Abstract
Background:
We evaluated agreement among several definitions of accelerated knee osteoarthritis (AKOA) and construct validity by comparing their individual associations with injury, age, obesity, and knee pain.
Methods:
We selected knees from the Osteoarthritis Initiative that had no radiographic knee osteoarthritis [Kellgren–Lawrence (KL) 0 or 1] at baseline and had high-quality quantitative medial joint space width (JSW) measures on two or more consecutive visits (n = 1655 knees, 1143 participants). Quantitative medial JSW was based on a semi-automated method and was location specific (x = 0.25). We compared six definitions of AKOA: stringent JSW (averaged): average JSW loss greater than 1.05 mm/year over 4 years; stringent JSW (consistent): JSW loss greater than 1.05 mm/year for at least 2 years; lenient JSW (averaged): average JSW loss greater than 0.25 mm/year over 4 years; lenient JSW (consistent): JSW loss greater than 0.25 mm/year for at least 2 years; comprehensive KL based: progression from no radiographic osteoarthritis to advance-stage osteoarthritis (KL 3 or 4; development of definite osteophyte and joint space narrowing) within 4 years; and lenient KL based: an increase of at least two KL grades within 4 years.
Results:
Over 4 years the incidence rate of AKOA was 0.4%, 0.8%, 15.5%, 22.1%, 12.4%, and 7.2% based on the stringent JSW (averaged and consistent), lenient JSW (averaged and consistent), lenient KL-based definition, and comprehensive KL-based definition. All but one knee that met the stringent JSW definition also met the comprehensive KL-based definition. There was fair substantial agreement between the lenient JSW (averaged), lenient KL-based, and comprehensive KL-based definitions. A comprehensive KL-based definition led to larger effect sizes for injury, age, body mass index, and average pain over 4 years.
Conclusions:
A comprehensive KL-based definition of AKOA may be ideal because it represents a broader definition of joint deterioration compared with those focused on just joint space or osteophytes alone.
Keywords: classification, phenotype, osteoarthritis, knee, radiography
Introduction
Accelerated knee osteoarthritis (AKOA) may be a unique subset of knee osteoarthritis that is associated with a knee injury [Driban et al. 2014] and greater age [Driban et al. 2014], body mass index (BMI) [Driban et al. 2014], and knee pain [Driban et al. 2015a]. Some investigators have suggested that recruiting individuals with AKOA for clinical trials could accelerate the development and testing of therapeutic interventions for osteoarthritis [Lohmander and Felson, 2004; Dam et al. 2009; Eckstein et al. 2009]. Conversely, some have raised concerns that if AKOA is a unique subset of knee osteoarthritis then selectively recruiting these participants may lead to results that are not generalizable to the overall population with osteoarthritis [Lohmander and Felson, 2004]. There is a pressing need to study individuals with AKOA to determine if we can identify individuals at risk for AKOA, a prerequisite if we want to include them in trials or implement prevention strategies. We must also clarify if AKOA is truly a unique subset of knee osteoarthritis, which may be characterized by distinct risk factors, pathological processes, and response to therapies. Unfortunately, there are several definitions of AKOA, which makes it challenging to understand this phenotype.
At least five research groups have offered a definition of AKOA, including two definitions based on changes in overall radiographic severity [changes in Kellgren–Lawrence (KL) grades] [Driban et al. 2014, 2015a, 2015b, 2016; Wesseling et al. 2015] and three definitions based on joint space width (JSW) changes [Bartlett et al. 2011; Neogi et al. 2012; Hochberg, 2015; Roemer et al. 2015]. A comprehensive KL-based definition requires a knee to progress from no radiographic osteoarthritis (KL 0 or 1) to advanced-stage knee osteoarthritis (presence of a definite osteophyte and joint space narrowing, KL 3 or 4) within 4 years [Driban et al. 2014, 2015a, 2015b, 2016]; however, a more lenient KL-based definition [Wesseling et al. 2015] also includes individuals who progress from KL 0 (no osteoarthritis) to KL 2 (presence of a definite osteophyte) within 4 years. The three JSW-based definitions require a knee to lose more than 0.25 mm/year of medial JSW [Neogi et al. 2012], more than 1.05 mm/year of medial JSW [Bartlett et al. 2011], or at least 2.00 mm/year of JSW [Hochberg, 2015; Roemer et al. 2015]. The implications of using these various definitions remains unclear; however, the prevalence of AKOA based on each definition varies: 3.4% of participants with a comprehensive KL-based definition [Driban et al. 2014, 2015a, 2015b, 2016], 10–15% with the lenient KL-based definition (Wesseling et al. 2015), 2% with more than 1.05 mm/year of medial JSW [Bartlett et al. 2011], and 23% with more than 0.25 mm/year of medial JSW [Neogi et al. 2012]. Since no study has examined all of these definitions in one study sample, it remains unclear how the various definitions influence prevalence rates, relate to each other, and influence effect estimates for known risk factors and knee pain.
