Abstract
The MHC class I chain–related molecules (MICs) have previously been shown to be induced on most epithelial tumor cells. Engagement of MIC by the activating immune receptor NKG2D triggers NK cells and augments antigen-specific CTL anti-tumor immunity. The MIC-NKG2D system was proposed to participate in epithelial tumor immune surveillance. Paradoxically, studies suggest that tumors may evade MIC-NKG2D–mediated immunity by MIC shedding–induced impairment of effector cell function. Here we demonstrate the first evidence to our knowledge of a significant correlation of MIC shedding and deficiency in NK cell function with the grade of disease in prostate cancer. MIC is widely expressed in prostate carcinoma. The presence of surface target MIC, however, is counteracted by shedding. A significant increase in serum levels of soluble MIC (sMIC) and deficiency in NK cell function was shown in patients with advanced cancer. Finally, the deficiency in NK cell function can be overcome by treatment with IL-2 or IL-15 in vitro. Our results suggest that (a) deficiency in MIC-NKG2D immune surveillance may contribute to prostate cancer progression, (b) sMIC may be a novel biomarker for prostate cancer, and (c) using cytokines to restore MIC-NKG2D–mediated immunity may have clinical significance for prostate cancer in cell-based adaptive immunotherapy.
Introduction
The C-type lectin–like stimulatory immune receptor NKG2D is expressed by all human NK and CD8+ T cells and by most γδ T cells (1–5). NKG2D-mediated immune activation can be triggered by interaction with its ligands (6–8): the family of stress-induced MHC class I chain–related molecules (MICs) MICA and MICB (5), and the UL16-binding protein (ULBP) family (9–11). In NK cells, the activation signal mediated by NKG2D was shown to dominate the inhibitory signal mediated by MHC class I binding to killer inhibitory receptors, leading to lysis of target cells that express NKG2D ligands (1–4). NKG2D-mediated activation signal also costimulates antigen-specific CD8+ T cell immunity and is necessary for activation of cytotoxic γδ T cells (5, 12–15).
According to current available data, MIC molecules are the best-characterized ligands and are the most frequently expressed ligands on epithelial tumors. MIC is induced on a broad range of epithelial tumor cells, such as melanoma, colon, breast, lung, ovary, renal, and hepatocellular carcinomas, but is absent from normal tissues (16–18). Cells expressing MIC on their surface are susceptible to NK and antigen-specific T cell immunity. Thus, surface expression of MIC on transformed cells is proposed to mark nascent tumors for immune surveillance (6–8). MIC proteins share structural homology with MHC class I molecules but have no role in antigen presentation (19). The two closely related MICs MICA and MICB share 84% amino acid sequence identity in the ectodomain and are suggested to be derived from recent gene duplication events (20).
Sequences directly related to MICA and MICB are conserved in the genomes of most if not all mammalian species with the exception of rodents (21, 22). In mice, the retinoic acid early inducible family of proteins RAE-1 (23–25), the minor histocompatibility antigen H60 (23–25), and the murine ULBP-like transcript 1 (26) were identified as mouse NKG2D ligands. Ectopic expression of RAE-1 or H60 on tumor cells resulted in potent tumor rejection by NK cells in syngeneic mice (27), which suggests a potential role for NKG2D-mediated activation of cytolytic effector cells in tumor immunity. However, little is known about the clinical significance of NKG2D-mediated immunity for human tumors. Studies have demonstrated that the MIC-NKG2D–mediated immunity is impaired in patients with progressive breast, lung, ovarian, or colon cancer (28, 29). In most of these cases, malignant tumors shed MIC and subsequently induce downregulation of surface NKG2D expression on NK cells and/or CTLs. Such a deficiency in NKG2D-mediated effector cell function has been proposed to be one of the mechanisms by which tumor cells evade NK cell and CTL immune surveillance and progress (28, 29). Nonetheless, to date, compelling evidence is lacking on how shedding or impairment of MIC in NKG2D-mediated effector function correlates with disease stages or progression.
Here we investigated MIC expression in primary prostate carcinoma and NKG2D-mediated NK cell function in prostate cancer patients with various degrees of disease. Biopsy studies showed that MIC expression was prevalent in prostate carcinoma, suggesting a role for MIC-NKG2D–mediated immunity in prostate cancer. However, membrane-bound MIC was predominant only in low-grade cancers and significant serum levels of soluble MIC (sMIC) were detected in higher-grade cancers, indicating that prostate tumors counteract MIC-stimulated, NKG2D-mediated immunity via MIC shedding. Importantly, serum levels of sMIC and a loss of NKG2D-mediated NK cell function significantly correlated with the degree of disease in prostate cancer patients. Furthermore, we investigated whether in vitro stimulation with cytokines can restore sMIC-impaired, NKG2D-mediated cytotoxic function in NK cells from prostate cancer patients.
Results
NKG2D-dependent NK cell cytotoxicity against prostate cancer cell lines.
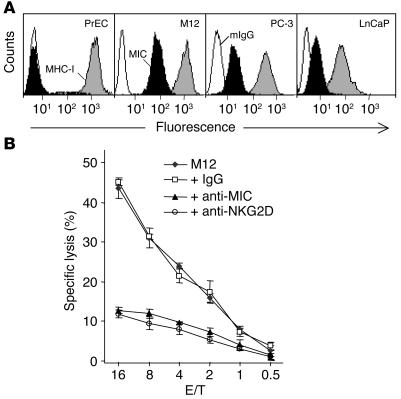
Transformation-associated MIC expression renders tumor cells more susceptible to NK cell cytotoxicity (1–8). Therefore, we first examined the expression of MIC molecules in prostate cancer cell lines. As shown by flow cytometry analysis with mAb BAMO1, which recognizes both MICA and MICB (together called MIC), MIC was abundantly expressed in the prostate cancer cell lines M12, PC-3, and LnCaP but was absent from normal prostate primary epithelial cells (PrECs) (Figure 1A).
