Abstract
Diabetes mellitus (DM) is a common chronic medical problem worldwide; one of its complications is painful peripheral neuropathy, which can substantially erode quality of life and increase the cost of management. Despite its clinical importance, the pathogenesis of painful diabetic neuropathy (PDN) is complex and incompletely understood. Voltage-gated sodium channels (VGSCs) link many physiological processes to electrical activity by controlling action potentials in all types of excitable cells. Two isoforms of VGSCs, NaV1.3 and NaV1.7, which are encoded by the sodium voltage-gated channel alpha subunit 3 and 9 (Scn3A and Scn9A) genes, respectively, have been identified in both peripheral nociceptive neurons of dorsal root ganglion (DRG) and pancreatic islet cells. Recent advances in our understanding of tetrodotoxin-sensitive (TTX-S) sodium channels NaV1.3 and NaV1.7 lead to the rational doubt about the cause–effect relation between diabetes and painful neuropathy. In this review, we summarize the roles of NaV1.3 and NaV1.7 in islet cells and DRG neurons, discuss the link between DM and painful neuropathy, and present a model, which may provide a starting point for further studies aimed at identifying the mechanisms underlying diabetes and painful neuropathy.
Keywords: diabetes mellitus, painful diabetic neuropathy, NaV1.3, NaV1.7, dorsal root ganglion neurons, pancreatic islet cells
1. Introduction
Diabetes mellitus (DM) is a chronic disease that affects more than 382 million people worldwide, and this number is expected to rise beyond 592 million by 2035 [1]. In a recent study that aimed to provide the number of deaths attributable to diabetes in the year 2013, it was estimated that 1 in 12 of global all-cause deaths were due to diabetes in adults [2]. With DM becoming increasingly prevalent over time, painful diabetic neuropathy (PDN), as one of its associated major complications, is also rapidly rising.
PDN affects almost 25% of the diabetic population and covers a wide variety of clinical presentations, which involve a significant risk in the quality of patients’ life [3,4,5,6]. The clinical characteristics of PDN range from spontaneous pain to allodynia (pain to a stimulus that is painless under normal conditions) and hyperalgesia (increased pain in response to a painful stimulus) [7,8,9]. The burning, shooting, tingling or lancinating pain of PDN impacts patients’ ability to perform daily activities, disturbs sleep, and causes negative influences on mood, such as anxiety and depression [4,6]. Patients with PDN are consumers of more health care resources, costing up to almost US $17,000 per year in patients with severe pain [10,11]. Even with frequent visits to medical professionals and use of prescription medications, it turns out that the clinical treatment of PDN is often unsatisfactory because the use of high doses of drugs is accompanied by abundant side effects. Therefore, the research of new, safe and effective strategies for the treatment of PDN is necessary. Additionally, the pathogenesis of PDN needs to be elucidated, even though it is complex and difficult. Although several pathogeneses, including metabolic, vascular, autoimmune and oxidative stress-related mechanisms for PDN, have been postulated, the precise cause of neuropathic pain in diabetes remains to be elucidated.
2. Main Cells Involved in Diabetes and Painful Diabetic Neuropathy
Diabetes mellitus, commonly referred to as diabetes, is characterized by hyperglycemia due to impaired insulin secretion and aberrant glucagon secretion. Insulin and glucagon are released into the blood by β cells and α cells of the pancreatic islets, respectively, in response to changes in plasma glucose levels [12]. Apparently, pancreatic islet cell dysfunction is critical for the development of hyperglycemia during DM and its associated complications, including PDN. Electrical activity, which is crucial for hormone release by the pancreatic islet cells, is organized by the concerted activity of several different types of ion channels [12,13].
The small diameter Aδ- and C-fibers of dorsal root ganglion (DRG) neurons, also known as pain-sensing sensory neurons (nociceptors), are the target cells of PDN, as they can be sensitized by a variety of mechanisms in response to different pathological conditions associated with diabetes. Increased ectopic discharges of sensory neurons are considered to contribute directly to the development and maintenance of PDN and changes of ion channel activities in DRG neurons play a significant role in peripheral sensitization and nociceptive sensation [14,15].
In previous research, voltage-gated ion channels, such as K+ and Ca2+ channels, or other factors that may contribute to the pathogenesis of insulin and glucagon secretion, as well as diabetic neuropathic pain, were discussed [12,13,14,15]. In this review, we present the current knowledge on the role of voltage-gated tetrodotoxin-sensitive sodium channels in the perception modulation of DM and PDN.
3. Review of Voltage-Gated Sodium Channels
Voltage-gated sodium channels (VGSCs) are integral membrane proteins that allow movement of sodium ions across cellular membranes and are present in many tissue types within human and rodent. VGSCs generate and conduct action potentials and regulate electrical signaling in all types of excitable cells [16,17,18]. The activity of these channels and the movement of charged sodium ions allow them to produce and respond to electrical signals within the excitable cells. Activation, deactivation, and inactivation in VGSCs link various physiological processes to electrical activity by controlling action potentials in different tissues. Because of their distribution throughout the body, VGSCs are implicated in a variety of diseases, including DM and PDN.
