Abstract
Inhibitors of the programmed cell death 1 (PD-1) signaling axis have recently demonstrated efficacy and are rapidly being incorporated into the treatment of non–small cell lung cancers (NSCLCs). Despite clear benefits to certain patients, the association of these responses with a predictive biomarker remains uncertain. Several different biomarkers have been proposed, with differing results and conclusions. This study compares multiple methods of biomarker testing for treatment of NSCLCs with PD1-axis inhibitors. Tissue microarrays of matched primary and metastatic NSCLCs were used to compare four different PD-1 ligand (PD-L1) IHC techniques, as well as RNA ISH. Additional cases with whole genome and transcriptome data were assessed for molecular correlates of PD-L1 overexpression. Eighty cases were included in the IHC study. Multiple IHC methodologies showed a high rate of agreement (Kappa = 0.67). When calibrated to RNA expression, agreement improved significantly (Kappa = 0.90, p=0.0049). PD-L1 status of primary and metastatic tumors was discordant in 17 (22%) cases. This study suggests that different IHC methodologies for PD-L1 assessment provide slightly different results. There is significant discordance between the PD-L1 status of primary tumors and lymph node metastases. RNA ISH may be a useful adjunct to complement PD-L1 IHC testing.
Keywords: biomarker, immunotherapy, lung cancer, non–small cell lung carcinoma, NSCLC, PD-1, PD-L1
Introduction
Immune checkpoint inhibitors have recently demonstrated marked efficacy in advanced non–small cell lung carcinoma (NSCLC)1–3 and a number of other advanced malignancies.4 These drugs, specifically programmed cell death 1 (PD-1) axis inhibitors, will have an increasing role in the treatment of advanced lung cancer. With the 2015 approvals of both nivolumab and pembrolizumab by the US Food and Drug Administration for previously treated NSCLC, and the development of other agents in this class, this therapeutic focus is rapidly expanding.5,6 Although clinical trials have demonstrated unequivocal benefits from this family of drugs, predictive biomarker testing is presently unclear. Many trials have attempted to place their results in the context of a predictive biomarker; however, these efforts have been uncoordinated and contribute to some confusion among oncologists and pathologists.7 Most efforts have focused on the PD-1 ligand (PD-L1) as a biomarker, which can be assessed by IHC.8 To date, trials have utilized different primary antibody clones and different cutoffs for determining positivity, and have, on some occasions, also assessed non-tumor cells within a tumor biopsy.9 Published studies comparing PD-L1 detection methods are somewhat limited.10–12
In general, tumors are known to aberrantly express PD-L1, which binds PD-1 expressed on immune cells. This results in downregulation of the antitumor immune response. Pharmacological blockade of this interaction results in the impressive responses documented in clinical trials.13–15
Currently, the molecular underpinnings of PD-L1 overexpression have not been elucidated. The lack of a single gene mutation, or rearrangement, leaves treatment response as the sole reference standard. To this end, investigators have explored the use of genomic mutational signatures such as that induced by tobacco,16 or mismatch repair deficiency,17 as alternative biomarkers. Elevated number of somatic mutations, from exposure to tobacco smoke, might contribute to the generation of novel and possibly immunogenic epitopes, which may increase the potency of the antitumor immune response.
Trial results have demonstrated that response to checkpoint inhibitors can be predicted by PD-L1 IHC assessment in lung adenocarcinoma.1,3,5 Enough evidence, and theory, exists to suggest that biomarker testing for immune checkpoint inhibition holds a great degree of promise and warrants more thorough investigation. The heterogeneity of IHC methodologies and analyses reported in the literature, coupled with the lack of a reproducible and measurable underlying genetic alteration, has created a challenging backdrop for the implementation of a PD-L1 biomarker program. In this report, we compare multiple PD-L1 IHC methods in a population of non-squamous NSCLC and explore the use of RNA ISH as a tool to help calibrate between different PD-L1 assays.
Materials and Methods
A study cohort was constructed by retrospectively reviewing the archives of the BC Cancer Agency from 2005 to 2011 for cases of non-squamous, NSCLC. Cases were included based upon treatment with primary surgical resection and the presence of pN1 or pN2 (American Joint Committee on Cancer [AJCC] seventh edition)18 nodal metastases. Patients were excluded based upon neoadjuvant radiation, neoadjuvant systemic therapy, or non-surgical treatment. Clinical parameters including demographics, staging criteria, treatment, and outcome were extracted from the patient’s medical chart.
