Abstract
Acetaminophen is a widely used antipyretic analgesic, reducing fever caused by bacterial and viral infections and by clinical trauma such as cancer or stroke. In rare cases in humans, e.g., in febrile children or HIV or stroke patients, acetaminophen causes hypothermia while therapeutic blood levels of the drug are maintained. In C57/BL6 mice, acetaminophen caused hypothermia that was dose related and maximum (>2°C below normal) with a dose of 300 mg/kg. The reduction and recovery of body temperature was paralleled by a fall of >90% and a subsequent rise of prostaglandin (PG)E2 concentrations in the brain. In cyclooxygenase (COX)-2–/– mice, acetaminophen (300 mg/kg) produced hypothermia accompanied by a reduction in brain PGE2 levels, whereas in COX-1–/– mice, the hypothermia to this dose of acetaminophen was attenuated. The brains of COX-1–/– mice had ≈70% lower levels of PGE2 than those of WT animals, and these levels were not reduced further by acetaminophen. The putative selective COX-3 inhibitors antipyrine and aminopyrine also reduced basal body temperature and brain PGE2 levels in normal mice. We propose that acetaminophen is a selective inhibitor of a COX-1 variant and this enzyme is involved in the continual synthesis of PGE2 that maintains a normal body temperature. Thus, acetaminophen reduces basal body temperature below normal in mice most likely by inhibiting COX-3.
Acetaminophen is a well established antipyretic analgesic, frequently administered therapeutically to alleviate pain and fever. It is often classified as a nonsteroid antiinflammatory drug (NSAID), but differs from other NSAIDs because it does not reduce inflammation or cause ulceration and bleeding of the stomach mucosa (1). In addition, unlike the aspirin-like drugs, acetaminophen does not inhibit platelet aggregation or increase bleeding time (1). Many of the symptoms of inflammation are caused by prostaglandins (PGs) synthesized by the inducible cyclooxygenase (COX)-2, whereas the PGs produced by the constitutive enzyme, COX-1, protect the stomach mucosal lining and initiate platelet aggregation when required (2). In in vitro assays on whole cells or cell homogenates, acetaminophen was shown to be a weak inhibitor of inducible COX-2, thus explaining its lack of antiinflammatory effects, and a weak inhibitor of constitutive COX-1, which explains the absence of gastrotoxicity and antiaggregatory action on platelets (3).
For many years the mechanism of action of acetaminophen in pain and fever remained a mystery. It reduced the excretion of PG metabolites when administered to humans in vivo (4), but inhibited only weakly PG synthesis by either COX-1 or COX-2 in vitro (5). Inhibition of PG synthesis by acetaminophen varied in different tissues so that activity of brain COX was reduced to a greater degree than activity of COX in the stomach mucosa (6). Flower and Vane (7) also showed that PG-synthesizing activity of dog brain was inhibited more than that of rabbit spleen. This finding led to the suggestion that COX-1 or COX-2 inhibition with acetaminophen only occurred in cells and tissues that had low concentrations of peroxides such as brain cells and that acetaminophen had no inhibitory activity on COX-1 or COX-2 in the presence of high peroxide levels such as exist in inflamed tissues (8, 9). An alternative explanation of the potent analgesic and antipyretic activity of acetaminophen is that it prevents PG synthesis by inhibiting another form of COX, different from COX-1 or COX-2 (10).
In 2002, Simmons and colleagues (11) cloned, characterized, and expressed a variant of COX-1 from dog and human brain, which they named COX-3. This enzyme, expressed in insect cells, was sensitive to inhibition with low concentrations of acetaminophen and other related antipyretic analgesic drugs such as antipyrine and aminopyrine. However, significantly increased concentrations of acetaminophen were needed to inhibit COX-1 and COX-2 under the same experimental conditions.
The mechanisms of fever have been extensively studied. In particular, fever induced with bacterial lipopolysaccharide (LPS) has been widely investigated in several species (12–14). Romanovsky et al. (12) found LPS-induced fever in rats to be triphasic and the increase in temperature to depend on the dose of LPS administered. It has been proposed that LPS, or other inflammatory mediators such as IL-1 circulating in the blood, stimulate the expression of COX-2 in endothelial cells of brain blood vessels (15, 16) and PGE2 synthesized by COX-2, then diffuses into the preoptic area of the anterior hypothalamus, which has no blood–brain barrier, acts on neurons in the organum vasculosum laminae terminalis and produces fever (13). This view is supported by experiments in COX-2–/– mice in which neither LPS nor IL-1 produce fever (17, 18). Also, selective COX-2 inhibitors reduced LPS-induced fever in squirrel monkeys (19) and human volunteers (20), indicating that it was associated with stimulation of COX-2.