Figure 1.
MIC expression in prostate cancer cell lines and susceptibility of prostate cancer cells to NK cell activation in an NKG2D-dependent fashion. (A) Flow cytometry analysis of MIC expression in PrECs and the prostate cancer cell lines M12, PC-3, and LnCaP. White profiles represent staining with control mouse IgG (mIgG); black profiles represent staining with the BAMO1 mAb against MIC (MIC); and gray profiles represent staining with the W6/32 mAb against MHC I (MHC I). (B) Cytotoxicity of NK cells isolated from a healthy individual against M12 target cells at the indicated effector/target (E/T) ratios. NK cell cytotoxicity was inhibited by masking of NKG2D on NK cells or of MIC on M12 cells with 10 μg/ml of antibody M585 (+ anti-NKG2D) (31) or BAMO1 (+ anti-MIC) (30), respectively, but was not affected by control mouse IgG (+ IgG). Data shown are mean ± SD of triplicates. Results shown are representative of three independent experiments.
Studies have shown that NK cell cytotoxicity against MIC-positive tumor cells may be NKG2D dependent or may be a result of combined activation of NKG2D and natural cytotoxicity receptors, depending upon the source of target cells (30). We thus tested whether the susceptibility of these MIC-expressing prostate cancer cells to NK cell cytotoxicity was NKG2D dependent. As shown in Figure 1B, M12 cells elicited NK cell target lytic activity and this response was substantially inhibited by either masking of MIC with mAb BAMO1 (31) or masking of NKG2D with mAb M585 (32). A similar NK cell lytic response was triggered by PC-3 or LnCaP cells but not by normal prostate primary epithelial cells (data not shown). Moreover, stimulating NK cells with these MIC-expressing prostate cancer cells resulted in a significant level of IFN-γ release (data not shown). These data indicate a MIC-specific, NKG2D-dependent activation of NK cells against prostate cancer cells.
MIC expression in primary prostate carcinomas.
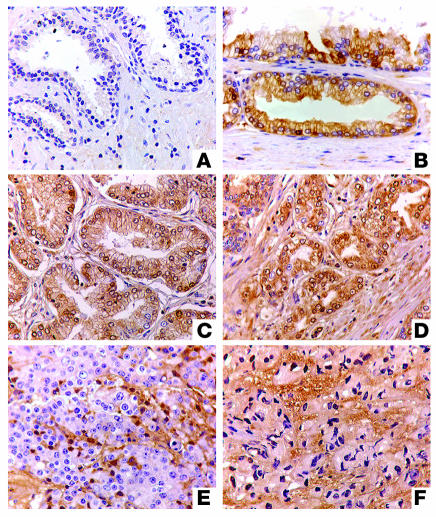
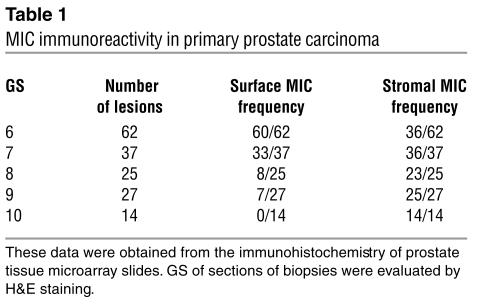
We investigated the expression of MIC by prostate secretory epithelial cells using a set of tissue microarray slides. Each slide was composed of the following: prostate biopsies from normal individuals and from individuals with prostatic neoplasia, carcinomas of varying Gleason scores (GS), and high-grade prostatic intraepithelial neoplasia (HGPIN). MIC immunoreactivity was seen only in neoplastic prostates but not in benign prostate glands (Figure 2A). Positive MIC immunoreactivity with varying patterns was exhibited in 95% of neoplastic prostate (Table 1). In HGPIN, MIC immunoreactivity was predominantly localized at the luminal surface of secretory epithelial cells (Figure 2B). In carcinomas with GS of 6–7, MIC immunoreactivity was no longer restricted to the epithelial cell surface but was diffusely distributed in the infiltrated glands (Figure 2C). In carcinomas with GS of 8–10, most tumor cells showed negative cell surface MIC immunoreactivity and diffuse MIC staining was shown in the stroma (Figure 2, D–F). These observations indicate that MIC is induced in the early stage of prostate luminal epithelial cell transformation (as early as HGPIN) and is expressed widely in prostate carcinoma. The data also suggest that loss of predominant surface localization of MIC may be associated with progression to invasive tumor or to progressively higher grades.
Figure 2.
Immunohistochemical staining of MIC on human prostate biopsies. (A) No MIC immune reactivity in a benign prostate. (B and C) Predominant surface MIC immune reactivity in prostate secretory epithelia of HGPIN (B) and GS 5–6 prostate carcinoma (C). (D–F) MIC staining was no longer abundantly present on carcinoma cell surface and diffuse stromal MIC immuoreactivity was shown in high-grade cancers (GS 8–10). Original magnification, ×40.