VGSCs mediate the influx of sodium ions into the cytosol of cells in response to local membrane depolarization, which results in the generation of the rising phase of an action potential [18]. All voltage-gated channels are formed by a long integral membrane polypeptide, α subunit, and one or more smaller auxiliary β subunits [19]. The ion-selective pore forming the α subunit is the main structure for channel function including voltage-dependent gating and conductance, whereas the kinetics and voltage dependence of channel gating are in part modulated by β subunits [20,21]. In mammals, nine α isoforms (NaV1.1–NaV1.9, encoded by the sodium voltage-gated channel alpha subunit genes Scn1A–Scn5A and Scn8A–Scn11A) and four β subunits (β1–β4, encoded by the sodium voltage-gated channel beta subunit genes Scn1B–Scn4B) have been identified. The distribution of sodium channel isoforms varies in different tissues and development stages. Based on differential sensitivity to tetrodotoxin (TTX), sodium currents are classified into TTX-sensitive (TTX-S) and TTX-resistant (TTX-R) components. Five members (NaV1.1–1.4, 1.6–1.7) of the VGSC family are TTX-S sodium channels, which can be blocked by TTX, while the other sodium channel isoforms are TTX-R sodium channels, which cannot be blocked by TTX [22].
In addition, many differences in sodium channel structure have been shown to arise from the extent of glycosylation. It appears that established mechanisms for structural heterogeneity within a species of ion channel could also contribute to the behavioral heterogeneity among Na+ channels. Glycosylation of ion channels may be altered in diabetes. TTX-S VGSCs within DRG neurons (including NaV1.3 and NaV1.7) can exhibit a spectrum of states of glycosylation [23]. In the previous study of voltage-dependent Na+ conductances in small adult rat DRG neurons, conducted by Rizzo et al., they found that, in all small neurons studied, there appeared to be a singular kinetic component of the current, based on sensitivity to the conditioning potential, voltage dependence of activation, and inactivation half-time [23]. The different properties of the slow Na+ conductance in different neurons are likely to reflect heterogeneity of the structure of the underlying channel molecule.
4. Roles of TTX-S NaV1.3 and NaV1.7 Channels in Painful Diabetic Neuropathy
The peripheral nociceptive neurons in DRG express a variety of sodium channel isoforms, particularly NaV1.3, NaV1.7, NaV1.8 and NaV1.9, each playing a key role in the physiology of nociception. Additionally, their encoding genes have been demonstrated to relate with neuropathic pain [24,25,26]. TTX-S currents induced by both fast and slow voltage ramps increase significantly in diabetic neurons. NaV1.3 and NaV1.7 are highly TTX-S and their expression levels increased in diabetic animals’ DRG homogenates. Pre-clinical data from non-specific blockers, knockouts and small interfering RNA for these specific subtypes of sodium channels have been revealed to be effective in attenuating hyperalgesia and allodynia.
5. NaV1.3
During embryonic stage, TTX-S NaV1.3 is broadly expressed in DRG neurons of the developing nervous system. Postnatally, the expression of NaV1.3 throughout the nervous system decreases dramatically to undetectable levels. However, NaV1.3 is reported to be re-expressed within peripheral DRG neurons under certain pathological conditions that involve peripheral nerve injuries such as inflammation and nerve transection [27,28,29,30,31]. A decrease in the expression of TTX-R α subunits and/or an increase in that of TTX-S α subunits (particularly NaV1.3) has been previously reported in nerve injury animal models [32,33,34,35]. Painful neuropathy may occur without the symptoms of DM. It is hard to determine to what extent diabetes and VGSC mutations contribute to PDN. Emerging evidence has demonstrated that NaV1.3 has a critical role in the development and maintenance of neuropathic pain of PDN. NaV1.3 produces sodium currents with rapid repriming kinetics and recovers quickly from inactivation. In addition, owing to the above distinct functional properties, the upregulated expression of NaV1.3 channels in diabetic DRG neurons would be expected to increase overall sodium channel density, decrease firing threshold and play an important role in the hyperexcitability of damaged or injured neurons [36,37,38,39]. A previous study has shown that high-level expression of NaV1.3 lasted for six months in streptozotocin (STZ)-induced diabetic rats with persistent mechanical allodynia [40]. In addition, long-term hyperglycemia exacerbated inflammatory reactions thus led to the upregulation of NaV1.3 [28,41].
Hoeijmakers et al. [42] reported that painful neuropathy is not necessarily a complication of diabetes, but may occur before DM, which was observed in two patients. The I739V mutation (c.2215A>G, p.Ile739Val) in NaV1.7 has been described in three patients with painful neuropathy, two of whom were found to have diabetes at least a year after the onset of neuropathy. As far as we know, there was no clinical report about painful neuropathy resulting from NaV1.3 mutation occurring prior to DM.
Many experts have considered NaV1.3 as a suitable target for pain therapeutics. Due to the absence of isoform-selective, effective and safe NaV1.3 blockers, research focused on gene therapy. Samad et al. provided evidence for a contribution of NaV1.3 to neuropathic pain, and demonstrated the therapeutic potential of NaV1.3 knockdown for pain treatment in a rat model. They suggested gene therapy as a potential therapeutic option [43]. In a recent study by Tan et al., they proved the efficacy of NaV1.3 knockdown by adeno-associated viral-mediated delivery of small hairpin RNA in an STZ-induced PDN rat model with reduced tactile allodynia, and a concomitant decrease in nociceptive dorsal horn neuron hyperexcitability [44]. Together, these findings demonstrate the functional relevance of NaV1.3 misexpression in diabetic neuropathic pain and provide groundwork for developing targeted gene therapy to manage PDN.
Additionally, altered levels of neurotrophin nerve growth factor (NGF) may also take part in the pathophysiology of PDN. NGF is known to regulate the expression of NaV1.3. In a previous study, performed by Black et al., they examined the hybridization signal of α-SNS (NaV1.8) and α-III (sodium channel III) mRNAs in small DRG neurons from adult rats that had been dissociated and maintained for seven days in the absence or presence of exogenous NGF. They found that NGF participates in the regulation of membrane excitability in small DRG neurons by pathways that include opposing effects on different sodium channel genes including Scn3A [45].
