Abstract
In the present work, potato-like silver molybdate (Ag2MoO4) microstructures were synthesized through a simple hydrothermal method. The microstructures of Ag2MoO4 were characterized by various analytical and spectroscopic techniques such as XRD, FTIR, Raman, SEM, EDX and XPS. Interestingly, the as-prepared Ag2MoO4 showed excellent photocatalytic and electrocatalytic activity for the degradation of ciprofloxacin (CIP) and electrochemical detection of hydrogen peroxide (H2O2), respectively. The ultraviolet-visible (UV-Vis) spectroscopy results revealed that the potato-like Ag2MoO4 microstructures could offer a high photocatalytic activity towards the degradation of CIP under UV-light illumination, leads to rapid degradation within 40 min with a degradation rate of above 98%. In addition, the cyclic voltammetry (CV) and amperometry studies were realized that the electrochemical performance of Ag2MoO4 modified electrode toward H2O2 detection. Our H2O2 sensor shows a wide linear range and lower detection limit of 0.04–240 μM and 0.03 μM, respectively. The Ag2MoO4 modified electrode exhibits a high selectivity towards the detection of H2O2 in the presence of different biological interferences. These results suggested that the development of potato-like Ag2MoO4 microstructure could be an efficient photocatalyst as well as electrocatalyst in the potential application of environmental, biomedical and pharmaceutical samples.
Nowadays, antibiotics are main class of antimicrobial drugs in today’s medicine and widely used to prevent the bacterial infection for both human and animals. In particular, ciprofloxacin (CIP), as an antibiotic drug, plays an important role for the treatment of urinary, digestive infections and pulmonary diseases1. The CIP can be entered into the aquatic environment through the intentional disposal of surplus drugs, the release of excreta from human and animals, malapropos treatment in the hospitals or in pharmaceutical industries, improper removal of waste water treatment plants and the use of animal’s feces as agricultural fertilizers2,3. Probably, the CIP drug is not completely metabolized and the continuous release of CIP into the environments displays the chronic toxicity to bacteria, which causes toxicity to the microorganism and retarding to aquatic vertebrates4,5. It also interacts with photosynthesis process and inhibits the growth of spinach plants6 and cause antibiotic-resistance bacteria growth in the environment7. Therefore, the removals of CIP from the water sources are major concern to protect the aquatic system and soil environment. Several methods have been reported for the removal of CIP from water including adsorption8, oxidation9, sonolysis10, sorption11, (photo)-Fenton process12, photocatalytic degradation13,14,15 and ozonation16. Among them, photocatalysis is an efficient, cost-effective and eco-friendly method for the environmental remediation, which degrades the hazardous organic pollutants into easily bio-degradable or nontoxic molecules17,18.
On the other hand, hydrogen peroxide (H2O2) is an essential intermediate in the various food manufacturing and also involved in our life process. The detection of H2O2 is a paramount issue because it is a major reactive oxygen species, generated by most oxidases in mitochondria and related to the several bodily disorders such as myocardial infarction, atherosclerosis, and Alzheimer’s disease, cancer, etc.19. H2O2 is also acts contrarily in cell growth, differentiation, physiological signaling pathways, migration and immune function system20. Moreover, it is most significant chemical in clinical, pharmaceutical industries and atomic power stations, which dramatically have an effect on the cloud and rainwater21 as well as a precursor to the formation of more reactive and potentially harmful hydroxyl radicals22. Therefore, the trace level detection of H2O2 is an important concern for the environment and medicinal fields. Up to now, various methods are available to detect the H2O2 and some of them have been explored a satisfactory results to the concern of H2O2 detection23. However, the electrochemical methods hold more advantages such as simplicity, portability, rapid analysis, high sensitivity and low-cost instrumentation. Due to these attractive features, the electrochemical methods are represent a promising alternative technique for the determination of H2O2.
Transition metal-based molybdates (M = Fe, Ni, Co, Ag, Mn etc.,) are considered as an important inorganic material which are widely explored in different applications such as Li-ion storage batteries24, birefringent filters25, supercapacitors26,27, optical fibers28, photoluminescence29, scintillation crystal30, photocatalyst31,32, humidity sensors33, magnetic properties34 and catalysts35. However, low dimensional metal molybdates have attracted more curiosity in current years. In particular, silver molybdate (Ag2MoO4) has attracted considerable attention because of its unique properties such as environmental friendly, photoluminescence, high electrical conductivity, excellent antimicrobial activity, good photocatalytic activity and remarkable electrochemical energy storage performance36. Due to these properties, the Ag2MoO4 is potentially used in several applications including ion-conducting glasses37, high-temperature lubrication38, gas sensor39, antibacterial material40, photoswitches41 and ceramics42. In photocatalysis, Ag2MoO4 has paid significant attention owing to it’s photosensitivity which make this material with high photocatalytic activity under UV or visible-light irradiation. Recently, few kinds of literature are reported based on Ag2MoO4 and its composite that act as a photocatalyst for the degradation of organic dyes into the wastewater43,44,45. The photocatalytic activity mainly depends on the crystal and electronic structures of materials that affect the energy band structure and the efficiency of charge carrier transfer. Moreover, to improve their physicochemical properties of the photocatalyst, researchers have growled a number of approaches to obtain the different morphologies of Ag2MoO4 including nanoparticles, nanorods, nanowires, wire-like nanostructures, nanoclusters, broom-like, flower-like microstructures and microcrystals36,41,46,47,48,49,50. However, to the best of our knowledge, we reported the synthesis of potato-like Ag2MoO4 microstructure, its applications for the photocatalytic degradation of CIP and electrochemical detection of H2O2 for the first time.
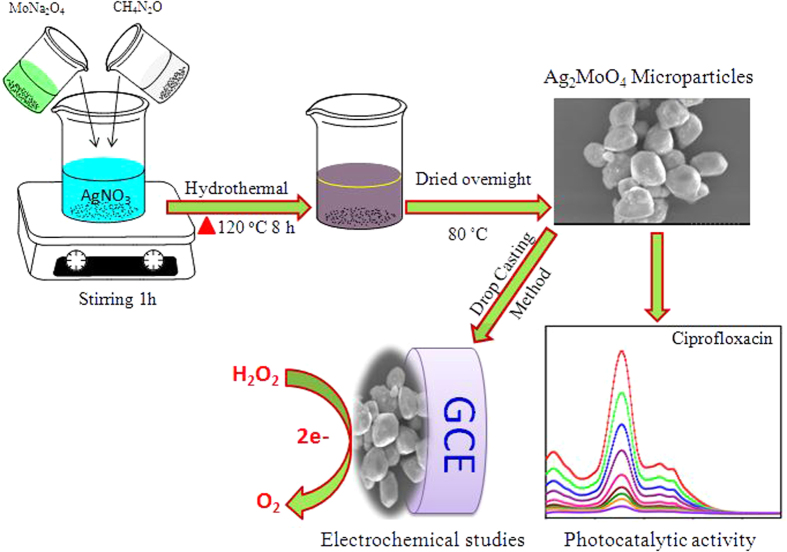
In this present work, we developed a simple one-pot hydrothermal synthesis of potato-like Ag2MoO4 microparticles with the assistance of urea and characterized using various analytical and spectroscopic techniques in detailed and further evaluated for electrochemical sensing and photocatalytic applications, as illustrated Fig. 1. Fascinatingly, we find that the as-prepared potato-like Ag2MoO4 microparticles exhibited a high-performance electrochemical sensor for the detection of H2O2. Moreover, their photocatalytic activity towards the removal of CIP antibiotic into the environment was also investigated with efficient degradation rate.
Figure 1. The synthesis route for Ag2MoO4 microparticles and its application for photocatalytic activity and electrochemical biosensor.
