Abstract
We recently reported the expression of matrix metalloproteinase 20 (MMP20), hitherto thought to be tooth specific, in the metabolically active ductal epithelial cells of human salivary glands. Furthermore, our report indicated that MMP20 co-expressed and potentially interacts with dentin sialophosphoprotein (DSPP), a member of the small integrin-binding ligand N-linked glycoproteins (SIBLINGs). Our earlier reports have shown the co-expression of three MMPs, MMP2, MMP3, and MMP9, with specific members of the SIBLING family: bone sialoprotein, osteopontin, and dentin matrix protein 1, respectively. This study investigated the expression of MMP20 and verified its co-expression with DSPP in human and monkey kidney sections and human mixed renal cells by IHC, in situ proximity ligation assay, and immunofluorescence. Our results show that MMP20 is expressed in all segments of the human and monkey nephron with marked intensity in the proximal and distal tubules, and was absent in the glomeruli. Furthermore, MMP20 co-expressed with DSPP in the proximal, distal, and collecting tubules, and in mixed renal cells. Consistent with other SIBLING–MMP pairs, the DSPP–MMP20 pair may play a role in the normal turnover of cell surface proteins and/or repair of pericellular matrix proteins of the basement membranes in the metabolically active duct epithelial system of the nephrons.
Keywords: dentin sialophosphoprotein, IHC, in situ proximity ligation assay, kidney, matrix metalloproteinase 20, metabolically active duct epithelia, MMPs, SIBLINGs
Introduction
Matrix metalloproteinases (MMPs) are notable for their proteolytic activities on a wide variety of proteins that include those of extracellular matrix (ECM).1 Currently, there are at least 28 family members of these zinc-dependent enzymes, and it is anticipated that new members of the family will continue to add to this number as they are discovered.1 Secreted as inactive proenzymes (pro-MMPs), the classical dogma for conversion to active MMPs requires cleaving of the propeptide domain to generate active MMP.2 About a decade ago, however, reported results of in vitro biochemical experiments showed that three members of the small integrin-binding ligand N-linked glycoproteins (SIBLINGs) family bind and activate specific pro-MMPs without the necessity to cleave the propeptides.3 It was further shown that resultant SIBLING/MMP pairs exhibit substantial resistance to the inhibitory activities of the tissue inhibitors of MMPs (TIMPs).3
The roles of MMPs in normal physiological as well as pathological processes are well documented and include their role in organ involution during development, wound healing, invasion, and metastasis of cancers.4 Their role in kidney development also has been reported.5 About a decade ago, we identified MMP2, MMP3, and MMP9 as the cognate MMP partners of bone sialoprotein (BSP), osteopontin (OPN), and dentin matrix protein 1 (DMP1), respectively, and reported their co-expression in specific segments of the nephron and other metabolically active ductal epithelia of the salivary glands and eccrine sweat gland.6–8 However, the MMP partners of the two other SIBLINGs, dentin sialophosphoprotein (DSPP) and matrix extracellular phosphoglycoprotein (MEPE), remained unknown until our very recent reports of the co-expression and interaction of MMP20 with DSPP in human oral squamous cell carcinoma (OSCC), and in normal human major salivary glands.9,10 Significantly, all the SIBLINGs with known MMP partners invariably have been co-expressed with their cognate MMP partners in all tissues and cells studied so far.6–11
Until our recent reports,9,10 the expression of MMP20 was deemed tooth specific.12–17 Because DSPP is expressed in human and monkey nephrons,7 we hypothesized in the present study that MMP20 is also expressed, and co-expresses with DSPP, in adult human and monkey kidneys. We investigated this hypothesis in tissue sections of normal adult human and monkey nephrons, and a mixed renal cell line, using IHC, immunofluorescence (IF), and in situ proximity ligation assay (iPLA) techniques.