Table 1.
MIC immunoreactivity in primary prostate carcinoma
Serum levels of sMIC in prostate cancer patients.
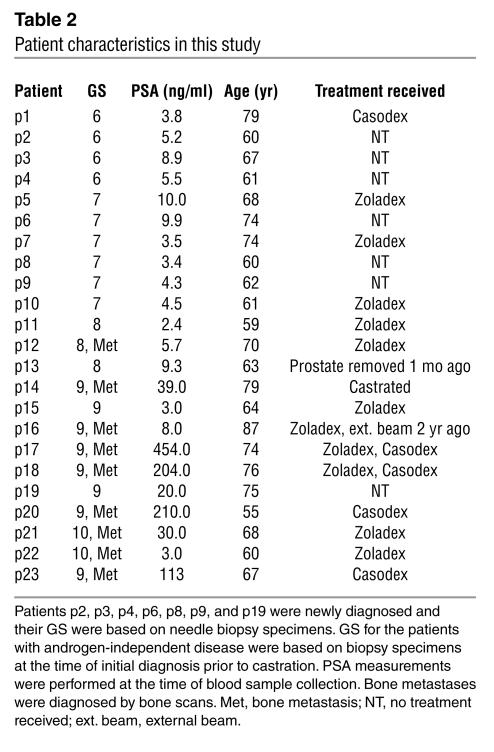
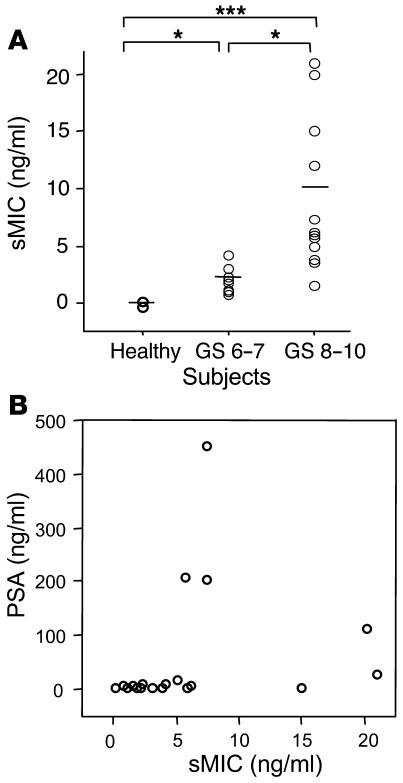
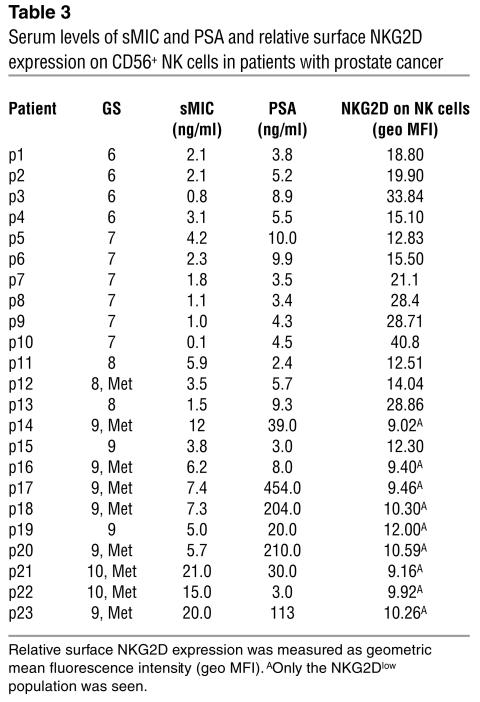
Our observations in prostate biopsy studies suggested an association of increased loss of cell surface MIC with higher grades of prostate cancer. We thus seek to investigate serum levels of sMIC in patients with prostate cancer of various grades. Sera from 23 characterized prostate cancer patients (Table 2) and from 10 age-matched healthy male donors were assayed for sMICA by noncompeting solid-phase ELISA. AMO1 (specific for the α1α2 domain of MICA) and BAMO3 (specific for the α3 domain of MICA and MICB) were used as capture and detection antibodies, respectively (30). Significant amounts of sMICA, as high as 21 ng/ml, were detected in sera from nearly all patients (Student’s t test, P < 0.05, Figure 3A). No significant levels of sMICA were detected in sera from healthy donors. Furthermore, significantly higher levels of sMICA were seen in sera from patients with more advanced disease, that is, with primary carcinoma at a GS greater than 7 at the time of diagnosis and/or with recurrent androgen-independent disease (Student’s t test, P < 0.05; Figure 3A). Interestingly, although in some cases patients with high serum levels of prostate-specific antigen (PSA) tended to have higher levels of sMICA (p14, p17, p18, p20, p21, and p23; Table 3), serum levels of sMICA did not seem to correlate significantly with PSA overall (Figure 3B; ANOVA, r = 0.2, P = 0.38).
Table 2.
Patient characteristics in this study
Figure 3.
Serum levels of sMIC and PSA. (A) Serum levels of sMIC in healthy subjects (Healthy; n = 10) and prostate cancer patients with primary carcinomas with a GS of 6–7 (n = 13) or a GS of 8–10 (n = 10). Horizontal lines indicate mean value of respective groups. *P < 0.05; ***P < 0.001. Data shown are mean values of three independent ELISA measurements. (B) Lack of correlation of serum levels of sMIC with PSA in prostate cancer patients (r = 0.02, P = 0.48).