Results and Discussion
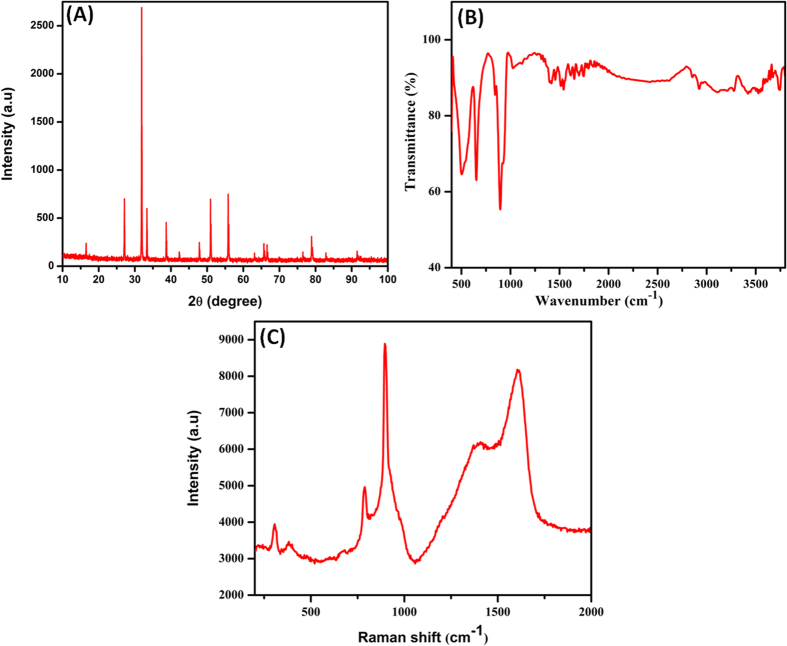
The crystalline structure and phase purity of the as-synthesized samples were determined by using XRD pattern as shown in Fig. 2A. The distinctive diffraction peaks obtained at 16.48°, 27.09°, 31.87°, 33.31°, 38.65°, 42.27°, 47.84°, 50.93°, 55.81°, 63.10°, 65.70°, 66.57°, 76.49° and 78.90° in the 2θ range which well agreed to the (111), (220), (311), (222), (400), (331), (422), (511), (440), (620), (533), (622), (642) and (731) reflection planes, respectively. Aforementioned planes are well related to the standard XRD report of cubic phase structured Ag2MoO4 with the space group of Fd m51. From the XRD pattern, it was clearly revealed that the as-synthesized product is β-Ag2MoO436. The appearance of sharp and high intense peaks demonstrated the higher crystalline nature of cubic Ag2MoO4 phase. There is no any other peaks were appeared which related to the Ag2O or MoO3 phase, indicates the as-synthesized Ag2MoO4 was homogeneous solid.
m51. From the XRD pattern, it was clearly revealed that the as-synthesized product is β-Ag2MoO436. The appearance of sharp and high intense peaks demonstrated the higher crystalline nature of cubic Ag2MoO4 phase. There is no any other peaks were appeared which related to the Ag2O or MoO3 phase, indicates the as-synthesized Ag2MoO4 was homogeneous solid.
Figure 2.
(A) XRD patterns (B) FTIR spectra and (C) Raman spectra of as-prepared Ag2MoO4.
FTIR and Raman spectroscopy is an important tool for analyzing the involvement of functional groups present in the as-synthesized Ag2MoO4. In the FTIR spectra (Fig. 2B), the absorption peaks at 3280 and 1650 cm−1 correspond to the O-H stretching and bending vibrations of the water molecules, respectively52. The peak at 645 cm−1 is confirmed the Ag-O stretching vibration of Ag2MoO4. The sharp peak at 891 cm−1 attributed to the anti-symmetric Mo-O stretching in tetrahedral MoO42− ion53. Raman spectra showed (Fig. 2C) the peaks at 896, 782, 382 and 305 cm−1 which are due to the ν1 (Ag), ν3 (Eg), ν4 (Bg) and ν2 (Ag) symmetric and asymmetric stretching vibration modes of Ag2MoO4, respectively. The vibration modes at 896 and 782 cm−1 could be ascribed to the symmetric stretching vibration of Mo-O bond of the MoO4 unit and the asymmetric stretching vibrations of the molybdate ion, respectively. The peaks at 382 and 305 cm−1 were clearly indicated the ν4 and ν2 bending vibration modes of tetrahedral MoO454.
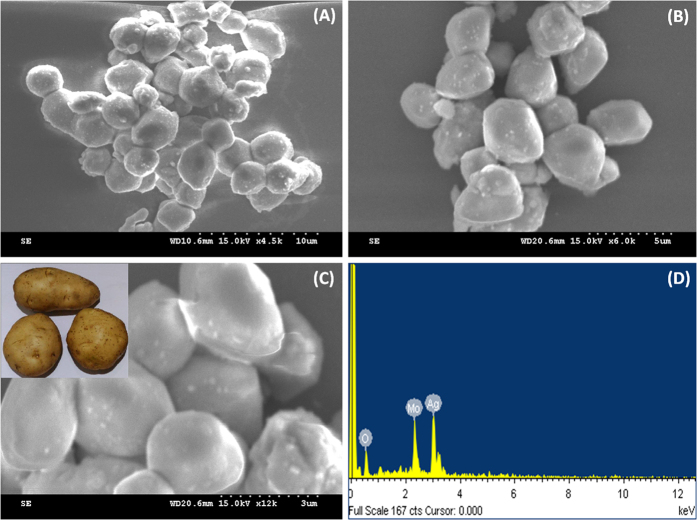
The surface morphology of the as-prepared microstructures was investigated by SEM. Figure 3(A–C) illustrated the general views of the different magnifications of the obtained Ag2MoO4 microstructures. The images are clearly displayed the potato-like structure of Ag2MoO4 which seems like bunches of potatoes with clean and fairly smooth surfaces, the average diameters of microstructures about 1–2 μm. Energy dispersive x-ray spectra (EDX) were used to identify the elements present in the as-prepared Ag2MoO4 microparticles, as depicted in Fig. 3D. The EDX spectra showed the peaks at approximately 0.5, 2.4 and 3 keV reveal the presence of O, Mo, and Ag elements in the material without any other significant impurities.
Figure 3.
(A–C) SEM images of Ag2MoO4 at different magnifications (A) 10 μm (B) 5 μm (C) 3 μm and (D) corresponding to EDX spectrum of Ag2MoO4.
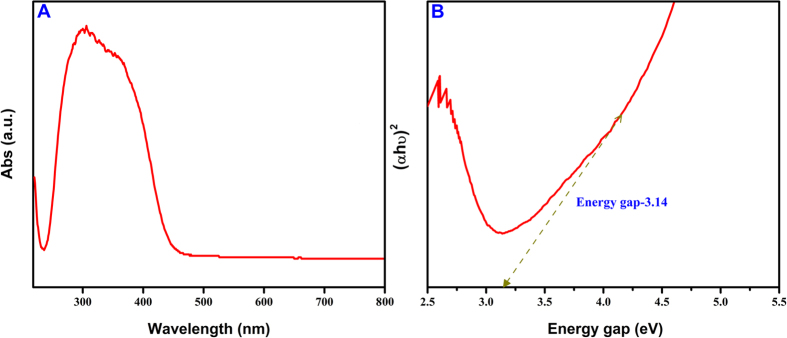
The bandgap energy of the Ag2MoO4 is an important parameter for the selection of suitable kind of light source needed for the degradation purposes. The UV-Vis (Diffuse reflectance) absorption spectrum of Ag2MoO4 microparticles is shown in Fig. 4A. The results shows that the relation between the normalized absorption of the photocatalyst and wavelength with a range of 200–800 nm. The most part of absorption spectra of Ag2MoO4 falls in the UV region, a broad steep from 300 to 420 nm which corresponds to the band gap energy value from 3–3.34 eV. The band gap value was calculated using Tauc’s equation and the graph plotted (αhν)2 against (hν) as can be seen in Fig. 4B. The calculated band gap energy value is 3.14 eV. On the other hand, the bandgap of Ag2MoO4 is significantly altered compared to that of previous reports36,43,44,50. The oxygen vacancy created in the crystal lattice of the Ag2MoO4 is leads to the distortion in the energy levels and influenced the bandgap which may be attributed to the effect of hydrothermal environment on the surface microstructures.
Figure 4.
(A) UV-Vis diffuse reflectance spectra (DRS) and (B) Energy gap spectra of Ag2MoO4.
