Abstract
Amyotrophic lateral sclerosis (ALS) is an adult-onset neurodegenerative disease characterized by selective loss of motor neurons and progressive muscle wasting. Growing evidence indicates that mitochondrial dysfunction, not only occurring in motor neurons but also in skeletal muscle, may play a crucial role in the pathogenesis. In this regard, the life expectancy of the ALS G93A mouse line is extended by creatine, an intracellular energy shuttle that ameliorates muscle function. Moreover, a population of patients with sporadic ALS exhibits a generalized hypermetabolic state of as yet unknown origin. Altogether, these findings led us to explore whether alterations in energy homeostasis may contribute to the disease process. Here, we show important variations in a number of metabolic indicators in transgenic ALS mice, which in all shows a metabolic deficit. These alterations were accompanied early in the asymptomatic phase of the disease by reduced adipose tissue accumulation, increased energy expenditure, and concomitant skeletal muscle hypermetabolism. Compensating this energetic imbalance with a highly energetic diet extended mean survival by 20%. In conclusion, we suggest that hypermetabolism, mainly of muscular origin, may represent by itself an additional driven force involved in increasing motor neuron vulnerability.
Amyotrophic lateral sclerosis (ALS) is the most common motor neuron disorder of adult onset. It is characterized by selective degeneration of upper and lower motor neurons, progressive decline of muscle strength, failure of pulmonary function and death usually within 3–5 years after the first symptoms (1). Although most cases occur sporadically, ≈10% of patients exhibit an autosomal dominant pattern of inheritance, and 10–20% of these are associated with missense mutations in the gene encoding Cu/Zn-superoxide dismutase (SOD1), the main free radical scavenging enzyme that protects cells against oxidative stress (2). Studies using transgenic mice expressing ALS-linked SOD1 mutations demonstrated that the deleterious effect of the mutant enzyme is related to a gain of function (3–5). However, the way in which this toxic property induces motor neuron degeneration is still unknown, and several mechanisms have been put forward, including oxidative stress, neurofilament disorganisation, protein misfolding, and glutamate excitotoxicity (6).
Growing evidence now points to mitochondria as playing a crucial role in ALS pathogenesis (7). In mice with the ALS-linked G37R SOD1 mutation, the presence in axons and dendrites of membrane-bound vacuoles, which seem to be derived from degenerating mitochondria (5), is noteworthy. Massive mitochondrial degeneration is also observed in motor neurons of G93A SOD1 mutant mice at the onset of the disease (8), when electron transfer chain activity and ATP synthesis appear severely injured (9). Furthermore, a selective decrease in the activity of cytochrome c oxidase, encoded by the mitochondrial genome, occurs in motor neurons of sporadic ALS patients (10), and the reduction in the amount of mitochondrial DNA in the spinal cord of ALS patients correlates with a decrease in the activities of citrate synthase and the respiratory chain complexes I+III, II+III, and IV (11).
Mitochondrial dysfunction is not only restricted to the nervous system but is also present in other tissues, including liver (12), lymphocytes (13), and muscle (14). Wiedemann et al. (15) found a deficiency of NADH:CoQ oxidoreductase in muscles of sporadic ALS. In a follow-up study, those authors postulated that the decreased activities of NADH:CoQ oxidoreductase and cytochrome C oxidase are associated with DNA abnormalities and reduced levels of the mitochondrial Mn-superoxide dismutase (16). Recently, we reported the early increase in the mRNA levels of the mitochondrial uncoupling protein-3 in skeletal muscles of ALS-linked G86R SOD1 mutant mice. In addition, isolated mitochondria from G86R muscle tissue exhibited a reduced respiratory control ratio, which is line with the existence of mitochondrial uncoupling (17).
Supporting the “mitochondrial dysfunction” hypothesis, the life expectancy of G93A mice is extended by creatine, an intracellular energy shuttle between mitochondria and sites of energy consumption that is known to ameliorate muscle function (18). Although clinical trials failed to show a beneficial effect of creatine in ALS patients (19), the findings in G93A mice invoke the existence of a characteristic energetic imbalance. In this respect, Desport et al. (20) showed a chronic hypermetabolism in a subset of sporadic ALS patients, which parallels a reduction in fat-free mass frequently observed in these patients. Kasarskis et al. (21) hypothesized that an increase in respiratory muscular work, and hence an exacerbated hypermetabolic trait, should take place in worsening patients to maintain an appropriate gas exchange. Although the origin of this ALS hypermetabolism still remains unsolved, these combined data point to metabolic perturbations as playing an important role in the disease. To gain insight into this question, (i) we performed a multidisciplinary study to determine whether energy homeostasis is defective in two transgenic ALS lines, the G86R and G93A mice, and (ii) we evaluated the way in which muscle could actively participate to a metabolic deficit.