Table 3.
Serum levels of sMIC and PSA and relative surface NKG2D expression on CD56+ NK cells in patients with prostate cancer
Deficiency in NKG2D-mediated NK cell function in advanced prostate cancer.
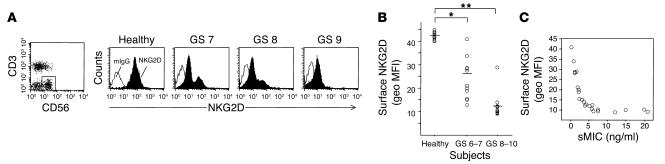
Our observation that MIC is induced frequently in transformed prostate epithelial cells suggests a potential role for NKG2D-mediated anti-tumor immunity in prostate cancer. However, the MIC-NKG2D immune surveillance may not be effective in prostate cancers, probably due to at least two factors. One is loss of the tumor cell surface target molecule MIC, as shown by our immunohistochemistry study of prostate tissue microarrays (Figure 2 and Table 1). The other is sMIC-induced downregulation of surface NKG2D expression on effector cells. The sMIC-induced impairment in NKG2D-mediated effector cell function has been demonstrated in other cancers (28, 29). To investigate whether deficiency in NKG2D-mediated effector cell function is associated with prostate cancer, we analyzed surface NKG2D expression on circulating NK cells freshly isolated from the 23 prostate cancer patients (Table 2) and from 10 healthy donors, using the M585 mAb against NKG2D in combination with anti-CD3 and anti-CD56. As demonstrated in Figure 4, A and B, significantly reduced surface NKG2D expression was seen in NK cells from prostate cancer patients compared with those of healthy donors (Student’s t test, P < 0.05). A more significant reduction in NK cell surface NKG2D expression was shown in patients with advanced disease (GS 8–10; P < 0.001). Interestingly, in nearly all patients with cancers with GS of 6–8 and nonmetastatic cancer with a GS of 9, two populations of CD3–CD56+ NK cells, NKG2Dlow and NKG2Dnormal, were seen and an increase in NKG2Dlow population was likely to be associated with cancer of a higher GS or with higher levels of serum sMIC. In all patients with cancer with a GS of 10 and metastatic cancer with a GS of 9, only the NKG2Dlow population was seen. In all cases, no difference in CD56 expression was noted between NKG2Dlow and NKG2Dnormal NK cells (data not shown). Nevertheless, a significant inverse correlation between serum levels of sMIC and levels of NK cell surface NKG2D expression was found in all patients by ANOVA (Figure 4C; r = 0.56, P = 0.0049).
Figure 4.
Surface NKG2D expression by NK cells from normal male donors and from prostate cancer patients. Cells were isolated and stained as described in Methods. (A) Plots show surface NKG2D expression of CD3–CD56+ NK cells from a representative healthy subject and three representative prostate cancer patients (GS 7, GS 8, and GS 9). Note that NKG2Dlow and NKG2Dnormal populations were present in the patients with cancer with GS of 7 and 8. (B) Geometric mean fluorescence intensity (geo MFI) of surface NKG2D on CD3–CD56+NK cells from 10 healthy subjects, 13 prostate cancer patients with primary carcinoma with a GS of 6–7, and 10 patients with prostate cancer with a GS of 8–10. Data shown are from three independent flow cytometry measurements. Horizontal lines indicate mean value of respective groups. *P < 0.05; **P < 0.01. Note that surface NKG2D expression on CD56+ cells was measured as geo MFI, due to the heterogeneous expression of surface NKG2D. (C) Inverse correlation of surface NKG2D expression on CD3–CD56+ NK cells with serum levels of sMIC in prostate cancer patients (r = 0.57, P = 0.0049).
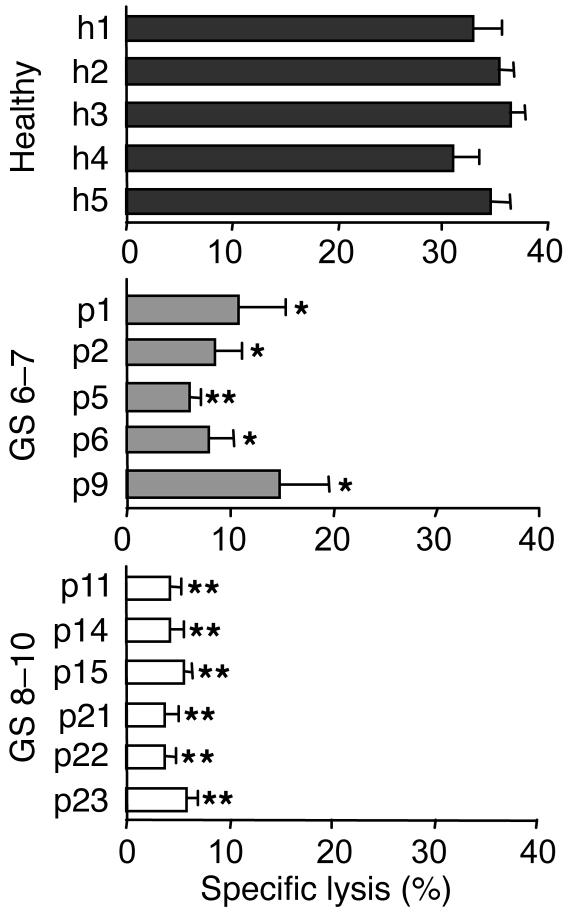
We next examined the NKG2D-dependent tumor lytic ability of freshly isolated polyclonal NK cells from representative prostate cancer patients and healthy donors. As presented in Figure 5, NK cell cytotoxicity against MIC-positive prostate cancer M12 cells was substantially decreased in all representative prostate cancer patients. A more pronounced decrease was seen in patients with high-grade cancers (GS 8–10). These cytotoxic responses were inhibited by pre-masking of NKG2D on NK cells with the antibody M585 or preincubation of M12 cells with the anti-MIC BAMO1 (data not shown). These results not only demonstrated deficiency of NKG2D-mediated NK cell anti-tumor function in prostate cancer patients but also demonstrated the correlation of severity of NK cell functional impairment with degree of disease.