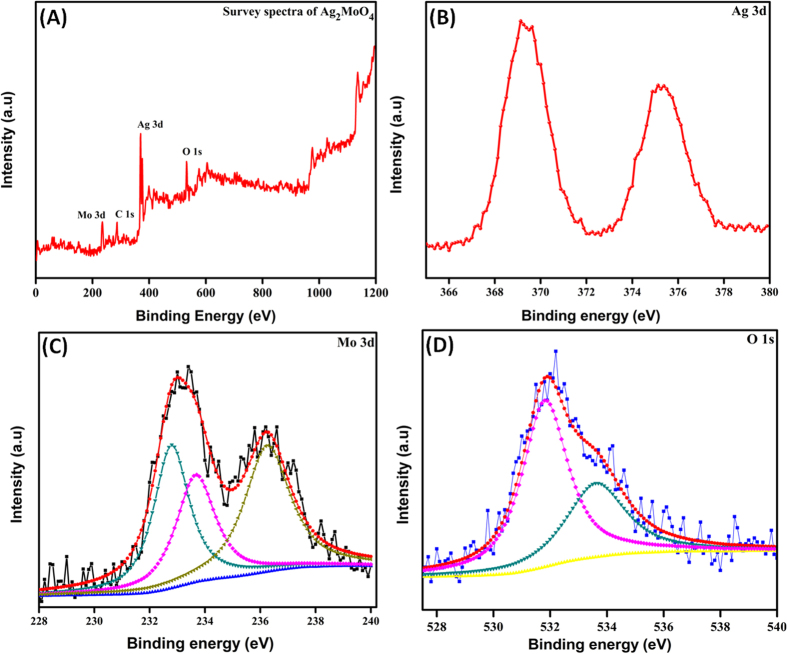
X-ray photoelectron spectroscopy (XPS) was used to evaluate the information about the chemical composition and chemical status of the as-synthesized Ag2MoO4, as shown in Fig. 5. The overall XPS spectrum in Fig. 5A shows the coexistence of elements Mo, C, Ag and O within the as-prepared Ag2MoO4 microparticles and no other impurities were detected, which are in good agreement with EDX report. In addition, the presence of C peak at 284.9 eV is ascribed to the adventitious hydrocarbon from the XPS instrument and it is inherent. High resolution scanning XPS spectra clearly confirms the Ag 3d, Mo 3d, and O 1s level, which is fitted by using the Gaussian fitting method, as shown in Fig. 5(B–D). In Fig. 5B, the Ag 3d spectra displays the two peaks at 368.7 and 374.4 eV attributed to the Ag 3d5/2 and Ag 3d3/2 electron binding energy in Ag2MoO4, respectively55. The peaks at 233.2 and 236.3 eV ascribed to the Mo 3d5/2 and Mo 3d3/2 binding energy of Mo 3d, respectively. The major binding energy peaks Mo 3d5/2 and Mo 3d3/2 are separated by 3.1 eV, which belongs to the Mo6+ oxidation state as depicted in Fig. 5C56. The high intense peaks at around in the range of 530.5–533.6 eV revealed the presence of O 1s core level57 in Ag2MoO4 (Fig. 5D). Hence, the obtained XPS results clearly confirmed that the valence of Ag, Mo and O are +1, +6 and −2, respectively, which is very good agreement with the phase structure of Ag2MoO4.
Figure 5.
(A) XPS survey spectra of Ag2MoO4, (B–D) High resolution XPS spectra of Ag 3d, Mo 3d and O 1s.
Electrochemical impedance spectroscopy (EIS) was used to investigate the changes of the electrode surface during the fabrication process. The nyquist curves of the EIS spectra was observed using bare GCE (a) and Ag2MoO4 modified GCE (b) in 0.1 M KCl containing 5.0 mM K3Fe(CN)6/K4Fe(CN)6 (Fig. 6). The diameter of the semicircle indicates the electron transfer resistance (Rct) of the electrode. This resistance controls the electron transfer kinetics of redox probe at the electrode interface. From the EIS results, the Ag2MoO4 modified GCE (b) shows larger semicircle than bare GCE (a) reveals that the Ag2MoO4 modified GCE can increase the electron transfer resistance on electrode surface, because it is hindered the electron transfer of K3Fe(CN)6/K4Fe(CN)6, thus confirming the successful modification of Ag2MoO4 on the GCE surface.
Figure 6.
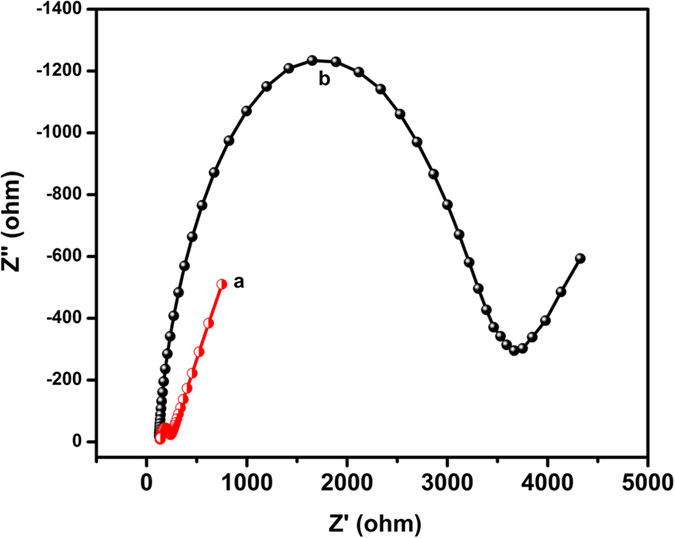
Electrochemical impedance spectroscopy of different modified electrodes (a) bare GCE (b) Ag2MoO4 modified GCE in 0.1 M KCl solution containing 5 mM [Fe(CN)6]3−/4−.
Photocatalytic activity
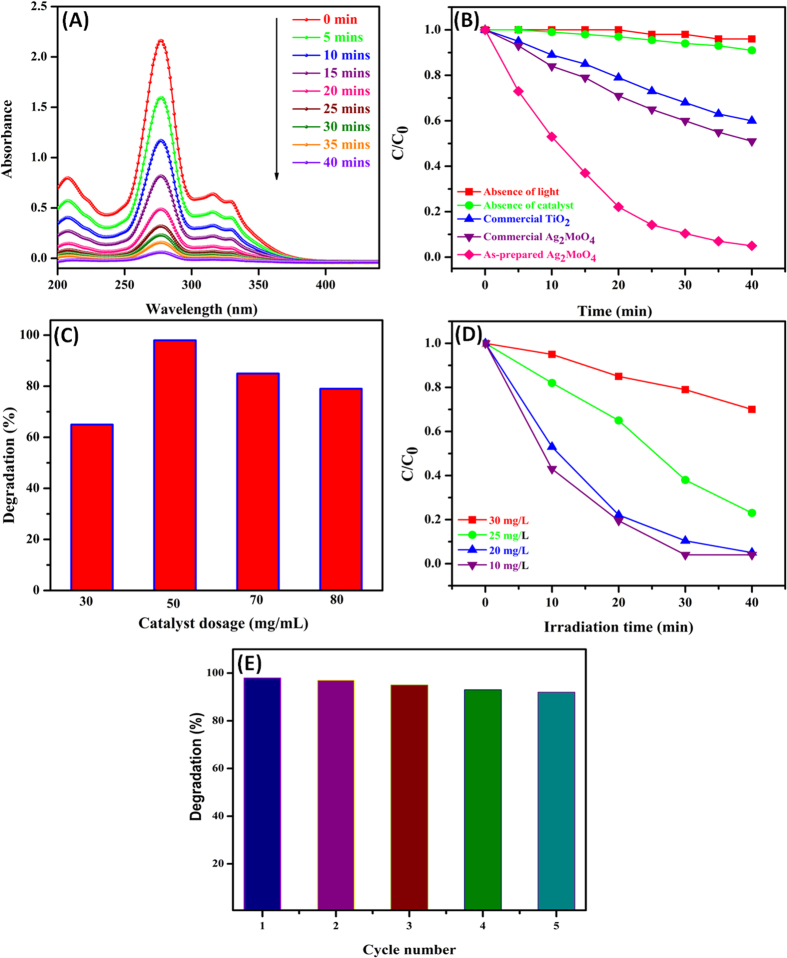
The photocatalytic behavior of as-prepared Ag2MoO4 microparticles was performed against the degradation of CIP under UV-light illumination, as illustrated in Fig. 7A. The absorbance spectrum shows the progressive degradation of CIP and the main absorption peak of CIP was observed at 276 nm and other small peaks were also completely diminished within 40 min. The degradation percentage of CIP solution was estimated from the relative intensity of absorbance in UV-visible spectra. The relative intensity of absorbance was decreased and reached almost zero within 40 min, reveals that the Ag2MoO4 microparticles degraded the 98% of the CIP solution. Initially, the utmost decrement of CIP was observed which could be attributed to the competence of CIP with hydroxyl radicals generated by UV-light photoexcitation of Ag2MoO4 microparticles. However, as the reaction proceeds, the formation of by-products from degradation might compete with the hydroxyl radicals and adsorption sites on the catalyst surface. Hence, the gradual degradation of CIP was observed.