Methods
Animals. Transgenic male mice with the G86R murine SOD1 mutation (4) were obtained from the animal facility at the Université Louis Pasteur. Transgenic male mice with the G93A human SOD1 mutation were obtained from The Jackson Laboratory. Both strains were bred with female littermates, and transgenic animals were identified by PCR amplification of DNA extracted from tail tissue. Nontransgenic age-matched male littermates served as control. Mice were maintained at 23°C with a 12-h light/dark cycle and had water and regular rodent chow (A04, UAR, Epinay sur Orge, France) ad libitum. Body mass was recorded every 10 days, and food intake was measured every 5 days. Peripheral nerve injury was accomplished by axotomy of sciatic nerve. Briefly, the right sciatic nerve of animals anesthetized with 250 mg/kg tribromoethanol i.p. was exposed at the midthigh level. Sciatic nerve was then dissected sharply with microscissors. Muscles and skin incision were sutured, and animals were allowed to recover. Sham-operated mice served as control. For the high-fat diet (HFD) experiments, mice were isolated at 4 weeks of age, and the regimen began at 6 weeks of age. HFD consisted of regular chow supplemented with 21% (wt/wt) butter fat and 0.15% (wt/wt) cholesterol. For the survival experiments, mice were killed when they could no longer roll over within 10 s of being pushed on their side, and this time point was used as the time of death. Tissues were carefully dissected, weighed, and stored at –80°C. Plasma from truncular blood collected in EDTA-containing tubes was stored at –20°C. Animal manipulation followed current European Union regulations.
Body Composition. We measured body composition by biochemical analysis of carcasses. Bodies were ground in liquid nitrogen, lyophilized, and further ground until a fine powder was obtained. Body nitrogen was then measured by the Kjeldahl's method and converted into protein content by multiplying by 6.25. Lipid content was determined gravimetrically by extraction in chloroform/methanol (2:1, vol/vol) and solvent evaporation. The mass of mineral ashes was measured after ignition in an oven at 800°C for 24 h.
Metabolite and Hormone Assays. Plasma concentrations of leptin and insulin (Linco Research, St. Charles, MO), corticosterone and T3 (ICN), β-hydroxybutyrate (Sigma), and free fatty acids (Oxoïd, Dardilly, France) were determined as indicated by the manufacturer. I.p. glucose tolerance tests were performed by injecting glucose at a dose of 2 g/kg into overnight-fasted mice. Glycemia was measured every 15 min with a glucometer (Glucomen, Menarini, Rungis, France). For the glucose uptake experiments, we applied the same protocol by using 10 μCi of radiolabeled 3H-2-deoxyglucose (NEN PerkinElmer) per mouse. After 1 h, mice were killed, and tissues were collected and counted for radioactivity by standard procedure.
Indirect Calorimetry. We measured O2 consumption and CO2 production by using an open-circuit indirect calorimetry system (Klogor, Lannion, France). Concentrations of O2 and CO2 in the outgoing air were successively measured in five different cages. The system was rinsed for 90 s between each measurement. Final values of gas concentrations were the mean of 10 measures obtained during 40 s. Each cage was sampled every 11 min, and one cage was left vacant as reference of ambient gas concentrations. Measurements were performed continuously over 23½h, a 30-min period being required for calibration of the O2 and CO2 analyzers. In total, 127 measures were collected per day and mouse. The average of the five lowest values of O2 consumption was considered as resting energy expenditure. Energy expenditure was obtained by using an energy equivalent of 20.1 J/ml O2. The respiratory quotient was the ratio of CO2 production over O2 consumption.
RT-PCR. Two micrograms of total RNA was reverse-transcribed by using 200 units of Moloney murine leukemia virus reverse transcriptase and 0.5 μg of random primers (Promega) by a standard procedure. After PCR amplification, products were separated with a 2% agarose gel and visualized by ethidium bromide staining. We used 18S rRNA or glyceraldehyde 3-phosphate dehydrogenase mRNA levels as internal control. We performed controls without reverse transcriptase and with different dilutions of cDNA template to ascertain the specificity and linearity of the amplification (data not shown). PCR primers are indicated in Table 2, which is published as supporting information on the PNAS web site.
White Adipose Tissue (WAT) Explant Preparation. Epididymal WAT was dissected, weighed, and minced with scissors in warm glucose-free Krebs–Ringer bicarbonate buffer, pH 7.4 containing 4% (wt/vol) BSA. Samples were rinsed three times and incubated in 3 ml of buffer for 90 min in the presence of 1 μM norepinephrine. Incubation medium was collected and assayed for glycerol content (Sigma).
Histological Analysis. Animals were anesthetized and perfused with 4% buffered paraformaldehyde. Lumbar spinal cords were dissected, further fixed for 24 h at 4°C, and cryoprotected in a 20% sucrose solution. The lumbar spinal cord regions corresponding to segments L3–L5 were cut on a cryostat into sections 20 μm thick and stained with 1% toluidine blue in 5% sodium borate. Neuronal counts were performed at a magnification of ×200 in the ventral horns of seven nonadjacent sections per animal (three mice per group). Cells were measured with National Institutes of Health image version 1.62 software, and then classified into three area groups of <300 μm2, between 300 and 600 μm2, and >600 μm2.
Statistical Analysis. Statistical comparisons were accomplished with the unpaired Student t test, unless otherwise indicated, or ANOVA followed by the post hoc Newman–Keuls multiple comparisons test (for data in Table 1), using prism version 2.0a software (GraphPad, San Diego).