Tissue microarrays (TMAs) were constructed from representative formalin-fixed paraffin-embedded blocks of the selected cases. Two TMAs were created, one with primary tumors and a second with nodal metastases. Both TMAs were constructed using duplicate 0.6-mm needle cores from each case. Each TMA included generic control tissues: tonsil, lung, uterus, and appendix.
IHC for PD-L1 was performed using four primary antibodies from two independent local laboratories (designated by primary clones SP142, E1L3N, RBT-PDL1, and 28-8). The 28-8 immunohistochemical assay is a US Food and Drug Administration–approved complementary diagnostic test for nivolumab, and staining was provided by Bristol-Myers Squibb Canada and performed according to the manufacturer’s recommendations. The 28-8 assay has been independently validated elsewhere.19 IHC methodologies are summarized in Table 1. Each staining protocol was independently developed and validated in the performing laboratory.
Table 1.
Summary of Four Immunohistochemical Methods Utilized in the Study.
| Antibody | Vendor | HIER (min) | Buffer | Dilution | Detection | Chromogen | Autostainer |
|---|---|---|---|---|---|---|---|
| SP142 | Spring Bioscience | 35 | DaVinci (BioCare, Concord, CA) | 1:100 | Polymer | DAB | Dako |
| E1L3N | Cell Signaling Technology | 35 | DaVinci (BioCare) | 1:200 | Polymer | DAB | Dako |
| RBT-PDL1 | Bio SB | 30 | Tris-EDTA (Thermo Fisher, Waltham, MA) | 1:50 | Polymer | DAB | Dako |
| 28-8a | Abcam | 20 | Unknown | Unknown | Polymer | DAB | Dako |
Abbreviation: HIER, heat-induced epitope retrieval.
28-8 Assay was performed by Bristol-Myers Squibb Canada (BMS) according to the manufacturer’s instruction using previously validated protocol.19
Slides were independently scored by three pathologists with expertise in pulmonary and molecular pathology (B.S.S., R.F., D.N.I.), and discordant cases were reviewed and consensus was achieved on all cases. PD-L1 immunoreactivity was quantified using an H score, calculated by intensity (0–3) multiplied by proportion (0–100).20 A case was considered PD-L1 positive if there was any detectable immunoreactivity (H score, ≥1). Membranous tumor cell staining was evaluated; all other signals, including immune cells, nuclear, cytoplasmic, and extracellular, were disregarded.
In situ detection of PD-L1 transcripts in formalin-fixed, paraffin-embedded (FFPE) TMA samples was performed using the RNAscope assay with probe Hs-CD274 (cat#600861; Advanced Cell Diagnostics [ACD], Newark, CA) and RNAscope 2.0 HD Reagent Kit (Brown, cat#310035; ACD) following protocols suggested by the manufacturer.
Scoring of the RNAscope PD-L1 assay was performed manually by two pathologists (B.S.S., R.F.), and consensus was achieved in all cases. Only tumor cells were scored. The intensity was assigned according to the scheme recommended by the vendor (ACD). Briefly, a score of 0 was given when no staining or less than 1 dot/10 cells was observed, 1 to 3 dots/cell was given a score of 1, 4 to 9 dots/cell was given a score of 2, 10 to 15 dots/cell was given a score of 3, and >15 dots/cell was given a score of 4. A composite score, analogous to an H score, was obtained by adding the products of each score (0–4) multiplied by the percentage of tumor cells staining at the corresponding score—for example, composite score = 0 × (%cells scored 0) + 1 × (%cells scored 1) + 2 × (%cells scored 2) + 3 × (%cells scored 3) + 4 × (%cells scored 4). RNA ISH composite score ranges from 0 to 400.
Preliminary analysis was made based on a separate cohort of patients retrospectively identified from Personalized OncoGenomics program at the BC Cancer Agency (2013–2015); cases were selected based on NSCLC diagnosis and available archival formalin-fixed, paraffin-embedded tissue for PD-L1 IHC. These cases all had available comprehensive, annotated whole-genome and whole-transcriptome sequencing, originally obtained to aid in clinical decision making.21 PD-L1 IHC using SP142 and E1L3N was performed and assessed (as described above) on whole-slide sections from selected cases. Molecular data were extracted for review, including the number of coding mutations, correlation with a previously described hypermutation signature,22 and PD-L1 mRNA expression in reads per kilobase per million (RPKM), extracted from RNA-seq data.
Cancer and matched normal whole-genome sequences were aligned to reference, and variant calling was performed using Strelka.23 Mutations were binned into 96 classes based on variant and trinucleotide context, and the tobacco signature contribution was determined using non-negative least squares, using 27 previously described signatures as a reference set.19 This approach is mathematically consistent with a previously proposed linear model.24
Using a cut-point of H score ≥1, we compared clinical parameters based upon primary tumor PD-L1 status and utilized a consensus score between the four IHC techniques using the chi-square test and Fisher’s exact test.