Figure 7.
(A) Absorption spectrum of CIP in the presence of 50 mg/mL Ag2MoO4 under UV-light illumination. (B) Photodegradation of CIP in the presence of different catalysts. (C) Effect of catalyst amount dosage on the photodegradation of CIP. (D) Effect of initial CIP concentration on the photodegradation and (E) reusability of the potato-like Ag2MoO4 photocatalyst.
Under similar degradation conditions, commercially available Ag2MoO4 and commercial TiO2 powder were also employed as a photocatalyst and their accurate comparison was carried out which depicted in Fig. 7B. The photocatalytic activity of commercial Ag2MoO4 and TiO2 on CIP degradation exhibited a poor efficiency and the corresponding degradation percentages are observed around 38% and 32%, respectively. Moreover, there is no significant degradation was observed either in the absence of light or in the absence of a catalyst. The results clearly confirmed the as-synthesized Ag2MoO4 shown a superior photocatalytic efficacy over the commercial Ag2MoO4 and TiO2 powder for the degradation of CIP solution.
Catalyst dosage is an important parameter that can significantly influence the rate of photodegradation. Figure 7C shows the efficiency of CIP degradation (%) against the effect of catalyst loading by varying the catalyst amount from 30 to 80 mg/mL, wherein the concentration of CIP and intensity of the light were maintained as constant. It can be seen that the rate of photocatalytic degradation was maximum at 50 mg/mL, due to the generation of more number of electron-hole pairs. However, the addition of excess amount of photocatalyst can blocks the existing active sites and interfere with the diffusion of photons. As a result, the photocatalytic degradation of CIP was decreased when increase the concentration of photocatalyst over 50 mg/mL58.
Effect of initial concentration of CIP solution on the photodegradation was also investigated and the concentration varied from 10 to 30 mg/L under identical conditions, the results are shown in Fig. 7D. In the present case ~98% degradation was achieved at 20 mg/L whereas 83% and 77% degradation were observed at 25 and 30 mg/L concentration of CIP, respectively. This is due to the screening of light by the CIP solution and the less number of photons to reach the Ag2MoO4 surface. Hence, the electron-hole pair generation is reduced greatly and consequently, the degradation efficiency decreased.
Generally, the reactive oxidative species (ROS) viz hydroxyl radical (·OH), superoxide radical anion (O2·−), hole (h+) and electron (e−) involved in the photocatalytic reaction59. The photocatalytic mechanism of the degradation of CIP is represented in the following equations
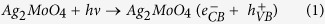 |
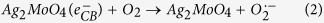 |
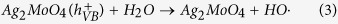 |
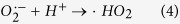 |
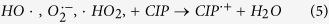 |
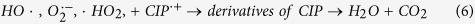 |
The semiconductor photocatalyst generally undergoes excitation under light illumination with energy greater than the bandgap while the e− excited from the valence band (VB) to the conduction band (CB) leaves h+ in the VB60. In the present study, Ag2MoO4 was irradiated by UV light which produced the e− in the CB and h+ in the VB, as illustrated in the Eq. 1. The e− in the CB were reacted with the oxygen molecule to form O2·− and the O2·− was reacted with the water molecule to form ·HO2, as shown in Eqs 2 and 4. Furthermore, h+ in the VB adsorbed water molecules and reacted to form ·OH, as given in Eq. 2. The ROS formed in the photocatalytic reaction facilitated the degradation of the CIP by the stepwise photocatalytic reduction process (Eqs 5 and 6).
The high photocatalytic activity as well as recycling ability of the catalyst is a vital issue for long-term use in practical applications. To evaluate the sustainability of Ag2MoO4, the recycling experiments were carried out for the degradation of CIP, as illustrated in Fig. 7E. The photocatalytic activity of the Ag2MoO4 on CIP degradation did not show a significant loss and attains more than 90% of degradation rate even after the five successive cycles with every cycle lasting for 40 min, which indicating that the potato-like Ag2MoO4 microstructure is a very effective and highly stable photocatalyst. Thus, the obtained results also indicated a good reusability of the catalyst. Moreover, the poor loss of catalytic activity during the recycle performance is due to the accumulation of impurities on the surfaces of the photocatalyst.
Electrochemical performance to hydrogen peroxide on Ag2MoO4 modified GCE
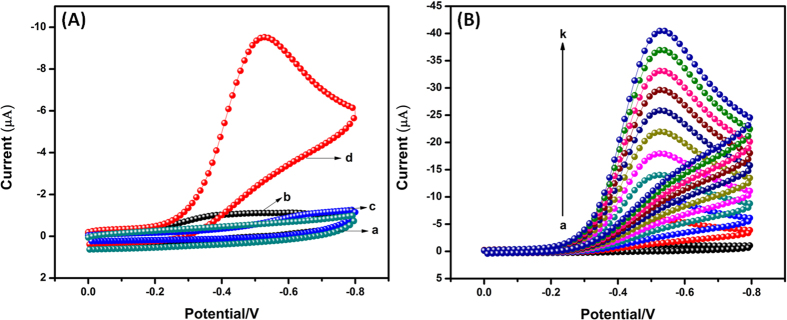
Cyclic voltammetry studies of the H2O2 sensor was demonstrated using bare GCE (a,c) and (b,d) Ag2MoO4 modified GCE in absence (curve a & b) and presence of 200 μM H2O2 (curve c & d) containing N2 saturated 0.05 M PBS (pH 7) at a scan rate 50 mV/s. In the absence of H2O2 (curve a & b), the results clearly indicates that there is no significant reduction peak appeared in the selected potential window. Whereas, in the presence of 200 μM H2O2 on Ag2MoO4 modified GCE (curve d) shows a strong and higher reduction peak current appeared at the lower onset potential −0.26 V due to the catalytic behavior of Ag2MoO4. Although, we also observed a slight and not considerable reduction peak in the bare GCE at the longer potential −0.7 V (curve c). The reduction peak current of H2O2 on Ag2MoO4 modified GCE is 10 times much higher than that of bare GCE. The above results confirmed that Ag2MoO4 modified GCE has high catalytic ability for H2O2 detection. Consequently, Ag2MoO4 are suitable as mediators to transfer electron between H2O2 and working electrode and make possible electrochemical regeneration following electron exchange with H2O2. The possible mechanisms for the electrochemical reduction of hydrogen peroxide as shown in Eqs (7, 8, 9). In order to investigate the electrocatalytic activity of Ag2MoO4 modified GCE, CVs were performed in the presence of different addition of H2O2 in 0.05 M PBS (pH 7), as shown in Fig. 8B. The reduction peak current of H2O2 was linearly increased with increasing the H2O2 concentration from 0 to 1 mM, which revealing electrocatalytic activity of Ag2MoO4 modified GCE toward the H2O2. Furthermore, the low level detection, sensitivity and linear range of H2O2 were clearly discussed in amperometric (i-t) section.
Figure 8.
(A) Cyclic voltammograms for the reduction of H2O2 on bare GCE (a,c) and Ag2MoO4 modified GCE (b,d) in absence (a,b) and presence (c,d) of 200 μM H2O2 containing N2 saturated 0.05 M PBS (pH 7) at a scan rate of 50 mV/s. (B) Cyclic voltammograms of Ag2MoO4 modified GCE in N2 saturated 0.05 M PBS (pH 7) in the absence and presence of H2O2 with different concentrations (a–k: 0 to 1 mM) at a scan rate of 50 mV/s.
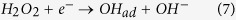 |
 |
 |
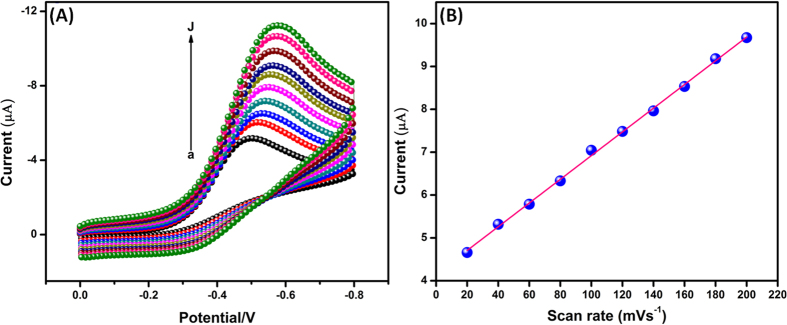
Furthermore, the electrocatalytic behavior of the Ag2MoO4 modified GCE towards H2O2 was studied with the change of scan rate. Figure 9A reveals the CVs responses of 200 μM of H2O2 detection at Ag2MoO4/GCE with different scan rate ranges from 20 to 200 mV/s and its denoted (a–j). When increasing the scan rate from 20 to 200 mV/s, the reduction peak current of H2O2 was increased and the peak potential was shifted towards the more negative side. The peak current of H2O2 reduction is directly proportional to the scan rate (Fig. 9B) (Correlation co-efficient R2 = 0.998), indicating that the electrode process is surface controlled process61.