However, this proposal does not explain the antipyretic action of acetaminophen, which has low inhibitory activity for COX-2, but easily penetrates into the CNS (21). Thus, acetaminophen has clear access to COX-3 in the brain, inhibition of which may also reduce PGE2 synthesis and abolish fever. A recent study (22) found expression of COX-3 mRNA in primary cultures of astrocytes, endothelial cells, pericytes, and choroidal epithelial cells of rat brain. Cerebral endothelial cells exhibited the highest COX-3 expression that was constitutive and not stimulated by LPS.
Acetaminophen is widely used to treat childhood fevers (23), as well as fever in cancer (24) and elderly patients (23). However, some clinical cases of hypothermia after therapeutic doses of acetaminophen have also been described (25–27). Hypothermia has also been reported when high doses of acetaminophen were administered orally to Swiss white mice (28) and after aminopyrine administration to rats (29).
The C57/BL6 strain of mice is sensitive to the hypothermic action of acetaminophen. Here, we have investigated the hypothermic effect of increasing doses of acetaminophen and its effect on brain PGE2 levels in C57/BL6 mice. We have also studied the effects of acetaminophen on body temperature in COX-1 and COX-2 knockout (KO) mice and in mice treated with antipyrine and aminopyrine.
Materials and Methods
Animals. Male C57/BL6 mice (20 ± 2 g) supplied from Harlan-Olac were maintained under a 12-h/12-h light/dark cycle at 22 ± 1°C. Food and water were provided ad libitum. Prostaglandin endoperoxide synthase (Ptgs1–/–) and Ptgs2–/– mice of the same strain as reported by Langenbach et al. (30) and Morham et al. (31) were used. Experimental procedures were conducted in accordance with the United Kingdom Home Office Guidelines. Some experiments were conducted with Ptgs1–/– and Ptgs2–/– mice as described by Ballou et al. (32).
Temperature Measurement. Microchip implants. To measure core temperature of freely moving mice, temperature-sensitive transponders (Plexx B.V. AB Elst, The Netherlands) were implanted s.c. under light anesthesia. Temperature measurements were made with a temperature-sensitive scanner held ≈3 cm above the back of the animal.
Rectal probe. A rectal probe for mice and a thermocouple monitoring thermometer were obtained from Harvard Apparatus. The probe was inserted into the rectum to a depth of 2 cm. All experiments began with the i.p. administration of acetaminophen at 10 a.m., and temperature measurements were made every 30 min or 1 h thereafter.
PGE2 Extraction and Measurement. Extraction of PGE2 from brain tissues. This procedure was carried out as a modification of the protocol described by Powell (33). Brain tissues were frozen in liquid nitrogen and pulverized with a nitrogen bomb (Biospec Products, Bartlesville, OK). One milliliter of 15% (vol/vol) ethanol in distilled water (pH 3) was added to each tissue sample. The tissue homogenates were left at 4°C for 10 min and then spun at 375 g for 10 min at 4°C. The columns (C-18 Sep-Pak cartridges) were conditioned with 4 ml of ethanol followed by 4 ml of distilled water at a flow rate of 5–10 ml/min. The supernatant from homogenates was then applied to the columns at a flow rate of 5 ml/min. The columns were then washed in 4 ml of distilled water followed by 4 ml of 15% (vol/vol) ethanol in distilled water. The samples were then eluted with 2 ml of ethyl acetate at a flow rate of 5 ml/min. The samples were freeze-dried overnight and then stored at –80°C, ready for PGE2 measurement.
PGE2 enzyme immunoassay kit. Measurements of brain PGE2 levels were carried out by using a commercial enzyme immunoassay (EIA) kit from Amersham Pharmacia. The kit was used according to the manufacturer's protocol. Extracted PGE2 was incubated on a goat anti-mouse IgG-coated plate along with anti-PGE2 antibody and horseradish peroxidase-labeled PGE2. The blue color developed with 3,3′,5,5′tetramethylbenzidine substrate was read colometrically at 630 nm. The concentration of PGE2 in the samples was determined by comparing the optical density of PGE2 in the samples with a standard PGE2 curve (0.05–6.4 ng/ml).
RT-PCR. Initially, total RNA was extracted from pulverized brain tissues by using guanidine isothiocyanate (Trizol reagent) according to the manufacturer's instructions (Invitrogen). An Oligotex midi kit (Qiagen, Valencia, CA) was then used according to the manufacturer's instructions to extract poly(A)+ mRNA from 0.02 mg total RNA.
Single-stranded complementary DNA (cDNA) was synthesized by RT-PCRs on the extracted mRNA using random primers and avian myleoblastosis virus reverse transcriptase (AMV-RT) from Promega. Reverse transcription was carried out at 42°C for 1 h followed by denaturation of AMV-RT at 99°C for 5 min. Single-stranded cDNA was diluted down in 80 μl of RNase-free water.