Table 1. Body composition and metabolite measurements in G86R mice.
| 75 days old (asymptomatic)
|
105 days old (early symptomatic)
|
|||
|---|---|---|---|---|
| WT | G86R | WT | G86R | |
| Body weight, g | 27.6 (24.3-31.3, 19) | 24.1 (20.3-28.0, 20)* | 31.8 (27.7-38.0, 36) | 21.5 (15.6-28.2, 34)*# |
| WAT, % | 2.14 (1.23-3.28, 19) | 1.24 (0.30-2.64, 20)* | 2.95 (0.92-6.16, 36) | 1.21 (0.01-4.43, 34)* |
| Retroperitoneal WAT, g | 0.23 (0.08-0.48, 19) | 0.10 (0.00-0.34, 20)* | 0.40 (0.10-1.16, 36) | 0.10 (0.00-0.44, 34)* |
| Epididymal WAT, g | 0.37 (0.24-0.56, 19) | 0.20 (0.06-0.40, 20)* | 0.56 (0.20-1.18 36) | 0.19 (0.00-0.64, 34)* |
| Liver, % | 5.00 (1.20-1.60, 19) | 5.30 (0.96-1.56, 20) | 4.80 (1.24-1.80, 36) | 4.70 (0.60-1.52, 36) |
| Water, % | 64.6 (14.5-19.0, 11) | 65.8 (13.1-16.4, 11) | 59.4 (15.8-19.7, 13) | 64.8 (9.4-16.3, 14,)* |
| Proteins, % | 20.9 (4.63-5.97, 11) | 20.7 (3.98-5.27, 11) | 19.9 (5.40-6.44, 13) | 22.7 (3.15-5.48, 14)*# |
| Lipids, g | 11.6 (2.04-4.18, 11) | 10.6 (1.74-3.30, 11) | 17.4 (2.03-10.1, 13) | 8.84 (0.62-3.33, 14)* |
| Minerals, g | 3.49 (0.85-1.04, 11) | 3.31 (0.69-0.89, 11) | 3.30 (0.92-1.23, 13) | 3.99 (0.62-1.17, 14)*# |
| Leptin, ng/ml | 2.63 (1.03-4.85, 7) | 1.62 (0.51-3.59, 7)* | 3.06 (0.86-8.14, 7) | 1.27 (0.00-4.75, 7)* |
| Insulin, ng/ml | ND | ND | 3.36 (2.39-4.33, 3) | 0.87 (0.45-1.35, 3)* |
| Corticosterone, ng/ml | 71 (25-123, 7) | 118 (32-293, 7) | 77 (25-165, 7) | 364 (92-754, 7)*# |
| Free T3, ng/ml | 1.18 (0.80-1.79, 11) | 1.01 (0.68-1.58, 11) | 1.69 (1.18-4.77, 11) | 0.63 (0.27-1.04, 11)* |
| β-Hydroxybutyrate, μM | 370 (13-713, 7) | 419 (65-737, 7) | 333 (32-694, 7) | 931 (51-3720, 7)*# |
| Free fatty acids, mM | 0.87 (0.42-1.84, 13) | 0.89 (0.52-1.45, 13) | 0.98 (0.43-1.42, 13) | 0.96 (0.44-1.77, 13) |
| Glucose, fed, mg/l | 195 (141-229, 25) | 182 (138-217, 25) | 143 (118-177, 12) | 116 (52-161, 13)*# |
| Glucose, fasted, mg/l | 80 (62-142, 30) | 101 (64-135, 30)* | ND | ND |
| Ob(leptin) mRNA, a.u.† | 1.00 (0.71-1.30, 4) | 0.85 (0.26-1.12, 4) | 1.00 (0.53-1.04, 4) | 0.50 (0.24-0.73, 4)* |
WAT is expressed as percentage of body weight. Data are means; values in parentheses are (i) the range of variability expressed either as in the same units as the corresponding parameter (e.g., body weight) or as grams for those measurements in percentage (e.g., WAT), and (ii) the number of animals. ND, nondetermined. *, P < 0.05, vs. corresponding WT; #, P < 0.05, vs. 75-day-old G86R mice.
mRNA levels are normalized relative to the corresponding WT littermates.
Results and Discussion
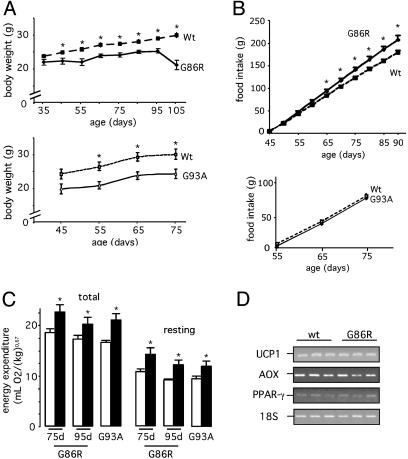
The two strains of transgenic ALS mice used here showed a lower body mass compared with their corresponding WT littermates (Fig. 1A). This paralleled an age-dependent alteration of body composition that resulted in a reduction of adiposity (Table 1). We observed that epididymal and retroperitoneal WAT was markedly reduced or almost nonexistent in the asymptomatic phase of the disease, and that plasma concentrations of leptin, mostly secreted by adipocytes (22), and leptin mRNA levels in WAT were concomitantly diminished (Table 1). These data clearly indicate the existence of a metabolic deficit. We also found that plasma concentrations of insulin decreased (consistent with a reduction in lipid stores) and those of corticosterone increased (indicative of muscle proteolysis) (Table 1). Altogether, these observations show an increased recruitment of body reserves already installed long before the appearance of any motor troubles.