Figure 9.
(A) Cyclic voltammograms of Ag2MoO4 modified electrode in 200 μM H2O2 containing N2 saturated 0.05 M PBS (pH 7) at different scan rates from 20 to 200 mV/s (a–j) and (B) Corresponding calibration plot of scan rate vs. Ipc of H2O2.
The effect of pH is very important phenomenon for the electrochemical sensor and biosensor. The electrocatalytic performance of Ag2MoO4 modified GCE for H2O2 reduction was examined in different pH solution. The CVs of Ag2MoO4 modified GCE in the presence of 200 μM H2O2 at different pH ranges (3 to 11) of 0.05 M PBS solution were studied and its calibration plot is depicted in Figure S1. When the pH value increased from 3 to 5, the reduction peak current increased and then decreased gradually while increasing the pH more than 7. However, higher peak currents are observed at pH 7. Therefore, Ag2MoO4 modified GCE has good electrocatalytic activity at pH 7 and the reduction of H2O2 is pH dependence. Therefore, we chosen pH 7 is optimized pH and this pH was used further electrochemical measurements.
Amperometric determination of H2O2 at Ag2MoO4 modified GCE
The amperometric i-t technique is one of the most important method to determine the electrocatalytic activity of modified electrodes in electrochemical sensor and biosensor applications. In the present work, we have utilized an amperometric method to estimate the performance of Ag2MoO4 modified GCE toward H2O2 detection. In this regards, Ag2MoO4 modified rotating disc glassy carbon electrode (RDGCE) was performed in continuously stirred pH 7 solution by applying constant potential at −0.5 V with rotation speed at 1200 rpm. Figure 10 reveals the amperometric i-t performance obtained at Ag2MoO4 modified RDGCE upon the different addition of H2O2 (0.049 to 247 μM) in the PBS solution. These results undoubtedly shows that Ag2MoO4 modified film demonstrates a fast and well-defined response obtained in each different addition of H2O2 concentration. The response time of H2O2 detection on Ag2MoO4 modified RDGCE was observed for 5 s, it’s clearly suggesting that the fast electron movement process was occurred in the electrolyte and electrode interface when introducing the H2O2. The linear response current increases with increasing the concentration of H2O2 (low concentration to high concentration) from 0.049 to 240 μM (linear range inset; Fig. 10), the obtained sensitivity and limit of detection (LOD) of the sensor is around 9.8 μAμM−1 cm−2 and 0.03 μM, respectively. The above results suggesting that the Ag2MoO4 modified RDGC electrode has good electrocatalytic activity towards H2O2. The analytical parameters, such as LOD, linear range, and sensitivity, of H2O2 sensor was compared with various modified electrodes are summarized in Table 1. Clearly, the Ag2MoO4 modified RDGCE, reported here, exhibits good sensitivity and LOD over a wide linear range of H2O2 concentration compared to other reports62,63,64,65,66,67,68,69,70,71,72,73,74,75.
Figure 10.
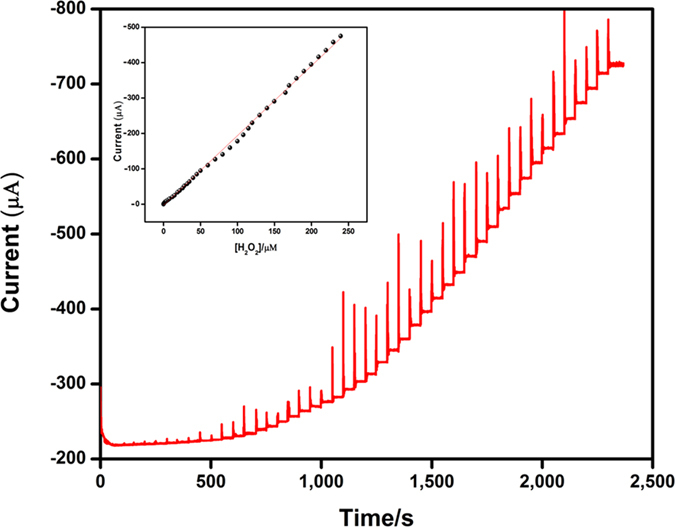
Amperometric i–t responses of H2O2 reduction at Ag2MoO4 film modified RDGCE upon successive additions of H2O2 from 0.04 to 247 μM into continuously stirred N2 saturated 0.05 M PBS (pH 7). Applied potential: −0.5 V; Rotation rate: 1200 rpm. Inset shows the calibration plot of response current vs. H2O2 concentration [0.04–240 μM].
Table 1.
| Modified electrode | Method of detection | LR (μM) | LOD (μM) | Ref. |
|---|---|---|---|---|
| AgNPs/DNA/GCE | Amperometry | 2.0–2500 | 0.6 | 62 |
| GNs/Ag/GCE | Amperometry | 100.0–40,000 | 28.0 | 63 |
| AgNWs/CS/GCE | Amperometry | 8.0–1350 | 2.0 | 64 |
| AgNWs/PtE | Amperometry | 0.5–3000 | 0.2 | 65 |
| ZnONRs/AgNPs/GCE | Amperometry | 0.9–983.0 | 0.9 | 66 |
| SiNWs/AgNPs/GCE | Amperometry | 0.2 −20,000 | 0.2 | 67 |
| AgNPs/GCE | Amperometry | 4.0–60.0 | 1.3 | 68 |
| PVP–AgNWs/GCE | Amperometry | 20.0–3620 | 2.3 | 69 |
| GR/AgNWs/GCE | Amperometry | 1 | 1.0 | 70 |
| CNT/AgNPs/GCE | Amperometry | 9.0–9000 | 1.6 | 71 |
| RGO/AgNPs/GCE | Amperometry | 100.0–8000 | 7.1 | 72 |
| Ag/GCE | Amperometry | 5.0–12,000 | 0.5 | 73 |
| Ag-AlOOH-rGO | Amperometry | 5.0 to 4200 | 1.8 | 74 |
| Ag-Bt/GCE | Amperometry | — | 9.1 | 75 |
| Ag2MoO4/GCE | Amperometry | 0.04–240 | 0.03 | This work |
Comparison of analytical performance of Ag2MoO4 modified electrode with previously reported similar modified electrodes for the detection of H2O2. Abbreviations: LR–linear response range; LOD–limit of detection; NPs–nanoparticles; NWs–nanowires; Ag- Silver nanoparticles; CS–chitosan; GCE–glassy carbon electrode; GR–graphene; PVP–polyvinylpyrolidone; GNs–graphene nanosheets; PtE–platinum electrode; CNT–carbon nanotubes; NRs–nanorods; RGO–reduced graphene oxide; AlOOH- Aluminum oxyhydroxide: Ag-Bt- silver nanoparticle-incorporated bentonite clay.
Selectivity is very important study in the electrochemical sensor. In order to investigate selectivity, the proposed sensor was used to detect the H2O2 in the presence of variety of biological interferences such as catechol (b), fructose (c), lactose (d), sucrose (e), glucose (f), hydroquinone (g), ascorbic acid (h), uric acid (i), dopamine (j) and epinephrine (k) with 50-fold excess concentration of each analytes, as shown in Fig. 11. The Ag2MoO4 modified RDGCE exhibited well-defined response towards each 100 μM H2O2 (a). No remarkable responses were monitored for the 50-fold excess concentration of biological interferences as mentioned above. Hence, the Ag2MoO4 modified RDGCE has excellent selectivity towards the determination of H2O2. Furthermore, we have studied the stability of our proposed sensor by amperometric (i-t) techniques and the results obtained as it can be seen in Figure S2. The prepared sensor retains its 98.2% of its initial response of H2O2 after prolongs runs up to 2600 s, which suggesting the good stability of the sensor.
Figure 11.