Recursive partitioning was used to determine the optimal antibody–specific cut-points using tumor-specific quantified RNA ISH scores as the independent variable. Validation of the resultant cut-points against the RNA ISH score was performed using the Wilcoxon rank sum test. The determination of the RNA ISH cut-point was subjectively derived through an examination of RNA ISH score densities for the group of cases that was classified as negative from the recursive partitioning exercise. This RNA ISH score cut-point was uniformly applied to all four antibodies. The resultant categorical data were subjected to contingency analysis, to further validate the association between binarized RNA and IHC values. Cohen’s Kappa statistic with 95% confidence interval (CI) was used to quantify the level of agreement for each antibody/RNA comparison. A p value of <0.05 was considered statistically significant for all analyses.
Research ethics board approval was obtained from the University of British Columbia and affiliated hospitals before the commencement of this work.
Results
Eighty cases were identified for inclusion in the TMA study set. Of these, 78 (98%) had matched lymph node (LN) metastases included for study. Two cases had insufficient nodal disease for IHC characterization. Demographic data for this study cohort are shown in Table 2.
Table 2.
Demographic, Clinical, Pathological, and Outcome Data Stratified by PD-L1 Status (Determined by Consensus of SP142, E1L3N, and 28-8 IHC).
| Characteristics | PD-L1 Positive | PD-L1 Negative | p Value |
|---|---|---|---|
| Female, n (%) | 14 (56) | 34 (62) | 0.632 |
| Male, n (%) | 11 (44) | 21 (38) | |
| Age (years), median | 56 | 70 | 0.744 |
| Performance status, n (%) | 0.480 | ||
| 0 | 2 (8) | 9 (16) | |
| 1 | 13 (52) | 27 (49) | |
| 2 | 3 (12) | 10 (18) | |
| Unknown | 7 (28) | 9 (16) | |
| Smoking status, n (%) | 1.000 | ||
| Never | 3 (12) | 7 (13) | |
| Former or current | 22 (88) | 48 (87) | |
| Tobacco consumption, n (%) | 0.040 | ||
| ≤40 pack years | 14 (56) | 43 (78) | |
| >40 pack years | 11 (44) | 12 (22) | |
| Tumor differentiation, n (%) | 0.103 | ||
| Well | 1 (4) | 8 (15) | |
| Moderate | 8 (32) | 25 (46) | |
| Poorly | 16 (64) | 22 (40) | |
| Lymphovascular invasion, n (%) | 16 (59) | 32 (60) | 1.000 |
| Pleural invasion, n (%) | 15 (60) | 33 (60) | 1.000 |
| Extranodal extension, n (%) | 7 (28) | 14 (26) | 0.791 |
| Mutational status, n (%) | |||
| EGFR positive | 0 (0) | 7 (13) | 0.092 |
| ALK positive | 0 (0) | 2 (4) | 1.000 |
| Disease-free survival (months) | 46 | 34 | 0.670 |
| Overall survival (months) | 76 | 68 | 0.744 |
| Total, n (%) | 25 (31) | 55 (69) |
Abbreviations: PD-L1, programmed cell death 1 ligand; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase.
Twenty-eight (35%) of the primary tumors showed PD-L1 positivity by consensus IHC (any two of four methods, H score, ≥1; 29 [36%] by SP142, 19 [24%] by E1L3N, 30 [38%] by RBT-PDL1, and 27 [34%] by 28-8). Twenty-eight (36%) LN metastases were PD-L1 positive by consensus score (25 [32%] by SP142, 18 [23%] by E1L3N, 31 [40%] by RBT-PDL1, and 31 [40%] by 28-8). Agreement between the four IHC techniques was high (Cohen’s Kappa, 0.67 [95% CI, 0.62–0.71]).
Univariate analysis showed an equal distribution of age, sex, grade, histological parameters, and clinical outcome (Table 2). A statistically significant association was identified for previous tobacco use, where heavy lifetime tobacco consumption (greater than 40 pack-year history) was associated with increased PD-L1 immunoreactivity in primary tumors (p=0.040, Table 2).
Comparison of primary tumor PD-L1 IHC status with LN metastasis IHC status using consensus scoring showed concordant results in 61 (78%) cases. Seventeen (22%) cases showed discordant staining, with eight (10%) cases presenting immunoreactivity in the primary tumor and featured an IHC-negative LN metastasis, and nine (12%) cases showing an IHC-negative primary tumor and PD-L1 immunoreactivity in the LN metastasis. Primary/LN PD-L1 status discordance was not unique to any of the IHC methodologies examined.