Figure 5.
Deficiency in NK cell anti-tumor cytotoxicity in prostate cancer patients. Freshly isolated NK cells from peripheral blood of healthy subjects and representative prostate cancer patients (GS 6–7 and GS 8–10) were used as effector cells against M12 target cells in a 4-hour 51Cr-release assay. Data showed a significant reduction in cancer patient NK cell cytotoxicity against M12 cells. Effector/target: 10/1. *P < 0.05; **P < 0.01.
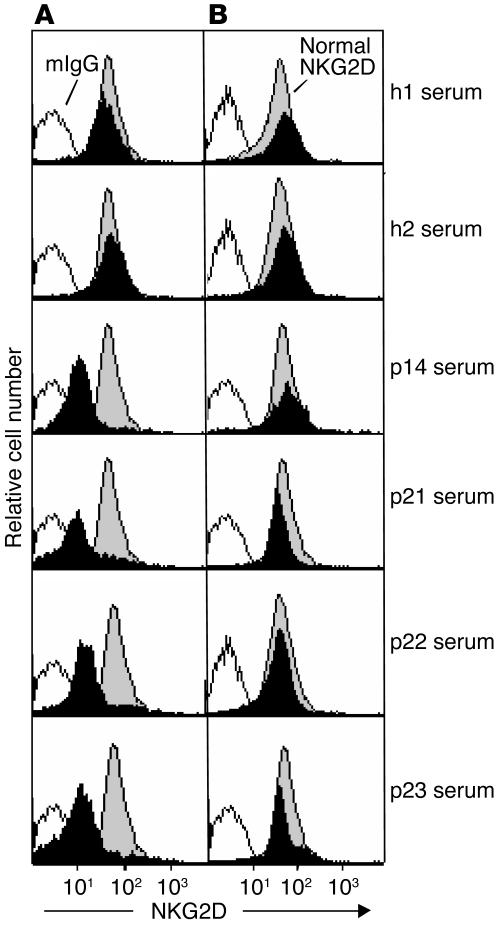
Downmodulation of normal NK cell surface NKG2D expression by sMIC in prostate cancer serum.
Studies have shown that several soluble factors in prostate cancer serum can inhibit NK cell function (33–36). To ascertain the association of cancer serum sMIC with the deficiency in NKG2D-dependent NK cell cytotoxicity, we investigated the effect of serum from patients with prostate cancer with a GS of 9 (p14 and p23) or a GS of 10 (p21 and p22) on normal NK cell surface NKG2D expression. A decrease in surface NKG2D expression was observed after 12 hours (data not shown) and a maximum reduction of 5- to 7-fold was observed after 48 hours (Figure 6A). When the sera were preincubated with mouse IgG, the same reduction in surface NKG2D expression as in Figure 6 was observed (data not shown). In contrast, when the sera were pretreated with the BAMO1 mAb against MIC, no significant reduction in surface NKG2D expression was observed, even at 48 hours (Figure 6B), suggesting that sMIC in the sera of prostate cancer patients was the main factor that downregulated NK cell surface NKG2D expression. Subsequently, after 48 hours of incubation with cancer serum of NK cells from a healthy donor, we observed a significant reduction in IFN-γ secretion and NKG2D-dependent cytotoxicity of these NK cells against M12 cells (data not shown).
Figure 6.
Effect of prostate cancer serum on normal NK cell surface NKG2D expression. Normal NK cells were cultured for 48 hours in media containing 20% serum from representative healthy donors (h1 and h2) and patients with prostate cancer with a GS of 9 (p14 and p23) or a GS of 10 (p21 and p22). (A) A reduction in NK cell surface NKG2D expression after cultured in serum from cancer patients was seen. (B) The effect on NKG2D expression was inhibited by pretreatment of serum from cancer patients with the BAMO1 mAb against MIC. Results shown are representative of three independent experiments.
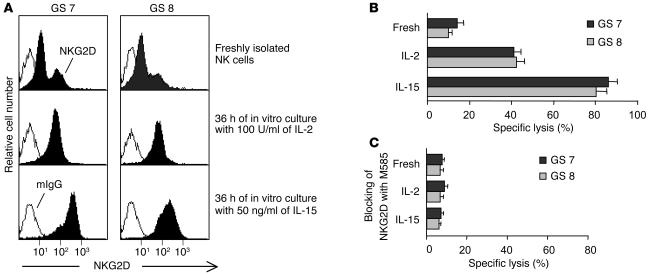
Restoring NKG2D-mediated NK cell function by in vitro cytokine stimulation.