Materials and Methods
Tissue and Cells, Culture Conditions, and Antibodies
Fresh surgical waste kidneys of monkey (Macaca fascicularis) were obtained from the Tissue Distribution Program of the National Primate Research Center (University of Washington, Seattle, WA) and routinely processed for IHC and other histological procedures as reported previously.7 Normal human kidney paraffin blocks without patient identifiers were obtained from the Mid-Atlantic Division of the Cooperative Human Tissue Network (Charlottesville, VA) under a National Institutes of Health–approved (exempt) protocol as previously reported.7 The normal human primary renal mixed epithelial cells (cat# PCS-400-012) were purchased from ATCC (Manassas, VA). Immortalized human oral keratinocyte, HOK16B,18 used as a negative cell control was a kind gift from Dr. Nadarajah Vigneswaran (University of Texas School of Dentistry at Houston). Human oral keratinocytes (HOKs) whole cell lysates (cat #2616) were purchased from ScienCell Research Laboratories (Carlsbad, CA); OSC2 cell line19 was a kind gift from Dr. Stephen Hsu (Georgia Regents University, GA). De-identified archived paraffin sections of human OSCC were used as positive controls for MMP20 expression.9 Renal cells were cultured in renal epithelial cell basal medium (cat# PCS-400-030; ATCC) supplemented with a renal epithelial cell growth kit (cat# PCS-400-040; ATCC) containing growth supplements. Culture conditions for HOK16B and OSC2 cells have been published earlier in detail.9 Antibodies used in this study have been published.10
Immunohistochemistry
Approximately 4- to 5-µm sections of paraffin-embedded, normal human and monkey kidney sections were used for IHC and iPLAs. Antibodies for the MMP20 (bs-5788R, rabbit polyclonal) and DSPP (LFMb-21, sc-73632, mouse monoclonal) were purchased from Bioss Inc. (Woburn, MA) and Santa Cruz Biotechnology (Dallas, TX), respectively. IHC was carried out on formalin-fixed paraffin-embedded normal human and monkey kidney sections with MACH 2 AP-Polymer Detection Kit (Mouse MALP521/Rabbit RALP525; Biocare Medical, Concord, CA) as recently described.9,10
Briefly, sections were warmed 15 min at 64C using a slide warmer before deparaffinizing in three washes of xylene (5 min each), and rehydrating through series of ethanol (100%, 95%, and 75%, 5 min each) and deionized water. Antigen retrieval was performed with decloaking chamber (Biocare Medical) using DIVA Decloaker solution (DV2004; Biocare Medical). Endogenous peroxidase activity was quenched by treating sections for 10 min with peroxidase blocking reagent, Peroxidase 1 (PX968; Biocare Medical), followed by Background Punisher (BP974; Biocare Medical) for 20 min to reduce nonspecific background staining. Sections were then incubated with DSPP (1:100), and MMP20 (1:100) diluted in Da Vinci Green diluent (PD900; Biocare Medical) overnight at 4C. Thereafter, sections were treated with mouse or rabbit AP polymer for DSPP and MMP20, respectively, for 30 min at room temperature before incubating with Warp Red chromogen (WR806; Biocare Medical) for 5 min and counterstaining with hematoxylin (SH26-500D; Fisher Scientific, Pittsburg, PA). Tris-buffered saline (BP2471; Fisher Scientific) with 0.05% Tween-20 (TBS-T) was used as washing buffer during the IHC protocol. For negative controls, similar protocol was performed except primary antibodies were replaced with Universal Negative Control Serum (NC498; Biocare Medical).
Multiple-label IHC was performed on consecutive normal human and monkey kidney sections using the MACH 2 Double Stain 2, Mouse-HRP + Rabbit-AP Kit (MRCT525; Biocare Medical) manually. Slides were deparaffinized, rehydrated, and antigen retrieved as described above. Endogenous peroxidase activity to reduce nonspecific background staining was accomplished as described above for single staining. Sections were then incubated for 1 hr with an antibody cocktail of DSPP (1:100), and MMP20 (1:100) diluted in Da Vinci Green diluent at room temperature, and with MACH 2 polymer for 30 min before treatment with DAB (3,3′-Diaminobenzidine; BDB2004; Biocare Medical) for 5 min and Warp Red for 7 min (Biocare Medical), or Deep Space Black (BRI4015; Biocare Medical) for 5 min. Counterstaining with hematoxylin was for 5 sec. All sections were washed with TBS-T buffer during the IHC protocol. For negative controls, similar steps were carried out except that Universal Negative Control Serum (Biocare Medical) substituted for primary antibodies. Photographs of representative results were captured using the Eclipse Ni-E microscope with Nikon DS-U3 digital camera and NIS-Elements Advanced Research software (Nikon, Melville, NY).
Immunofluorescence
Primary mixed renal cells, OSC2 cells (positive control), and HOK16B cells (negative controls) were plated on coverslips in a 6-well dish overnight before washing three times with 1× PBS and fixed in 3% paraformaldehyde for 20 min. Thereafter, cells were permeabilized in 0.1% Triton X-100 for 10 min and treated with blocking buffer (1× PBS, 3% goat serum) for 1 hr at room temperature, followed by overnight incubation at 4C with primary antibodies to MMP20 and DSPP diluted in blocking buffer. Coverslips were washed three times with 1× PBS before incubating with secondary antibodies for 1 hr. Cells were then mounted with Prolong Gold anti fade reagent with 4′,6-diamidino-2-phenylindole, dilactate (DAPI; cat# P36931; Life Technologies, Grand Island, NY).
