Abstract
Understanding the mechanisms underlying the poor immunogenicity of human self/tumor antigens is challenging because of experimental limitations in humans. Here, we developed a human-mouse chimeric model that allows us to investigate the roles of the frequency and self-reactivity of antigen-specific T cells in determination of the immunogenicity of an epitope (amino acids 209–217) derived from a human melanoma antigen, gp100. In these transgenic mice, CD8+ T cells express the variable regions of a human T cell receptor (hTCR) specific for an HLA-A*0201–restricted gp100209–217. Immunization of hTCR-transgenic mice with gp100209–217 peptide elicited minimal T cell responses, even in mice in which the epitope was knocked out. Conversely, a modified epitope, gp100209–217(2M), was significantly more immunogenic. Both biological and physical assays revealed a fast rate of dissociation of the native peptide from the HLA-A*0201 molecule and a considerably slower rate of dissociation of the modified peptide. In vivo, the time allowed for dissociation of peptide-MHC complexes on APCs prior to their exposure to T cells significantly affected the induction of immune responses. These findings indicate that the poor immunogenicity of some self/tumor antigens is due to the instability of the peptide-MHC complex rather than to the continual deletion or tolerization of self-reactive T cells.
Introduction
Significant progress has been made in the past decade in the identification of tumor-associated antigens. More than 170 antigenic peptides derived from 60 human tumor antigens are expressed in the context of MHC molecules and are recognizable by cells in the available T cell repertoire (1). It is surprising that so many of these are nonmutated “self” antigens (2). Many candidate peptides have been used in clinical trials in efforts to develop therapeutic cancer vaccines, but most of these have failed to elicit measurable immune responses in the majority of the patients immunized, even when highly sensitive techniques for measuring these responses are used. One example of this phenomenon is illustrated in studies using a native peptide epitope derived from the human melanoma differentiation antigen, gp100. The epitope corresponding to amino acids 209–217 (ITDQVPFSV) of gp100 protein (gp100209–217) is restricted by HLA-A*0201 and has been studied extensively in tumors from patients with metastatic malignant melanoma (3–6).
The gp100 protein is abundantly expressed in most human melanomas. High-avidity T cell precursors specific to gp100209–217 can often be identified in circulating blood and within the tumor bed (3, 4). Recognition of the gp100209–217 epitope by adoptively transferred tumor-infiltrating lymphocytes has been correlated with tumor regression (3, 4). The nonamer gp100209–217 peptide binds to HLA-A*0201, the most commonly expressed MHC class I molecule in patients with melanoma, with an intermediate affinity of approximately 100 nM (5, 7). Hence, this peptide was a very attractive candidate for active immunization in melanoma patients. However, attempts at immunization of HLA-A*0201–positive melanoma patients using peptide emulsified in Montanide (Seppic, Paris, France) or recombinant adenoviruses or poxviruses encoding gp100 had a minimal effect on activating the immune system to recognize the tumor antigen (6, 8, 9). We have previously shown that native gp100209–217 peptide is also a poor immunogen in mice transgenic for a chimeric HLA-A2/Kb molecule (10).
The reasons underlying the poor immunogenicity of many candidate antigens used in clinical trials, including the gp100209–217 epitope, remain unelucidated. Nevertheless, it is clear that a modified version of this epitope (IMDQVPFSV), gp100209–217(2M), in which the threonine at position 2 has been changed to a methionine, is more immunogenic in melanoma patients and in mice transgenic for the HLA-A*0201/Kb molecule (6, 8, 10–12). The molecular bases for why modifications to a tumor-associated epitope improve immunogenicity have been the subject of some speculation. One hypothesis is that gp100209–217–specific T cells are present in at very low frequencies and that immunization using the altered peptide ligand gp100209–217(2M) enables the activation of a subset of T cells that are cross-reactive to the native epitope, as shown in autoimmune diseases (13) as well as in tumor immunity (12). A second possibility is that the immunogenicity of a self antigen is dampened because of reduction in the number of high-affinity self-reactive T cells, which result from tolerating mechanisms such as clonal deletion, ignorance, anergy, or suppression in the host (14). A third theory stems from the recent understanding of the role of immunological synapses in T cell activation (15), in which a stable peptide-MHC complex may facilitate the formation of the synapses between T cells and APCs (16). The altered peptide may thus allow full T cell activation through sustained signaling and therefore an increased peptide immunogenicity (17), while the native peptide forms an unstable complex that could fail to fully sustain signaling. Recent studies have suggested that the immunogenicity of MHC-I–binding viral and tumor peptides were dependent on MHC-peptide complex stability (18–21). Unfortunately, without a defined T cell receptor (TCR) specificity, these studies had to assume that the frequencies and affinity of T cell precursors with different antigen specificities were equal in the open TCR repertoires.
Because of the clinical importance of the gp100209–217 epitope, we sought to precisely study the molecular interaction of this relevant TCR–MHC-I–peptide complex in vivo. Numerous gp100209–217–specific CD8+ CTL clones have been successfully raised from melanoma patients. One of the representative clones, R6C12, was selected for this study because it was highly reactive to both native and modified gp100209–217 peptide–pulsed T2 cells and gp100+HLA-A*0201+ melanoma cells. We genetically chimerized the variable regions of human TCR (hTCR) with the constant regions of mouse TCR. In addition, the peptide-binding domains of the human HLA-A*0201 molecule were combined with mouse Kb α3 domains, which allow interactions with mouse CD8 coreceptors in these mice. Because the gp100209–217 sequence is identical in mouse and man, we were able to study the in vivo activities of the transgenic T cells with a defined specificity to a true, “noninduced” self/melanoma antigen. This also provided us a unique opportunity to investigate the self-tolerance mechanisms that regulate the transgenic T cells in both target epitope–expressing and knockout mice. Furthermore, our transgenic model allowed us to compare the immunogenicity of the native and HLA-A*0201 anchor–modified gp100209–217 peptides, which are both recognized by the parental human T cell clone. In this study, our goal was to understand the nature of the poor immunogenicity of the gp100209–217 epitope and the precise reason why this poor immunogenicity is reversed when using the modified peptide.
Results
T cells from the chimeric TCR-transgenic mouse are functional.
“Humanized” TCR-transgenic mice have been previously reported only to model human CD4+ T cells specific to myelin basic protein in the context of HLA-DR2 (22). Our model is the first to graft human self/tumor–reactive, HLA-A2–restricted CD8+ T cells into mice (Figure 1A). To validate the bioimmunological functions of the transgenic T cells in JR209 transgenic (JR209-Tg) mice, we examined the number, antigen specificity, and MHC restriction of the transgenic T cells.