Single-stranded complementary DNA for COX-1, COX-2, and COX-3 was amplified by PCR using pure Taq Ready-To-Go PCR beads (Amersham Pharmacia). When the total reaction volume was brought to 25 μl each reaction contained 0.2 μM sense and antisense-specific oligonucleotides. COX-1 oligonucleotides, sense 5′-AGG AGA TGG CTG CTG AGT TGG-3′ and antisense 5′-AAT CTG ACT TTC TGA GTT GCC-3′, amplified a product size of 602 bp; COX-2 oligonucleotides, sense 5′-ACA CAC TCT ATC ACT GGC ACC-3′ and antisense 5′-TTC AGG GAG AAG CGT TTG C-3′, amplified a product size of 274 bp; and those for COX-3, sense primer in intron-1, 5′-ATG AGT CGT GAG TCC GAC CCC AGT-3′ and antisense 5′TGT CGA GGC CAA AGC GGA-3′, amplified a product size of 290 bp. The quality of RNA samples was evaluated by RT-PCR using GAPDH-specific oligonucleotides, sense 5′-AAG GTG AAG GTC GGA GTC AAC G-3′ and antisense 5′-GGC AGA GAT GAT GAC CCT TTT GGC-3′ (363 bp). After denaturation at 94°C for 3 min, the following cycling conditions were performed: for COX-1, 32 cycles (94°C, 15 sec; 60°C, 15 sec; 65°C, 1 min) with final extension at 72°C for 7 min; for COX-2, 40 cycles (94°C, 15 sec; 55°C, 15 sec; 72°C, 1 min) with final extension at 72°C for 7 min; for COX-3, 35 cycles (95°C, 30 sec; 56°C, 1 min; 72°C, 1 min); and for GAPDH, 30 cycles (94°C, 30 sec; 60°C, 30 sec; 72°C, 1 min) with final extension at 72°C for 10 min. The amplified cDNA products were visualized on 2% (wt/vol) agarose gels.
Materials. All chemicals and biochemicals used were of the highest purity. Acetaminophen (N-acetyl-p-aminophenol, Sigma) was dissolved in 12.5% (vol/vol) 1,2-propanediol. Antipyrine (Sigma) and aminopyrine (Sigma) were dissolved in 0.9% saline. PGE2 enzyme immunoassay kits were obtained from Amersham Pharmacia. An Oligotex mRNA Midi kit (Qiagen) was also used.
Results
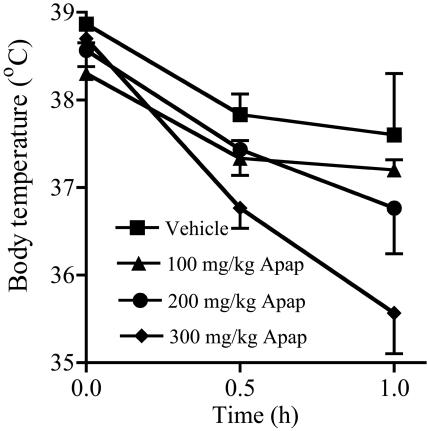
The Effect of Acetaminophen on Basal Body Temperature and Brain PGE2 Levels. Acetaminophen (100–300 mg/kg) administered i.p. to C57/Bl6 mice produced a dose-dependent hypothermic effect measured as reduction in the rectal temperature (Fig. 1). Compared with the vehicle control group, acetaminophen after 1 h produced a 0.4°C, 0.8°C, and 2°C fall in basal body temperature at doses of 100, 200, and 300 mg/kg, respectively. The mean initial body temperature in the vehicle group was 37.6 ± 0.7°C.
Fig. 1.
The effect of increasing doses of acetaminophen (Apap) on body temperature in male C57/BL6 mice. A dose-related fall in temperature was obtained with 100–300 mg/kg acetaminophen with a maximum effect at 300 mg/kg; n = 3.
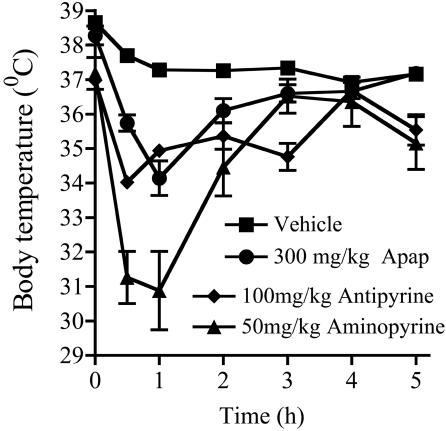
The hypothermic effect of 300 mg/kg acetaminophen (Fig. 2) was maximum 1 h after drug administration with the temperature returning to normal after 4–5 h. These data correlate with the plasma concentration levels for acetaminophen in mice (34), demonstrating that hypothermia is directly related to the blood level of acetaminophen. A similar time course was shown for the hypothermic effects of 100 mg/kg antipyrine and 50 mg/kg aminopyrine.