In Situ Proximity Ligation Assay
iPLA was used to investigate the proximity and potential specific cellular interactions of MMP20 with DSPP in human and monkey kidney tissue sections. Before iPLA, fixed, paraffin-embedded human and monkey kidney tissue sections were deparaffinized and antigen retrieved. iPLA was carried out using the HRP/NovaRed detection kit from Olink Bioscience according to the manufacturer’s protocol (cat# DUO92012; Sigma-Aldrich, St. Louis, MO). Briefly, tissue sections were incubated with primary anti-MMP20 polyclonal (rabbit) antibody and anti-DSPP monoclonal (mouse) antibody, or with normal rabbit/mouse IgG before incubation with corresponding secondary antibodies conjugated to oligonucleotides (PLA probes; MINUS and PLUS) at 37C for 1 hr. T4-DNA ligase (Olink Bioscience), rolling circle amplification (RCA) was accomplished following the manufacturer’s instruction. Thereafter, HRP/NovaRed oligonucleotide (Sigma-Aldrich) was used to detect RCA products on sections. Protein–protein interaction was evidenced as brownish-red punctate, dust-like signals. Images were captured using the Nikon Eclipse Ni-E microscope and NIS-Elements Advanced Research software (Nikon).
Results
IHC Analysis of MMP20 and DSPP Co-expression in Normal Kidney Tissues and Cells
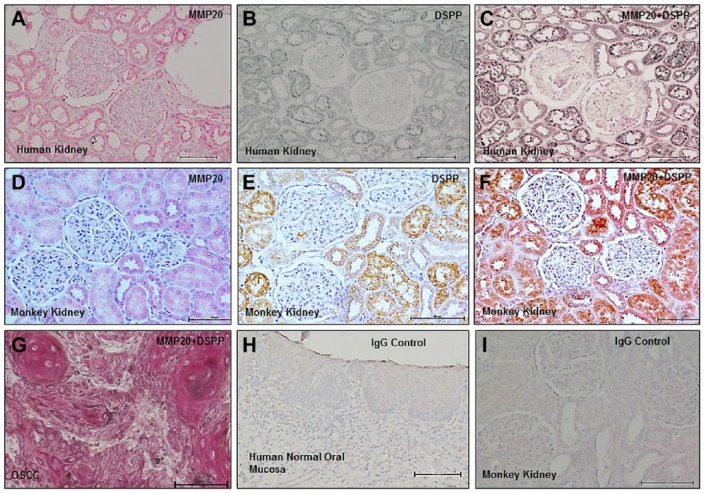
IHC on normal human and monkey kidney sections and IF on primary mixed renal cells were analyzed for the expression of MMP20 and its co-localization with DSPP. Immunoreactivity for MMP20 was observed in all segments of the nephron with more intense expression in the proximal and distal tubules in both human (Fig. 1A) and monkey (Fig. 1D) kidneys, whereas the glomeruli showed negative immunoreactivity for MMP20. Consistent with previous reports,7 DSPP immunoreactivity was observed in the proximal and distal tubules as well as the collecting ducts (Fig. 1B and E). Double immunolabeling showed co-localization of MMP20 with DSPP at the proximal, distal, and collecting tubules of the nephron on serial sections (Fig. 1C and F). The positive control consisted of tissue section of human OSCC (Fig. 1G),9 whereas normal human mucosa was used as the negative control (Fig. 1H).9 Figure 1I represents preimmune IgG negative controls on monkey kidney tissue sections.
Figure 1.
MMP20 and DSPP co-localize in normal human and monkey kidney tissue. IHC on normal human kidney sections showed immunoreactivity of MMP20 (A, red, alkaline phosphatase chromogen), DSPP (B, black, HRP chromogen), and their co-localization (C, reddish black) in proximal, distal, and collecting tubules. Similar to human kidneys, IHC on monkey kidney sections showed immunoreactivity of MMP20 (D, red, alkaline phosphatase chromogen), DSPP (E, brown, HRP chromogen), and their co-localization (F, reddish brown) in proximal, distal, and collecting tubules. Double-labeled IHC of OSCC served as positive (G, reddish black; MMP20, red alkaline phosphatase chromogen; and DSPP, black HRP chromogen) control. Normal human oral mucosa served as tissue negative control (H), whereas preimmune IgG negative control on monkey sections is shown in panel I. Abbreviations: MMP20, matrix metalloproteinase 20; DSPP, dentin sialophosphoprotein; OSCC, oral squamous cell carcinoma. Scale bars = 100 µm.