PD-L1 RNA ISH studies showed positive signal above threshold (score of 50 or higher) in 18 (23%) primary tumors and 22 (28%) LN metastases. Comparison of RNA ISH scores with global IHC H scores confirmed that an RNA ISH score of 50 was associated with significant changes in protein expressivity. RNA ISH and IHC were generally consistent; two (3%) cases showed positive RNA ISH with negative IHC, and three (4%) cases showed negative RNA ISH in the setting of significant IHC positivity (Fig. 1).
Figure 1.
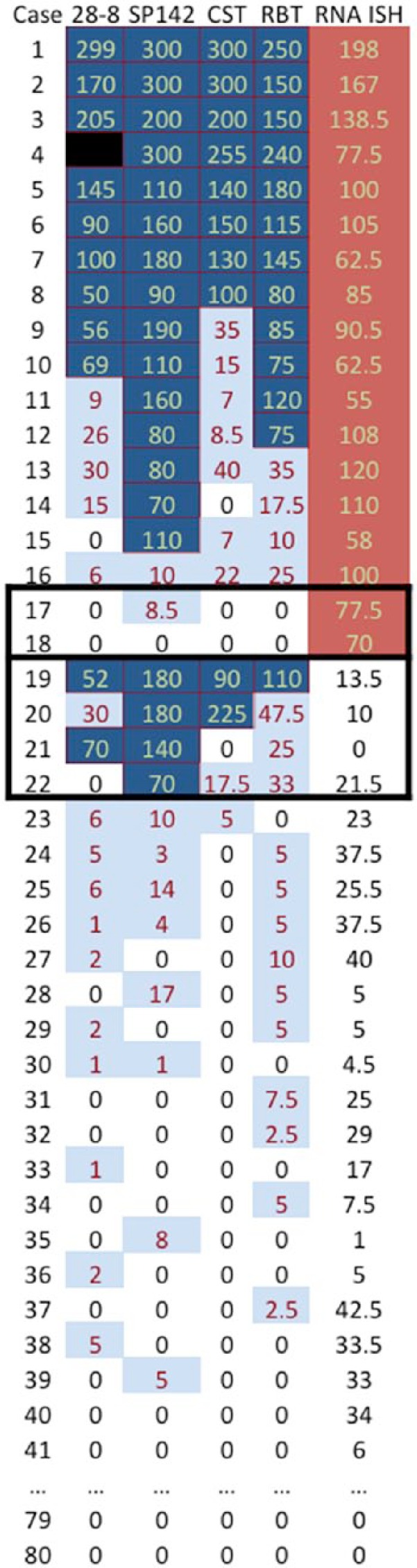
Heatmap of IHC staining by four antibodies measured in H score (percentage [0–100] × intensity [0–3]) and RNA ISH score (percentage [0–100] × intensity [0–4]). Cases 42 to 78 omitted for brevity; all omitted cases show scores of 0 for all methodologies. IHC with H score >50 colored dark blue, H score between 50 and 1 in light blue, and 0 no shading. ISH scores >50 colored in red. Note ISH positivity and IHC negativity in cores 17 to 18 and IHC positivity with ISH negativity in cases 19 to 22.
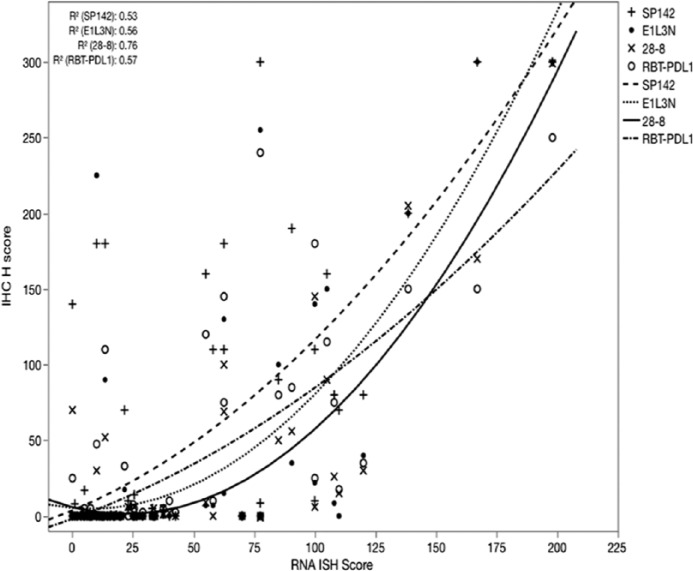
Protein expression (IHC H score) was compared with RNA expression (RNA ISH) using a quadratic regression model for each antibody. This showed a very good to excellent correlation between each of the four IHC methods and RNA ISH (R2 = 0.53 [SP142], 0.56 [E1L3N], 0.76 [28-8], 0.57 [RBT-PDL1]; Fig. 2).