Fig. 2.
Time course of the hypothermic effect of 300 mg/kg acetaminophen (Apap) i.p. in male C57/BL6 mice compared with 100 mg/kg antipyrine, 50 mg/kg aminopyrine, and vehicle control; n = 3–5.
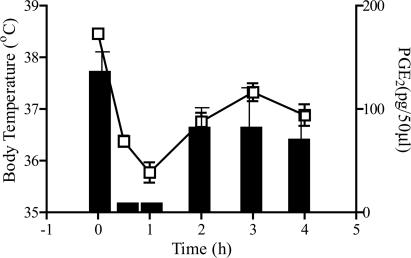
There was clear correlation between reduction in body temperature and fall in brain PGE2 levels over 5 h after administration of 300 mg/kg acetaminophen. Thus, the hypothermic effect of 300 mg/kg acetaminophen appeared to be caused by reduction of PGE2 levels in brain tissues (Fig. 3). The greatest fall in both body temperature and brain PGE2 levels occurred after 1 h, when the drop in temperature was 2.7°C and the decrease in PGE2 was 96%. Brain PGE2 levels were also reduced by ≈60% 30 min after treatment with antipyrine or aminopyrine (data not shown).
Fig. 3.
The effect of 300 mg/kg acetaminophen i.p. on basal body temperature and brain PGE2 levels in male C57/BL6 mice. Brains were removed for PGE2 determinations at intervals for 4 h after administration of acetaminophen. Body temperature and brain PGE2 were reduced in parallel and recovered together. □, Body temperature; histograms represent brain PGE2; n = 6.
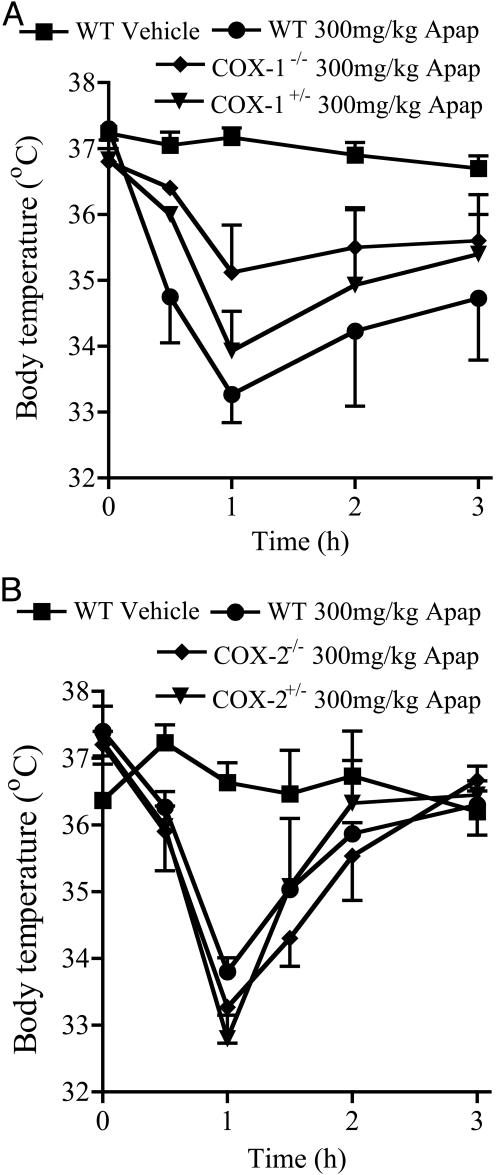
The Effect of Acetaminophen on the Basal Body Temperature and Brain PGE2 Levels in COX-1 and COX-2 KO Mice. In COX-1 KO mice, the hypothermic effect of 300 mg/kg acetaminophen was reduced in parallel with the loss of Ptgs1 (Fig. 4A). No difference was observed in the hypothermic action of a similar dose of acetaminophen in COX-2+/– or COX-2–/– mice (Fig. 4B). This finding suggests that the hypothermic action of acetaminophen is caused by inhibition of a Ptgs1 product. However, the hypothermic effect of acetaminophen was not completely abolished in COX-1–/– animals (–2.05°C in COX-1–/– compared with –3.9°C in WT controls). This finding suggests that another mechanism may also operate in acetaminophen hypothermia, perhaps unrelated to COX inhibition.
Fig. 4.