IF Analysis of MMP20–DSPP Co-expression in Human Mixed Renal Cells
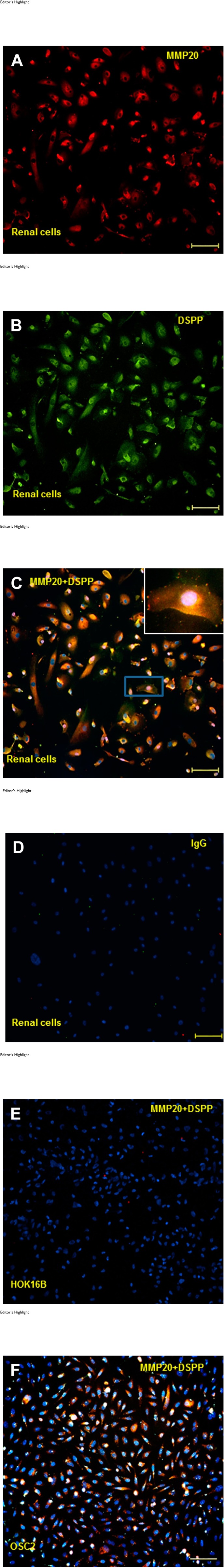
We used IF to investigate the co-localization of MMP20 and DSPP in normal human mixed renal cells. As shown in Fig. 2A to C, MMP20, DSPP, and MMP20 co-localized with DSPP in normal human primary renal mixed epithelial cells. Preimmune IgG negative control is shown in Fig. 1D, whereas HOK16B and OSC2 cells served as negative (Fig. 1E) and positive (Fig. 1F) cell controls, respectively.9,10
Figure 2.
Editor’s Highlight
MMP20 and DSPP co-localization in human mixed renal cells. Immunofluorescence for MMP20 (red; A), DSPP (green; B), and their co-localization (yellow hue; C) in mixed renal cells is shown. IgG negative control is shown in panel D. Immortalized human oral keratinocytes, keratinocyte (HOK16B) cells, known to be negative for MMP20 and DSPP were used as cell negative control for MMP20–DSPP (E), whereas OSC2 cells known to co-localize MMP20–DSPP were used as cell positive control (F). Abbreviations: MMP20, matrix metalloproteinase 20; DSPP, dentin sialophosphoprotein. Scale bars = 100 µm.
iPLA Analyses of MMP20–DSPP Interaction in Human and Monkey Kidneys
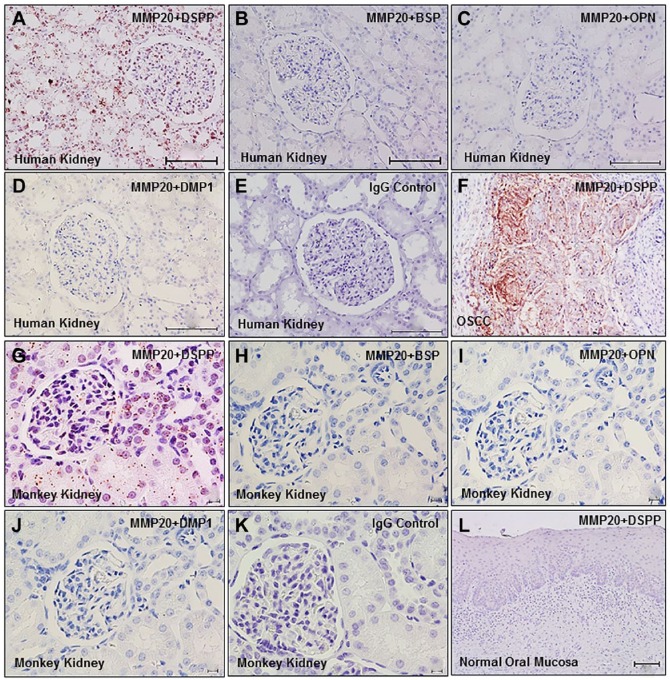
The recently described iPLA was developed as a means of visualizing pairs of proteins in sufficient proximity to indicate interacting pairs represented as dot signals in conventional fluorescent light microscopy (see also “Materials and Methods” section).9,10 To determine any potential MMP20–DSPP interaction, we performed iPLA in normal human and monkey kidney tissue sections. As shown in Fig. 3, iPLA on human (Fig. 3A–D) and monkey (Fig. 3G–J) kidney tissue sections confirmed perinuclear and nuclear duct signals (intense brown dusts and dots), indicating MMP20 and DSPP co-localization and interaction. iPLA showed MMP20–DSPP interaction to be specific in both human (Fig. 3A) and monkey (Fig. 3G) kidney sections, and precluded MMP20 pairing with the other SIBLINGs, BSP (Fig. 3B and H), OPN (Fig. 3C and I), and DMP1 (Fig. 3D and J) with known specific MMP partners, MMP2, MMP3, and MMP9, respectively. iPLA on sections of human OSCC known to co-express MMP20 and DSPP served as the tissue positive control (Fig. 3F), whereas normal oral mucosa known to express neither MMP20 nor DSPP was used as the tissue negative control (Fig. 3L). Preimmune IgG negative controls for human and monkey kidneys are shown in Fig. 3E and K.