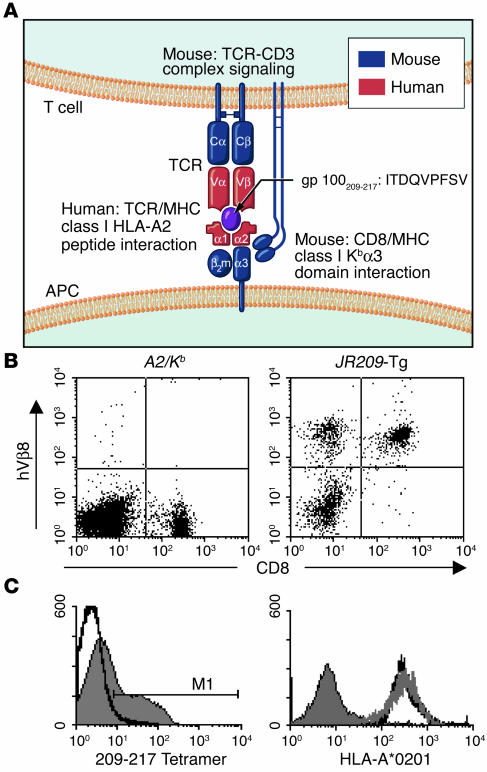
Figure 1.
Characterization of the human-mouse TCR–HLA-A2/Kb transgenic mouse model. (A) Illustration of a hTCR–HLA-A*0201–gp100209–217 complex in the JR209-Tg mouse model. (B) hTCR Vβ8 and CD8 expression on lymphocyte-gated population from spleens of non–TCR-transgenic A2/Kb (A2/Kb) and JR209-Tg littermate mice. The percentage of hVβ8+CD8+ lymphocytes was approximately 0 in A2/Kb mice and 41 in JR209-Tg littermates. (C) Binding of HLA-A*0201–gp100209–217 tetramer to the lymphocyte-gated population from spleens of A2/Kb (area under thick black line) and JR209-Tg (gray area) mice. M1 (tetramer positive gate) represented 42% of the gated population. (D) HLA-A*0201 expression on lymphocyte-gated population from splenocytes of C57BL/6 (gray area), A2/Kb (area under gray line), and JR209-Tg (area under thick black line) mice.
Like conventional TCR-transgenic mice, the human-mouse chimeras had an increased percentage of CD8+ lymphocytes (40%) in the spleen compared with their TCR transgene–negative littermates (20%) (Figure 1B), and had a similar number of total lymphocytes (not shown). More than 95% of the CD8+ T cells from JR209-Tg mice expressed human Vβ8 (hVβ8) (Figure 1B). To confirm the proper TCR Vα and Vβ configurations on the transgenic T cells, we used HLA-A*0201–gp100209–217 tetramer to label the lymphocytes. We found that approximately 40% of lymphocytes stained positive with the tetramer (Figure 1C), which was similar to the percentage of CD8+hVβ8+ lymphocytes (Figure 1B). The expression of HLA-A*0201 in mice with and without the transgenic TCR was similar (Figure 1D).
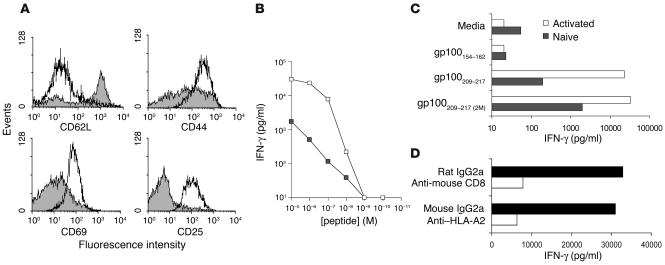
CD8+ T cells from naive JR209-Tg mice were in an inactivated state, expressing low levels of CD69 and CD44 and a high level of CD62L, but no CD25 (Figure 2A). gp100209–217 peptide–pulsed APCs induced IFN-γ production by naive JR209-Tg T cells at the concentration of 10–8 M (Figure 2B). After culturing in media containing 1 μM of gp100209–217 peptide and 30 IU/ml of IL-2 for 7 days, JR209-Tg T cells were fully activated. They not only phenotypically expressed high levels of CD25, CD69, and CD44 and a low level of CD62L (Figure 2A), but also secreted more IFN-γ upon antigen stimulation (Figure 2B). These activities were peptide-specific (Figure 2C) and could be blocked by mAb’s against HLA-A2 and mouse CD8 (Figure 2D).
Figure 2.
T cells from JR209-Tg mice are functional. (A) Expression of CD62L, CD44, CD69, and CD25 on freshly isolated (gray area) and ex vivo peptide-stimulated JR209-Tg (area under thick black line) CD8+ T cells. The florescence intensities of cells labeled with isotype Ab’s were less than 102 (not shown). (B) IFN-γ release in 24-hour coculture of 1 × 105 naive (filled squares) or gp100209–217 peptide–activated (open squares) CD8+ JR209-Tg T cells and 1 × 105 A2/Kb splenocytes pulsed with titrated gp100209–217 peptides. Data represented the mean of duplicate testing samples. (C) IFN-γ release in 24-hour coculture of 1 × 105 freshly isolated (gray bars) or ex vivo peptide-stimulated (white bars) CD8+ JR209-Tg T cells and 1 × 105 A2/Kb splenocytes pulsed with 1 μM of gp100209–217(2M), gp100209–217, and gp100154–162 (irrelevant control) peptide. (D) IFN-γ release in 24-hour coculture of 1 μM gp100209–217 peptide–pulsed A2/Kb splenocytes and activated JR209-Tg T cells and their blockade by anti–HLA-A2 and anti-mCD8 mAb’s. Data represent the mean of duplicate testing samples.
We noticed that about 20% of lymphocytes in the spleen of the JR209-Tg mice were CD4+ T cells that also expressed human Vβ8 transgene. These CD4+ T cells did not proliferate in response to in vitro stimulation with gp100209–217 peptide (data not shown), and their functions were not known.
Tolerance is observed in JR209-Tg mice with the gp100209–217 epitope knockout.
Despite large numbers of circulating antigen-specific CD8+ T cells and the expression of target antigens in normal melanocytes and other pigmented cells in eyes and brain, no significant changes in the color or appearance of hair, skin, or eyes were observed in JR209-Tg mice compared with nontransgenic littermates. To test whether these self-reactive T cells were tolerized, we compared the lymphocytes in JR209-Tg mice and JR209-Tg mice in which the gp100209–217 epitope was disrupted by insertion of the neomycin gene into exon 4 (corresponding to cDNA sequence 636–902 bp) of the gp100 gene. This resulted a truncation of gp100 protein from amino acid 212.
