent alcohol use. Longitudinal studies are clearly required to dissociate trait characteristics from the effects of substance use on the brain. The current sample of CHR individuals is highly representative of recreational cannabis users rather than heavy and problematic users, and this should be considered when comparing the present results to previously published studies in schizophrenia. Unfortunately, we did not have a representative sample size of healthy control cannabis users to evaluate whether observed differences are due to a combination of cannabis use and CHR status, or of cannabis use alone. Future research should aim to address this important question by recruiting healthy controls who also endorse cannabis use. The present analysis did not factor in the contribution of other SCID-IV diagnoses to the results, and thus we are unable to identify to the specific role of other diagnoses or their interaction with cannabis use patterns and subcortical brain structure in the CHR sample. The current work extends knowledge on the relationship between cannabis and brain structure by showing that cannabis use is associated with smaller hippocampal volumes after controlling for alcohol use in youth at CHR of psychosis. However this manuscript also highlights the importance of controlling for effects of other substance use when evaluating the association between cannabis and neural structure.
Supplementary Material
Highlights.
Cannabis use measured in youth at clinical high risk (CHR) of psychosis
Subcortical volumes compared in CHR subjects who used cannabis vs. non-users
CHR cannabis users showed significantly smaller amygdala compared to non-users
Among light alcohol users, smaller hippocampi in CHR cannabis users vs. non-users
Controlling for alcohol and tobacco rendered these effects non-significant
Acknowledgments
This study was supported by the National Institute of Mental Health (grant U01MH081984 to Dr Addington; grants U01 MH081928; P50 MH080272; Commonwealth of Massachusetts SCDMH82101008006 to Dr Seidman; grants R01 MH60720, U01 MH082022 and K24 MH76191 to Dr Cadenhead; grant U01MH081902 to Dr Cannon; P50 MH066286 (Prodromal Core) to Dr Bearden; grant U01MH082004 to Dr Perkins; grant U01MH081988 to Dr Walker; grant U01MH082022 to Dr Woods; and UO1 MH081857-05 grant to Dr Cornblatt. The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors declare no conflict of interest.
Authors’ contributions
LB was responsible for conceptualizing this study, performing analyses and writing the manuscript. JA, TDC, KSC, BAC, DOP, LJS, THM, MTT, EFW, SWW, were responsible for all aspects of the NAPLS-2 study including study design, obtaining funding, data collection, and all contributed to the writing of the final version of the manuscript. TDC was responsible for the neuroimaging analysis. DHM assisted in the conceptualization for this study and assisted in performing the data analysis and writing the manuscript. CEB was responsible for managing NAPLS-2 at the UCLA Site and contributed to the writing of the final version of the manuscript. All authors read and approved the final version of the manuscript.
References
- Addington J, Cadenhead KS, Cornblatt BA, Mathalon DH, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Addington JA, Cannon TD. North American Prodrome Longitudinal Study (NAPLS 2): overview and recruitment. Schizophrenia research. 2012;142:77–82. doi: 10.1016/j.schres.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Case N, Saleem MM, Auther AM, Cornblatt BA, Cadenhead KS. Substance use in clinical high risk for psychosis: a review of the literature. Early Interv Psychiatry. 2014;8:104–112. doi: 10.1111/eip.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. Bmj. 2002;325:1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangalore SS, Prasad KM, Montrose DM, Goradia DD, Diwadkar VA, Keshavan MS. Cannabis use and brain structural alterations in first episode schizophrenia–a region of interest, voxel based morphometric study. Schizophrenia research. 2008;99:1–6. doi: 10.1016/j.schres.2007.11.029. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, Yucel M, Velakoulis D, Pantelis C. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophrenia research. 2011;127:46–57. doi: 10.1016/j.schres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Buchy L, Cadenhead KS, Cannon TD, Cornblatt BA, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Heinssen R, Bearden CE, Mathalon DH, Addington J. Substance Use in Individuals at Clinical High Risk of Psychosis. Psychological Medicine. 2015a;45:2275–2284. doi: 10.1017/S0033291715000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchy L, Cannon T, Lyngberg K, Cadenhead K, Cornblatt B, McGlashan T, Perkins D, Seidman L, Tsuang M, Walker E, Woods SW, Bearden C, Mathalon DH, Addington J. Biological Psychiatry. Toronto, ON: 2015b. Impact of cannabis use on thalamic connectivity in youth at clinical high risk of psychosis; p. 251S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TG, McEwen S, Addington J, Bearden CE, Cadenhead K, Cornblatt B, Mathalon DH, McGlashan T, Perkins D, Jeffries C, Seidman LJ, Tsuang M, Walker E, Woods SW, Heinssen R, North American Prodrome Longitudinal Study, C Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77:147–157. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Sun F, McEwen SJ, Papademetris X, He G, van Erp TG, Jacobson A, Bearden CE, Walker E, Hu X, Zhou L, Seidman LJ, Thermenos HW, Cornblatt B, Olvet DM, Perkins D, Belger A, Cadenhead K, Tsuang M, Mirzakhanian H, Addington J, Frayne R, Woods SW, McGlashan TH, Constable RT, Qiu M, Mathalon DH, Thompson P, Toga AW. Reliability of neuroanatomical measurements in a multisite longitudinal study of youth at risk for psychosis. Hum Brain Mapp. 2014;35:2424–2434. doi: 10.1002/hbm.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol EE, Mittal VA. Self-reported cannabis use is inconsistent with the results from drug-screening in youth at ultra high-risk for psychosis in Colorado. Schizophrenia research. 2014;157:317–318. doi: 10.1016/j.schres.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, Taylor A, Arseneault L, Williams B, Braithwaite A, Poulton R, Craig IW. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Chan RC, Di X, McAlonan GM, Gong QY. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull. 2011;37:177–188. doi: 10.1093/schbul/sbp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake RE, Mueser K, McHugo G. Clinical Rating Scales. In: Sederer L, Dickey B, editors. Outcomes assessment in clinical practice. Williams and Wilkins; Baltimore: 1996. pp. 113–116. [Google Scholar]
- Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. The American journal of psychiatry. 2008;165:1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, Barale F, Caverzasi E, McGuire P. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Archives of general psychiatry. 2012a;69:220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr Bull. 2012b;38:1297–1307. doi: 10.1093/schbul/sbr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Habets P, Marcelis M, Gronenschild E, Drukker M, van Os J, Genetic R, Outcome of P. Reduced cortical thickness as an outcome of differential sensitivity to environmental risks in schizophrenia. Biol Psychiatry. 2011;69:487–494. doi: 10.1016/j.biopsych.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Kuepper R, van Os J, Lieb R, Wittchen HU, Hofler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. Bmj. 2011;342:d738. doi: 10.1136/bmj.d738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Solowij N, Whittle S, Fornito A, Lubman DI, Pantelis C, Yucel M. Gross morphological brain changes with chronic, heavy cannabis use. Br J Psychiatry. 2015;206:77–78. doi: 10.1192/bjp.bp.114.151407. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Pfefferbaum A, Lim KO, Rosenbloom MJ, Sullivan EV. Compounded brain volume deficits in schizophrenia-alcoholism comorbidity. Archives of general psychiatry. 2003;60:245–252. doi: 10.1001/archpsyc.60.3.245. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Walsh BC, Woods SW. The psychosis risk syndrome: Handbook for diagnosis and follow-up. Oxford University Press; New York, NY: 2010. [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Olabi B, Ellison-Wright I, McIntosh AM, Wood SJ, Bullmore E, Lawrie SM. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011;70:88–96. doi: 10.1016/j.biopsych.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Cobia DJ, Wang L, Alpert KI, Cronenwett WJ, Goldman MB, Mamah D, Barch DM, Breiter HC, Csernansky JG. Cannabis-related working memory deficits and associated subcortical morphological differences in healthy individuals and schizophrenia subjects. Schizophr Bull. 2014;40:287–299. doi: 10.1093/schbul/sbt176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Walterfang M, Lubman DI, Whittle S, Lorenzetti V, Styner M, Velakoulis D, Pantelis C, Yucel M. Alteration to hippocampal shape in cannabis users with and without schizophrenia. Schizophrenia research. 2013;143:179–184. doi: 10.1016/j.schres.2012.10.040. [DOI] [PubMed] [Google Scholar]
- Valmaggia LR, Day FL, Jones C, Bissoli S, Pugh C, Hall D, Bhattacharyya S, Howes O, Stone J, Fusar-Poli P, Byrne M, McGuire PK. Cannabis use and transition to psychosis in people at ultra-high risk. Psychol Med. 2014;44:2503–2512. doi: 10.1017/S0033291714000117. [DOI] [PubMed] [Google Scholar]
- Welch KA, McIntosh AM, Job DE, Whalley HC, Moorhead TW, Hall J, Owens DG, Lawrie SM, Johnstone EC. The impact of substance use on brain structure in people at high risk of developing schizophrenia. Schizophr Bull. 2011a;37:1066–1076. doi: 10.1093/schbul/sbq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch KA, Moorhead TW, McIntosh AM, Owens DG, Johnstone EC, Lawrie SM. Tensor-based morphometry of cannabis use on brain structure in individuals at elevated genetic risk of schizophrenia. Psychol Med. 2013;43:2087–2096. doi: 10.1017/S0033291712002668. [DOI] [PubMed] [Google Scholar]
- Welch KA, Stanfield AC, McIntosh AM, Whalley HC, Job DE, Moorhead TW, Owens DG, Lawrie SM, Johnstone EC. Impact of cannabis use on thalamic volume in people at familial high risk of schizophrenia. Br J Psychiatry. 2011b;199:386–390. doi: 10.1192/bjp.bp.110.090175. [DOI] [PubMed] [Google Scholar]
- Yucel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, Lubman DI. Regional brain abnormalities associated with long-term heavy cannabis use. Archives of general psychiatry. 2008;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