Figure 3.
Interaction of MMP20 and DSPP in normal human and monkey kidneys. In situ proximity ligation assay shows matrix metalloproteinase interactions (brown dusty dots) with DSPP in human (A) and monkey (G) kidney tissue sections. There was no interaction of MMP20 with BSP (B, H), osteopontin (OPN; C, I), or DMP1 (D, J), validating the specificity of the MMP20–DSPP interaction previously reported.9,20 Sections of OSCC served as tissue positive control (F), whereas normal oral mucosa served as tissue negative control (L). IgG negative controls for human and monkey sections are shown in panels E and K, respectively. Abbreviations: MMP20, matrix metalloproteinase 20; DSPP, dentin sialophosphoprotein; BSP, bone sialoprotein; OPN, osteopontin; DMP1, dentin matrix protein 1; OSCC, oral squamous cell carcinoma. Scale bars = 100 µm.
Discussion
To our knowledge, this is the first report of in situ expression of MMP20 in tissues and cell lines of normal adult kidneys by IHC, iPLA, and IF. MMP20 expression has, until our very recent reports, been assumed to be tooth specific.12–17,21 Results of studies suggesting a possible role for MMP20 in renal function in the context of aging,22–24 and a recent report of the presence of MMP20 mRNA in the human retina and human retinal pigment epithelium (RPE)/choroid, have been published.25
Following reports that three pro-MMPs, pro-MMP2, pro-MMP3, and pro-MMP9, each bind with high affinity (nM) to specific SIBLINGs: BSP, OPN, and DMP1, respectively, and are activated in the process in the in vitro biochemical system,3 we reported the possible biological relevance of the SIBLING–MMP pairings in duct epithelial systems of the salivary glands and nephrons.6,7 Our most recent reports of the expression of MMP20 in the duct systems of human major salivary glands and its co-expression with DSPP established MMP20 as the cognate MMP partner of DSPP.10 This finding prompted us to investigate the expression of MMP20 in the kidney. As anticipated, MMP20 co-expressed with DSPP whose expression in the kidney was reported over 8 years ago,7 thereby presenting additional confirmation of the MMP20–DSPP cognate pairing in a third biological system.
As indicated in our earlier report, the expressions of BSP, OPN, and DMP1 (three SIBLINGs with known cognate MMPs) were localized to specific segments of the duct epithelial cells of the normal human and monkey nephrons.7 Although SIBLINGs always co-expressed with their respective cognate MMP partners, the MMPs also occasionally expressed at segments where a SIBLING partner is absent. We postulated that the segmental localization of SIBLING–MMP pairs may reflect their relevance to metabolic function characteristics of such segment.7 The co-expression of BSP–MMP2 was confined to the proximal and distal tubules, whereas OPN–MMP3 co-expression was distinctly confined to distal tubules. MMP3 also was expressed in both the proximal and distal tubules.7 DMP1–MMP9 co-expressed throughout the nephron, including the parietal cells of Bowman’s capsule and the thin limb of the loop of Henle.7 MEPE expression was distinctly confined the proximal tubule.7
Earlier reports of the localization of MMP2 and MMP9 in the collecting ducts in the normal rabbit kidney, using sheep polyclonal antisera made against the corresponding human proteins, led the authors to suggest MMP2 as a specific marker for the collecting tubules.26 However, our subsequent investigations using more robustly sensitive MMP2 antibody showed localization of MMP2 at both the proximal and distal tubules, indicating that MMP2 could not be specific for normal collecting ducts.7 There also are reports of in vitro studies of MMP expressions by cells designated as distinct segments of the nephron27–31 during nephrogenesis, and in specific pathologies of the kidney.27,30,32–35 The generally assumed roles of MMPs in other tissues and organ systems—breakdown of ECM in normal physiological processes such as embryonic development, reproduction, tissue remodeling, and in metastatic processes—also have been ascribed to these kidney conditions and pathologies.