6. NaV1.7
NaV1.7, also known as hNE9 or PN1, one of the TTX-S sodium channels, is proved to be an important contributor to pain signaling. Altered expression and gain-of-function mutations of NaV1.7 have been described in many studies of neuropathy pain, including PDN. Because of its slow open state and slow closed state inactivation and relatively hyperpolarized activation voltage dependence, NaV1.7 amplifies small depolarization below the threshold for the all-or-none action potential [46], thereby setting the gain on action potential electrogenesis and pain signaling by DRG neurons [47]. In PDN, it has been reported that NaV1.7 channel expression increased robustly in the DRG neurons of rats and triggered development of hyperalgesia and allodynia [48,49]. The link of NaV1.7 and PDN has been supported by the decrease of pain-related behaviors after reduction of NaV1.7 in DRG neurons induced via vector-mediated microRNA against NaV α subunits [50], vector-mediated release of γ-aminobutyric acid (GABA) [51], activation of delta opioid receptor [52], or administration of gabapentin [53]. In addition, NaV1.7 and NaV1.8 are believed to operate in tandem within DRG neurons, with NaV1.7 amplifying small stimuli to bring membrane potential to the threshold for activation of NaV1.8, which conducts the majority of the inward transmembrane current of an action potential upstroke during repetitive firing [54,55]. In a diabetic model, methylglyoxal depolarized neurons and induced posttranslational modifications of NaV1.8, however, it also promoted the slow inactivation of NaV1.7 [56].
Gain- or loss-of-function mutations in the Scn9A gene, which codes for NaV1.7, might be the determining pathogenic factor in the development of PDN. To date, at least 19 mutations in the Scn9A gene have been reported relating to primary erythromelalgia, which is an exceptionally painful disorder characterized by intermittent severe burning pain, erythema and elevation of temperature in the extremities [57]. Furthermore, gain-of-function mutations in the Scn9A gene were found to be linked with paroxysmal extreme pain disorder (PEPD) [58] and idiopathic small fiber neuropathy [59]; whereas, loss-of-function NaV1.7 mutations produce congenital insensitivity (or indifference) to pain (CIP), which is a disease in which patients experience painless fractures, lacerations, burns, and tooth extractions, for example [60]. Given that NaV1.7 channels are present in both pancreatic β cells and DRG neurons, a new concept, which might explain why some patients have neuropathy before diabetes onset, proposed by Hoeijmakers et al. [42], links the beginning of pancreatic β cell failure and PDN with genetic disruptions on NaV1.7 channels. A susceptible genetic background could facilitate generation of NaV1.7 mutations, leading to gain-of-function that evokes β cell lesions, and, thereafter, diabetes and hyperexcitability in DRG neurons [42,61].
7. Roles of TTX-S NaV1.3 and NaV1.7 Channels in Diabetes
Pancreatic islet cells express TTX-S VGSCs, especially NaV1.3 and NaV1.7 [62,63,64], which supports the generation of electrical activity. It is demonstrated that Nav1.3 and Nav1.7 channels are expressed within both α and β cells in different amounts, which explains the different properties of Na+ currents in both cells [62]. In particular, Zhang et al. found that NaV1.3 was the functionally important channel in both types of islet cells, whereas, due to an islet cell-specific factor, NaV1.7 channels were locked in an inactive state in mouse islet cells [62].
8. Pancreatic α Cells
Because glucagon secretion depends on the generation of Na+-dependent action potentials, TTX-S voltage-gated Na+ channels play a key role in regulating α cell function [64,65]. In DM, regulation of glucagon release is impaired with its levels inappropriately elevated at high glucose and reduced at low glucose, which might lead to fatal hypoglycemia. Some research indicated that the dysfunction of sodium channels in pancreatic α cells was associated with dysregulation of glucagon secretion in diabetes. In the islet α cells of STZ-induced diabetic mice with hyperglycemia, glucagon content and release was reported to increase due to enhanced Na+ current (INa), action potential duration and firing frequency [66]. In contrast, Na+ currents were inactivated under hypoglycemic conditions with reduced action potential height which inhibited glucagon secretion [67]. To investigate the underlying mechanism of the antidiabetic effect of VGSC blockers, Dhalla et al. found that glucagon release was mediated by the NaV1.3 channels, and selective NaV1.3 blockers might provide a novel approach for the treatment of diabetes [68]. Dusaulcy et al. demonstrated that some intrinsic defects were found in α cells and identified the Scn9A gene to be involved in glucagon biosynthesis and secretion [69]. The expression of the Scn9A gene was decreased in α cells from STZ-induced diabetic mice and insulin treatment normalized NaV1.7 [69].
Hoeijmakers et al. [42] hypothesized that NaV1.7 mutations chronically depolarize membrane potential, thereby increasing susceptibility to injury of pancreatic β cells and, thus, predisposing the individual to the development of diabetes. According to this hypothesis, diabetes does not necessarily cause peripheral neuropathy, but, on the contrary, both diabetes and neuropathy can occur as a result of NaV1.7 mutations, which increase vulnerability to injury in both small nerve fibers and β cells. In fact, NaV1.3 mutations possibly play a similar role. NaV1.3 channels are present in both pancreatic α and β cells and DRG neurons. The roles of VGSCs in pancreatic cells’ electrical activity are not yet completely understood, but it is likely that VGSCs have important roles in these cells. The chronic membrane depolarization or homeostatic overload and glycosylation influence the pancreatic cells’ activity and the hyperexcitability of DRG neurons. All together, these findings suggest that NaV1.3 dysregulation does not necessarily form a direct link between DM and PDN; both diabetes and neuropathy might occur as a result of NaV1.3 mutations, which increase vulnerability to injury in small nerve fibers and pancreatic α cells and β cells.