Fig. 1.
Mutant SOD1 mice exhibit an altered metabolic status. (A) Typical curves showing the body mass of G86R (Upper) and G93A (Lower) mice, and their corresponding WT littermates. *, P < 0.05 vs. WT (n = 7). (B) Food intake in the same animals as in A. *, P < 0.05 vs. WT (n = 7). (C) Total (Left) and resting (Right) energy expenditure in G86R mice (75 and 95 days old; filled bars), G93A (75 days old; filled bars), and their corresponding WT littermates (open bars). *, P < 0.05 vs. WT (n = 8). (D) Representative RT-PCR showing uncoupling protein-1 (UCP1), acetylCoA oxidase (AOX), and PPAR-γ mRNA levels in BAT from 75-day-old WT and G86R mice. Identical results were obtained in 105-day-old mice (data not shown).
Several mechanisms may account for this energetic deficiency, including a lowering of food intake or an increase of the metabolic rate (23). Despite having lower body mass, mutant SOD1 mice consumed the same, or even higher, amounts of food as their WT littermates (Fig. 1B), which excludes the possibility that the observed metabolic deficit is caused by a lower energy intake. Alternatively, an increased energy expenditure could also explain the energy deficit. Indeed, we found that G86R and G93A mice showed higher rates of total O2 consumption than their corresponding WT littermates. Energy expenditure was also increased at rest (Fig. 1C), indicating that the augmented O2 consumption is not linked to higher locomotor activity. These observations may account for the reduced ability of transgenic ALS mice to gain weight and are in line with the existence of hypermetabolism as has been described to occur in a population of sporadic ALS patients (20, 21).
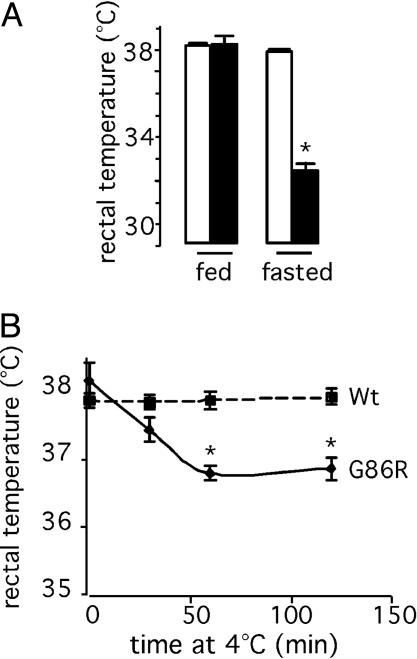
Several stimuli may up-regulate energy expenditure. Thermogenesis is an important adaptative mechanism that consumes energy to produce heat in response to changes in environmental temperature and diet. In rodents, brown adipose tissue (BAT) is a major site of thermogenesis, where oxidation of fatty acids stimulates uncoupling protein-1 (UCP1), a mitochondrial innermembrane protein that uncouples oxidative phosphorylation from ATP synthesis therefore releasing energy in the form of heat (24). To determine whether increased thermogenesis occurs in transgenic ALS mice, we examined the mRNA levels of UCP1, and those of acetylCoA oxidase and peroxisome proliferator-activated receptor-γ (PPAR-γ), two proteins involved in fatty acid metabolism. We found no differences of expression in BAT from WT and G86R mice (Fig. 1D), thus suggesting that the thermogenic machinery appears unaltered at least under normal conditions. It should be noted that these animals were not febrile (Fig. 2A), a condition also known to stimulate energy expenditure. However, G86R mice were unable to maintain their body temperature when they were placed under conditions in which adaptative thermogenesis was challenged. We observed that G86R mice had a lower rectal temperature than WT littermates when fasted for 24 h (Fig. 2 A) or exposed to 4°C (Fig. 2B). Finally, because energy expenditure can be up-regulated by thyroid hormone (25), we measured levels of T3 in plasma and found that they were not increased but rather decreased in G86R mice (Table 1), which seems to exclude any role for thyroid hormone in inducing the observed augmentation in energy expenditure. Altogether, these data demonstrate how deteriorated is energy balance in such fragile animals and provide persuasive evidence that they develop a characteristic hypermetabolism that seems to be specifically associated with the disease process.
Fig. 2.
Adaptative thermogenesis is compromised in G86R mice. Rectal temperature in 105-day-old WT (open bars) and G86R (filled bars) mice fasted for 24 h (A) or exposed to 4°C(B) is shown. *, P < 0.05 vs. WT (n = 8 for fasted; n = 7 for cold-exposed).