Consistent with our previous reports regarding the three MMPs (MMP2, MMP3, and MMP9) with known cognate SIBLING partners,7 our current results also show that MMP20 was expressed in nephron segments where its cognate SIBLING, DSPP, was not localized. However, the SIBLINGs continue to localize with their known cognate MMPs in the kidney as in other biological systems thus far studied.6–8,11 This observation remains a very compelling evidence of a potential critical role for the SIBLING–MMP interaction in physiological and pathological conditions. In duct epithelia such as those of the salivary glands, nephron, and eccrine sweat ducts, this role is postulated to be that of repairing pericellular matrix proteins of the basement membranes damaged in the course of the activities of these highly metabolically active duct systems.6–8 It is noteworthy that, in contrast, the SIBLINGs (except for MEPE) are not expressed in such a passive duct system as that of the normal lacrimal duct.8
Activation of these MMPs classically has required the removal of their inhibitory propeptides. However, it was demonstrated, over a decade ago, that the three SIBLINGs, BSP, OPN, and DMP1, bind and activate their respective MMP partners without removal of the propeptides.3 With respect to the nephrons, this fact shows that every time a nephron cell produces a specific SIBLING, it also invariably made its cognate MMP partner, reinforcing earlier notion that the active SIBLING–MMP complex is formed locally.7 Furthermore, the differential expression of the four SIBLING–MMP complexes, including DSPP–MMP20, in the nephron suggests that there are different target proteins that each cell is modifying.
Reports of MMP20 gene mediating kidney aging is thought to be related to two single nucleotide polymorphisms seen in age-related decline in kidney functions, notably, decline in glomerular filtration rate.22–24 Given the general role of MMPs in ECM activities, the finding that MMP20 is involved in kidney aging aligns with this role as similar ECM changes—glomerular basement membrane thickening, increase in the volume of mesangial matrix, and interstitial fibrosis due to increase in matrix and fibrillar collagen accumulation in the subintimal space22,36,37—are also evident in aging kidney. Earlier reports, indeed, suggested that certain activities of MMP20 may contribute to the interstitial fibrosis resulting from increased matrix in aging kidney.24–37 As speculated for other MMPs present in the kidney,7 MMP20 may be involved in the homeostasis of normal tissue elements. Overall, a definitive role for MMP20 in renal function is yet to be described.
In summary, our current report of the expression of MMP20, and its co-expression and potential interaction with DSPP in the kidney, lays additional foundation to investigate specific protein substrates for MMP20–DSPP partnering in physiological and various pathological conditions of the kidney. In pathological conditions, future findings may offer new opportunities for intervention and prevention of kidney diseases. For example, in diseases in which both the MMP and its SIBLING partner are upregulated, any synthetic MMP protease inhibitor that may be proposed to be used to treat the disorder should first be shown to work in the presence of that MMP’s SIBLING partner.
Footnotes
Author Contributions: KUEO: Conceived the manuscript, designed all experiments, analyzed results of study, produced initial draft of manuscript, and reviewed final manuscript; KK: Contributed to design of IHC experiment, carried out IHC, analyzed results of study, contributed to draft manuscript, and reviewed final draft of manuscript; GS: Contributed to design of in situ proximity ligation assay (iPLA) experiment, carried out iPLA and immunofluorescence experiments, analyzed results of study, contributed to draft manuscript, and reviewed final draft of manuscript.
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Faculty start-up research funding (to K.U.E.O.) by the School of Dentistry, University of Texas Health Science Center at Houston, Texas.
Literature Cited
- 1. Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–4. [DOI] [PubMed] [Google Scholar]