It is important to compare these definitions of AKOA so we can understand how they may influence the results. Therefore, we evaluated agreement among several definitions of AKOA and construct validity by comparing their individual associations with injury, age, obesity, and knee pain. We hypothesized that a definition of AKOA that requires changes throughout a joint, development of a definite osteophyte and joint space narrowing, would have moderate agreement with other definitions. This comprehensive definition will offer the greatest effect estimates for known risk factors for the condition and knee pain.
Methods
Participants
We used data from the Osteoarthritis Initiative (OAI), which is a longitudinal multicenter observational study in the United States. Between February 2004 and May 2006, 4796 men and women (45–79 years of age) with or at risk for knee osteoarthritis were recruited at four clinical sites: Memorial Hospital of Rhode Island, The Ohio State University, University of Maryland and Johns Hopkins University, and the University of Pittsburgh. The clinical staff also recruited a small subsample (n = 122) of individuals without symptomatic knee osteoarthritis and no risk factors for osteoarthritis. The OAI protocol and eligibility criteria are publicly available at the OAI website (https://oai.epi-ucsf.org, accessed June 27, 2016). Institutional review boards at each OAI clinical site and the coordinating center (University of California, San Francisco) approved the OAI study. All participants provided informed consent.
To evaluate agreement among several definitions of AKOA and construct validity we selected 1655 knees (1143 participants) that had no radiographic knee osteoarthritis (KL 0 or 1) at baseline and had quantitative medial JSW measures on high-quality radiographs on at least two consecutive visits. We defined a high-quality knee radiograph as a radiograph with a beam angle between 5° and 15° and inter-tibial rim distance change between adjacent visits of ±2 mm. The latter criterion helped to ensure that the knee position was consistent between visits.
Quantitative joint space width
Bilateral, weight-bearing, fixed-flexion posterior-anterior knee radiographs were obtained at baseline and the first four annual follow-up visits. Quantitative medial JSW was based on a well validated semi-automated method that uses location-specific measures [Duryea, 2003; Neumann et al. 2009]. For our analyses we focused on the location in the medial tibiofemoral compartment that is most responsive to change (x = 0.250) [Neumann et al. 2009]. The cross-sectional and longitudinal reliability are excellent (intra-class correlation coefficient ⩾ 0.93). All JSW data, reliability reports, and protocols are publicly available at the OAI website (files: kxr_qjsw_duryea##, versions: 0.6, 1.6, 3.5, 5.5, and 6.3).
Semi-quantitative radiographic scores
Central readers, who were blinded to the order of follow-up radiographs scored the aforementioned radiographs for KL grades (0–4). The agreement for these readings (read–reread) was good [weighted κ (intra-rater reliability) = 0.70–0.80]. These KL grades are publicly available (files: kXR_SQ_BU##_SAS, versions 0.6, 1.6, 3.5, 5.5, and 6.3).
Clinical data
A priori we selected four key clinical variables to assess the various definitions of AKOA. Baseline age [Driban et al. 2014], BMI [Driban et al. 2014], recent knee injury [Driban et al. 2014], and average WOMAC knee pain score (averaged across all visits) [Driban et al. 2015a] were selected because these variables are different between people with AKOA and those with more gradual onset of knee osteoarthritis or no knee osteoarthritis. At each follow-up visit participants were asked ‘Since your last annual visit to the OAI clinic about 12 months ago, have you injured your right knee badly enough to limit your ability to walk for at least two days?’. A similar question was asked for the left knee. If a participant answered yes to one of these questions at any follow-up visit then the knee had a recent knee injury.
Definitions of accelerated knee osteoarthritis
We considered six definitions of AKOA: two KL-based definitions and four JSW-based definitions. A comprehensive KL definition was based on a knee progressing from no radiographic osteoarthritis (KL 0 or 1) to advanced-stage knee osteoarthritis (KL 3 or 4) within 4 years, which would require the development of a definite osteophyte and joint space narrowing [Driban et al. 2014, 2015a, 2015b]. A lenient KL definition was defined by a knee with no radiographic osteoarthritis at baseline but the KL score increased two grades within 4 years [Wesseling et al. 2015]. The two KL-based definitions are similar except that the lenient KL definition would include an individual who progressed from KL 0 (no radiographic osteoarthritis) to KL 2 (presence of a definite osteophyte).