Figure 2.
IHC (H score) versus RNA ISH score for four different antibody clones. Very good to excellent quadratic relation for all four antibodies. RNA ISH cut-point of 50 was independently validated by density distribution of IHC-negative cases.
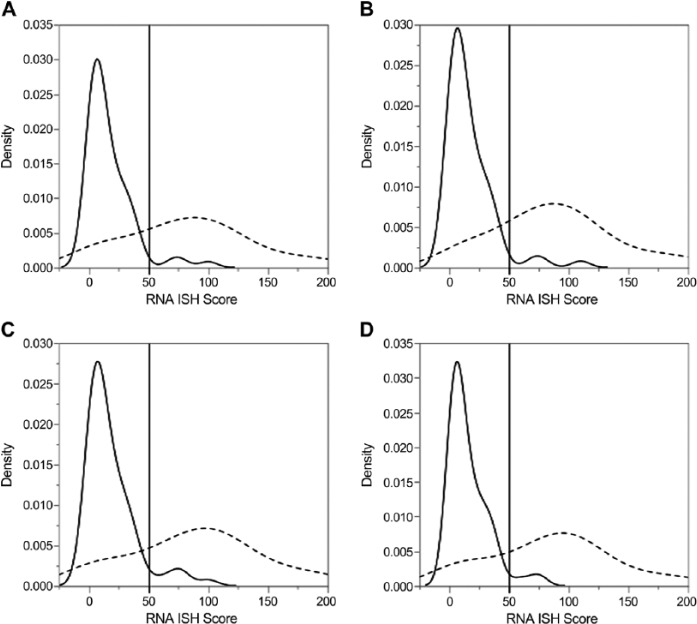
Determination of the optimal cut-point for each antibody in primary tumor samples using recursive partitioning yielded divisions for positivity at the following H scores: SP142, ≥70; E1L3N, ≥7; DAKO 28-8, ≥9; and RBT-PDL1, ≥17. This binarization yielded between a 19.8% to 23.2% positive case rate for each antibody. An internal validation experiment comparing the binarized PD-L1 IHC for the four antibodies against the raw PD-L1 ISH data demonstrated highly significant segregation of high and low mRNA expressers (p<0.0001). Subjective examination of the densities of H scores for each category revealed that for each antibody, an RNA ISH score of approximately 50 corresponded to the beginning of the posterior tail of the density distribution for the cases classified as negative by IHC, given the cut-points derived through the recursive partitioning procedure (Fig. 3).
Figure 3.
Density distributions of RNA scores for each binarized programmed cell death 1 ligand antibody, where A = 28-8, B = SP142, C = E1L3N, and D = RBT-PDL1. A reference line is placed at an RNA ISH score of 50 to illustrate the rationale for the establishment of RNA binarization at that value. Note, solid and dashed lines correspond to IHC-negative (H score = 0) and IHC-positive (H score, >0) cases, respectively.
Using these individualized H-score cut-points, interantibody agreement improved to a Cohen’s Kappa of 0.90 (p=0.049, compared with 0.67 using the global cut-point of ≥1).
Nine cases with comprehensive whole-genome and whole-transcriptome sequence were identified (Table 3) for preliminary analysis. None of the cases showed any mutations or copy number changes in the PD-1 or PD-L1 genes. One (11%) case was positive for PD-L1 by IHC with SP142 and E1L3N. This case showed a high expression of PD-L1 mRNA (5.8 RPKM) on RNA-seq but did not exhibit “hypermutation” nor a hypermutation signature associated with tobacco. Three (33%) cases were relatively hypermutated (325, 305, 273 coding mutations [mean, 169]), and two (22%) cases showed a strong association with the tobacco-induced hypermutation signature.19 None of the cases with relative hypermutation or tobacco-induced hypermutation signature showed detectable PD-L1 expression by IHC.
Table 3.