Studies have shown that IL-15 can upregulate NKG2D expression in vitro in both CD8+ T cells (37) and NK cells (32). Very recent studies have demonstrated a similar effect of IL-2 on CD8+ T cells in vitro (38). We thus addressed whether in vitro cytokine stimulation could overcome the NKG2D-mediated functional deficiency of NK cells from prostate cancer patients. We cultured NK cells from patients with cancers with a GS of 7 (p6) or a GS of 8 (p11) in normal media containing 100 U/ml of IL-2 or 20 ng/ml of IL-15. After 24 hours, an increase in surface NKG2D expression on these NK cell was observed (data not shown). A maximum of increase was shown after 36 hours of culture (Figure 7A). In the presence of IL-15, a more pronounced increase in NKG2D expression was seen (Figure 7A). Moreover, these NK cells exhibited substantially enhanced cytotoxicity against M12 cells (Figure 7B). These lytic responses were inhibited when surface NKG2D was masked with mAb M585 (Figure 7C), indicating an NKG2D-dependent enhancement in NK cell cytotoxicity.
Figure 7.
Recovery of the NKG2D-mediated anti-tumor function of NK cells from prostate cancer patients by in vitro stimulation. NK cells from representative patients with cancer with a GS of 7 (p4) or a GS of 8 (p11) were cultured for 36 hours with 100 U/ml of IL-2 or 50 ng/ml of IL-15. (A) Increased NK cell surface NKG2D expression after IL-2 or IL-15 stimulation. (B) Increased NK cell cytotoxicity against M12 cells. Effector/target: 10/1. Data shown are representative of three independent experiments. (C) NK cell cytotoxicity against M12 cells were blocked by preincubation with the M585 mAb against NKG2D. Effector/target: 10/1. Fresh, freshly isolated NK cells.
Discussion
In this study, we determined the significance of MIC-specific, NKG2D-mediated immune surveillance in prostate cancer for the first time, to our knowledge. Our data showed prevalent MIC expression in prostate carcinoma and the susceptibility of MIC-expressing prostate cancer cells to NK cell activation, suggesting that MIC-NKG2D tumor immune surveillance can play an important role in the eradication of prostate cancer cells. Consistent with studies in other epithelial tumors, our data demonstrated negative effects resulting from MIC shedding. One effect was the loss of predominant surface localization of MIC in high-grade prostate carcinomas. The data showed that although it was highly expressed on prostate carcinomas, membrane-bound surface MIC was prevalent only in HGPIN and low-grade cancers. Indeed, serum levels of sMIC in prostate cancer patients were found to correlate significantly with the grade of the disease. The second effect was sMIC-induced impairment of NK cell surface NKG2D expression. According to our preliminary data, surface expression of NKG2D was also impaired in peripheral CD8+ T cells from prostate cancer patients (J.D. Wu, unpublished observations). An sMIC-induced deficiency in effector function has been shown in other cancers (28, 29); however, we are the first, to our knowledge, to correlate the levels of sMIC and deficiency in NK cell function with the degree of disease in prostate cancer.
Our cross-sectional clinical data showed a significant increase in serum levels of sMIC in patients with high-grade and/or invasive prostate cancers. Although the GS were characterized at the time of diagnosis of the primary carcinomas, most of the patients with high-grade cancers had recurrent androgen-independent disease, including bone metastasis. Profoundly higher levels of sMIC were shown in the sera of patients with bone metastasis. The serum levels of sMIC did not show significant correlation with PSA; however, most if not all patients with androgen-independent metastatic disease tended to have high serum levels of sMIC and PSA (Table 3). This suggests that serum levels of sMIC may be a potential serum marker complementary to PSA for prostate cancer prognosis. A systematic and intensive clinical investigation to evaluate the correlation between serum levels of sMIC with PSA and cancer progression is currently underway in our laboratory.
Notably, our data showed that surface NKG2D expression on polyclonal NK cells from prostate cancer patients was not uniform. This heterogeneity of NK cells was also observed previously in colon cancer patients, and most of the NKG2Dlow NK cells were shown to lack the homing receptor CXCR1, which can also be downmodulated by serum sMIC (29). The fact that we did not observe any difference in CD56 expression between NKG2Dlow and NKG2Dnormal NK cells indicates this discrepancy is independent of the two main subsets of human NK cells. In contrast, we observed that the numbers of NKG2Dlow NK cells increased in patients with more-advanced cancers, including bone metastases. In all cases, higher levels of serum sMIC were detected. As both surface expression of NKG2D and CXCR1 can be impaired by sMIC (29), the possibility of competition for sMIC cannot be ruled out. Furthermore, sMIC-induced downmodulation of NKG2D expression was shown to be required for internalization of surface CXCR1 (29), suggesting multiple and complex mechanisms are involved, which remain to be explored.
Long-term immunosuppression increases the incidence of many forms of malignancies. Systemic and local immune defects have been demonstrated in prostate cancer progression (33–36, 39). Several possible tumor-associated candidate substances within the prostate were found to be associated with suppression of host immune responses to cancer cells. The best described substance is TGF-β1, which is expressed during progression of prostate cancer to androgen independence and is highest in the most poorly differentiated, highest-grade primary human cancer samples (40). TGF-β1 was proposed to function as lymphocyte proliferation inhibitor in prostate cancer (40). Other substances, such as PSA and inducible nitric oxide synthases, have also been found to suppress T lymphocyte proliferation (41, 42). Recent studies have shown that TGF-β downregulates NKG2D expression at the transcriptional level in some but not in all NK clones or in polyclonal NK cells (43). The mechanism by which TGF-β regulates NKG2D expression remains unclear. It should be emphasized that according to our data, should high levels of TGF-β in the serum of prostate cancer patients downregulate NKG2D expression on NK cells, the effect would be secondary to the prevalent sMIC-induced and presumably direct internalization of surface NKG2D. Our study has identified a new form of tumor-mediated immunosuppression in prostate cancer.