The hypothermic response to 300 mg/kg acetaminophen (Apap) i.p. in COX-1 KO mice and COX-2 KO mice. Deletion of Ptgs1 reduced (A), whereas deletion of Ptgs2 had no effect on (B) the hypothermic action of acetaminophen; n = 3–6.
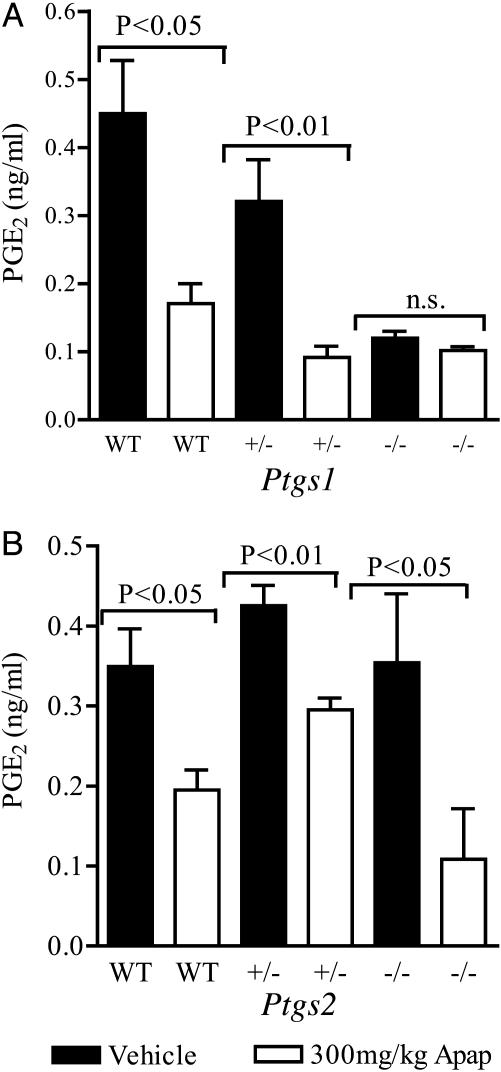
Determination of the brain levels of PGE2 1 h after the administration of 300 mg/kg acetaminophen to COX-1 and COX-2 KO mice provided further evidence that acetaminophen produces hypothermia by inhibition of a Ptgs1-derived protein. The reduction of PGE2 in brains of COX-1–/– mice was completely abolished (Fig. 5A). However, there was no difference in the reduction of PGE2 levels by acetaminophen in COX-2+/– or COX-2–/– mice compared with WT litter mates (Fig. 5B). In addition, the resting levels of PGE2 in COX-1–/– animals were ≈30% of those in WT litter mates, suggesting that most of the brain PGE2 is produced by products of Ptgs1.
Fig. 5.
The effect of 300 mg/kg acetaminophen (Apap) on brain PGE2 levels in COX-1 KO and COX-2 KO mice. Brains of COX-1 KO mice (A) and COX-2 KO mice (B) were removed and frozen 1 h after administration of acetaminophen to male or female animals. Acetaminophen reduced PGE2 in WT and KO mice except Ptgs1–/–. Control PGE2 levels in Ptgs1–/– animals were ≈30% of those in WT; n = 4–7; n.s., not significant.
Both male and female COX-1 and COX-2 KO animals were used in these experiments. However, no difference was apparent between the two sexes in terms of hypothermia or brain PGE2 levels.
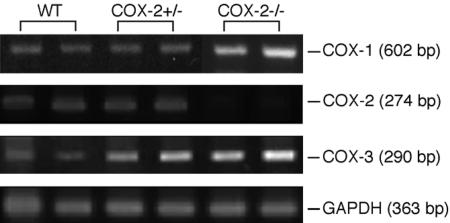
Expression of COX-1, COX-2, and COX-3 mRNA with RT-PCR. Analysis of the expression of COX-1, COX-2, and COX-3 mRNA in whole brain tissues of COX-1 and COX-2 KO mice, using RT-PCR, was used to demonstrate the presence of these enzymes in WT and KO animals.
The expression of COX-1 mRNA was unchanged in all genotypes of COX-2 animals (Fig. 6). The loss in expression of COX-2 mRNA in brain tissues of COX-2–/– mice was paralleled by an apparent increase in COX-3 mRNA (Fig. 6). However, quantitative PCR would be needed to validate this observation.
Fig. 6.
Expression of COX-1, COX-2, and COX-3 mRNA in brain tissues of COX-2 KO mice.