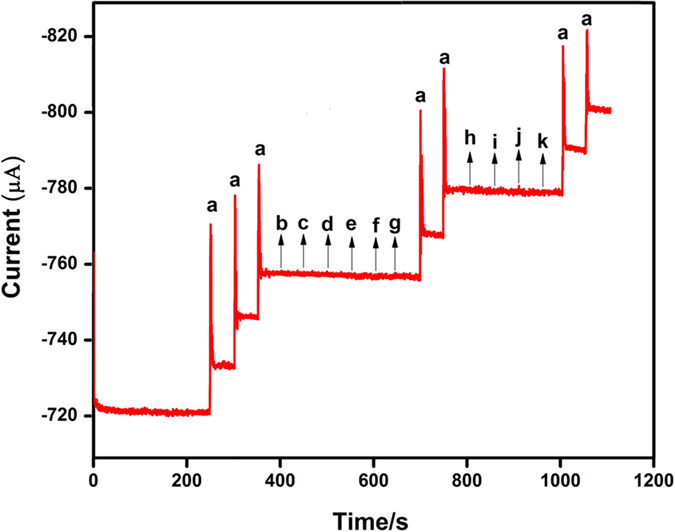
Amperometric i–t responses of H2O2 reduction at Ag2MoO4 modified RDGCE for the successive addition of 100 μM of H2O2 (a), and 50-fold excess concentration of catechol (b), fructose (c), lactose (d), sucrose (e), glucose (f), hydroquinone (g), ascorbic acid (h), uric acid (i), dopamine (j) and epinephrine (k) in continuously stirred N2 saturated 0.05 M PBS (pH 7). Applied potential: −0.5 V; Rotation rate: 1200 rpm.
In summary, a potato-like Ag2MoO4 microparticles were successfully prepared through a simple hydrothermal method. Different analytical and spectroscopic methods were used to confirm the structural nature of Ag2MoO4 microparticles. All the obtained results are strongly evidenced that the prepared compound shows like pristine Ag2MoO4 without any other impurities. The as-prepared Ag2MoO4 microparticles explored excellent photocatalytic activity towards the degradation of chronic toxicity CIP under UV-light illumination and 98% of the CIP was degraded within 40 min. On the other hand, the Ag2MoO4 microparticles were used to fabricate the sensor electrode for the detection of H2O2 in the potentially interfering biological substances. The Ag2MoO4 modified GCE revealed a high electrocatalytic activity with wide linear range, good stability and low detection limit than the previous reports. Finally, the Ag2MoO4 represents an interesting and promising candidate for photocatalytic and electrochemical applications with the advantages of one-pot preparation, unique features, excellent catalytic activity and high sensing performance.
Experimental Section
Materials
Silver nitrate (AgNO3), sodium molybdate dihydrate (Na2MoO4.2H2O), urea (CH4N2O), hydrogen peroxide (H2O2) and all chemicals were purchased from Sigma-Aldrich and used further purification. Ciprofloxacin drug was purchased from Thiruvengadam Medicals, Virudhunagar, India. All other chemicals were of analytical grade and used without further purification. The phosphate buffer solution (PBS) was prepared using 0.05 M Na2HPO4 and NaH2PO4 and all the required solutions were prepared using deionized water (DI).
Synthesis of silver molybdate
In a typical synthesis, 0.5 M of Na2MoO4.2H2O and 0.1 M of AgNO3 were dissolved in 60 mL DI water. Then, 0.3 g of urea (10 mL) was gradually added into the above mixture under vigorous stirring at room temperature for 30 min. The mixture was transferred into 100 mL Teflon-lined autoclave and maintained at 120 °C for 8 h in an oven. The autoclave was then cooled down to room temperature naturally, and the obtained yellow product was collected by centrifugation and washed thoroughly with DI water and ethanol for three times and dried overnight at 80 °C.
Characterization
The powder X-ray diffraction (XRD) analysis was carried out using PANalytical X’Pert PRO X-ray diffractometer measured with Cu-Kα radiation (λ = 1.54178 Å) in the 2θ range of 10–100°. X-ray photoelectron spectroscopy (XPS) measurements were carried out using ULVAC-PHI 5000 VersaProb instrument. Scanning electron microscope (SEM) and Energy dispersive X-ray spectra (EDX) were probed using Hitachi S-3000 H and HORIBA EMAX X-ACT, respectively. Raman spectra were collected in an NT-MDT confocal Raman microscopic system with an exciting laser wavelength of 488 nm and the laser spot-size is around 0.5 μm. Fourier transform infrared spectroscopy (FTIR) was recorded by Shimadzu FT-IR 3000 spectrometer in the diffuse reflectance mode at a resolution of 4 cm−1, the sample was pressed into KBr disc with a weight ratio of sample to KBr of 1:100 in the range of 4000-400 cm−1. UV-Visible diffused reflectance (DRS) spectrum of the sample was taken from Shimadzu UV-2600 spectrophotometer and BaSO4 was used as a reflectance reference material. The absorption spectra in the photocatalytic degradation process were monitored by Shimadzu 2100 UV-Visible spectrometer. The electrochemical impedance spectroscopy (EIS) was performed by XPOT (ZAHNER elektrik instrument). Cyclic voltammetry (CV) and Amperometric (i-t) experiments were carried out using CHI 405a work station and PINE instrument, respectively. All the electrochemical studies were carried out in three-electrode cell system, glassy carbon electrode (GCE surface area = 0.071 cm2) was used as a working electrode, platinum wire and standard Ag/AgCl electrodes were used as a counter and reference electrode, respectively.
Photocatalytic activity
The photocatalytic activity of the as-prepared Ag2MoO4 was evaluated via degradation of CIP solution under UV light illumination (λ = 200~400 nm). In a typical activity, 50 mg/mL of catalyst was dispersed in 100 mL aqueous solutions of CIP (20 mg/L). Prior to illumination, the solution mixture was stirred magnetically for 30 min in the dark to establish the adsorption-desorption equilibrium between CIP and as-prepared Ag2MoO4 photocatalyst. Then, the solutions were illuminated by UV light (λmax = 365 nm) to induce photocatalytic reaction. During the irradiation, 5 mL of the solution was withdrawn at 5 min time intervals and centrifuged to remove the catalyst. The obtained clear liquor was analyzed by UV-Vis spectrometer to determine the concentration changes of CIP.
Fabrication of silver molybdate modified GCE
Before surface modification, the GCE was mirror like polished with 0.05 μm alumina slurry and rinsed with DI to remove the alumina particles on the GCE surface. After that the GCE was sonicated for 1 min containing ethanol and water (1:1 ratio). About 5 mg/mL of the as-prepared Ag2MoO4 was re-dispersed in DI water and about 8 μL (optimized concentration) of the dispersed Ag2MoO4 was drop casted on the GCE surface. Then it was allowed to dry at room temperature, followed by the dried Ag2MoO4 modified GCE was rinsed with DI water to remove the loosely attached catalyst on the GCE surface. The obtained Ag2MoO4 modified GCE was used to further electrochemical experiments. Then, it was stored in room temperature when not in use.
Additional Information
How to cite this article: Kumar, J. V. et al. Fabrication of potato-like silver molybdate microstructures for photocatalytic degradation of chronic toxicity ciprofloxacin and highly selective electrochemical detection of H2O2. Sci. Rep. 6, 34149; doi: 10.1038/srep34149 (2016).
Supplementary Material
Acknowledgments
Financial supports of this work by the Ministry of Science and Technology, Taiwan (NSC101-2113-M-027- 001-MY3 to SMC) are gratefully acknowledged. We are grateful to thank the University of Grant Commission (UGC-F. No. 42-348/2013 (SR) & 01.04.2013), New Delhi, India. We also express our gratitude to the College Managing Board, Principal and Head of the Department of Chemistry, VHNSN College, Virudhunagar for providing research facilities.
Footnotes
Author Contributions J.V.K. and V.M. conceived the synthesis method and fabricated the Ag2MoO4 samples. They also performed photocatalytic activity and characterization of the materials and composed the manuscript. R.K. and S.-M.C. performed the electrochemical studies, analyzed the data and wrote the manuscript. C.K. performed the material characterization using XPS and Raman spectroscopy and also corrected the manuscript. S.-M.C. and V.M. supervised and finalized the project. All authors discussed the results and contributed to the final paper.