- 2. Carmeliet P, Moons L, Lijnen R, Baes M, Lemaître V, Tipping P, Drew A, Eeckhout Y, Shapiro S, Lupu F, Collen D. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat Genet. 1997;17:439–44. [DOI] [PubMed] [Google Scholar]
- 3. Fedarko NS, Jain A, Karadag A, Fisher LW. Three small integrin binding ligand N-linked glycoproteins (SIBLINGs) bind and activate specific matrix metalloproteinases. FASEB J. 2004;18:734–36. [DOI] [PubMed] [Google Scholar]
- 4. McCawley LJ, Matrisan LM. Matrix metalloproteinases: They’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–40. [DOI] [PubMed] [Google Scholar]
- 5. Tanney DC, Feng L, Pollock AS, Lovett DH. Regulated expression of matrix metalloproteinases and TIMP in nephrogenesis. Dev Dyn. 1998;213:121–9. [DOI] [PubMed] [Google Scholar]
- 6. Ogbureke KU, Fisher LW. Expression of SIBLINGs and their partner MMPs in salivary glands. J Dent Res. 2004;83:664–70. [DOI] [PubMed] [Google Scholar]
- 7. Ogbureke KU, Fisher LW. Renal expression of SIBLING proteins and their partner matrix metalloproteinases (MMPs). Kidney Int. 2005;68:155–66. [DOI] [PubMed] [Google Scholar]
- 8. Ogbureke KU, Fisher LW. SIBLING expression patterns in duct epithelia reflect the degree of metabolic activity. J Histochem Cytochem. 2007;55:403–9. [DOI] [PubMed] [Google Scholar]
- 9. Saxena G, Koli K, de la Garza J, Ogbureke KU. Matrix metalloproteinase 20-dentin sialophosphoprotein interaction in oral cancer. J Dent Res. 2015;94:584–93. [DOI] [PubMed] [Google Scholar]
- 10. Koli K, Saxena G, Ogbureke KU. Expression of matrix metalloproteinase (MMP)-20 and potential interaction with dentin sialophosphoprotein (DSPP) in human major salivary glands. J Histochem Cytochem. 2015;63:524–33. [DOI] [PubMed] [Google Scholar]
- 11. Ogbureke KU, Nikitakis NG, Warburton G, Ord RA, Sauk JJ, Waller JL, Fisher LW. Up-regulation of SIBLING proteins and correlation with cognate MMP expression in oral cancer. Oral Oncol. 2007;43:920–32. [DOI] [PubMed] [Google Scholar]
- 12. Llano E, Pendás AM, Knäuper V, Sorsa T, Salo T, Salido E, Murphy G, Simmer JP, Bartlett JD, López-Otín C. Identification and structural and functional characterization of human enamelysin. Biochemistry. 1997;36:15101–8. [DOI] [PubMed] [Google Scholar]
- 13. Bartlett JD, Ryu OH, Xue J, Simmer JP, Margolis HC. Enamelysin mRNA displays a developmentally defined pattern of expression and encodes a protein which degrades amelogenin. Connect Tissue Res. 1998;39:101–9. [DOI] [PubMed] [Google Scholar]
- 14. Bartlett JD, Simmer JP. Proteinases in developing dental enamel. Crit Rev Oral Biol Med. 1999;10:425–41. [DOI] [PubMed] [Google Scholar]
- 15. Bègue-Kirn C, Krebsbach PH, Bartlett JD, Butler WT. Dentin sialoprotein, dentin phosphoprotein, enamelysin and ameloblastin: tooth-specific molecules that are distinctively expressed during murine dental differentiation. Eur J Oral Sci. 1998;106:963–70. [DOI] [PubMed] [Google Scholar]
- 16. Grant GM, Giambernardi TA, Grant AM, Klebe RJ. Overview of expression of matrix metalloproteinases (MMP-17, MMP-18, and MMP-20) in cultured human cells. Matrix Biol. 1999;18:145–8. [DOI] [PubMed] [Google Scholar]
- 17. Turk BE, Lee DH, Yamakoshi Y, Klingenhoff A, Reichenberger E, Wright JT, Simmer JP, Komisarof JA, Cantley LC, Bartlett JD. MMP-20 is predominately a tooth-specific enzyme with a deep catalytic pocket that hydrolyzes type V collagen. Biochemistry. 2006;45:3863–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park NH, Gujuluva CN, Baek JH, Cherrick HM, Shin KH, Min BM. Combined oral carcinogenicity of HPV-16 and benzo(a)pyrene: an in vitro multistep carcinogenesis model. Oncogene. 1995;10:2145–53. [PubMed] [Google Scholar]
- 19. Osaki T, Tatemoto Y, Yoneda K, Yamamoto T. Tumorigenicity of cell lines established from oral squamous cell carcinoma and its metastatic lymph nodes. Eur J Cancer B Oral Oncol. 1994;30B:296–301. [DOI] [PubMed] [Google Scholar]
- 20. Koli K, Saxena G, Ogbureke KU. Expression of matrix metalloproteinase (MMP)-20 and potential interaction with dentin sialophosphoprotein (DSPP) in human major salivary glands. J Histochem Cytochem. 2015;63:524–33. [DOI] [PubMed] [Google Scholar]