Discussion
The recent discovery by the group of Daniel Simmons (11) of the COX-1 variant protein, COX-3, which can be inhibited by acetaminophen, in canine brain tissues, is thought to provide the long-sought target for the analgesic and antipyretic actions of acetaminophen (7). Similarly to COX-1 and COX-2, COX-3 was shown to possess COX and peroxidase active sites and to have the same cellular localization. COX-3 was also shown to be glycosylated and have the capacity to metabolize arachidonic acid, leading to the formation of PGE2. The major difference between COX-3 and COX-1 is that at the mRNA level COX-3 retains intron-1, which encodes a 30-aa sequence inserted into the N-terminal hydrophobic signal peptide of the enzyme. Although in canine COX-3 mRNA intron-1 is within frame, in the mouse there is a frameshift in intron-1 of the COX-3 transcript.
The aim of the present study was to investigate the mechanism of the hypothermic action produced by acetaminophen in mice. The hypothermia was dose dependent and reached a maximum in 1 h, and the body temperature returned to normal levels within 5 h. Furthermore, the action of acetaminophen was closely linked to a reduction of the biosynthesis of brain PGE2, which is indicative of inhibition of COX activity. Drug doses in mice are frequently higher than in humans to produce the same effect, and high doses of acetaminophen were used in this study. There is even a wide variation in sensitivity to the same drug between different strains of mice (35).
Our studies provide evidence that the COX enzyme inhibited by acetaminophen to produce hypothermia is likely to be COX-3. In COX-1 KO mice, both the hypothermia and reduction of brain PGE2 by acetaminophen were greatly reduced, whereas in COX-2 KO mice the hypothermic action of acetaminophen was unaltered. In addition, the selective COX-3 inhibitors, antipyrine and aminopyrine, produced hypothermia (11). Like acetaminophen, these antipyretic analgesic drugs are gastric-sparing and lack antithrombotic actions (1).
The role of PGE2 in central temperature regulation has been extensively studied (36, 37), and PGE2 has been described as the ultimate mediator to generate hyperthemia during fever (38). During pyresis, an inducible COX-2 protein that forms PGE2 (36) is produced in hypothalamic endothelial cells (39) in response to systemically administered pyrogens such as LPS or IL-1 (18, 40). However, LPS-induced fever is triphasic (12) and the first phase is likely to be mediated by pyrogenic PGE2 synthesized by a constitutive COX, such as COX-3. Thus, inhibition of COX-3 would explain the potent antipyretic action of acetaminophen in fever.
The hypothermic effect of acetaminophen, which we have recorded in mice, has occasionally been observed in humans (25–27). The hypothermia observed in human studies is of short duration and does not present serious problems. Thus, it is an acute effect that disappears ≈3 h after drug administration, which coincides with the plasma half-life of acetaminophen (41). Some sources believe that this hypothermia could have adverse consequences, especially when it occurs in children under the age of 5 years (27), whereas others consider it to be beneficial (25).
In the clinical trial conducted by Dippel et al. (25), acetaminophen given to patients with acute ischemic stroke produced 0.3°C reduction in body temperature when compared with placebo. This effect was predicted to be beneficial as increased temperatures in stroke patients have been linked to the pathogenesis of stroke (42–44). Thus, if further evidence is obtained in larger trials, acetaminophen hypothermia is likely to be of clinical benefit to stroke patients.
We propose that the target for the hypothermic action of acetaminophen is COX-3 and that constitutive COX-3, most likely expressed in endothelial cells in brain tissue, functions under physiological conditions to maintain a constant body temperature in mice. Although it has been suggested that PGE2 in the brain has no role in the control of physiological temperature in cats (45), rabbits (46), or humans (47), we report that in the mouse PGE2 from COX-3 may regulate body temperature. The thermoneutral zone for mice has been determined as 31–34°C (48, 49), which is higher than for other mammalian species except rats (50). Because our studies in mice were conducted at 22°C, a constant release of PGE2 in this species may function to maintain thermostatic brain mechanisms.
Acknowledgments
We thank Dr. Robert Hannon for his expert consultation concerning RT-PCR techniques used in this study, Dr. Wenkei Dou for expert assistance in dissecting mouse brains for analysis, and Dr. Daniel Simmons for valuable consultation. The financial support for this project is a Ph.D. Case studentship from Glaxo SmithKline (to S.S.A.) and the Biotechnological and Biological Sciences Research Council.
Abbreviations: COX, cyclooxygenase; PG, prostaglandin; LPS, lipopolysaccharide; Ptgs, prostaglandin endoperoxide synthase; KO, knockout.
References
- 1.Roberts, L. J., II & Morrow, J. D. (2001) in The Pharmacological Basis of Therapeutics, eds. Hardman, J. G., Limbird, L. E. & Gilman, A. G. (McGraw–Hill, New York), pp. 687–731.