There is in vivo evidence that implantation of functional human NK cells into mice xenografted with MIC-positive human tumors or ectopic overexpression of MIC in human tumor cells induces potent tumor cell sensitization to NK cells (29, 44). In this study, we showed that MIC-expressing prostate cancer cells are susceptible to NKG2D-mediated NK cell destruction in vitro. This suggests that MIC could serve as a tumor antigen target in prostate cancer for immunotherapy, tagging tumor cells for immune destruction. We also demonstrated loss of the tumor cell surface antigen MIC and impaired NKG2D-mediated anti-tumor responses in patients with prostate cancer of high grades. Therefore, counteracting tumor shedding of surface MIC and sustaining the NKG2D-mediated immune response could be a new therapeutic option for prostate cancer. Recent evidence has shown that production of sMIC results from MMP activity (31). Our preliminary investigations have also suggested that inhibition of MMP activity would inhibit shedding of MIC in prostate cancer cell lines (J.D. Wu, unpublished observations). Upregulation of MMP-9 and MMP-2 and downregulation of tissue MMP inhibitors TIMP-1 and TIMP-2 have been found to be associated with malignant progression of prostate cancer (45, 46). Therefore, identifying and targeting the specific MMPs that contribute to the shedding of MIC in prostate cancer would be a potential strategy for enhancing the presence of cell surface MIC and for rendering tumors susceptible to MIC-specific, NKG2D-mediated immune destruction.
MIC expression is induced on many epithelial tumors (16–18) and, as presented here, on 95% of prostate carcinomas. The MIC gene transcriptional regulatory sequences contain heat shock elements similar to those in the hsp70 promoter, and studies have shown that MIC expression can be upregulated by heat shock treatment (47). Limited information is available about how MIC expression is regulated, particularly in specific tumors. Recently, Jinushi et al. (18) showed that retinoic acid upregulates MIC expression in hepatoma cells, and Molinero et al. (48) showed that activation of the MAPK intracellular signaling pathway upregulates MICA expression on activated T lymphocytes. However, the specific mechanisms of the induction of MIC expression remain unclear.
In summary, our study has suggested that a deficiency in MIC-NKG2D immune surveillance may contribute to prostate cancer progression. Our study also suggests that sMIC may potentially be an additional marker for prostate cancer. Our results using cytokines to enhance NKG2D-mediated NK cell function in vitro have provided an alternative for cell-based immune therapy for prostate cancer.
Methods
PBMCs and sera.
Blood samples were obtained from 23 prostate cancer patients (age 58–80) with primary tumors with a GS of 6 or greater (Table 2) and 10 healthy male donors (age 50–65). Whole blood was collected in heparinized tubes. Serum was collected and PBMCs were separated by Ficoll-Hypaque density gradient centrifugation. This study was approved by University of Washington Human Subject Review Board (Seattle, Washington, USA) and all subjects gave written informed consent.
Cell culture.
The human prostate cancer epithelial cell line M12 was previously established in our laboratory (49). The human prostate cancer epithelial cell lines PC-3 and LnCaP were purchased from American Type Culture Collection (Manassas, Virginia, USA). Normal human PrECs were purchased from Cambrex BioWhittaker (Walkersville, Maryland, USA). All cells were cultured at 37°C with 5% CO2 in RPMI 1640 medium supplemented with 5% FBS, 10 ng/ml EGF, 0.02 mM dexamethasone, 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml selenium, fungizone, and gentamycin.
Antibodies and cytokines.
BAMO1 and M585 are mAb’s specific for MIC and NKG2D, respectively (31, 32). The W6/32 mAb against MHC I was from Sigma-Aldrich (Saint Louis, Missouri, USA). Immunohistochemistry-specific rabbit polyclonal anti-MIC antiserum H-300 was from Santa Cruz Biotechnology Inc. (Santa Cruz, California, USA). Isotype control mouse IgG and anti-CD3 were from BD Biosciences (San Diego, California, USA). Anti-CD56 and phycoerythrin-conjugated (PE-conjugated) goat anti-mouse IgG were from SouthernBiotech (Birmingham, Alabama, USA). IL-2 and IL-15 were from PeproTECH (London, United Kingdom).
Flow cytometry.
For detection of surface MIC expression on epithelial cells, cells were trypsinized and washed and were incubated with a specific primary antibody followed by PE-conjugated goat anti-mouse IgG. For assays of surface NKG2D expression on PBMCs, cells were incubated on ice for 30 minutes with the M585 mAb against NKG2D or with isotype control mouse IgG1, followed by PE-conjugated goat anti-mouse secondary reagent. Cells were washed, blocked with 10 μg/ml of mouse IgG, and further stained with peridinin chlorophyll protein–conjugated anti-CD3 in combination with FITC-conjugated anti-CD56. Cells were analyzed with a BD FACScan cytometer and CellQuest software from BD Biosciences (San Jose, California, USA).
Immunohistochemistry.