The four JSW-based definitions were derived from previous latent class analyses [Bartlett et al. 2011; Neogi et al. 2012]: stringent JSW (averaged): average JSW loss greater than 1.05 mm/year over 4 years [Bartlett et al. 2011]; stringent JSW (consistent): JSW loss greater than 1.05 mm/year for at least 2 years [Bartlett et al. 2011]; lenient JSW (averaged): average JSW loss greater than 0.25 mm/year over 4 years [Neogi et al. 2012]; and lenient JSW (consistent): JSW loss greater than 0.25 mm/year for at least 2 years [Neogi et al. 2012]. We focused on medial JSW for these definitions because the prior studies only examined the medial tibiofemoral compartment [Bartlett et al. 2011; Neogi et al. 2012]. One other definition of AKOA, which requires at least 2.00 mm JSW loss in 1 year, has been published [Hochberg, 2015; Roemer et al. 2015], but based on the small sample size that met the stringent JSW definition we did not explore this definition. Figure 1 shows a knee that met the criteria for all definitions.
Figure 1.
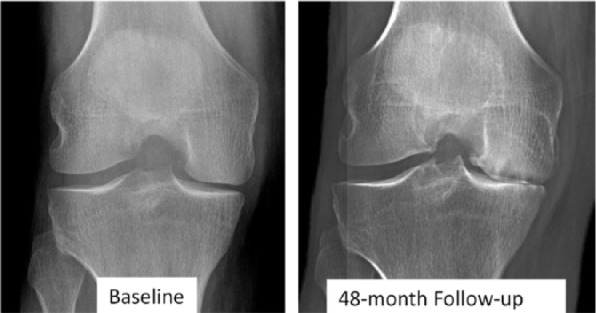
Example of accelerated knee osteoarthritis based on all of the definitions.
Statistical analyses
All analyses were knee based and considered each knee as independent observations. We calculated κ statistics between the JSW-based definitions, lenient KL definition, and the comprehensive KL-based definition of AKOA. We interpreted the κ statistics as poor (κ < 0.00), slight (κ = 0.00–0.20), fair (κ = 0.21–0.40), moderate (κ = 0.41–0.60), substantial (κ = 0.61–0.80), and almost perfect (κ = 0.81–1.00) [Landis and Koch, 1977]. As a form of construct validity, we examined how the different definitions influenced the effect size of group differences (AKOA or no AKOA) of an injury during the first 4 years of the OAI, baseline age, baseline BMI, and average WOMAC pain score from baseline and the first four annual visits. Based on the calculated effect sizes we estimated the sample size that would be required to detect a significant difference using a two-tailed independent t test (power = 0.90,α = 0.05; G*Power 3.1.2) or odds ratio (PASS 14). To follow up on our primary analyses, we replicated the primary analyses using person-level data (n = 512). For the person-level analyses, we selected people as described above but limited the sample to those who had no radiographic osteoarthritis in either knee at baseline (KL 0 or 1).
Results
Over 4 years the incidence rate of AKOA ranged from 0.4% to 22.1%: comprehensive KL = 7.2%, lenient KL = 12.4%, stringent JSW = 0.4% (averaged) and 0.8% (consistent), and lenient JSW = 15.5% (averaged) and 22.1% (consistent). All but one knee that met the stringent JSW definition (averaged) also met the comprehensive KL-based definition (see Figure 2). There was fair to substantial agreement between the lenient JSW (averaged κ = 0.36), lenient KL-based (κ = 0.71), and comprehensive KL-based definitions (Figure 2).
Figure 2.
Agreement between five definitions of accelerated knee osteoarthritis (AKOA; blue circles) and a comprehensive Kellgren–Lawrence (KL)-based definition (red circles). Circle size represents the sample size for each definition of AKOA. Superscripts refer to Table 1. JSW, joint space width.
‡lenient KL definition was defined by a knee with no radiographic osteoarthritis at baseline but the KL score increased 2 grades within 4 years [Wesseling et al., 2015].
§lenient JSW (averaged) definition was defined by an average JSW loss >0.25 mm/year over 4 years [Neogi et al., 2012].