- 2.Vane, J. R., Bakhle, Y. S. & Botting, R. M. (1998) Annu. Rev. Pharmacol. Toxicol. 38, 97–120. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell, J. A., Akarasereenont, P., Thiemermann, C., Flower, R. J. & Vane, J. R. (1993) Proc. Natl. Acad. Sci. USA 90, 11693–11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grèen, K., Drvota, V. & Vesterqvist, O. (1989) Prostaglandins 37, 311–315. [DOI] [PubMed] [Google Scholar]
- 5.Warner, T. D., Giuliano, F., Vojnovic, I., Bukasa, A., Mitchell, J. A. & Vane, J. R. (1999) Proc. Natl. Acad. Sci. USA 96, 7563–7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swierkosz, T. A., Jordan, L., McBride, M., McGough, K., Devlin, J. & Botting, R.M. (2002) Med. Sci. Monit. 8, BR496–BR503. [PubMed] [Google Scholar]
- 7.Flower, R. J. & Vane, J. R. (1972) Nature 240, 410–411. [DOI] [PubMed] [Google Scholar]
- 8.Ouellet, M. & Percival, M. D. (2001) Arch. Biochem. Biophys. 387, 273–280. [DOI] [PubMed] [Google Scholar]
- 9.Boutaud, O., Aronoff, D. M., Richardson, J. H., Marnett, L. J. & Oates, J. A. (2002) Proc. Natl. Acad. Sci. USA 99, 7130–7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botting, R. M. (2000) Clin. Infect. Dis. 31, Suppl. 5, S202–S210. [DOI] [PubMed] [Google Scholar]
- 11.Chandrasekharan, N. V., Hu Dai, K., Roos, L. T., Evanson, N. K., Tomsik, J., Elton, T. S. & Simmons, D. L. (2002) Proc. Natl. Acad. Sci. USA 99, 13926–13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romanovsky, A. A., Simons, C. T. & Kulchitsky, V. A. (1998) Am. J. Physiol. 275, R323–R331. [DOI] [PubMed] [Google Scholar]
- 13.Sehic, E., Székely, M., Ungar, A. L., Oladehin, A. & Blatteis, C. M. (1996) Brain Res. Bull. 39, 391–399. [DOI] [PubMed] [Google Scholar]
- 14.Kozak, W., Conn, C. A. & Kluger, M. J. (1994) Am. J. Physiol. 266, R125–R135. [DOI] [PubMed] [Google Scholar]
- 15.Matsumura, K., Cao, C., Ozaki, M., Morii, H., Nakadate, K. & Watanabe, Y. (1998) J. Neurosci. 18, 6279–6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ek, M., Engblom, D., Saha, S., Blomqvist, A., Jakobsson, P.-J. & Ericsson-Dahlstrand, A. (2001) Nature 410, 430–431. [DOI] [PubMed] [Google Scholar]
- 17.Li, S., Wang, Y., Matsumura, K., Ballou, L. R., Morham, S. G. & Blatteis, C. M. (1999) Brain Res. 825, 86–94. [DOI] [PubMed] [Google Scholar]
- 18.Li, S., Ballou, L. R., Morham, S. G. & Blatteis, C. M. (2001) Brain Res. 910, 163–173. [DOI] [PubMed] [Google Scholar]
- 19.Chan, C.-C., Panneton, M., Taylor, A. M., Therien, M. & Rodger, I. W. (1997) Eur. J. Pharmacol. 327, 221–225. [DOI] [PubMed] [Google Scholar]
- 20.McAdam, B. F., Mardini, I. A., Habib, A., Burke, A., Lawson, J. A., Kapoor, S. & FitzGerald, G. A. (2000) J. Clin. Invest. 105, 1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Courade, J.-P., Besse, D., Delchambre, C., Hanoun, N., Hamon, M., Eschalier, A., Caussade, F. & Cloarec, A. (2001) Life Sci. 69, 1455–1464. [DOI] [PubMed] [Google Scholar]
- 22.Kis, B., Snipes, J. A., Isse, T., Nagy, K. & Busija, D. W. (2003) J. Cereb. Blood Flow Metab. 23, 1287–1292. [DOI] [PubMed] [Google Scholar]
- 23.Prescott, L. F. (2000) Am. J. Ther. 7, 143–147. [PubMed] [Google Scholar]
- 24.Oborilova, A., Mayer, J., Pospisil, Z., Koristek, J., Vorlicek, J. & Havranova, D. (1999) Klin. Onkol. 12, 200–205. [Google Scholar]