References
- Glassmeyer S. T. et al. Transport of chemical and microbial compounds from known wastewater discharges: Potential for use as indicators of human fecal contamination. Environ. Sci. Technol. 39, 5157–5169 (2005). [DOI] [PubMed] [Google Scholar]
- Beier S. et al. Treatment of hospital wastewater effluent by nanofiltration and reverse osmosis. Water Sci. Technol. 61, 1691–1698 (2010). [DOI] [PubMed] [Google Scholar]
- Wei X. Y. et al. Advanced treatment of a complex pharmaceutical wastewater by nanofiltration: Membrane foulant identification and cleaning. Desalination. 251, 167–175 (2010). [Google Scholar]
- Dodd M. C., Shah A. D., Von Gunten U. & Huang C. H. Interactions of fluoroquinolone antibacterial agents with aqueous chlorine: reaction kinetics, mechanisms, and transformation pathways. Environ. Sci. Technol. 39, 7065–7076 (2005). [DOI] [PubMed] [Google Scholar]
- Carlsson G., Orn S. & Larsson D. G. J. Effluent from bulk drug production is toxic to aquatic vertebrates. Environ. Toxicol. Chem. 28, 2656–2662 (2009). [DOI] [PubMed] [Google Scholar]
- Aristilde L., Melis A. & Sposito G. Inhibition of Photosynthesis by a Fluoroquinolone Antibiotic. Environ. Sci. Technol. 44, 1444–1450 (2010). [DOI] [PubMed] [Google Scholar]
- Belden J. B., Maul J. D. & Lydy M. J. Partitioning and photodegradation of ciprofloxacin in aqueous systems in the presence of organic matter. Chemosphere. 66, 1390–1395 (2007). [DOI] [PubMed] [Google Scholar]
- Tan Y. Y., Guo Y., Gu X. Y. & Gu C. Effects of metal cations and fulvic acid on the adsorption of ciprofloxacin onto goethite. Environ. Sci. Pollut. Res. 22, 609–617 (2015). [DOI] [PubMed] [Google Scholar]
- Wang P., He Y. L. & Huang C. H. Oxidation of fluoroquinolone antibiotics and structurally related amines by chlorine dioxide: reaction kinetics, product and pathway evaluation. Water Res. 44, 5989–5998 (2010). [DOI] [PubMed] [Google Scholar]
- De Bel E., Dewulf J., Van Lagenhove H. & Janssen C. Influence of pH on the sonolysis of ciprofloxacin: biodegradability, ecotoxicity and antibiotic activity of its degradation products. Chemosphere. 77, 291–295 (2009). [DOI] [PubMed] [Google Scholar]
- Chen H., Gao B. & Li H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J. Hazard. Mater. 282, 201–207 (2015). [DOI] [PubMed] [Google Scholar]
- De Lima Perini J. A., Perez-Moya M. & Nogueira R. F. P. Photo-Fenton degradation kinetics of low ciprofloxacin concentration using different iron sources and pH. J. Photochem. Photobiol. A. 259, 53–58 (2013). [Google Scholar]
- El-Kemary M., El-Shamy H. & El-Mehasseb I. Photocatalytic degradation of ciprofloxacin drug in water using ZnO nanoparticles. J. Lumin. 130, 2327–2331 (2010). [Google Scholar]
- Jiang Z. et al. In situ synthesis of bimetallic Ag/Pt loaded single-crystalline anatase TiO2 hollow nano-hemispheres and their improved photocatalytic properties. Cryst Eng Comm. 16, 2384–2394 (2014). [Google Scholar]
- Jiang Z. et al. Natural leaves assisted synthesis of nitrogen-doped, carbon-rich nanodots sensitized, Ag-loaded anatase TiO2 square nanosheets with dominant 001 facets and their enhanced catalytic applications. J. Mater. Chem. A. 47, 14963–14972 (2013). [Google Scholar]
- Sui M. H. et al. Heterogeneous catalytic ozonation of ciprofloxacin in water with carbon nanotube supported manganese oxides as catalyst. J. Hazard. Mater. 227–228, 227–236 (2012). [DOI] [PubMed] [Google Scholar]
- Zangeneh H. et al. Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: a comparative review. J. Ind. Eng. Chem. 26, 1–36 (2015). [Google Scholar]
- Byrne J. A. et al. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules. 20, 5574–5615 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. W. et al. Boronate-Based Fluorescent Probes for Imaging Cellular Hydrogen Peroxide. J. Am. Chem. Soc. 127, 16652–16659 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. G. H2O2, a Necessary Evil for Cell Signaling. Science. 312, 1882–1883 (2006). [DOI] [PubMed] [Google Scholar]
- Penkett S. A., Jones B. M. R., Brice K. A. & Eggleton A. E. J. The importance of atmospheric ozone and hydrogen peroxide in oxidising sulphur dioxide in cloud and rainwater. Atmos. Environ. 41, 154–168 (2007). [Google Scholar]
- Laloi C., Apel K. & Danon A. Reactive oxygen signalling: the latest news. Curr. Opin. Plant Biol. 7, 323–328 (2004). [DOI] [PubMed] [Google Scholar]
- Karuppiah C. et al. An Ultrahigh Selective and Sensitive Enzyme-Free Hydrogen Peroxide Sensor Based on Palladium Nanoparticles and Nafion-Modified Electrode. Electrocatalysis. 5, 177–185 (2014). [Google Scholar]
- Xiao W. et al. Synthesis, Characterization, and Lithium Storage Capability of AMoO4 (A = Ni, Co) Nanorods. Chem. Mater. 22, 746–754 (2010). [Google Scholar]
- Tournois P. Acousto-optic programmable dispersive filter for adaptive compensation of group delay time dispersion in laser systems. Opt. Commun. 140, 245–249 (1997). [Google Scholar]
- Zhang Z. et al. Facile hydrothermal synthesis of NiMoO4@CoMoO4 hierarchical nanospheres for supercapacitor applications. Phys Chem Chem Phys. 17, 20795–20804 (2015). [DOI] [PubMed] [Google Scholar]
- Senthilkumar B. et al. Nano α-NiMoO4 as a new electrode for electrochemical supercapacitors. RSC Adv. 3, 352–357 (2013). [Google Scholar]
- Rushbrooke J. G. & Ansorge R. E. Optical Fiber Readout and Performance of Small Scintillating Crystals for a Fine- Grained Gamma Detector. Nucl. Instrum. Methods Phys.Res. Sect A. 280, 83–90 (1989). [Google Scholar]
- Ryu J. H. et al. Microwave-assisted synthesis of CaMoO4 nano-powders by a citrate complex method and its photoluminescence property. J. Alloys Compd. 390, 245–249 (2005). [Google Scholar]
- Mikhailik V. B. & Kraus H. Performance of scintillation materials at cryogenic temperatures. Phys. Status Solidi. B. 247, 1583–1599 (2010). [Google Scholar]
- Nasab A. S., Maddahfar M. & Mashkani S. M. H. Ce(MoO4)2 nanostructures: Synthesis, characterization, and its photocatalyst application through the ultrasonic method. J. Mol. Liq. 216, 1–5 (2016). [Google Scholar]
- Shen M. et al. Hierarchical PbMoO4 microspheres: hydrothermal synthesis, formation mechanism and photocatalytic properties. Cryst Eng Comm. 15, 1146–1152 (2013). [Google Scholar]
- Sundaram R. & Nagaraja K. S. Solid state electrical conductivity and humidity sensing studies on metal molybdate–molybdenum trioxide composites (M = Ni2+, Cu2+ and Pb2+). Sens. Actuators B. 101, 353–360 (2004). [Google Scholar]
- Ding Y., Yu S. H., Liu C. & Zang Z. A. 3D Architectures of Iron Molybdate: Phase Selective Synthesis, Growth Mechanism, and Magnetic Properties. Chem. Eur. J. 13, 746–753 (2007). [DOI] [PubMed] [Google Scholar]
- Driscoll S. & Ozkan U. S. Effect of O2 Concentration in Selective and Complete Oxidation of 1,3-Butadiene, Furan, and Maleic Anhydride over MnMoO4/MoO3 Catalysts. Stud. Surf. Sci. Catal. 82, 367–375 (1994). [Google Scholar]
- Cunha F. S. et al. Structural, morphological and optical investigation of β-Ag2MoO4 microcrystals obtained with different polar solvents. Cryst Eng Comm. 17, 8207–8211 (2015). [Google Scholar]
- Bhattacharya S. & Ghosh A. Silver molybdate nanoparticles, nanowires, and nanorods embedded in glass nanocomposites. Phys Rev B. 75, 092103 (2007). [Google Scholar]
- Arora A. K., Nithya R., Misra S. & Yagi T. Behavior of silver molybdate at high-pressure. J. Solid State Chem. 196, 391–397 (2012). [Google Scholar]