9. Pancreatic β Cells
The role of sodium channels in the generation of action potential and effective blockage of the channels using the specific VGSC inhibitor TTX determined TTX-S sodium channels critical for insulin secretion in pancreatic β cells [70,71]. A study showed that insulin secretion by β cells was affected by TTX-S in the mitochondrial membrane, which shaped both global Ca2+ and metabolism signals [72]. Furthermore, sodium channels were identified to be potential therapeutic targets in diabetes by analyzing the expression and characteristics of Na+ currents in β cells from mice that express green fluorescent protein under the control of the mouse insulin I gene promoter (MIP-GFP mice) [73]. Even though the relative expression of NaV1.3 and NaV1.7 differs in insulin-secreting β cells, with NaV1.7 being the dominant subtype, it has been shown that knocking out the sodium voltage-gated channel alpha subunit 3 Scn3A (part of NaV1.3 channel) reduces glucose-stimulated insulin secretion in mice [62]. Salunkhe et al. investigated whether modulation of the expression of various VGSC subunits could have an impact on insulin secretion, and found out that VGSCs, especially NaV1.3 (encoded by Scn3A), are regulated by microRNA-375 in rat insulinoma INS-1 832/13 cells and in primary mouse β cells [74]. Szabat et al. validated the role of the Scn9A gene in insulin production by examining NaV1.7 knockout mice, and insulin content of islet β cells from these animals had dramatically elevated [75]. Carbamazepine, a sodium channel inhibitor, has been confirmed as a positive modulator, which has protective effects in islet β cells [73,75].
Special cases, as follows, suggest that the presence of VGSC mutations is not only associated with painful neuropathy, but also with DM, where sodium channel genes are involved in insulin or glucagon secretion. Scn1B is a major regulatory subunit expressed with NaV1.7 protein in mouse pancreatic islets. It was demonstrated that in an Scn1B null genetic mouse model, pancreatic glucose-stimulated insulin and glucagon secretion reduced and resulted in severe hypoglycemia [76]. In two particular cases, patients with the NaV1.7 I739V mutation (c.2215A>G, p.Ile739Val) were found to have diabetes after the onset of peripheral neuropathy [42,77].
10. Conclusions
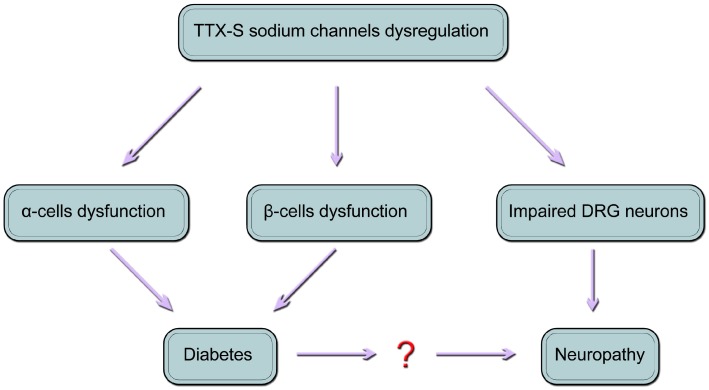
VGSCs are required for the initiation of action potentials in DRG neurons and pancreatic islet cells, including α cells and β cells. Usually, the roles of TTX-S channels in these three types of cells are separately studied. However, the association between diabetes and neuropathic pain has yet to be analyzed when it comes to the fact that painful form has no relationship to diabetes duration, metabolic control, or the severity of the neuropathy [3]. Based on all the aforementioned studies, it is reasonable to present a model (Figure 1), which is modified from the paper of Hoeijmakers et al. [42]. In this model, we propose that diabetes and peripheral neuropathy are the result of dysregulated TTX-S sodium channels, particularly TTX-S NaV1.3 and NaV1.7; both diabetes and painful neuropathy might occur as a result of NaV1.3 and/or NaV1.7 mutations or dysfunction, which increase vulnerability to injury in both small nerve fibers and pancreatic α and β cells.
Figure 1.
A model of the relationship between diabetes and neuropathy. The dysregulation of tetrodotoxin-sensitive (TTX-S) sodium channels, which are expressed in pancreatic α cells, β cells and dorsal root ganglion (DRG) neurons, leads to diabetes and periphery neuropathy. This model is modified from Hoeijmakers et al. [42].
To what extent the roles of NaV1.3 and NaV1.7 channels contribute to DM and PDN is unclear, however, it raises doubts as to whether painful neuropathy is caused by diabetes [42,77]. It is necessary to build an adequate stratification of DM patients with neuropathic pain. This new model may provide a starting point for further studies aimed at elucidating the molecular mechanisms of diabetes and painful neuropathy.
There are some limitations in this review. It would be more innovative with evidence based on functional tests. Investigation concerning the functional testing, such as voltage-clamp, patch-clamp and counter-flow of NaV1.3 and NaV1.7 to support the theories is worthy of future study.