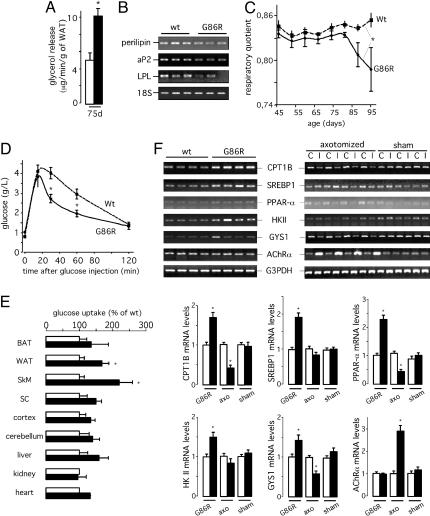
At this point, we sought to determine the way in which lipid metabolism was altered in transgenic ALS mice. Using explants of WAT from WT and G86R animals, we found that norepinephrine-stimulated glycerol release was higher in G86R mice than in WT littermates (Fig. 3A), thus providing in vitro evidence for an increase of activated lipolysis in G86R WAT. Consistent with the loss of adipose tissue in vivo, we found that the gene expression of enzymes involved in fatty acid uptake and storage in epididymal WAT, such as perilipin, fatty acid binding protein (aP2), and lipoprotein lipase, was lower in G86R mice than in WT littermates (Fig. 3B), which is indicative of decreased lipid storage (26). In fact, the respiratory quotient was lower in G86R mice that in WT littermates (Fig. 3C), and the levels of circulating ketone bodies, an indicator of fatty acid oxidation, increased with age in G86R mice (Table 1). In all, this finding means that mutant SOD1 mice fuel more intensively fatty acids than glucose as they become older. It should be noted, however, that the amount of plasma free fatty acids did not increase, as usually expected from stimulated lipolysis (Table 1). A rapid turnover of free fatty acids in G86R mice may explain this finding.
Fig. 3.
G86R mice exhibit increased lipolysis and skeletal muscle hypermetabolism. (A) Norepinephrine-stimulated glycerol release in epididymal WAT explants from 75-day-old WT (open bars) and G86R (filled bars) mice. *, P < 0.05 vs. WT (n = 5). (B) Representative RT-PCR showing perilipin, aP2, and lipoprotein lipase (LPL) mRNA levels in epididymal WAT from 75-day-old WT and G86R mice. (C) Respiratory quotient of WT and G86R mice. Paired t test; *, P < 0.05 vs. WT (n = 8). (D) Glucose tolerance test in overnight-fasted WT and G86R mice at 60–80 days of age after i.p. injection of 2 g/kg glucose at time 0. *, P < 0.05 vs. WT (n = 20). (E) Glucose uptake in tissues from 75-day-old WT (open bars) and G86R (filled bars) mice treated as in D in the presence of radiolabeled 2-deoxyglucose. Tissues included BAT, WAT, spinal cord (SC), hindlimb skeletal muscle (SkM), cortex, cerebellum, liver, kidney, and heart. Data are expressed as percentage relative to the corresponding WT tissue. *, P < 0.05 vs. WT (n = 6). (F)(Upper) Representative RT-PCR showing carnitine palmityl-transferase 1B (CPT1B), serum-response element binding protein 1c (SREBP1), PPAR-α, hexokinase II (HKII), and glycogen synthase 1 (GYS1) mRNA levels in hindlimb skeletal muscle from 75-day-old WT and G86R (Left) and sciatic nerve axotomized (axo) (Right) mice. The expression levels in the skeletal muscle ipsi-(I) and contralateral (C) to the lesion are shown. Sham-operated animals (sham) served as control. The degree of denervation in G86R and axotomized mice was assessed by monitoring the mRNA levels of AChRα. (Lower) The quantitative analysis of the data in WT or contralateral (open bars) and G86R or ipsilateral hindlimb skeletal muscle (filled bars). *, P < 0.05 vs. WT or contralateral (n = 4 for G86R; n = 5 for axo).
We wanted to determine whether the increased use of lipid stores was somehow linked to altered carbohydrate metabolism. We found that glycemia in asymptomatic G86R mice was similar to that in WT littermates (Table 1). Furthermore, using RT-PCR we measured the hepatic gene expression of glucose 6-phosphatase and phosphoenolpyruvate carboxykinase, two enzymes involved in gluconeogenesis, and found that their mRNA levels were also similar between G86R and WT animals (data not shown). According to this finding, we can suggest, at least until the end stage of the disease, the absence of major perturbations in carbohydrate metabolism under normal diet. However, expression of these same enzymes was significantly up-regulated in fasted G86R mice (data not shown), and this finding was consistent with a concomitant increase in fasting glycemia (Table 1). Although an increased gluconeogenesis may explain these results, one can speculate that an associated decrease in glucose uptake could also take place. To explore this possibility, we injected i.p. a glucose load in fasted animals and followed glycemia. Unexpectedly, we found that G86R mice cleared glucose more efficiently than WT littermates (Fig. 3D), which contrasted with their higher fasting glycemia. This finding shows that glucose turnover in fasted animals is augmented and prompted us to determine which were the tissue(s) implicated in glucose clearance. Fasted G86R mice rapidly incorporated the nonmetabolizable glucose analog 2-deoxyglucose in two significant tissues, WAT and, to a greater extent, skeletal muscle (Fig. 3E). Altogether, these data point to skeletal muscle as a major site of avid consumption of any kind of nutrients, either lipids or carbohydrates. Skeletal muscle is thus a strong candidate tissue to support the characteristic hypermetabolic phenotype of transgenic ALS mice.