- Misra S., Jayaraman V. & Gnanasekaran T. Trace Level Gas Sensing Characteristics of Nano-Crystalline Silver Decamolybdate. Soft Nanosci. Lett. 3, 39–42 (2013). [Google Scholar]
- Tang H. et al. Highly antibacterial materials constructed from silver molybdate nanoparticles immobilized in chitin matrix. Chem. Eng. J. 234, 124–131 (2013). [Google Scholar]
- Cheng L. et al. Photoswitches of one-dimensional Ag2MO4 (M = Cr, Mo, and W). J. Phys. Chem. C. 113, 1764–1768 (2009). [Google Scholar]
- Zhou D. et al. Sintering Behavior and Dielectric Properties of Ultra-Low Temperature Fired Silver Molybdate Ceramics. J. Am. Ceram. Soc. 97, 3597–3601 (2014). [Google Scholar]
- Bai Y., Lu Y. & Liu J. K. An efficient photocatalyst for degradation of various organic dyes: Ag@Ag2MoO4-AgBr composite. J. Hazard. Mater. 307, 26–35 (2016). [DOI] [PubMed] [Google Scholar]
- ZhaoQian L., XueTai C. & Ling X. Z. Microwave-assisted hydrothermal synthesis of cube-like Ag-Ag2MoO4 with visible-light photocatalytic activity. Sci. China. Chem. 56, 443–450 (2013). [Google Scholar]
- Feng M. et al. Ultralong Silver Trimolybdate Nanowires: Synthesis, Phase Transformation, Stability, and Their Photocatalytic, Optical, and Electrical Properties. ACS Nano. 5, 6726–6735 (2011). [DOI] [PubMed] [Google Scholar]
- Nagaraju G., Chandrappa G. T. & Livage J. Synthesis and characterization of silver molybdate nanowires, nanorods and multipods. Bull. Mater. Sci. 31, 367–371 (2008). [Google Scholar]
- Saito K. et al. Monoclinic Ag2Mo2O7 nanowire: a new Ag-Mo-O nanophotocatalyst material. Inorg. Chem. 52, 8297–8299 (2013). [DOI] [PubMed] [Google Scholar]
- Jiang H. et al. Thermal perturbation nucleation and growth of silver molybdate nanoclusters by a dynamic template route. Cryst Eng Comm. 17, 5511–5521 (2015). [Google Scholar]
- Singh D. P. et al. Broom-like and flower-like heterostructures of silver molybdate through pH controlled self assembly. J. Nanopart. Res. 14, 660–671 (2012). [Google Scholar]
- Fabbro M. T. et al. Identifying and rationalizing the morphological, structural, and optical properties of β-Ag2MoO4 microcrystals, and the formation process of Ag nanoparticles on their surfaces: combining experimental data and first-principles calculations. Sci. Technol. Adv. Mater. 16, 065002 (1–10) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan A. et al. A combined experimental and theorectical study on the formation of Ag filaments on β-Ag2MoO4 induced by electron irradiation. Part. Part. Syst. Charact. 32, 646–651 (2015). [Google Scholar]
- Saravanakumar K., Ramjan M. M., Suresh P. & Muthuraj V. Fabrication of highly efficient visible light driven Ag/CeO2 photocatalyst for degradation of organic pollutants. J. Alloy. Compd. 664, 149–160 (2016). [Google Scholar]
- Subcik J. et al. Structure and properties of MoO3-containing zinc borophosphate glasses. J Non-Cryst Solids. 355, 970–975 (2009). [Google Scholar]
- Arora A. K. Amorphization and decomposition of scandium molybdate at high pressure. J. Apply. Phys. 97, 013508 (2005). [Google Scholar]
- Jiang Z. & Xie J. In-situ growth of Ag/Ag2O nanoparticles on g-C3N4 by a natural carbon nanodots-assisted green method for synergistic photocatalytic activity. RSC Adv. 6, 3186–3197 (2016). [Google Scholar]
- Xu Z. et al. Supercapacitive carbon nanotube-cobalt molybdate nanocomposites prepared via solvent-free microwave synthesis. RSC Adv. 2, 2753–2755 (2012). [Google Scholar]
- Yang L. et al. Structure and effective visible-light-driven photocatalytic activity of α-NiMoO4 for degradation of methylene blue dye. J. Alloys Compd. 664, 756–763 (2016). [Google Scholar]
- Chowdhury P. R. & Bhattacharyya K. G. Ni/Ti layered double hydroxide: synthesis, characterization and application as a photocatalyst for visible light degradation of aqueous methylene blue. Dalton Trans. 44, 6809–6824 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li Z., Cong S. & Xu Y., Brookite vs Anatase TiO2 in the Photocatalytic Activity for Organic Degradation in Water. ACS Catal. 4, 3273–3280 (2014). [Google Scholar]
- Kumar P. S. et al. Novel CuO/chitosan nanocomposite thin film: facile hand-picking recoverable, efficient and reusable heterogeneous photocatalyst. RSC Adv. 5, 57493–57501 (2015). [Google Scholar]
- Fotouhi L., Fatollahzadeh M. & Heravi M. M. Electrochemical Behavior and Voltammetric Determination of Sulfaguanidine at a Glassy Carbon Electrode Modified With a Multi-Walled Carbon Nanotube. Int. J. Electrochem. Sci. 7, 3919–3928 (2012). [Google Scholar]
- Wu S. et al. Electrodeposition of silver–DNA hybrid nanoparticles for electrochemical sensing of hydrogen peroxide and glucose. Electrochem. Commun. 8, 1197–1203 (2006). [Google Scholar]
- Liu S. et al. Stable Aqueous Dispersion of Graphene Nanosheets: Noncovalent Functionalization by a Polymeric Reducing Agent and Their Subsequent Decoration with Ag Nanoparticles for Enzymeless Hydrogen Peroxide Detection. Macromolecules. 43, 10078–10083 (2010). [Google Scholar]
- Gao X. et al. A Nonenzymatic Hydrogen Peroxide Sensor Based on Silver Nanowires and Chitosan Film. Electroanal. 24, 1771–1777 (2012). [Google Scholar]
- Qin X. et al. A novel non-enzyme hydrogen peroxide sensor based on catalytic reduction property of silver nanowires. Talanta. 139, 56–61 (2015). [DOI] [PubMed] [Google Scholar]
- Lin C. Y. et al. Electrode modified with a composite film of ZnO nanorods and Ag nanoparticles as a sensor for hydrogen peroxide. Talanta. 82, 340–347 (2010). [DOI] [PubMed] [Google Scholar]
- Yin J. et al. A hydrogen peroxide electrochemical sensor based on silver nanoparticles decorated silicon nanowire arrays. Electrochim. Acta. 56, 3884–3889 (2011). [Google Scholar]
- Liao K. et al. A promising method for fabricating Ag nanoparticle modified non-enzyme hydrogen peroxide sensors. Sens. Actuators B. 181, 125–129 (2013). [Google Scholar]
- Yang X. et al. Hydrogen peroxide and glucose biosensor based on silver nanowires synthesized by polyol process. Analyst. 137, 4362–4367 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang M. & Wang Z. Nanostructured silver nanowires-graphene hybrids for enhanced electrochemical detection of hydrogen peroxide. Appl. Phys. Lett. 102, 213104 (2013). [Google Scholar]
- Shi Y. et al. Carbon nanotube decorated with silver nanoparticles via noncovalent interaction for a novel nonenzymatic sensor towards hydrogen peroxide reduction. J. Electroanal. Chem. 656, 29–33 (2011). [Google Scholar]
- Liu S. et al. Aniline as a dispersing and stabilizing agent for reduced graphene oxide and its subsequent decoration with Ag nanoparticles for enzymeless hydrogen peroxide detection. J. Colloid Interface Sci. 363, 615–619 (2011). [DOI] [PubMed] [Google Scholar]
- Qin X. et al. Synthesis of dendritic silver nanostructures and their application in hydrogen peroxide electro reduction. Electrochim. Acta. 56, 3170–3174 (2011). [Google Scholar]
- Ziyin Y. et al. Sensing hydrogen peroxide with a glassy carbon electrode modified with silver nanoparticles, AlOOH and reduced graphene oxide. Microchim. Acta. 183, 1131–1136 (2016). [Google Scholar]
- Dharmendra K. Y. et al. Electrochemical sensing platform for hydrogen peroxide determination at low reduction potential using silver nanoparticle-incorporated bentonite clay. J. Appl. Electrochem. 46, 103–112 (2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.