Based on the comprehensive KL definition, lenient KL definition, and lenient JSW (averaged) definition, baseline age, BMI, average WOMAC knee pain, and recent knee injury were statistically different between those with and without AKOA (see Table 1). Qualitatively, the comprehensive KL-based definition led to larger effect sizes for injury, age, BMI, and average knee pain.
Table 1.
Differences between those with and without accelerated knee osteoarthritis (AKOA) based on five definitions of AKOA.
| Key variables by definition of AKOA | No AKOA | AKOA | Effect size* | Estimated sample size per group for p < 0.05 |
|---|---|---|---|---|
| Comprehensive KL definition | (n = 1536) | (n = 119) | ||
| Baseline age (years) | 60 (9) | 62 (8)$ | d = 0.29 (0.19, 0.38) | 251 |
| Baseline BMI (kg/m2) | 27.9 (4.5) | 29.9 (4.7)$ | d = 0.45 (0.35, 0.55) | 105 |
| Average WOMAC pain | 1.5 (2.1) | 2.7 (2.3)$ | d = 0.59 (0.50, 0.69) | 62 |
| Recent knee injury, n (%) | 120 (8%) | 40 (34%)$ | OR = 5.8 (3.8, 8.9) | 66 |
| Lenient KL definition‡ | (n = 1449) | (n = 206) | ||
| Baseline age (years) | 60 (9) | 61 (9)$ | d = 0.19 (0.09, 0.28) | 584 |
| Baseline BMI (kg/m2) | 27.8 (4.5) | 29.6 (4.7)$ | d = 0.40 (0.30, 0.50) | 133 |
| Average WOMAC pain | 1.5 (2.1) | 2.3 (2.1)$ | d = 0.41 (0.31, 0.50) | 126 |
| Recent knee injury, n (%) | 100 (7%) | 60 (30%)$ | OR = 5.4 (3.8, 7.8) | 94 |
|
Lenient KL definition
(excluding those with comprehensive KL) |
(n = 1449) | (n = 87) | ||
| Baseline age (years) | 60 (9) | 60 (9) | d = 0.05 (−0.06, 0.15) | 8407 |
| Baseline BMI (kg/m2) | 27.8 (4.5) | 29.2 (4.8)$ | d = 0.31 (0.21, 0.41) | 220 |
| Average WOMAC pain | 1.5 (2.1) | 1.8 (1.7) | d = 0.14 (0.04, 0.24) | 1074 |
| Recent knee injury, n (%) | 100 (7%) | 20 (24%)$ | OR = 4.0 (2.3, 6.9) | 246 |
| Lenient JSW (averaged) Definition§ | (n = 1398) | (n = 257) | ||
| Baseline age (years) | 60 (9) | 61 (9)$ | d = 0.17 (0.08, 0.27) | 729 |
| Baseline BMI (kg/m2) | 27.8 (4.5) | 28.9 (4.5)$ | d = 0.24 (0.14, 0.34) | 366 |
| Average WOMAC pain | 1.5 (2.0) | 2.2 (2.5)$ | d = 0.33 (0.24, 0.43) | 194 |
| Recent knee injury, n (%) | 102 (8%) | 58 (24%)$ | OR = 3.8 (2.7, 5.5) | 125 |
|
Lenient JSW (averaged) definition
(excluding those with comprehensive KL) |
(n = 1398) | (n = 177) | ||
| Baseline age (years) | 60 (9) | 61 (9) | d = 0.14 (0.04, 0.24) | 1074 |
| Baseline BMI (kg/m2) | 27.8 (4.5) | 28.3 (4.4) | d = 0.11 (0.01, 0.21) | 1738 |
| Average WOMAC pain | 1.5 (2.0) | 1.7 (2.3) | d = 0.12 (0.02, 0.22) | 1461 |
| Recent knee injury, n (%) | 102 (8%) | 28 (17%)$ | OR = 2.5 (1.6, 4.0) | 470 |
Average WOMAC pain across the first five visits. Mean (standard deviation) unless noted otherwise.
Effect size: d = mean difference/standard deviation for continuous measurements and OR = odds ratio for recent knee injury.
p < 0.05 with an independent sample t test.
lenient KL definition was defined by a knee with no radiographic osteoarthritis at baseline but the KL score increased 2 grades within 4 years [Wesseling et al., 2015].
lenient JSW (averaged) definition was defined by an average JSW loss →0.25 mm/year over 4 years [Neogi et al., 2012].