Although these previous results were obtained under fasting conditions, the muscle expression in fed animals of a set of genes involved in lipid (carnitine palmityl-transferase 1B, PPAR-α, and serum-response element binding protein 1c) and carbohydrate (hexokinase II and glycogen synthase 1) metabolism appeared up-regulated (Fig. 3F), thus suggesting that muscular hypermetabolism is present in transgenic ALS mice irrespective of their nutritional status. Importantly, we observed that sciatic nerve axotomy, a dramatic neurogenic insult known to influence muscle metabolism and gene expression, rather caused the opposite effect on the expression of these same genes (Fig. 3F), which further reinforces the specific hypermetabolic trait of ALS.
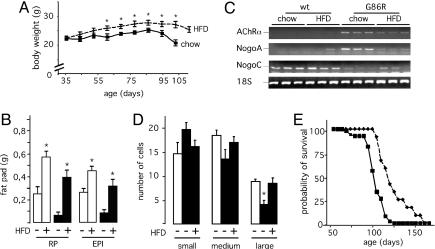
If the hypermetabolism we observe plays an active role in ALS, then compensating the increase in energy expenditure may reverse some of the pathological features of the disease. Administration of a fat-enriched high-energy diet to G86R mice induced, as predicted, an increase in body mass (Fig. 4A) and adipose tissue accumulation (Fig. 4B), thus showing that increasing energy intake is sufficient to reduce the energetic deficit. Moreover, the high-fat regimen partially reversed the expression of certain prototype markers of muscle denervation (Fig. 4C): mRNA levels of nicotinic acetylcholine receptor α subunit (AChRα) and Nogo-A, characteristically elevated in G86R mice (27), were down-regulated, whereas the levels of Nogo-C mRNA, almost nonexistent in G86R mice, were restored. When we looked at the number of cells in the ventral horns of the lumbar spinal cord, we found that the significant loss of large-sized cells, most probably representing motor neurons (28), in symptomatic G86R mice was prevented in HFD-fed animals of the same age (Fig. 4D). Concomitant with this, HFD also extended mean survival by 20% (122.5 ± 4.5 days for HFD-fed G86R mice versus 102.3 ± 1.6 days for normally fed animals; P < 0.05, n = 13) (Fig. 4E), a significant effect when compared to other beneficial treatments (29–32). Although it cannot be excluded that increased fat storage caused by the high-fat regimen may facilitate the activity of the still preserved innervated muscle fibers, our data rather suggest that HFD not only ameliorates the metabolic status of G86R mice but also improves their motor neuronal function.
Fig. 4.
HFD improves the altered metabolic phenotype of G86R mice and delays disease onset. (A) Typical curves showing the body weight of G86R mice fed a regular diet (chow) or HFD. Similar increases in body weight were observed in WT animals fed HFD (data not shown). *, P < 0.05 vs. chow (n = 12 for chow; n = 13 for HFD). (B) Mass of retroperitoneal (RP) and epididymal WAT (EPI) of WT (open bars) and G86R (filled bars) mice fed as in A. Animals were killed at 90 days of age before the onset of any motor symptoms, and white fat pads were dissected and weighed. *, P < 0.05 vs. HFD–(n = 6). (C) Representative RT-PCR showing AChRα, Nogo-A, and Nogo-C mRNA levels in hindlimb skeletal muscle from WT and G86R mice fed as in A and killed at 90 days of age. (D) Cell counts in the ventral horns of the lumbar spinal cord of WT (open bars) and G86R (filled bars) mice fed a regular chow diet (–) or HFD (+). Cells were classified into three area groups of <300 μm2 (small), between 300 and 600 μm2 (medium), and >600 μm2 (large). *, P < 0.05 vs. HFD+ or WT (n = 21); ANOVA followed by Newman–Keuls test. (E) Cumulative probability of survival of G86R mice fed a regular diet (solid line) or HFD (dashed line).
We provide compelling evidence that transgenic ALS mice suffer from a dramatic defect in energy homeostasis, and that this condition is likely to be linked to a hypermetabolism mainly of muscular origin. Because many of the metabolic alterations occur during the asymptomatic phase of the disease, it is conceivable that they may constitute an additional driven force to increase motor neuron vulnerability. Indeed, targeted expression of mutant SOD1 in neurons or astrocytes does not lead to ALS, thus showing that these cell types may not be primarily involved in the disease process (33–35). It is thus more likely that a concerted action of several cellular interplayers is a requisite step to develop the pathology (36). From our data, one can suggest that skeletal muscle is not merely a passive actor irrevocably suffering from motor neuron loss, and that failure in this metabolically important tissue may accelerate the disease.