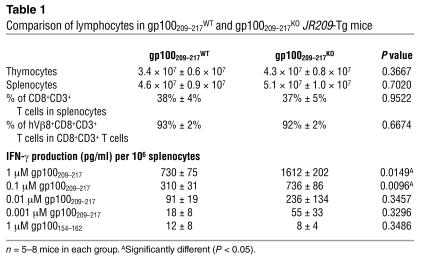
Although the numbers of thymocytes and splenocytes in both types of JR209-Tg mice were similar, naive splenocytes from JR209-Tg–gp100209–217KO mice produced more IFN-γ within 24 hours of peptide–APC stimulation than did those from JR209-Tg–gp100209–217WT mice expressing the epitope (Table 1). The differences between the naive self and non-self JR209-Tg T cells seemed to be more obvious when the antigen-specific proliferative responses were examined. In the absence of exogenous IL-2 in the culture media, more than 70% of JR209-Tg T cells from naive non–epitope-expressing mice had divided at least once within 48 hours of gp100209–217 stimulation. In comparison, less than 20% of JR209-Tg T cells from epitope-expressing mice had divided in the same period of time (Figure 3A). The sensitivity of JR209-Tg T cells to gp100209–217 epitope concentrations in ex vivo assays was similar regardless of the expression of the epitope (Table 1), which indicated unchanged TCR affinity in both types of mice. When JR209-Tg T cells were cultured ex vivo with antigen and IL-2 (known to reverse anergy of self-reactive T cells; see ref. 23), the differences in IFN-γ production and proliferation between the cells from epitope-expressing and nonexpressing mice diminished (data not shown). Our experimental results indicated that self-tolerance mechanisms partially abrogate the early T cell activation events.
Table 1.
Comparison of lymphocytes in gp100209–217WT and gp100209–217KO JR209-Tg mice
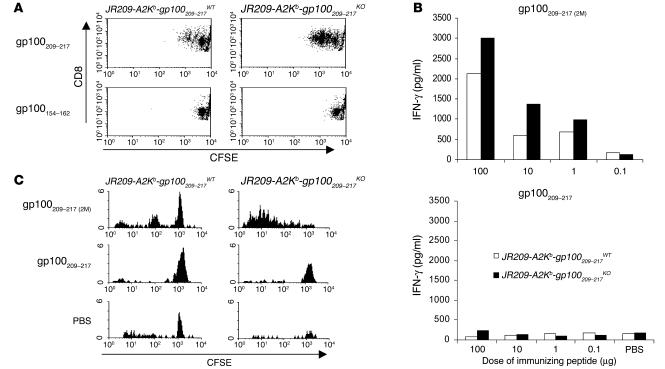
Figure 3.
Native gp100209–217 peptide fails to activate JR209-Tg T cells in both gp100209–217WT and gp100209–217KO mice. (A) Ex vivo antigen-specific proliferative responses of freshly isolated splenocytes from JR209-Tg mice with and without gp100209–217 epitope expression. CFSE-labeled splenocytes were cultured in media containing 1 μM of gp100209–217 or gp100154–162 peptide for 48 hours before FACS. The dot plots represent 10,000 total events in each sample. (B) gp100209–217 peptide–specific IFN-γ release in cells from draining lymph nodes (pooled from two mice in each group) after various doses of gp100209–217(2M) and gp100209–217 peptide immunization in JR209-Tg mice with (white bars) and without (black bars) the epitope expression. Draining lymph nodes were collected 7 days after immunization. One micromole of gp100209–217 peptide was added to 1 × 105 cells in 200 μl of culture media and incubated for 24 hours. IFN-γ concentrations in the supernatant were determined by ELISA. (C) In vivo antigen-specific proliferative responses of adoptively transferred freshly isolated splenocytes from JR209-Tg mice with and without gp100209–217 epitope expression. CFSE-labeled splenocytes (1 × 107) from JR209-Tg–gp100209–217WT or JR209-Tg–gp100209–217KO mice were intravenously injected into A2/Kb recipient mice, followed by immunization (into the footpad) with 100 μg of gp100209–217(2M), gp100209–217 peptide, or PBS (in IFA). Four days after immunization, the cells of draining lymph nodes were pooled from two mice in each group and gated on hVβ8+CD8+ T cells for FACS analysis.
Native gp100209–217 antigen fails to immunize JR209-Tg T cells in the epitope-expressing and knockout mice.
To investigate whether the poor immunogenicity of the gp100209–217 epitope was due to the low frequency of self-reactive T cells, we compared immune responses induced by peptides in JR209 TCR-transgenic and non–TCR-transgenic A2/Kb mice (or A2/Kb mice). In A2/Kb mice, immunization with neither the native nor the modified gp100209–217 peptide could elicit antigen-specific CD8+ T cell responses, even at a 100-μg dose (data not shown). However, immunization of JR209-Tg mice with as little as 10 μg of the modified peptide enhanced gp100209–217 peptide–specific IFN-γ production in draining lymph nodes compared with PBS controls (Figure 3B). Clearly, in the case of the modified gp100209–217 peptide, there was a positive correlation between the frequency of antigen-specific T cells and peptide immunogenicity. When we examined this correlation in JR209-Tg mice immunized with the native peptide, we found that none of the tested doses activated JR209-Tg T cells in draining lymph nodes compared with PBS controls (Figure 3B). Our data indicated that the quantity of tumor-specific T cells was not a determining factor for the failure of the native gp100209–217 peptide to immunize.
To investigate whether self-tolerance was responsible for the inferior immunogenicity of the native gp100209–217 epitope, we immunized JR209-Tg–gp100209–217KO mice with the peptides. Seven days after immunization, none of the tested doses activated JR209-Tg T cells in draining lymph nodes to produce antigen-specific IFN-γ (Figure 3B). In contrast, the JR209-Tg–gp100209–217KO mice immunized with the modified gp100209–217(2M) peptide responded better than JR209-Tg–gp100209–217WT mice did (Figure 3B). When CFSE-labeled naive JR209-Tg T cells from gp100209–217WT or gp100209–217KO mice were adoptively transferred into HLA-A2/Kb recipient mice and immunized with gp100209–217(2M) peptide, significant numbers of the transferred JR209-Tg T cells were proliferative 4 days after immunization. In addition, JR209-Tg T cells from gp100209–217KO mice seemed to proliferate more than those from gp100209–217WT mice (Figure 3C). However, 4 days after recipient mice were immunized with the native peptide, neither JR209-Tg T cells from gp100209–217WT mice nor those from gp100209–217KO mice had divided (Figure 3C). These results led us to consider factors other than self-tolerance that might contribute to the poor immunogenicity of the native epitope.
Native gp100209–217–MHC-I complexes are metastable.