Author Contributions
Linlin Yang and Quanmin Li conceived the review. Linlin Yang wrote the paper. Xinming Liu and Shiguang Liu contributed in the collection of literature.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Guariguata L., Whiting D., Hambleton I., Beagley J., Linnenkamp U., Shaw J. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.IDF Diabetes Atlas Group Update of mortality attributable to diabetes for the IDF Diabetes Atlas: Estimates for the year 2013. Diabetes Res. Clin. Pract. 2015;109:461–465. doi: 10.1016/j.diabres.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 3.Abbott C.A., Malik R.A., van Ross E.R., Kulkarni J., Boulton A.J. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the UK. Diabetes Care. 2011;34:2220–2224. doi: 10.2337/dc11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies M., Brophy S., Williams R., Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29:1518–1522. doi: 10.2337/dc05-2228. [DOI] [PubMed] [Google Scholar]
- 5.Spallone V., Greco C. Painful and painless diabetic neuropathy: One disease or two? Curr. Diabetes Rep. 2013;13:533–549. doi: 10.1007/s11892-013-0387-7. [DOI] [PubMed] [Google Scholar]
- 6.Van Acker K., Bouhassira D., de Bacquer D., Weiss S., Matthys K., Raemen H., Mathieu C., Colin I.M. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab. 2009;35:206–213. doi: 10.1016/j.diabet.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Brown M.J., Asbury A.K. Diabetic neuropathy. Ann. Neurol. 1984;15:2–12. doi: 10.1002/ana.410150103. [DOI] [PubMed] [Google Scholar]
- 8.Gooch C., Podwall D. The diabetic neuropathies. Neurologist. 2004;10:311–322. doi: 10.1097/01.nrl.0000144733.61110.25. [DOI] [PubMed] [Google Scholar]
- 9.Pabbidi R.M., Cao D.-S., Parihar A., Pauza M.E., Premkumar L.S. Direct role of streptozotocin in inducing thermal hyperalgesia by enhanced expression of transient receptor potential vanilloid 1 in sensory neurons. Mol. Pharmacol. 2008;73:995–1004. doi: 10.1124/mol.107.041707. [DOI] [PubMed] [Google Scholar]
- 10.Cappelleri J.C., Joshi A.V. Association between pain severity and health care resource use, health status, productivity and related costs in painful diabetic peripheral neuropathy patients. Pain Med. 2011;12:799–807. doi: 10.1111/j.1526-4637.2011.01103.x. [DOI] [PubMed] [Google Scholar]
- 11.Ritzwoller D.P., Ellis J.L., Korner E.J., Hartsfield C.L., Sadosky A. Comorbidities, healthcare service utilization and costs for patients identified with painful DPN in a managed-care setting. Curr. Med. Res. Opin. 2009;25:1319–1328. doi: 10.1185/03007990902864749. [DOI] [PubMed] [Google Scholar]
- 12.Ashcroft F.M., Rorsman P. KATP channels and islet hormone secretion: New insights and controversies. Nat. Rev. Endocrinol. 2013;9:660–669. doi: 10.1038/nrendo.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rorsman P., Eliasson L., Kanno T., Zhang Q., Gopel S. Electrophysiology of pancreatic β-cells in intact mouse islets of Langerhans. Prog. Biophys. Mol. Biol. 2011;107:224–235. doi: 10.1016/j.pbiomolbio.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Grabauskas G., Heldsinger A., Wu X., Xu D., Zhou S., Owyang C. Diabetic visceral hypersensitivity is associated with activation of mitogen-activated kinase in rat dorsal root ganglia. Diabetes. 2011;60:1743–1751. doi: 10.2337/db10-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Said G. Diabetic neuropathy: An update. J. Neurol. 1996;243:431–440. doi: 10.1007/BF00900495. [DOI] [PubMed] [Google Scholar]
- 16.Mantegazza M., Curia G., Biagini G., Ragsdale D.S., Avoli M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010;9:413–424. doi: 10.1016/S1474-4422(10)70059-4. [DOI] [PubMed] [Google Scholar]
- 17.Dib-Hajj S.D., Black J.A., Waxman S.G. Voltage-Gated Sodium Channels: Therapeutic Targets for Pain. Pain Med. 2009;10:1260–1269. doi: 10.1111/j.1526-4637.2009.00719.x. [DOI] [PubMed] [Google Scholar]
- 18.Catterall W.A. Voltage-gated sodium channels at 60: Structure, function and pathophysiology. J. Physiol. 2012;590:2577–2589. doi: 10.1113/jphysiol.2011.224204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldin A.L. Diversity of Mammalian Voltage-Gated Sodium Channels. Ann. N. Y. Acad. Sci. 1999;868:38–50. doi: 10.1111/j.1749-6632.1999.tb11272.x. [DOI] [PubMed] [Google Scholar]
- 20.Waxman S.G. Painful Na-channelopathies: An expanding universe. Trends Mol. Med. 2013;19:406–409. doi: 10.1016/j.molmed.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Waxman S.G. Neuroscience: Channelopathies have many faces. Nature. 2011;472:173–174. doi: 10.1038/472173a. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M.M., Wilson M.J., Gajewiak J., Rivier J.E., Bulaj G., Olivera B.M., Yoshikami D. Pharmacological fractionation of tetrodotoxin-sensitive sodium currents in rat dorsal root ganglion neurons by μ-conotoxins. Br. J. Pharmacol. 2013;169:102–114. doi: 10.1111/bph.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizzo M.A., Kocsis J.D., Waxman S.G. Slow sodium conductances of dorsal root ganglion neurons: Intraneuronal homogeneity and interneuronal heterogeneity. J. Neurophysiol. 1995;72:2796–2815. doi: 10.1152/jn.1994.72.6.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dib-Hajj S.D., Cummins T.R., Black J.A., Waxman S.G. Sodium channels in normal and pathological pain. Annu. Rev. Neurosci. 2010;33:325–347. doi: 10.1146/annurev-neuro-060909-153234. [DOI] [PubMed] [Google Scholar]