It seems increasingly clear that the classical concept of neurodegenerative diseases affecting discrete neuronal populations is turning into a more widespread view in which such diseases might be considered as multisystem disorders. Whether the metabolic defects found in mice might also occur in sporadic ALS still needs exhaustive research. It is worth emphasizing, however, that at least a subset of ALS patients show a characteristic hypermetabolic phenotype (20) reminiscent of that observed in our mice. From a clinical point of view, the nutritional status is a prognostic factor for survival in ALS (37), and growing evidence now supports that the appropriate, individualized nutritional management of patients may constitute a primary symptomatic treatment for the disease (38). In this scenario, our present findings may lend clues to further improve our knowledge about the pathogenic mechanisms underlying ALS and to explore new approaches of nutritional intervention.
Supplementary Material
Acknowledgments
We thank MariJo Ruivo and Alexandre Henriques for skilful technical help. J.-P.L.'s laboratory is funded by grants from the Amyotrophic Lateral Sclerosis Association, Association pour la Recherche sur la Sclérose Latérale Amyotrophique, Association pour la Recherche contre les Maladies Neurodégénératives, and Association pour la Recherche contre le Cancer.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ALS, amyotrophic lateral sclerosis; SOD1, Cu/Zn-superoxide dismutase; HFD, high-fat diet; WAT, white adipose tissue; BAT, brown adipose tissue; PPAR, peroxisome proliferator-activated receptor; AChRα, acetylcholine receptor α.
References
- 1.Brooks, B. R., Sanjak, M., Belden, D., Juhasz-Poscine, K. & Waclawik, A. (2000) in Amyotrophic Lateral Sclerosis, eds. Brown, R. H., Jr., Meininger, V. & Swash, M. (Dunitz, London), pp. 31–58.
- 2.Rosen, D. R., Siddique, T., Patterson, D., Figlewicz, D. A., Sapp, P., Hentati, A., Donaldson, D., Goto, J., O'Regan, J. P., Deng, H. X., et al. (1993) Nature 362, 59–62. [DOI] [PubMed] [Google Scholar]
- 3.Gurney, M. E., Pu, H., Chiu, A. Y., Dal Canto, M. C., Polchow, C. Y., Alexander, D. D., Caliendo, J., Hentati, A., Kwon, Y. W., Deng, H. X., et al. (1994) Science 264, 1772–1775. [DOI] [PubMed] [Google Scholar]
- 4.Ripps, M. E., Huntley, G. W., Hof, P. R., Morrison, J. H. & Gordon, J. W. (1995) Proc. Natl. Acad. Sci. USA 92, 689–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong, P. C., Pardo, C. A., Borchelt, D. R., Lee, M. K., Copeland, N. G., Jenkins, N. A., Sisodia, S. S., Cleveland, D. W. & Price, D. L. (1995) Neuron 14, 1105–1116. [DOI] [PubMed] [Google Scholar]
- 6.Cleveland, D. W. & Rothstein, J. D. (2001) Nat. Rev. Neurosci. 2, 806–819. [DOI] [PubMed] [Google Scholar]
- 7.Menzies, F. M., Ince, P. G. & Shaw, P. J. (2002) Neurochem. Int. 40, 543–551. [DOI] [PubMed] [Google Scholar]
- 8.Kong, J. & Xu, Z. (1998) J. Neurosci. 18, 3241–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattiazzi, M., D'Aurelio, M., Gajewski, C. D., Martushova, K., Kiaei, M., Beal, M. F. & Manfredi, G. (2002) J. Biol. Chem. 277, 29626–29633. [DOI] [PubMed] [Google Scholar]
- 10.Borthwick, G. M., Johnson, M. A., Ince, P. G., Shaw, P. J. & Turnbull, D. M. (1999) Ann. Neurol. 46, 787–790. [DOI] [PubMed] [Google Scholar]
- 11.Wiedemann, F. R., Manfredi, G., Mawrin, C., Beal, M. F. & Schon, E. A. (2002) J. Neurochem. 80, 616–625. [DOI] [PubMed] [Google Scholar]
- 12.Nakano, K., Hirayama, K. & Terao, K. (1987) Arch. Neurol. 44, 103–106. [DOI] [PubMed] [Google Scholar]
- 13.Curti, D., Malaspina, A., Facchetti, G., Camana, C., Mazzini, L., Tosca, P., Zerbi, F. & Ceroni, M. (1996) Neurology 47, 1060–1064. [DOI] [PubMed] [Google Scholar]
- 14.Leclerc, N., Ribera, F., Zoll, J., Warter, J. M., Poindron, P., Lampert, E. & Borg, J. (2001) Neuromuscul. Disord. 11, 722–727. [DOI] [PubMed] [Google Scholar]