Initiation and maintenance of immunological synapses and TCR signaling following interaction with peptide-MHC complexes are essential for full activation of T cells (15, 17, 24). Crystal structures of both native and modified HLA-A*0201–gp100209–217 complexes have been recently resolved (O.Y. Borbulevych and B.M. Baker, unpublished data). Preliminary analyses indicated minimal differences in peptide binding and TCR interface structure between the native and modified gp100209–217 peptides. This data was consistent with the observations that human T cell clones induced by either the native or modified peptide had no fine specificity enabling them to distinguish between the two forms of the peptide. Therefore, it is unlikely that two peptide–MHC-I complexes would result in different interactions with the TCR.
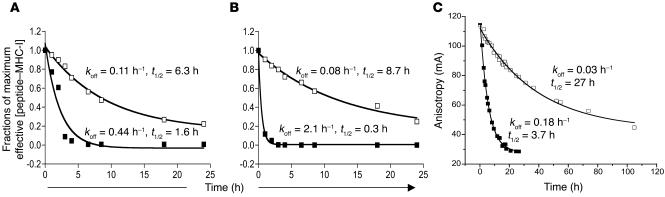
We decided to address whether the stability of the peptide–MHC-I complexes correlated to peptide immunogenicity. We determined the peptide–MHC-I dissociation rate by measuring the ability of a gp100209–217–specific T cell clone to recognize peptide-pulsed target cells at different time points after the peptide–MHC-I binding occurred. A human T cell clone specific for gp100209–217 (CK3H6) recognized gp100209–217(2M) peptide–pulsed T2 cells 24 hours after binding. In contrast, recognition of gp100209–217 peptide–pulsed T2 cells diminished 4 hours after binding. The calculated dissociation rate of the modified peptide–HLA-A2 complexes was significantly slower (P < 0.05) than that of native peptide–HLA-A2 (Figure 4A). Using cultured JR209-Tg T cells in which the TCR–peptide–MHC-I interaction was independent of CD8 coreceptors, we found similar results as when we used human T cell clones (Figure 4B). Therefore, the native peptide dissociated from HLA-A2 molecules at a much faster rate than the modified peptide, which was likely to be detrimental to activation of naive CD8+ T cells.
Figure 4.
Native gp100209–217–MHC-I complex is metastable. (A and B) Determination of peptide dissociation rates from target cell surface. Calculated amounts of gp100209–217 (filled squares) and gp100209–217(2M) (open squares) peptide bound to HLA-A2 molecules were plotted over time. Apparent koff values were determined by fitting to a single exponential decay of all points above an undetectable concentration of peptide (1 × 10–10 M using human T cell clone for A and 1 × 10–9 M using JR209-Tg T cells for B). t1/2 was determined from the relationship t1/2 = 0.693/koff. (C) Direct assay of peptide dissociation from purified peptide-MHC complexes using fluorescence anisotropy. Dissociation rates and t1/2of gp100209–217and gp100209–217(2M) peptides are indicated. Experiments were repeated in triplicate; experimental errors for the reported parameters were 3% for gp100209–217 and 7% for gp100209–217(2M). mA, millianisotropy.
The above experiments report biologically on peptide dissociation and provide apparent dissociation rates unique to the T cell–APC pair, yet do not directly measure the effect of modification on peptide dissociation from the HLA-A2 molecule. To investigate this, we measured peptide dissociation directly from purified, recombinant HLA-A2 using an in vitro fluorescence assay (Figure 4C). The results clearly indicated that position 2 modification resulted in slower peptide dissociation, with a dissociation rate (off-rate, or koff) at 37°C of 0.18 h–1 for gp100209–217 and 0.03 h–1 for gp100209–217(2M) (i.e., the modified peptide dissociates 6-fold slower). The difference in these rates was especially clear when we considered the t1/2 of the peptide-MHC complexes, or the time required for 50% of the complex to decay. The t1/2 for the parental peptide was 3.7 hours, whereas the t1/2 for the modified peptide was considerably greater at 27 hours. The rates from in vitro assays were slower than those reported for these biological measurements, as expected given the time required to form an immunological synapse, initiate downstream signaling events, and release IFN-γ. However, the relative differences between the rates were consistent with the biological measurements.
The affinity of the modified gp100209–217 peptide for HLA-A2 has been reported to be approximately 9-fold stronger than that of the parental peptide (5, 7). As Kd is proportional to koff/kon, the koff measurements predict an approximate 2-fold increase in the peptide association rate for the modified peptide. Thus the increased affinity resulting from the position 2 modification was predominantly due to slower peptide dissociation. As peptide binding to HLA-A2 is rate-limited by a transition in the heavy chain from a “peptide-inaccessible” to a “peptide-accessible” conformation (25–27), direct calculation of kon values from the current data are not possible. However, preliminary measurements of association rates for the two peptides supported the conclusion that the association rate for the modified peptide was slightly faster than that of the parental peptide (T.K. Baxter and B.M. Baker, unpublished data).
Stable peptide–MHC-I complexes are required for successful induction of antitumor responses in vivo.
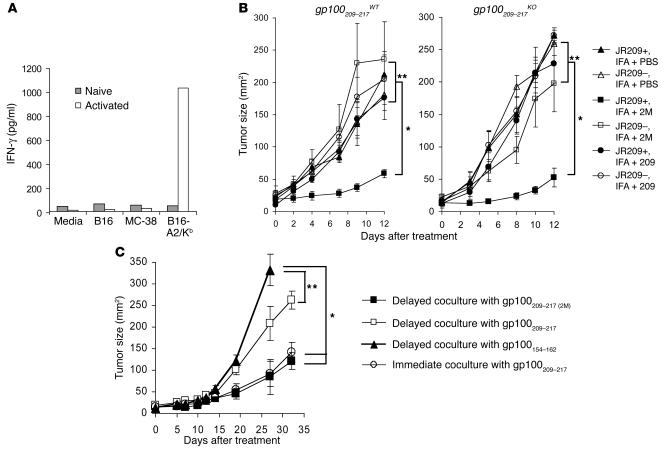
Mouse melanoma cells expressing HLA-A2/Kb molecules were not recognized by naive JR209-Tg T cells, but were recognized by activated JR209-Tg T cells (Figure 5A) due to the increased avidity for sensing the low-density antigen–MHC complexes on tumor cells (28). In JR209-Tg mice, growth of cutaneously implanted B16-A2/Kb tumor was comparable to growth in TCR transgene–negative, A2/Kb mice. Only when JR209-Tg mice were immunized with both gp100209–217(2M) peptide and IL-2 was B16-A2/Kb tumor growth inhibited (Figure 5B). As shown in an early experiment, the native peptide failed to activate JR209-Tg T cells in vivo, and immunization of JR209-Tg mice with the native peptide and IL-2 had no impact on B16-A2/Kb tumors (Figure 5B). In JR209-Tg–gp100209–217KO mice, B16-A2/Kb tumor also had a similar growth rate to that in TCR transgene–negative littermates. Immunizing these mice with native peptide and IL-2 did not result in the significant tumor reduction seen with the modified peptide.