BMI, body mass index; KL, Kellgren Lawrence; JSW, joint space width.
We also explored the subset who met the lenient KL definition and not the comprehensive KL definition (i.e. those who progressed from KL 0 to KL 2; developed a definite osteophyte but not joint space narrowing). Among these 87 knees, average WOMAC knee pain was no longer different between those with and without AKOA and the effect estimates were smaller than those found using the comprehensive KL definition. To differentiate between those with and those without AKOA based on WOMAC knee pain, an investigator would need 1074 participants/group if they only included those who progressed from KL 0 to KL 2. In comparison, if an investigator included everyone who met the lenient KL definition, they would need 126 participants/group or 62 participants/group if an investigator used the comprehensive KL definition.
Finally, we explored the subset who met the lenient JSW definition (averaged) and not the comprehensive KL definition (i.e. those who primarily had JSW change). Among these 177 knees, average WOMAC knee pain was no longer different between those with and those without AKOA and the effect estimates were smaller than those found using the comprehensive KL definition. To differentiate between those with and those without AKOA based on WOMAC knee pain, an investigator would need 1461 participants/group if they only included those with primarily JSW change versus 194 participants/group using everyone who met the lenient JSW (averaged) definition or 62 participants/group based on the comprehensive KL definition.
The secondary analyses, which were based on person-level data, supported the primary findings (see Table 1).
Discussion
This is the first study to assess various definitions of AKOA. We found fair to substantial agreement among some definitions of AKOA but the discordance between definitions may be important. A comprehensive KL-based definition of AKOA that accounts for osteophyte formation and joint space narrowing may be ideal because it offers greater effect estimates for known risk factors for the condition and knee pain. The other definitions offer a larger sample size but at the cost of less specificity, which dilutes the effect estimates.
The performance of the comprehensive KL-based definition may be related to a requirement for changes in JSW and osteophytes compared with definitions that include people with only JSW change or osteophyte development. JSW measurements and the presence of an osteophyte are associated with knee pain and obesity [Spector et al. 1993; Lanyon et al. 1998] but requiring changes in both may lead to a stronger association. Given that osteoarthritis is a whole organ disease involving changes in bone, cartilage, menisci, and other structures, it makes sense to define AKOA based on the combination of JSW and osteophytes.
This study helped to clarify how the different definitions agree and influence results, but there were some limitations. For example, there is no gold-standard definition for AKOA to serve as a reference. It is possible that the comprehensive KL-based definition includes a more specific smaller subset of AKOA. For example, a knee that progresses from no radiographic OA to advanced stage OA within 12 months or that experiences JSW loss of at least 2 mm/year. Future studies should explore the implications of these more aggressive forms of progression to determine if they are different than the other knees that the comprehensive KL-based definition identifies.
We found fair to substantial agreement among some definitions of AKOA, but the discordance between definitions may be important. A KL-based definition of AKOA that accounts for osteophyte formation and joint space narrowing may be ideal because it offers greater effect estimates for known risk factors for the condition and knee pain. Alternative definitions of AKOA may have less specificity. The performance of the comprehensive KL-based definition may be related to the broader definition of joint changes compared with those focused on just joint space or osteophytes alone.
Footnotes
Funding: Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01-AR065977-01A1. The Osteoarthritis Initiative is a public–private partnership composed of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories, Novartis Pharmaceuticals Corporation, GlaxoSmithKline, and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use dataset and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners.
This work was also supported in part by the Houston Veterans Affairs Health Services Research and Development Center of Excellence (HFP90-020). The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Jeffrey B. Driban, Division of Rheumatology, Tufts Medical Center, 800 Washington Street, Box 406, Boston, MA 02111, USA.
Alina C. Stout, Division of Rheumatology, Tufts Medical Center, Boston, MA, USA
Grace H. Lo, Medical Care Line and Research Care Line, Houston Health Services Research and Development (HSR&D) Center of Excellence Michael E. DeBakey VAMC, Houston, TX, USA Section of Immunology, Allergy, and Rheumatology, Baylor College of Medicine, Houston, TX, USA.
Charles B. Eaton, Center for Primary Care and Prevention, Alpert Medical School of Brown University, Pawtucket, RI, USA
Lori Lyn Price, The Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, Boston, MA, USA; Tufts Clinical and Translational Science Institute, Tufts University, Boston, MA, USA.
Bing Lu, Brigham & Women’s Hospital and Harvard Medical School, Boston, MA, USA.