Molecular Correlates of PD-L1 Protein Overexpression.
| Case | Tobacco History | Coding Mutations | Hypermutation Signature | PD-L1 mRNA (RPKM)a | PD-L1 Protein (IHC, H Score)b |
|---|---|---|---|---|---|
| 1 | Yes | 305 | Strong | 1.8 | 0 |
| 2 | Yes | 325 | Weak | 0.71 | 0 |
| 3 | Yes | 161 | Strong | 2.1 | 0 |
| 4 | No | 42 | None | 2.2 | 0 |
| 5 | No | 81 | None | 5.8 | 140 |
| 6 | Yes | 133 | Weak | 1.8 | 0 |
| 7 | No | 145 | None | 0.1 | 0 |
| 8 | Yes | 273 | Weak | 0.2 | 0 |
| 9 | No | 52 | None | 1.5 | 0 |
Abbreviations: PD-L1, programmed cell death 1 ligand; RPKM, reads per kilobase per million.
mRNA expression is measured in RPKM.
Protein expression as measured by IHC H score (intensity [0–3] × proportion [0–100]).
Discussion
This study explores and compares multiple methods for PD-L1 biomarker testing. We show a head-to-head comparison of four primary IHC antibodies directed toward PD-L1. These identify a small, but significant, rate of discordance. Determination of diagnostic test characteristics is challenging due to the lack of a reference standard. The intermethod agreement seen in this study is similar to what has been reported for other class 2 IHC biomarkers.25
These data show a 22% discordance rate between primary tumors and LN metastases. Other studies in NSCLC have found similar rates as our study, with Mitchell et al.26 reporting 11% and Kowanetz et al.27 noting only 18% discordance between primary tumors and lymph nodes. The discordance seen here could possibly be attributed to intratumoral heterogeneity and a result of TMA sampling bias; however, this sampling issue would be present in the setting of small biopsies or cytology specimens in clinical practice.28 Similar findings have been reported for PD-L1 assessment in renal cell carcinomas.29 A PD-L1 biomarker testing strategy that tests multiple tumor samples from different sites may improve the sensitivity of this test based on tumor heterogeneity. The heterogeneity detected between IHC methodologies is similar to those previously reported by Velcheti et al.10 and McLaughlin et al.11
The lack of a molecular gold standard for PD-L1 overexpression is problematic, making it difficult to unequivocally identify positive cases and challenging to definitively evaluate primary screening techniques such as IHC. To this end, we queried a series of cases with comprehensive molecular profiling as a starting point to identify an assessable molecular correlate of PD-L1 overexpression. Although PD-L1 overexpression by IHC was detected only in one of these nine cases, we believe this to be informative. It is of note that this case was not hypermutated, nor did it harbor a tobacco-associated hypermutation signature. Furthermore, three cases in this series were relatively hypermutated, and two cases showed a strong tobacco-associated genomic hypermutation signature, yet none of these cases featured PD-L1 protein overexpression.
This finding is somewhat different than that published by Rizvi et al.16 who reported an association between hypermutation signature and response to PD-1 axis inhibition. It should be noted that the current study uses a different definition of tobacco-associated genomic hypermutation signature19 and is comparing this with PD-L1 protein and RNA expression, whereas previously reported results are based upon exome sequencing and comparison of DNA transitions with transversions.
Hypermutation and associated signatures may yet play a role in prediction of immunotherapy response; however, based upon these data, their current role remains unclear. Both of these parameters require a large amount of DNA sequencing (whole exome or, preferably, genome) and are currently not feasible for delivery of results in meaningful clinical timelines. In the absence of a surrogate for hypermutation and hypermutation signatures (eg, tobacco history), these measures are unlikely to play a useful role in a biomarker testing strategy. The data shown in Table 2 suggest that tobacco history, hypermutation, or tobacco-associated hypermutation signature may not be useful predictors of PD-L1 overexpression.
The correlation between PD-L1 protein overexpression and PD-L1 mRNA expression is of interest and potentially exploitable. Although this correlation was identified through transcriptomic sequencing, mRNA expression can additionally be interrogated via real-time PCR and slide-based RNA ISH techniques, as demonstrated in this study.
RNA ISH allows a pathologist to assess, and semiquantify, the expression of a specific mRNA molecule on a glass slide. This is particularly useful in the setting of PD-L1 as the pathologist can specifically identify expression levels within tumor cells which would not be possible using next-generation sequencing or PCR-based techniques (Fig. 4). The target, PD-L1 mRNA transcript, is different than the protein epitope target of IHC, yet their intracellular concentrations are intimately linked. PD-L1 RNA ISH was previously demonstrated to be a useful technique by Velcheti et al.,10 where high PD-L1 expression by RNA ISH was associated with improved outcome in NSCLC. Our data indicate that PD-L1 RNA ISH may be useful in discriminating cases of borderline positive PD-L1 IHC. Our study suggests that PD-L1 RNA ISH is an attractive candidate to help refine a possible PD-L1 biomarker strategy.
Figure 4.