Figure 5.
Stable peptide–MHC-I complex is required for in vivo induction of antitumor responses in JR209-Tg mice. (A) Tumor-specific IFN-γ production by freshly isolated (gray bars) or ex vivo peptide-stimulated (white bars) CD8+ JR209-Tg T cells in 24-hour coculture with target cells from B16-A2/Kb, its parental B16 melanoma, and MC-38 murine adenocarcinoma. Data represent the mean of duplicate testing samples. (B) Treatment of B16-A2/Kb tumor in A2/Kb (open symbols) and JR209-Tg (filled symbols) mice by peptide immunization. One hundred micrograms of gp100209–217 (circles), gp100209–217(2M) (squares) peptides or PBS (triangles) in IFA was subcutaneously injected into mice 13 days after tumor inoculation. Five mice were in each group. *Significantly different (P < 0.05). **Not significantly different (P > 0.05). (C) Treatment of B16-A2/Kb tumor in A2/Kb transgenic mice receiving adoptive transfer of ex vivo immunized JR209-Tg splenocytes. Peptide-pulsed splenocytes were separated from JR209-Tg T cells for 24 hours before being cocultured overnight and then injected into tumor-bearing mice. In a control group, gp100209–217 peptide–pulsed splenocytes were directly cocultured with JR209-Tg T cells overnight and injected into tumor-bearing mice. Seven mice were in each group.
Because in vivo activation of naive tumor-specific CD8+ T cells is known to occur in the draining lymph nodes where APCs cross-present tumor antigens to T cells (29), we hypothesized that after peptide immunization, the stability of peptide–MHC-I complexes during the migration from immunization sites to draining lymph nodes could determine the efficiency of the induction of immune responses. To test this, we used an ex vivo model in which naive JR209-Tg T cells and peptide-pulsed A2/Kb splenocytes were separated for 24 hours before they were mixed in culture and transferred into B16-A2/Kb tumor–bearing HLA-A2/Kb mice. Tumor growth was significantly inhibited only by ex vivo immunization with the modified peptide, not the native peptide (Figure 5C). In contrast, adoptively transferred JR209-Tg T cells that were stimulated ex vivo with HLA-A2/Kb splenocytes immediately after native peptide pulsing inhibited tumor growth (Figure 5C). Therefore, the level of available gp100209–217–MHC complexes on APCs at the time they encounter CD8+ T cells could have a dramatic impact on an antigen’s ability to sensitize T cells.
Discussion
The JR209-Tg mouse model presents a magnified picture of the interactions between a human self/tumor antigen–specific CD8+ T cell and its cognate peptide–MHC-I ligand. This model clearly shows that a stable peptide–MHC-I complex is the first requirement for activating self-reactive CD8+ T cells during peptide immunization. It not only explains the poor immunogenicity of vaccines based on nonmodified tumor antigens, but also elucidates a plausible escape mechanism for autoreactive T cells with intermediate affinity to self/tumor antigens.
One mechanism by which autoreactive T cells escape negative selection is the lack of tissue-specific antigen expression in the thymus (30). This is unlikely in the case of JR209-Tg T cells because gp100 is highly expressed in mouse thymic epithelial and dendritic cells (31). We have shown that the number of JR209-Tg T cells in gp100209–217WT mice is comparable to the number found in gp100209–217KO mice. Therefore, the majority of JR209-Tg T cells escape thymic selection regardless of antigen expression. A possible explanation is that with a very fast rate of dissociation, the gp100209–217 epitope–MHC-I complex is not stable enough to initiate TCR signaling in the thymus and thus fails to clonally delete the self-reactive T cells.
However, in the periphery, JR209-Tg T cells have reduced responsiveness to self-antigen stimulation compared with those that mature in a self antigen–free environment. If the self-reactive T cells do not “see” the antigen, there is no need to downregulate their response to it. Therefore, ignorance cannot explain the reduced responsiveness of tolerized JR209-Tg T cells. There are fewer CD4+ T cells in JR209-Tg mice than in A2/Kb mice, but it is not clear whether there are suppressive CD4+ T cells in the JR209-Tg mice. Anergy can result in tolerization of T cells. Although it is not known how anergy is induced in vivo, the lack of costimulatory signals during TCR-antigen recognition is the common speculation (32). Because gp100 is abundantly expressed in melanoma and normal melanocytes, there are apparently still enough peptide-MHC complexes on the cell surface at any given time, even with a fast dissociation rate from MHC complexes. This could provide the cognate T cell signal 1. In addition, we have evidence that IL-2, which has been shown to reverse T cell anergy (23), can restore the responsiveness of JR209-Tg T cells from gp100209–217WT mice to self-antigen stimulation to the same level as that of JR209-Tg T cells from gp100209–217KO mice. If this mouse model mimics the situation in man and many of the tumor-reactive T cells in the host are anergized, it is important to combine de-anergizing solutions such as common γ-chain cytokines or costimulatory factors with immunization protocols to achieve effective antitumor immune responses.
The key to understanding the poor immunogenicity of the gp100209–217 epitope becomes apparent following analysis of the dissociation rate of peptide–MHC-I complexes. The “anchor-modified” gp100209–217 peptide, in which a methionine replaces the natural threonine at position 2 of the nanomer, was predicted to fit well into the HLA-A2 binding pocket based on resolved peptide–HLA-A2 crystal structures. This prediction also indicated minimal differences of the peptide surface structures facing the TCR, which agreed with the clinical findings that human T cell clones do not distinguish the two peptides in the context of HLA-A2 molecules (5, 6). Thus, it is unlikely that the gp100209–217(2M) peptide could stimulate a subset of T cells that cross-react to the native antigen. We are in the process of resolving the crystal structure of the TCR–peptide–MHC-I complex to further test this prediction. The parental peptide has a 9-fold weaker binding affinity than the modified peptide (5, 7), due primarily to faster peptide dissociation. Thus, at any given time after initial presentation on the cell surface, a greater amount of the modified peptide remains bound to MHC-I than does its parental peptide. The correlation between this difference in dissociation rate and immunological potency was clearly demonstrated when peptide-pulsed APCs were separated for 24 hours before mixing; in this experiment only those cells pulsed with the modified peptide remained active. The expected 2-fold difference in association rates was not likely to influence these results, since no exogenous peptide was present during the 24-hour incubation, ensuring that the peptide concentration outside the cell remained extremely low. Note that the 24-hour time period is not even a single half-life for the modified peptide (t1/2 of 27 hours), yet is over six half-lives for the parental peptide (t1/2 of 3.7 hours). Thus, the 100-fold greater potency of the modified peptide can best be attributed to slower peptide dissociation.