Mary F. Barbe, Department of Anatomy and Cell Biology, Temple University School of Medicine, Philadelphia, PA, USA
Timothy E. McAlindon, Division of Rheumatology, Tufts Medical Center, Boston, MA, USA
References
- Bartlett S., Ling S., Mayo N., Scott S., Bingham C., 3rd (2011) Identifying common trajectories of joint space narrowing over two years in knee osteoarthritis. Arthritis Care Res 63: 1722–1728. [DOI] [PubMed] [Google Scholar]
- Dam E., Loog M., Christiansen C., Byrjalsen I., Folkesson J., Nielsen M., et al. (2009) Identification of progressors in osteoarthritis by combining biochemical and MRI-based markers. Arthritis Res Ther 11: R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driban J., Eaton C., Lo G., Price L., Lu B., Barbe M., et al. (2016) Overweight older adults, particularly after an injury, are at high risk for accelerated knee osteoarthritis: data from the Osteoarthritis Initiative. Clin Rheumatol 35: 1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driban J., Eaton C., Lo G., Ward R., Lu B., McAlindon T. (2014) Association of knee injuries with accelerated knee osteoarthritis progression: data from the Osteoarthritis Initiative. Arthritis Care Res 66: 1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driban J., Price L., Eaton C., Lu B., Lo G., Lapane K., et al. (2015a) Individuals with incident accelerated knee osteoarthritis have greater pain than those with common knee osteoarthritis progression: data from the Osteoarthritis Initiative. Clin Rheumatol 35: 1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driban J., Ward R., Eaton C., Lo G., Price L., Lu B., et al. (2015b) Meniscal extrusion or subchondral damage characterize incident accelerated osteoarthritis: data from the Osteoarthritis Initiative. Clin Anat 28: 792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duryea J. (2003) New radiographic-based surrogate outcome measures for osteoarthritis of the knee. Osteoarthritis Cartilage 11: 102–110. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Benichou O., Wirth W., Nelson D., Maschek S., Hudelmaier M., et al. (2009) Magnetic resonance imaging-based cartilage loss in painful contralateral knees with and without radiographic joint space narrowing: data from the Osteoarthritis Initiative. Arthritis Rheum 61: 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg M. (2015) Serious joint-related adverse events in randomized controlled trials of anti-nerve growth factor monoclonal antibodies. Osteoarthritis Cartilage 23(Suppl. 1): S18–S21. [DOI] [PubMed] [Google Scholar]
- Landis J., Koch G. (1977) The measurement of observer agreement for categorical data. Biometrics 33: 159–174. [PubMed] [Google Scholar]
- Lanyon P., O’Reilly S., Jones A., Doherty M. (1998) Radiographic assessment of symptomatic knee osteoarthritis in the community: definitions and normal joint space. Annals Rheum Dis 57: 595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmander L., Felson D. (2004) Can we identify a ‘high risk’ patient profile to determine who will experience rapid progression of osteoarthritis? Osteoarthritis Cartilage 12(Suppl. A): S49–S52. [DOI] [PubMed] [Google Scholar]
- Neogi T., Niu J., Duryea J., Lynch J., Zhang Y. (2012) Identifying trajectories of medial joint-space width loss and associated risk factors. Osteoarthritis Cartilage 20(Suppl. 1): S182-S183. [Google Scholar]
- Neumann G., Hunter D., Nevitt M., Chibnik L., Kwoh K., Chen H., et al. (2009) Location specific radiographic joint space width for osteoarthritis progression. Osteoarthritis Cartilage 17: 761–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer F., Hayes C., Miller C., Hoover K., Guermazi A. (2015) Imaging atlas for eligibility and on-study safety of potential knee adverse events in anti-NGF studies (part 1). Osteoarthritis Cartilage 23(Suppl. 1): S22–S42. [DOI] [PubMed] [Google Scholar]
- Spector T., Hart D., Byrne J., Harris P., Dacre J., Doyle D. (1993) Definition of osteoarthritis of the knee for epidemiological studies. Ann Rheum Dis 52: 790–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling J., Bierma-Zeinstra S., Kloppenburg M., Meijer R., Bijlsma J. (2015) Worsening of pain and function over 5 years in individuals with ‘early’ OA is related to structural damage: data from the Osteoarthritis Initiative and CHECK (Cohort Hip & Cohort Knee) Study. Ann Rheum Dis 74: 347–353. [DOI] [PubMed] [Google Scholar]