Editor’s Highlight
Positive IHC (A) and RNA ISH (B) (from case 3, Fig. 1). Negative IHC (C) and RNA ISH (D) (from case 79, Fig. 1).
There are several limitations to our study: The number of patients included in the TMA was small due to tissue constraints, archival specimens were used which may affect the quality of the testing, and none of the patients included were treated with PD1-axis inhibitors. Although PD1-axis inhibition is typically utilized for advanced-stage patients, this study is performed on locally advanced (pN1/pN2) cases due to inherent tissue constraints. The most important limitation is the lack of a true reference standard for PD-L1 status. Previous clinical trials have utilized a surrogate reference standard of clinical response to PD1-axis inhibition. Although response to treatment is arguably the most clinically relevant reference standard, there is no true gold standard identified for this biomarker. The lack of a true reference standard hampers the ability to calculate test characteristics and perform true comparisons between diagnostic laboratory tests. Despite this, these data offer much insight into the intricacies of PD-L1 biomarker testing. Many of these findings, including the improved concordance when using individually calculated cutoffs, and the discordance between primary and metastatic tissues, warrant further investigation.
These data may shed light on the convoluted status of PD-L1 biomarker testing. Here, we have shown that there is an appreciable discordance in PD-L1 status of primary tumors and lymph nodes using multiple methodologies. Multiple IHC assays for PD-L1 have slightly differing results, the clinical significance of which is not currently clear. PD-L1 mRNA expression is a useful molecular correlate of PD-L1 positivity by IHC, and further exploitation of this fact may be clinically useful. Finally, PD-L1 RNA ISH may be a very useful tool to help laboratories calibrate and maintain precision in PD-L1 biomarker testing.
Acknowledgments
The authors acknowledge Bristol-Myers Squibb Canada for providing the 28-8 immunohistochemical stain studied in this work.
Footnotes
Author Contributions: BSS: Drafted the manuscript, coordinated the study, interpreted results, and built the tissue microarrays; RF: Interpreted stains, contributed to the manuscript, and interpreted results; SEK: Performed statistical analysis and contributed to the manuscript; KM: Performed immunohistochemical stains and contributed to the manuscript; GG: Assembled clinical data on patient cohorts and contributed to the manuscript; MJ: Performed bioinformatics analysis on preliminary study cohort and contributed to the manuscript; CJ: Performed RNA ISH studies and contributed to the manuscript; SZ: Built tissue microarray and contributed to the manuscript; EZ: Performed mutation signature analysis on the preliminary study cohort and contributed to the manuscript; EP: Assembled the preliminary cohort and performed bioinformatics analysis on the preliminary cohort; JL: Assembled clinical cohort and provided supervision and oversight to the preliminary study cohort; SJMJ and MAM: Provided supervision and oversight to the preliminary study cohort; SY: Provided supervision and oversight to the preliminary study cohort and contributed to the manuscript; BHN: Contributed reagents and labor and provided supervision and oversight of immunohistochemical staining; AMG: Contributed reagents and labor and provided supervision and oversight of immunohistochemical staining and ISH; CH: Assembled the clinical cohort, provided supervision and oversight of the entire project, and contributed to the manuscript; DNI: Assembled the clinical cohort, interpreted stains, provided supervision and oversight of the entire project, and contributed to the manuscript.
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by the University of British Columbia Anatomical Pathology program. Reagents were contributed by the BC Cancer Agency, PhenoPath Laboratories, and Bristol-Myers Squibb Canada. Additional support was received from the British Columbia Personalized Onco-Genomics program.