- 25.Dippel, D. W. J., van Breda, E. J., van der Worp, H. B., van Gemert, H. M. A., Meijer, R. J., Kappelle, L. J. & Koudstaal, P. J. (2003) BMC Cardiovasc. Disorders 3, 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denes, E., Amaniou, M., Rogez, J.-P., Weinbreck, P. & Merle, L. (2002) Ann. Med. Intern. 153, 411–413. [PubMed] [Google Scholar]
- 27.Tittelboom, T. V. & Govaerts-Lepicard, M. (1988) Vet. Hum. Toxicol. 31, 57–59. [PubMed] [Google Scholar]
- 28.Walker, R. M., Massey, T. E., McElligott, T. F. & Racz, W. J. (1981) Toxicol. Appl. Pharmacol. 59, 500–507. [DOI] [PubMed] [Google Scholar]
- 29.Polk, D. L. & Lipton, J. M. (1975) Pharmacol. Biochem. Behav. 3, 167–172. [DOI] [PubMed] [Google Scholar]
- 30.Langenbach, R., Morham, S. G., Tiano, H. F., Loftin, C. D., Ghanayem, B. I., Chulada, P. C., Mahler, J. F., Lee, C. A., Goulding, E. H., Kluckman, K. D., et al. (1995) Cell 83, 483–492. [DOI] [PubMed] [Google Scholar]
- 31.Morham, S. G., Langenbach, R., Loftin, C. D., Tiano, H. F., Vouloumanos, N., Jenette, J. C., Mahler, J. F., Kluckman, K. D., Ledford, A., Lee, C. A. & Smithies, O. (1995) Cell 83, 473–482. [DOI] [PubMed] [Google Scholar]
- 32.Ballou, L. R., Botting, R. M., Goorha, S., Zhang, J. & Vane, J. R. (2000) Proc. Natl. Acad. Sci. USA 97, 10272–10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powell, W. S. (1980) Prostaglandins 20, 947–957. [DOI] [PubMed] [Google Scholar]
- 34.Fischer, L. J., Green, M. D. & Harman, A. W. (1981) J. Pharmacol. Exp. Ther. 219, 281–286. [PubMed] [Google Scholar]
- 35.Wilson, S. G., Bryant, C. D., Lariviere, W. R., Olsen, M. S., Giles, B. E., Chesler, E. J. & Mogil, J. S. (2003) J. Pharmacol. Exp. Ther. 305, 755–764. [DOI] [PubMed] [Google Scholar]
- 36.Feldberg, W., Gupta, K. P., Milton, A. S. & Wendlandt, S. (1972) Br. J. Pharmacol. 46, 550P–551P. [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumura, K., Watanabe, Y., Onoe, H., Watanabe, Y. & Hayaishi, O. (1990) Brain Res. 533, 147–151. [DOI] [PubMed] [Google Scholar]
- 38.Coceani, F. (1991) in Fever: Basic Mechanisms and Management, ed. Mackowiak, P. (Raven, New York), pp. 59–70.
- 39.Yamagata, K., Matsumura, K., Inoue, W., Takuma, S., Suzuki, K., Yasuda, S., Sugiura, H., Cao, C., Watanabe, Y. & Kobayashi, S. (2001) J. Neurosci. 21, 2669–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quan, N., Whiteside, M. & Herkenham, M. (1998) Brain Res. 802, 189–197. [DOI] [PubMed] [Google Scholar]
- 41.Meredith, T. J. & Goulding, R. (1980) Postgrad. Med. J. 56, 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castillo, J., Martinez, F., Leira, R., Prieto, J. M., Lema, M. & Noya, M. (1994) Cerebrovasc. Dis. 4, 66–71. [Google Scholar]
- 43.Hindfelt, B. (1976) Acta Neurol. Scand. 53, 72–79. [DOI] [PubMed] [Google Scholar]
- 44.Reith, J., Jorgensen, H. S., Pedersen, P. M., Nakayama, H., Raaschou, H. O. & Jeppesen, L. L. (1996) Lancet 347, 422–423. [DOI] [PubMed] [Google Scholar]
- 45.Cammock, S., Dascombe, M. J. & Milton, A. S. (1976) Adv. Prostaglandin Thromboxane Res. 1, 375–380. [PubMed] [Google Scholar]
- 46.Bernheim, H. A., Gilbert, T. M. & Stitt, J. T. (1980) J. Physiol. (London) 301, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker, F. C., Driver, H. S., Paiker, J., Rogers, G. G. & Mitchell, D. (2002) J. Appl. Physiol. 92, 1684–1691. [DOI] [PubMed] [Google Scholar]
- 48.Herrington, L. P. (1940) Am. J. Physiol. 129, 123–139. [Google Scholar]
- 49.Gordon, C. J. (1985) Physiol. Behav. 34, 687–690. [DOI] [PubMed] [Google Scholar]
- 50.Romanovsky, A. A., Ivanov, A. I. & Shimansky, Y. P. (2002) J. Appl. Physiol. 92, 2667–2679. [DOI] [PubMed] [Google Scholar]