It has been recently demonstrated by Spiotto et al. and Nguyen et al. that tumor antigens are cross-presented to CD8+ T cells in draining lymph nodes by professional APCs (29, 33). Using real-time two-photon microscopy, Bousso et al. elegantly illustrated the dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Contrary to conventional wisdom, it takes hours, not minutes, for APCs to activate naive CD8+ T cells (34). Antigen uptake by an APC either at the tumor site or at the peptide immunization site, migration to draining lymph nodes, and stable contact with an antigen-specific T cell could take hours and possibly days. Therefore, the stability of peptide–MHC-I complexes becomes an important determinant of the immunogenicity of a given antigen.
In our transgenic mouse model, because the variable regions of the TCR were fixed, we could not address the issue brought by cross-reactive T cells. However, clinically it has become evident that T cells reactive to self/melanoma antigens with high avidity (capable of recognizing 1 nM or less peptide) do exist in humans (18, 35–37), which perhaps explains why immunotherapy is more efficient in treating patients with melanoma than those with other cancers. We have successfully isolated and cloned a CD8+ T cell specific for the gp100 mouse homologue Pmel-17, residues 25–33 from normal C57BL/6 mice that were immunized with recombinant vaccinia virus expressing human gp100. This clone was able to recognize target cells pulsed with self-peptide at a level as low as 1 pM (38). Transgenic T cells derived from this clone, antigenic stimulation, and the administration of IL-2 have been demonstrated to be three inseparable necessities to successfully destroy cutaneously implanted solid syngeneic murine melanoma (39). Clearly, there are mechanisms regulating the function of these high-avidity autoreactive T cells in the host to prevent autoimmune diseases; however, in cancer immunotherapy, the goal is the opposite. In our study, we demonstrated that the strength of the stable peptide–MHC-I complexes could determine an antigen’s ability to break the self-tolerance in the host. As numerous approaches are undergoing investigation in the field, we strongly recommend modifying the selected epitope to achieve the maximum stability of peptide–MHC-I complexes for effective immunization against self/tumor antigens.
Methods
Peptide synthesis.
Native and modified gp100209–217 peptides (ITDQVPFSV and IMDQVPFSV, respectively) were synthesized by Macromolecular Resources (Colorado State University, Fort Collins, Colorado, USA). They were more than 95% pure by HPLC analysis. All the peptides were dissolved in DMSO at 10 mg/ml as stock for ex vivo use. Fluorescently labeled peptides, modified at position 5 with a fluorescein-derivatized lysine [ITDQ(K-Flc)PFSV and IMDQ(K-Flc)PFSV], were synthesized by SynPep Corp. (Dublin, California, USA) and were HPLC-purified to greater than 95% purity.
Production of transgenic mice expressing human gp100209–217–specific TCRs.
R6C12, an HLA-A*0201–restricted, gp100209–217–specific CD8+ T cell clone derived from a melanoma patient was selected for hTCR cloning. The basic procedures used to identify and clone the TCR were adopted from the methods described by Kouskoff et al. (40). Briefly, the VJ fragments of the α chain and the VDJ fragments of the β chain were cloned by 5′ rapid amplification of cDNA ends and identified by DNA sequencing as Vα41S1, Jα54, Vβ8S1, Dβ2, and Jβ2S1. Genomic DNA of the TCR α and β chain, from the 10–12 bp upstream of the start codon and up to the 200-bp intronic sequences downstream of the junction regions, were PCR amplified and inserted into pTα and pTβ cassette vectors, respectively (kindly provided by Diane Mathis of Harvard University School of Medicine, Boston, Massachusetts, USA). The inserted genomic DNA fragments were verified by restriction enzyme digestion. After removing the prokaryote DNA sequences, the linearized pTα and pTβ cassettes were coinjected into fertilized eggs from C57BL/6 mice. Founder mice carrying both the α and β chains of the transgenic TCR were identified by Southern blot analysis. To positively select the hTCR in mice, the TCR-transgenic mice were bred to mice expressing HLA-A2/Kb MHC molecules (41) (purchased from The Jackson Laboratory, Bar Harbor, Maine, USA). Transgenic mice expressing both TCR and HLA-A2/Kb transgenes were named JR209-Tg mice.
Expression of human gp100209–217–specific TCR in epitope-knockout mice.
JR209-Tg mice were crossed with C56BL/6 mice lacking expression of the gp100209–217 epitope (provided by Byoung Kwon, Indiana University, Indianapolis, Indiana, USA). Epitope-knockout mice were generated by insertion of a neomycin gene into exon 4 (corresponding to cDNA sequence 636–902 bp) of the gp100 gene. This genetic interruption resulted in a truncation of gp100 protein amino acid residue 212 and therefore eliminated the target epitope. Mice were housed at the NIH-10A animal facility and at Biocon Inc. (Rockville, Maryland, USA). All animal study protocols were approved by the NIH institutional review board.
Murine melanoma cell line engineered to express HLA-A2/Kb molecules.
B16 murine melanoma cells were stably transfected with plasmid DNA encoding HLA-A2/Kb as described previously (10). The expression of the transgene on the B16-A2/Kb tumor cells was monitored by FACS analysis of HLA-A2 on the cell surface.
Evaluation of JR209-Tg T cells.
Freshly isolated splenocytes (5 × 106 cells) from JR209-Tg mice were cultured at 37°C with 5% CO2 in 2 ml of RPMI culture media as described previously (10), containing 30 IU/ml of human recombinant IL-2 (Chiron Corp., Emeryville, California, USA) and 1 μM of gp100209–217 native or modified peptide in a 24-well plate. Once the cells grew confluent, they were subdivided in culture media containing IL-2.
To detect activation markers on JR209-Tg T cells upon antigen stimulation, 1 × 105 to 1 × 106 splenocytes or cultured T cells were stained with fluorescence-labeled mAb against hVβ8 (clone JR-2), CD25 (clone PC-61), CD62L (clone MEL-14), CD69 (clone H1.2F3), CD44 (clone TM-1), or their isotype controls (BD Biosciences – Pharmingen, San Diego, California, USA) and analyzed by FACS. In antigen-specific IFN-γ release assays, 1 × 105 naive or peptide-stimulated JR209-Tg T cells were cocultured with 1 × 105 peptide-pulsed, irradiated (30 Gy) HLA-A2/Kb splenocytes or tumor cells from B16-A2/Kb, its parental B16 murine melanoma, and MC-38 murine colon adenocarcinoma (Southern Research Institute, Birmingham, Alabama, USA) in 200 μl of culture media/well of a 96-well U-bottom plate for 24 hours.