Literature Cited
- 1. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L; KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28. [DOI] [PubMed] [Google Scholar]
- 2. Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman DM, Sequist LV, Smith DC, Leming P, Carbone DP, Pinder-Schenck MC, Topalian SL, Hodi FS, Sosman JA, Sznol M, McDermott DF, Pardoll DM, Sankar V, Ahlers CM, Salvati M, Wigginton JM, Hellmann MD, Kollia GD, Gupta AK, Brahmer JR. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, Otterson GA, Campos LT, Gandara DR, Levy BP, Nair SG, Zalcman G, Wolf J, Souquet PJ, Baldini E, Cappuzzo F, Chouaid C, Dowlati A, Sanborn R, Lopez-Chavez A, Grohe C, Huber RM, Harbison CT, Baudelet C, Lestini BJ, Ramalingam SS. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR, Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Eng J Med. 2015;373(2):123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kerr KM, Tsao MS, Nicholson AG, Yatabe Y, Wistuba II, Hirsch FR; IASLC Pathology Committee. Programmed death-ligand 1 immunohistochemistry in lung cancer: in what state is this art? J Thorac Oncol. 2015;10(7):985–9. [DOI] [PubMed] [Google Scholar]
- 8. Cai G, Karni A, Oliveira EM, Weiner HL, Hafler DA, Freeman GJ. PD-1 ligands, negative regulators for activation of naïve, memory, and recently activated human CD4+ T cells. Cell Immunol. 2014;230(2):89–98. [DOI] [PubMed] [Google Scholar]
- 9. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L, Rimm DL. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94(1):107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McLaughlin J, Han G, Schalper KA, Carvajal-Hausdorf D, Pelekanou V, Rehman J, Velcheti V, Herbst R, LoRusso P, Rimm DL. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol. 2016;2(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vassilakopoulou M, Avgeris M, Velcheti V, Kotoula V, Rampias T, Chatzopoulos K, Perisanidis C, Kontos CK, Giotakis AI, Scorilas A, Rimm D, Sasaki C, Fountzilas G, Psyrri A. Evaluation of PD-L1 expression and associated tumor-infiltrating lymphocytes in laryngeal squamous cell carcinoma. Clin Cancer Res. 2016;22(3):704–13. [DOI] [PubMed] [Google Scholar]
- 13. Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. [DOI] [PubMed] [Google Scholar]
- 14. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;248(6230):124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA., Jr PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Edge SP, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4. [DOI] [PubMed] [Google Scholar]
- 19. Phillips T, Simmons P, Inzunza HD, Cogswell J, Novotny J, Jr, Taylor C, Zhang X. Development of an automated PD-L1 immunohistochemistry (IHC) assay for non-small cell lung cancer. Appl Immunohistochem Mol Morphol. 2015;23(8):541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48(9):876–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sheffield BS, Tinker AV, Shen Y, Hwang H, Li-Chang HH, Pleasance E, Ch’ng C, Lum A, Lorette J, McConnell YJ, Sun S, Jones SJ, Gown AM, Huntsman DG, Schaeffer DF, Churg A, Yip S, Laskin J, Marra MA. Personalized oncogenomics: clinical experience with malignant peritoneal mesothelioma using whole genome sequencing. PLoS ONE. 2015;10(3):e0119689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjörd JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinski M, Jäger N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, López-Otín C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdés-Mas R, van Buuren MM, van ’tVeer L, Vincent-Salomon A, Waddell N, Yates LR; Australian Pancreatic Cancer Genome Initiative; ICGC Breast Cancer Consortium; ICGC MMML-Seq Consortium; ICGC PedBrain; Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saunders CT, Wong WS, Swamy S, Becq J, Murray LJ, Cheetham RK. Strelka: accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics. 2012;28(14):1811–7. [DOI] [PubMed] [Google Scholar]
- 24. Alexandrov LB, Nik-Zainal S, Wedge DC, Campbell PJ, Stratton MR. Deciphering signatures of mutational processes operative in human cancer. Cell Rep. 2013;3(1):246–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sheffield BS, Garratt J, Kalloger SE, Li-Chang HH, Torlakovic EE, Gilks CB, Schaeffer DF. HER2/neu testing in gastric cancer by immunohistochemistry: assessment of interlaboratory variation. Arch Pathol Lab Med. 2014;138(11):1495–502. [DOI] [PubMed] [Google Scholar]
- 26. Mitchell P, Murone C, Asadi K, et al. PDL-1 expression in NSCLC: analysis of a large early stage cohort; and concordance of expression in primary, nodes and metastasis. J Thorac Oncol. 2015;10(9 Suppl 2):S199. [Google Scholar]
- 27. Kowanetz M, Koeppen H, Boe M, et al. Spatiotemporal effects on programmed death ligand 1 (PD-L1) expression and immunophenotype of non-small cell lung cancer (NSCLC). J Thorac Oncol. 2015;10(9 Suppl 2):S199. [Google Scholar]
- 28. Ilie M, Long-Mira E, Bence C, Butori C, Lassalle S, Bouhlel L, Fazzalari L, Zahaf K, Lalvée S, Washetine K, Mouroux J, Vénissac N, Poudenx M, Otto J, Sabourin JC, Marquette CH, Hofman V, Hofman P. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol. 2016;27(1):147–53. [DOI] [PubMed] [Google Scholar]
- 29. Callea M, Albiges L, Gupta M, Cheng SC, Genega EM, Fay AP, Song J, Carvo I, Bhatt RS, Atkins MB, Hodi FS, Choueiri TK, McDermott DF, Freeman GJ, Signoretti S. Differential expression of PD-L1 between primary and metastatic sites in clear cell renal cell carcinoma. Cancer Immunol Res. 2015;3(10):1158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]