- 25.Waxman S.G., Cummins T.R., Dib-Hajj S.D., Black J.A. Voltage-gated sodium channels and the molecular pathogenesis of pain: A review. J. Rehabil. Res. Dev. 2000;37:517–528. [PubMed] [Google Scholar]
- 26.Cummins T.R., Sheets P.L., Waxman S.G. The roles of sodium channels in nociception: Implications for mechanisms of pain. Pain. 2007;131:243–257. doi: 10.1016/j.pain.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waxman S.G., Kocsis J.D., Black J.A. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is re-expressed following axotomy. J. Neurophysiol. 1994;72:466–470. doi: 10.1152/jn.1994.72.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Black J.A., Liu S., Tanaka M., Cummins T.R., Waxman S.G. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain. 2004;108:237–247. doi: 10.1016/j.pain.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 29.Hains B.C., Klein J.P., Saab C.Y., Craner M.J., Black J.A., Waxman S.G. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J. Neurosci. 2003;23:8881–8892. doi: 10.1523/JNEUROSCI.23-26-08881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black J.A., Cummins T.R., Plumpton C., Chen Y.H., Hormuzdiar W., Clare J.J., Waxman S.G. Upregulation of a silent sodium channel after peripheral, but not central, nerve injury in DRG neurons. J. Neurophysiol. 1999;82:2776–2785. doi: 10.1152/jn.1999.82.5.2776. [DOI] [PubMed] [Google Scholar]
- 31.Yin R., Liu D., Chhoa M., Li C.M., Luo Y., Zhang M., Lehto S.G., Immke D.C., Moyer B.D. Voltage-gated sodium channel function and expression in injured and uninjured rat dorsal root ganglia neurons. Int. J. Neurosci. 2016;126:182–192. doi: 10.3109/00207454.2015.1004172. [DOI] [PubMed] [Google Scholar]
- 32.Cummins T.R., Waxman S.G. Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury. J. Neurosci. 1997;17:3503–3514. doi: 10.1523/JNEUROSCI.17-10-03503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai J., Gold M.S., Kim C.S., Di B.A., Ossipov M.H., Porreca F. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain. 2002;95:143–152. doi: 10.1016/S0304-3959(01)00391-8. [DOI] [PubMed] [Google Scholar]
- 34.Lindia J.A., Köhler M.G., Martin W.J., Abbadie C. Relationship between sodium channel NaV1.3 expression and neuropathic pain behavior in rats. Pain. 2005;117:145–153. doi: 10.1016/j.pain.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 35.Sleeper A.A., Cummins T.R., Dib-Hajj S.D., Hormuzdiar W., Tyrrell L., Waxman S.G., Black J.A. Changes in expression of two tetrodotoxin-resistant sodium channels and their currents in dorsal root ganglion neurons after sciatic nerve injury but not rhizotomy. J. Neurosci. 2000;20:7279–7289. doi: 10.1523/JNEUROSCI.20-19-07279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waxman S.G., Hains B.C. Fire and phantoms after spinal cord injury: Na+ channels and central pain. Trends Neurosci. 2006;29:207–215. doi: 10.1016/j.tins.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Cummins T.R., Aglieco F., Renganathan M., Herzog R.I., Dib-Hajj S.D., Waxman S.G. Nav1.3 sodium channels: Rapid repriming and slow closed-state inactivation display quantitative differences after expression in a mammalian cell line and in spinal sensory neurons. J. Neurosci. 2001;21:5952–5961. doi: 10.1523/JNEUROSCI.21-16-05952.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Craner M.J., Klein J.P., Renganathan M., Black J.A., Waxman S.G. Changes of sodium channel expression in experimental painful diabetic neuropathy. Ann. Neurol. 2002;52:786–792. doi: 10.1002/ana.10364. [DOI] [PubMed] [Google Scholar]
- 39.Shah B.S., Gonzalez M.I., Bramwell S., Pinnock R.D., Lee K., Dixon A.K. β3, a novel auxiliary subunit for the voltage gated sodium channel is upregulated in sensory neurones following streptozocin induced diabetic neuropathy in rat. Neurosci. Lett. 2001;309:1–4. doi: 10.1016/S0304-3940(01)01976-0. [DOI] [PubMed] [Google Scholar]
- 40.Cheng K.I., Wang H.C., Chuang Y.T., Chou C.W., Tu H.P., Yu Y.C., Chang L.L., Lai C.S. Persistent mechanical allodynia positively correlates with an increase in activated microglia and increased P-p38 mitogen-activated protein kinase activation in streptozotocin-induced diabetic rats. Eur. J. Pain. 2014;18:162–173. doi: 10.1002/j.1532-2149.2013.00356.x. [DOI] [PubMed] [Google Scholar]