To test MHC restriction, 10 μg mAb against HLA-A2 (clone KS-21; Surgery Branch, National Cancer Institute, Bethesda, Maryland, USA), CD8 (clone 53-6.7, BD Biosciences – Pharmingen), and their isotype controls were added to target cells 30 minutes before coculture. The coculture supernatants were collected 24 hours later and assayed for IFN-γ by ELISA.
To measure the proliferation of JR209-Tg T cells in response to antigen stimulation, 1 × 106 freshly isolated splenocytes from naive JR209-Tg mice were labeled with 1 nM of CFSE (Molecular Probes Inc., Eugene, Oregon, USA) and cultured in culture media with 1 μM of peptide for 1–5 days before FACS for CFSE on CD8+hVβ8+ populations.
To examine the antigen-specific proliferation in vivo of naive JR209-Tg T cells, 1 × 107 CFSE-labeled naive JR209-Tg splenocytes were injected intravenously into each recipient mouse, which was then immunized with 100 μg of peptide in incomplete Freund’s adjuvant (IFA; Sigma-Aldrich, St. Louis, Missouri, USA) in the footpad. Three to five days later, the draining lymph nodes of immunized mice were harvested and FACS for CFSE on CD8+hVβ8+ populations was performed.
Tumor treatment.
B16-A2/Kb melanoma cells (2 × 105 cells in 100 μl of PBS) were injected intradermally into the flank region of mice. Seven to 14 days after tumor injection, mice were immunized (by injection into the footpads) with various doses of peptides emulsified in 100 μl of IFA. In adoptive-transfer experiments, tumor-bearing recipient mice were given whole-body sublethal irradiation (5 Gy) followed by intravenous injection of 1 × 107 cultured JR209-Tg T cells. All mice in the tumor treatment experiments were given recombinant human IL-2 (Chiron Corp.) starting the day of treatment, at 600,000 IU/dose twice daily for 3 days. Tumor size was measured in a randomized, blinded fashion. Vitiligo was determined by shaving the coat hair on the abdominal area of the mice and examining the return of depigmented hair at the site.
Ex vivo modeling of peptide immunization.
Splenocytes from HLA-A2/Kb transgenic mice were pulsed with 1 μM of peptide for 3 hours at 37°C, washed three times with media, plated in 24-well plates, and incubated at 37°C overnight. Equal numbers of freshly isolated JR209-Tg splenocytes were then added to the wells and cocultured in media containing 30 IU/ml of IL-2 for 24 hours before adoptive transfer. As a control, freshly JR209-Tg splenocytes were cocultured with APCs immediately after peptide pulsing.
Determination of peptide dissociation rates from APCs.
T2 cells, derived from human lymphoma and lacking transporter associated with antigen processing, were pulsed with various concentrations of peptide for 3 hours at 37°C and washed three times. Peptide-pulsed T2 cells were then suspended in culture media and incubated at 37°C for various time periods (see Figure 4 for time points) before they were cocultured for 24 hours with a human HLA-A2–restricted CD8+ T cell clone specific for gp100209–217 (CK3H6; Surgery Branch, National Cancer Institute) or JR209-Tg T cells stimulated with peptide for 7 days. The supernatants from the cocultures were assayed for IFN-γ by ELISA. The correlation between the concentration of peptide-MHC complexes and IFN-γ levels was calculated in the following manner. A regression analysis of peptide concentrations and IFN-γ production was determined immediately after peptide pulsing. Using this correlation, the relative amount of peptide–MHC-I complexes remaining on the cell surface at each time point after peptide pulsing was determined. Data were fit to a first-order rate equation of the form y = A0 + exp(–1/kofft) + y0, where A0 is the initial amplitude, koff is the dissociation rate, t is time, and y0 is a baseline offset.
In vitro determination of peptide dissociation rates.
Recombinant, soluble HLA-A2 and β2m were refolded from bacterially expressed inclusion bodies as previously described (42) in the presence of fluorescently labeled native or position 2–modified gp100209–217 peptide. Refolded peptide–MHC-I was purified chromatographically. Dissociation rates were measured using a fluorescence anisotropy assay as previously described (26). Briefly, about 7 nM HLA-A2 loaded with fluorescent peptide was incubated at 37°C with a 1,000-fold excess of unlabeled peptide, and the anisotropy was measured as a function of time using a Beacon 2000 fluorescence polarization instrument (Invitrogen Corp., Carlsbad, California, USA). The decay in anisotropy was fit to the first-order rate equation y = A0 + exp(–1/kofft) + y0, described above. The assay solution consisted of 10 mM HEPES and 150 mM NaCl, pH 7.4. A second kinetic phase, attributed in other reports to initial dissociation of β2m prior to dissociation of the peptide (26), was not observed for dissociation of the gp100-based peptides. Measurements were performed in triplicate.
Statistical analysis.
Statistical differences in tumor growth for different treatment groups were analyzed by Wilcoxon rank sum test. A Student’s t test was used to compare the average cell numbers or IFN-γ concentrations in different groups.
Acknowledgments
The authors wish to thank Diane Mathis and Byoung Kwon for generously providing transgenic mouse reagents for this study, and Mark Dudley for providing us the human CD8+ T cell clones and helpful discussion during the manuscript preparation. The study was supported in part by grant GM-067079 (to B.M. Baker) from the National Institute of General Medical Sciences, NIH.
Footnotes
See the related Commentary beginning on page 468.
Nonstandard abbreviations used: human TCR (hTCR); human Vβ8 (hVβ8); incomplete Freund’s adjuvant (IFA); JR209 transgenic ( JR209-Tg); T cell receptor (TCR).
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Van den Eynde, B., and van der Bruggen, P. 2003. Peptide database of T-cell defined tumor antigens. Cancer Immunity. [serial online]. http://www.cancerimmunity.org/peptidedatabase/Tcellepitopes.htm#text.