- 15.Wiedemann, F. R., Winkler, K., Kuznetsov, A. V., Bartels, C., Vielhaber, S., Feistner, H. & Kunz, W. S. (1998) J. Neurol. Sci. 156, 65–72. [DOI] [PubMed] [Google Scholar]
- 16.Vielhaber, S., Kunz, D., Winkler, K., Wiedemann, F. R., Kirches, E., Feistner, H., Heinze, H. J., Elger, C. E., Schubert, W. & Kunz, W.S. (2000) Brain 123, 1339–1348. [DOI] [PubMed] [Google Scholar]
- 17.Dupuis, L., di Scala, F., Rene, F., de Tapia, M., Oudart, H., Pradat, P. F., Meininger, V. & Loeffler, J. P. (2003) FASEB J. 17, 2091–2093. [DOI] [PubMed] [Google Scholar]
- 18.Klivenyi, P., Ferrante, R. J., Matthews, R. T., Bogdanov, M. B., Klein, A. M., Andreassen, O. A., Mueller, G., Wermer, M., Kaddurah-Daouk, R. & Beal, M. F. (1999) Nat. Med. 5, 347–350. [DOI] [PubMed] [Google Scholar]
- 19.Groeneveld, G. J., Veldink, J. H., van der Tweel, I., Kalmijn, S., Beijer, C., de Visser, M., Wokke, J. H. J., Franssen, H. & van den Berg, L. H. (2003) Ann. Neurol. 53, 437–445. [DOI] [PubMed] [Google Scholar]
- 20.Desport, J. C., Preux, P. M., Magy, L., Boirie, Y., Vallat, J. M., Beaufrere, B. & Couratier, P. (2001) Am. J. Clin. Nutr. 74, 328–334. [DOI] [PubMed] [Google Scholar]
- 21.Kasarskis, E. J., Berryman, S., Vanderleest, J. G., Schneider, A. R. & McClain, C. J. (1996) Am. J. Clin. Nutr. 63, 130–137. [DOI] [PubMed] [Google Scholar]
- 22.Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L. & Friedman, J. M. (1994) Nature 372, 425–432. [DOI] [PubMed] [Google Scholar]
- 23.Spiegelman, B. M. & Flier, J. S. (2001) Cell 104, 531–543. [DOI] [PubMed] [Google Scholar]
- 24.Lowell, B. B. & Spiegelman, B. M. (2000) Nature 404, 652–660. [DOI] [PubMed] [Google Scholar]
- 25.Al-Adsani, H., Hoffer, L. J. & Silva, J. E. (1997) J. Clin. Endocrinol. Metab. 82, 1118–1125. [DOI] [PubMed] [Google Scholar]
- 26.Picard, F., Gehin, M., Annicotte, J., Rocchi, S., Champy, M. F., O'Malley, B. W., Chambon, P. & Auwerx, J. (2002) Cell 111, 931–941. [DOI] [PubMed] [Google Scholar]
- 27.Dupuis, L., Gonzalez de Aguilar, J. L., di Scala, F., Rene, F., de Tapia, M., Pradat, P. F., Lacomblez, L., Seihlan, D., Prinjha, R., Walsh, F. S., et al. (2002) Neurobiol. Dis. 10, 358–365. [DOI] [PubMed] [Google Scholar]
- 28.Morrison, B. M., Janssen, W. G., Gordon, J. W. & Morrison, J. H. (1998) J. Comp. Neurol. 391, 64–77. [DOI] [PubMed] [Google Scholar]
- 29.Gurney, M. E., Cutting, F. B., Zhai, P., Doble, A., Taylor, C. P., Andrus, P. K. & Hall, E. D. (1996) Ann. Neurol. 39, 147–157. [DOI] [PubMed] [Google Scholar]
- 30.Dugan, L. L., Turetsky, D. M., Du, C., Lobner, D., Wheeler, M., Almli, C. R., Shen, C. K., Luh, T. Y., Choi, D. W. & Lin, T. S. (1997) Proc. Natl. Acad. Sci. USA 94, 9434–9439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hottinger, A. F., Fine, E. G., Gurney, M. E., Zurn, A. D. & Aebischer, P. (1997) Eur. J. Neurosci. 9, 1548–1551. [DOI] [PubMed] [Google Scholar]
- 32.Zhu, S., Stavrovskaya, I. G., Drozda, M., Kim, B. Y., Ona, V., Li, M., Sarang, S., Liu, A. S., Hartley, D. M., Wu-Du, C., et al. (2002) Nature 417, 74–78. [DOI] [PubMed] [Google Scholar]
- 33.Pramatarova, A., Laganiere, J., Roussel, J., Brisebois, K. & Rouleau, G. A. (2001) J. Neurosci. 21, 3369–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lino, M. M., Schneider, C. & Caroni, P. (2002) J. Neurosci. 22, 4825–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong, Y. H., Parsadanian, A. S., Andreeva, A., Snider, W. D. & Elliott, J. L. (2000) J. Neurosci. 20, 660–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clement, A. M., Nguyen, M. D., Roberts, E. A., Garcia, M. L., Boillee, S., Rule, M., McMahon, A. P., Doucette, W., Siwek, D., Ferrante, R. J., et al. (2003) Science 302, 113–117. [DOI] [PubMed] [Google Scholar]
- 37.Desport, J. C., Preux, P. M., Truong, C. T., Vallat, J. M., Sautereau, D. & Couratier, P. (1999) Neurology 53, 1059–1063. [DOI] [PubMed] [Google Scholar]
- 38.Cameron, A. & Rosenfeld, J. (2002) Curr. Opin. Clin. Nutr. Metab. Care 5, 631–643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.