- 41.He X.-H., Zang Y., Chen X., Pang R.-P., Xu J.-T., Zhou X., Wei X.H., Li Y.Y., Xin W.J., Qin Z.H., et al. TNF-α contributes to up-regulation of Nav1.3 and Nav1.8 in DRG neurons following motor fiber injury. Pain. 2010;151:266–279. doi: 10.1016/j.pain.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Rush A.M., Cummins T.R., Waxman S.G. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J. Physiol. 2007;579:1–14. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samad O.A., Tan A.M., Cheng X., Foster E., Dib-Hajj S.D., Waxman S.G. Virus-mediated shRNA knockdown of Nav1.3 in rat dorsal root ganglion attenuates nerve injury-induced neuropathic pain. Mol. Ther. 2013;21:49–56. doi: 10.1038/mt.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan A.M., Samad O.A., Dib-Hajj S.D., Waxman S.G. Virus-Mediated Knockdown of Nav1.3 in Dorsal Root Ganglia of STZ-Induced Diabetic Rats Alleviates Tactile Allodynia. Mol. Med. 2015;21:544–552. doi: 10.2119/molmed.2015.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Black J.A., Langworthy K., Hinson A.W., Dib-Hajj S.D., Waxman S.G. NGF has opposing effects on Na+ channel III and SNS gene expression in spinal sensory neurons. Neuroreport. 1997;8:2331–2335. doi: 10.1097/00001756-199707070-00046. [DOI] [PubMed] [Google Scholar]
- 46.Cummins T.R., Howe J.R., Waxman S.G. Slow closed-state inactivation: A novel mechanism underlying ramp currents in cells expressing the hNE/PN1 sodium channel. J. Neurosci. 1998;18:9607–9619. doi: 10.1523/JNEUROSCI.18-23-09607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waxman S.G. Neurobiology: A channel sets the gain on pain. Nature. 2006;444:831–832. doi: 10.1038/444831a. [DOI] [PubMed] [Google Scholar]
- 48.Hong S., Morrow T.J., Paulson P.E., Isom L.L., Wiley J.W. Early painful diabetic neuropathy is associated with differential changes in tetrodotoxin-sensitive and-resistant sodium channels in dorsal root ganglion neurons in the rat. J. Biol. Chem. 2004;279:29341–29350. doi: 10.1074/jbc.M404167200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang Y., Zang Y., Zhou L., Gui W., Liu X., Zhong Y. The role of TNF-alpha/NF-kappa B pathway on the up-regulation of voltage-gated sodium channel Nav1.7 in DRG neurons of rats with diabetic neuropathy. Neurochem. Int. 2014;75:112–119. doi: 10.1016/j.neuint.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 50.Chattopadhyay M., Zhou Z., Hao S., Mata M., Fink D.J. Reduction of voltage gated sodium channel protein in DRG by vector mediated miRNA reduces pain in rats with painful diabetic neuropathy. Mol. Pain. 2012;8:17. doi: 10.1186/1744-8069-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chattopadhyay M., Mata M., Fink D.J. Vector-mediated release of GABA attenuates pain-related behaviors and reduces NaV1.7 in DRG neurons. Eur. J. Pain. 2011;15:913–920. doi: 10.1016/j.ejpain.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chattopadhyay M., Mata M., Fink D.J. Continuous δ-opioid receptor activation reduces neuronal voltage-gated sodium channel (NaV1.7) levels through activation of protein kinase C in painful diabetic neuropathy. J. Neurosci. 2008;28:6652–6658. doi: 10.1523/JNEUROSCI.5530-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J.L., Yang J.P., Zhang J.-R., Li R.Q., Wang J., Jan J.J., Zhuang Q. Gabapentin reduces allodynia and hyperalgesia in painful diabetic neuropathy rats by decreasing expression level of Nav1.7 and p-ERK1/2 in DRG neurons. Brain Res. 2013;1493:13–18. doi: 10.1016/j.brainres.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 54.Renganathan M., Cummins T.R., Waxman S.G. Contribution of Nav1.8 sodium channels to action potential electrogenesis in DRG neurons. J. Neurophysiol. 2001;86:629–640. doi: 10.1152/jn.2001.86.2.629. [DOI] [PubMed] [Google Scholar]
- 55.Bierhaus A., Fleming T., Stoyanov S., Leffler A., Babes A., Neacsu C., Sauer S.K., Eberhardt M., Schnölzer M., Lasitschka F., et al. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat. Med. 2012;18:926–933. doi: 10.1038/nm.2750. [DOI] [PubMed] [Google Scholar]
- 56.Wu M.T., Huang P.Y., Yen C.T., Chen C.C., Lee M.J. A Novel SCN9A Mutation Responsible for Primary Erythromelalgia and Is Resistant to the Treatment of Sodium Channel Blockers. PLoS ONE. 2013;8:1479. doi: 10.1371/journal.pone.0055212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fertleman C., Ferrie C. What’s in a name—Familial rectal pain syndrome becomes paroxysmal extreme pain disorder. J. Neurol. Neurosurg. Psychiatry. 2006;77:1294–1295. doi: 10.1136/jnnp.2006.089664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Faber C.G., Hoeijmakers J.G., Ahn H.S., Cheng X., Han C., Choi J.S., Estacion M., Lauria G., Vanhoutte E.K., Gerrits M.M., et al. Gain of function Nav1.7 mutations in idiopathic small fiber neuropathy. Ann. Neurol. 2012;71:26–39. doi: 10.1002/ana.22485. [DOI] [PubMed] [Google Scholar]