- 2.Renkvist N, Castelli C, Robbins PF, Parmiani G. A listing of human tumor antigens recognized by T cells. Cancer Immunol. Immunother. 2001;50:3–15. doi: 10.1007/s002620000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawakami Y, et al. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J. Immunol. 1995;154:3961–3968. [PubMed] [Google Scholar]
- 4.Kawakami Y, et al. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc. Natl. Acad. Sci. U. S. A. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parkhurst MR, et al. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J. Immunol. 1996;157:2539–2548. [PubMed] [Google Scholar]
- 6.Rosenberg SA, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat. Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelhard VH, Bullock TN, Colella TA, Sheasley SL, Mullins DW. Antigens derived from melanocyte differentiation proteins: self-tolerance, autoimmunity, and use for cancer immunotherapy. Immunol. Rev. 2002;188:136–146. doi: 10.1034/j.1600-065x.2002.18812.x. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg SA, et al. Recombinant fowlpox viruses encoding the anchor-modified gp100 melanoma antigen can generate antitumor immune responses in patients with metastatic melanoma. Clin. Cancer Res. 2003;9:2973–2980. [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg SA, et al. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J. Natl. Cancer Inst. 1998;90:1894–1900. doi: 10.1093/jnci/90.24.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irvine KR, et al. Recombinant virus vaccination against “self” antigens using anchor-fixed immunogens. Cancer Res. 1999;59:2536–2540. [PMC free article] [PubMed] [Google Scholar]
- 11.Phan GQ, et al. Immunization of patients with metastatic melanoma using both class I- and class II-restricted peptides from melanoma-associated antigens. J. Immunother. 2003;26:349–356. doi: 10.1097/00002371-200307000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bullock TN, Mullins DW, Colella TA, Engelhard VH. Manipulation of avidity to improve effectiveness of adoptively transferred CD8(+) T cells for melanoma immunotherapy in human MHC class I-transgenic mice. J. Immunol. 2001;167:5824–5831. doi: 10.4049/jimmunol.167.10.5824. [DOI] [PubMed] [Google Scholar]
- 13.Oldstone MB. Molecular mimicry and autoimmune disease. Cell. 1987;50:819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- 14.Lee PP, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat. Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 15.Dustin ML. Coordination of T cell activation and migration through formation of the immunological synapse. Ann. N. Y. Acad. Sci. 2003;987:51–59. doi: 10.1111/j.1749-6632.2003.tb06032.x. [DOI] [PubMed] [Google Scholar]
- 16.Slansky JE, et al. Enhanced antigen-specific antitumor immunity with altered peptide ligands that stabilize the MHC-peptide-TCR complex. Immunity. 2000;13:529–538. doi: 10.1016/s1074-7613(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 17.Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat. Immunol. 2003;4:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 18.Stanislawski T, et al. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat. Immunol. 2001;2:962–970. doi: 10.1038/ni1001-962. [DOI] [PubMed] [Google Scholar]
- 19.Dyall R, et al. Heteroclitic immunization induces tumor immunity. J. Exp. Med. 1998;188:1553–1561. doi: 10.1084/jem.188.9.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gold JS, et al. A single heteroclitic epitope determines cancer immunity after xenogeneic DNA immunization against a tumor differentiation antigen. J. Immunol. 2003;170:5188–5194. doi: 10.4049/jimmunol.170.10.5188. [DOI] [PubMed] [Google Scholar]
- 21.Chen ZW, et al. Simian immunodeficiency virus evades a dominant epitope-specific cytotoxic T lymphocyte response through a mutation resulting in the accelerated dissociation of viral peptide and MHC class I. J. Immunol. 2000;164:6474–6479. doi: 10.4049/jimmunol.164.12.6474. [DOI] [PubMed] [Google Scholar]
- 22.Madsen LS, et al. A humanized model for multiple sclerosis using HLA-DR2 and a human T-cell receptor. Nat. Genet. 1999;23:343–347. doi: 10.1038/15525. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz RH. Models of T cell anergy: is there a common molecular mechanism? J. Exp. Med. 1996;184:1–8. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faroudi M, Zaru R, Paulet P, Muller S, Valitutti S. Cutting edge: T lymphocyte activation by repeated immunological synapse formation and intermittent signaling. J. Immunol. 2003;171:1128–1132. doi: 10.4049/jimmunol.171.3.1128. [DOI] [PubMed] [Google Scholar]
- 25.Gakamsky DM, Davis DM, Strominger JL, Pecht I. Assembly and dissociation of human leukocyte antigen (HLA)-A2 studied by real-time fluorescence resonance energy transfer. Biochemistry. 2000;39:11163–11169. doi: 10.1021/bi000763z. [DOI] [PubMed] [Google Scholar]
- 26.Binz AK, Rodriguez RC, Biddison WE, Baker BM. Thermodynamic and kinetic analysis of a peptide-class I MHC interaction highlights the noncovalent nature and conformational dynamics of the class I heterotrimer. Biochemistry. 2003;42:4954–4961. doi: 10.1021/bi034077m. [DOI] [PubMed] [Google Scholar]
- 27.Springer S, Doring K, Skipper JC, Townsend AR, Cerundolo V. Fast association rates suggest a conformational change in the MHC class I molecule H-2Db upon peptide binding. Biochemistry. 1998;37:3001–3012. doi: 10.1021/bi9717441. [DOI] [PubMed] [Google Scholar]
- 28.Fahmy TM, Bieler JG, Edidin M, Schneck JP. Increased TCR avidity after T cell activation: a mechanism for sensing low-density antigen. Immunity. 2001;14:135–143. [PubMed] [Google Scholar]
- 29.Spiotto MT, et al. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 2002;17:737–747. doi: 10.1016/s1074-7613(02)00480-6. [DOI] [PubMed] [Google Scholar]
- 30.Sprent J, Kishimoto H. The thymus and negative selection. Immunol. Rev. 2002;185:126–135. doi: 10.1034/j.1600-065x.2002.18512.x. [DOI] [PubMed] [Google Scholar]
- 31.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat. Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz RH. T cell anergy. Annu. Rev. Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen LT, et al. Tumor growth enhances cross-presentation leading to limited T cell activation without tolerance. J. Exp. Med. 2002;195:423–435. doi: 10.1084/jem.20010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat. Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 35.Dudley ME, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeh HJ, III, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J. Immunol. 1999;162:989–994. [PubMed] [Google Scholar]
- 37.Yee C, Savage PA, Lee PP, Davis MM, Greenberg PD. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J. Immunol. 1999;162:2227–2234. [PubMed] [Google Scholar]
- 38.Overwijk WW, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J. Exp. Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Overwijk WW, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kouskoff V, Signorelli K, Benoist C, Mathis D. Cassette vectors directing expression of T cell receptor genes in transgenic mice. J. Immunol. Methods. 1995;180:273–280. doi: 10.1016/0022-1759(95)00002-r. [DOI] [PubMed] [Google Scholar]
- 41.LaFace DM, et al. Human CD8 transgene regulation of HLA recognition by murine T cells. J. Exp. Med. 1995;182:1315–1325. doi: 10.1084/jem.182.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garboczi DN, Hung DT, Wiley DC. HLA-A2-peptide complexes: refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides. Proc. Natl. Acad. Sci. U. S. A. 1992;89:3429–3433. doi: 10.1073/pnas.89.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]