- 21. Fukae M, Tanabe T, Uchida T, Lee SK, Ryu OH, Murakami C, Wakida K, Simmer JP, Yamada Y, Bartlett JD. Enamelysin (matrix metalloproteinase-20): localization in the developing tooth and effects of pH and calcium on amelogenin hydrolysis. J Dent Res. 1998;77:1580–8. [DOI] [PubMed] [Google Scholar]
- 22. Wheeler HE, Metter EJ, Tanaka T, Absher D, Higgins J, Zahn JM, Wilhelmy J, Davis RW, Singleton A, Myers RM, Ferrucci L, Kim SK. Sequential use of transcriptional profiling, expression quantitative trait mapping, and gene association implicates MMP20 in human kidney aging. PLoS Genet. 2009;5:e1000685. doi: 10.1371/journal.pgen.1000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wheeler HE, Kim SK. Genetics and genomics of human ageing. Philos Trans R Soc Lond B Biol Sci. 2011;366:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Han SS, Lee H, Oh YJ, Lee JP, Kim S, Ha J, Kim SJ, Park MH, Kim YS, Kim DK. Identification of the effects of aging-related gene-matrix metalloproteinase on allograft outcomes in kidney transplantation. Transplant Proc. 2013;45:2158–64. [DOI] [PubMed] [Google Scholar]
- 25. Akagi-Kurashige Y, Yamashiro K, Gotoh N, Miyake M, Morooka S, Yoshikawa M, Nakata I, Kumagai K, Tsujikawa A, Yamada R, Matsuda F, Saito M, Iida T, Sugahara M, Kurimoto Y, Cheng CY, Khor CC, Wong TY, Yoshimura N; Nagahama Cohort Research Group. MMP20 and ARMS2/HTRA1 are associated with neovascular lesion size in age-related macular degeneration. Ophthalmology. 2015;122:2295–302.e2. [DOI] [PubMed] [Google Scholar]
- 26. Piedagnel R, Murphy G, Ronco PM, Lelongt B. Matrix metalloproteinase 2 (MMP2) and MMP9 are produced by kidney collecting duct principal cells but are differentially regulated by SV40 large-T, arginine vasopressin, and epidermal growth factor. J Biol Chem. 1999;274:1614–20. [DOI] [PubMed] [Google Scholar]
- 27. Norman JT, Gatti L, Wilson PD, Lewis M. Matrix metalloproteinases and tissue inhibitor of matrix metalloproteinases expression by tubular epithelia and interstitial fibroblasts in the normal kidney and in fibrosis. Exp Nephrol. 1995;3:88–89. [PubMed] [Google Scholar]
- 28. Junaid A, Amara FM. Osteopontin: correlation with interstitial fibrosis in human diabetic kidney and PI3-kinase-mediated enhancement of expression by glucose in human proximal tubular epithelial cells. Histopathology. 2004;44:136–46. [DOI] [PubMed] [Google Scholar]
- 29. Norman JT, Lewis MP. Matrix metalloproteinases (MMPs) in renal fibrosis. Kidney Int. 1996;54 Suppl:S61–3. [PubMed] [Google Scholar]
- 30. Lelongt B, Trugnan G, Murphy G, Ronco PM. Matrix metalloproteinases MMP2 and MMP9 are produced in early stages of kidney morphogenesis but only MMP9 is required for renal organogenesis in vitro. J Cell Biol. 1997;136:1363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Verhulst A, Persy VP, Van Rompay AR, Verstrepen WA, Helbert MF, De Broe ME. Osteopontin synthesis and localization along the human nephron. J Am Soc Nephrol. 2002;13:1210–8. [DOI] [PubMed] [Google Scholar]
- 32. Carome MA, Striker LJ, Peten EP, Elliot SJ, Yang CW, Stetler-Stevenson WG, Reponen P, Tryggvason K, Striker GE. Assessment of 72-kilodalton gelatinase and TIMP-1 gene expression in normal and sclerotic murine glomeruli. J Am Soc Nephrol. 1994;5:1391–9. [DOI] [PubMed] [Google Scholar]
- 33. Kallakury BV, Karikehalli S, Haholu A, Sheehan CE, Azumi N, Ross JS. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin Cancer Res. 2001;7:3113–9. [PubMed] [Google Scholar]
- 34. McMillan JI, Riordan JW, Couser WG, Pollock AS, Lovett DH. Characterization of a glomerular epithelial cell metalloproteinase as matrix metalloproteinase-9 with enhanced expression in a model of membranous nephropathy. J Clin Invest. 1996;97:1094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suzuki D, Miyazaki M, Jinde K, Koji T, Yagame M, Endoh M, Nomoto Y, Sakai H. In situ hybridization studies of matrix metalloproteinase-3, tissue inhibitor of metalloproteinase-1 and type IV collagen in diabetic nephropathy. Kidney Int. 1997;52:111–9. [DOI] [PubMed] [Google Scholar]
- 36. McLachlan MS, Guthrie JC, Anderson CK, Fulker MJ. Vascular and glomerular changes in the ageing kidney. J Pathol. 1977;121:65–78. [DOI] [PubMed] [Google Scholar]
- 37. Abrass CK, Adcox MJ, Raugi GJ. Aging-associated changes in renal extracellular matrix. Am J Pathol. 1995;146:742–52. [PMC free article] [PubMed] [Google Scholar]