- 59.Cox J.J., Sheynin J., Shorer Z., Reimann F., Nicholas A.K., Zubovic L., Baralle M., Wraige E., Manor E., Levy J., et al. Congenital insensitivity to pain: Novel SCN9A missense and in-frame deletion mutations. Hum. Mutat. 2010;31:E1670–E1686. doi: 10.1002/humu.21325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoeijmakers J.G., Faber C.G., Merkies I.S., Waxman S.G. Channelopathies, painful neuropathy, and diabetes: Which way does the causal arrow point? Trends Mol. Med. 2014;20:544–550. doi: 10.1016/j.molmed.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 61.Schreiber A.K., Nones C.F., Reis R.C., Chichorro J.G., Cunha J.M. Diabetic neuropathic pain: Physiopathology and treatment. World J. Diabetes. 2015;6:432. doi: 10.4239/wjd.v6.i3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Q., Chibalina M.V., Bengtsson M., Groschner L.N., Ramracheya R., Rorsman N.J., Leiss V., Nassar M.A., Welling A., Gribble F.M., et al. Na+ current properties in islet α-and β-cells reflect cell-specific Scn3a and Scn9a expression. J. Physiol. 2014;592:4677–4696. doi: 10.1113/jphysiol.2014.274209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vignali S., Leiss V., Karl R., Hofmann F., Welling A. Characterization of voltage-dependent sodium and calcium channels in mouse pancreatic A-and B-cells. J. Physiol. 2006;572:691–706. doi: 10.1113/jphysiol.2005.102368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Göpel S., Kanno T., Barg S., Weng X.G., Gromada J., Rorsman P. Regulation of glucagon release in mouse α-cells by KATP channels and inactivation of TTX-sensitive Na+ channels. J. Physiol. 2000;528:509–520. doi: 10.1111/j.1469-7793.2000.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramracheya R., Ward C., Shigeto M., Walker J.N., Amisten S., Zhang Q., Johnson P.R., Rorsman P., Braun M. Membrane potential-dependent inactivation of voltage-gated ion channels in α-cells inhibits glucagon secretion from human islets. Diabetes. 2010;59:2198–2208. doi: 10.2337/db09-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang Y.C., Rupnik M.S., Karimian N., Herrera P.L., Gilon P., Feng Z.P., Gaisano H.Y. In situ electrophysiological examination of pancreatic α cells in the streptozotocin-induced diabetes model, revealing the cellular basis of glucagon hypersecretion. Diabetes. 2013;62:519–530. doi: 10.2337/db11-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Q., Ramracheya R., Lahmann C., Tarasov A., Bengtsson M., Braha O., Braun M., Brereton M., Collins S., Galvanovskis J., et al. Role of KATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes. Cell Metab. 2013;18:871–882. doi: 10.1016/j.cmet.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dhalla A.K., Yang M., Ning Y., Kahlig K.M., Krause M., Rajamani S., Belardinelli L. Blockade of Na+ channels in pancreatic α-cells has antidiabetic effects. Diabetes. 2014;63:3545–3556. doi: 10.2337/db13-1562. [DOI] [PubMed] [Google Scholar]
- 69.Dusaulcy R., Handgraaf S., Heddad-Masson M., Visentin F., Vesin C., Reimann F., Gribble F., Philippe J., Gosmain Y. α-Cell Dysfunctions and Molecular Alterations in Male Insulinopenic Diabetic Mice Are Not Completely Corrected by Insulin. Endocrinology. 2016;157:536–547. doi: 10.1210/en.2015-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Braun M., Ramracheya R., Bengtsson M., Zhang Q., Karanauskaite J., Partridge C., Johnson P.R., Rorsman P. Voltage-gated ion channels in human pancreatic β-cells: Electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57:1618–1628. doi: 10.2337/db07-0991. [DOI] [PubMed] [Google Scholar]
- 71.MacDonald P.E. Signal integration at the level of ion channel and exocytotic function in pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 2011;301:E1065–E1069. doi: 10.1152/ajpendo.00426.2011. [DOI] [PubMed] [Google Scholar]
- 72.Nita I.I., Hershfinkel M., Kantor C., Rutter G.A., Lewis E.C., Sekler I. Pancreatic β-cell Na+ channels control global Ca2+ signaling and oxidative metabolism by inducing Na+ and Ca2+ responses that are propagated into mitochondria. FASEB J. 2014;28:3301–3312. doi: 10.1096/fj.13-248161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang Y.H.C., Vilin Y.Y., Roberge M., Kurata H.T., Johnson J.D. Multiparameter Screening Reveals a Role for Na+ Channels in Cytokine-Induced β-Cell Death. Mol. Endocrinol. 2014;28:406–417. doi: 10.1210/me.2013-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salunkhe V., Esguerra J., Ofori J., Mollet I., Braun M., Stoffel M., Wendt A., Eliasson L. Modulation of microRNA-375 expression alters voltage-gated Na+ channel properties and exocytosis in insulin-secreting cells. Acta Physiol. 2015;213:882–892. doi: 10.1111/apha.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Szabat M., Modi H., Ramracheya R., Girbinger V., Chan F., Lee J.T., Piske M., Kamal S., Carol Yang Y.H., Welling A. High-content screening identifies a role for Na+ channels in insulin production. R. Soc. Open Sci. 2015;2:150306. doi: 10.1098/rsos.150306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ernst S.J., Aguilar-Bryan L., Noebels J.L. Sodium channel β1 regulatory subunit deficiency reduces pancreatic islet glucose-stimulated insulin and glucagon secretion. Endocrinology. 2009;150:1132–1139. doi: 10.1210/en.2008-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han C., Hoeijmakers J.G., Liu S., Gerrits M.M., te Morsche R.H., Lauria G., Dib-Hajj S.D., Drenth J.P., Faber C.G., Merkies I.S., et al. Functional profiles of SCN9A variants in dorsal root ganglion neurons and superior cervical ganglion neurons correlate with autonomic symptoms in small fibre neuropathy. Brain. 2012;135:2613–2628. doi: 10.1093/brain/aws187. [DOI] [PubMed] [Google Scholar]