Tissue microarray slides containing formalin-fixed, paraffin-embedded sections of prostate lesions (a generous gift from J.L. Ware, Virginia Commonwealth University, Richmond, Virginia, USA) were dewaxed in Citrosolv (Fisher Scientific, Fair Lawn, New Jersey, USA) and were rehydrated in graded ethanol alcohol. Antigens were unmasked with 0.01 M citric acid (pH 6.0) at 95°C for two 5-minute incubations. Slides were allowed to cool for 30 minutes followed by sequential rinsing with PBS. Endogenous peroxidase activity was quenched by incubation with 0.3% H2O2 in methanol for 15 minutes. After being blocked for 1 hour with 1.5% normal goat serum in PBS containing 0.05% Triton X-100, slides were incubated with rabbit polyclonal anti-MIC antiserum H-300 (1 μg/ml) for 1 hour followed by sequential incubation with biotinylated goat anti-rabbit IgG for 30 minutes, peroxidase-labeled avidin for 30 minutes (Santa Cruz Biotechnology) and diaminobenzidine/hydrogen peroxide chromogen substrate (Vector Laboratories, Burlingame, California, USA) for 5–10 minutes. All incubation steps were performed at room temperature. Slides were counterstained with hematoxylin and mounted with Permount (Fisher Scientific). As a negative control, rabbit IgG (Vector Laboratories) was used instead of the primary anti-MIC. Slides were examined with a Zeiss microscope (Carl Zeiss, Jena, Germany) and digital images were obtained. The pathology of H&E–stained tissue microarray slides was reviewed for assignment of GS.
ELISA.
A human MICA ELISA kit (IMMATICS Biotechnologies, Tubigen, Germany) was used to detect sMIC in sera following a protocol described previously (31). Briefly, plates were coated with the AMO1 capture mAb against MICA at a concentration of 2 μg/ml in PBS, then were blocked with 15% BSA overnight at 4°C and washed. Standard recombinant sMICA and serum samples (diluted 1:3) were added to the plates, which were then incubated at room temperature for 2 hours. After washing, detection mAb BAMO3 (IgG2a, specific for MICA and MICB) was added, followed by incubation at room temperature for 2 hours, then 1 hour of incubation at room temperature with HRP-conjugated anti–mouse IgG2a (SouthernBiotech). Color was developed with the tetramethylbenzidine system (KPL, Gaithersburg, Maryland, USA) and the reaction was terminated with 1 M phosphoric acid. Absorbance was measured at 450 nm. A standard curve of the logarithmic relationship between concentration and absorbance was used to calculate the sMIC concentration in serum samples.
Cytotoxicity and cytokine-release assay.
NK cells were isolated by depletion of T cells and B cells from fresh blood with the RosetteSep NK cell enrichment cocktail (Stem Cell Technologies, Vancouver, British Columbia, Canada) according to the manufacturer’s protocol. The isolated CD56+ NK cell purity was more than 80%. For Figures 1 and 6, freshly isolated polyclonal NK cells were used. For Figure 4, polyclonal NK cells isolated from healthy donors were cultured for 48 hours at 37°C with 5% CO2 in RPMI 1640 medium in the presence of 20% serum from prostate cancer patients, 2 mM L-glutamine, and antibiotics and then were used for cytotoxicity and cytokine-release assays. Cytotoxicity was assessed in triplicate with a 4-hour 51Cr-release assay using labeled M12 cells, as described previously (14). For the IFN-γ–release assay, 1 × 105 NK cells were cocultured in a 96-well plate for 24 hours with an equal number of γ-irradiated M12 cells. Supernatants were combined from three replicates and IFN-γ was measured by ELISA at the Cytokine Analysis Laboratory of the Fred Hutchinson Cancer Research Center (Seattle, Washington, USA).
Statistical analysis.
Differences among subject groups were assessed by ANOVA. Statistical significance between means of two paired groups was assayed using Student’s t test with Bonferroni correction. A 95% confidence interval (P < 0.05) was considered significant.
Acknowledgments
This work was supported by NIH grants RO1DK52683 and PO1 CA85859 and a Department of Veterans Affairs Research Service Award to S.R. Plymate and Department of Defense New Investigator’s Award W81XWH-04-1-0577 to J.D. Wu. We are grateful to Joy L. Ware (Department of Pathology, Virginia Commonwealth University, Richmond, Virginia, USA) for the generous gift of prostate tissue microarray slides. We thank Lawrence True and Jeff Virgin for their help with histopathological evaluation of prostate tissue slides. We thank R. Bruce Montgomery and David Koelle for critical reading of the manuscript.
Footnotes
Nonstandard abbreviations used: Gleason score(s) (GS); high-grade prostatic intraepithelial neoplasia (HGPIN); MHC class I chain–related molecule (MIC); phycoerythrin (PE); primary epithelial cell (PrEC); prostate-specific antigen (PSA); soluble MIC (sMIC); UL16-binding protein (ULBP).
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat. Rev. Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 2.Watzl C. The NKG2D receptor and its ligands-recognition beyond the “missing self”? Microbes Infect. 2003;5:31–37. doi: 10.1016/s1286-4579(02)00057-6. [DOI] [PubMed] [Google Scholar]
- 3.Vivier E, Tomasello E, Paul P. Lymphocyte activation via NKG2D: towards a new paradigm in immune recognition? Curr. Opin. Immunol. 2002;14:306–311. doi: 10.1016/s0952-7915(02)00337-0. [DOI] [PubMed] [Google Scholar]
- 4.Lanier LL. On guard–activating NK cell receptors. Nat. Immunol. 2000;2:23–27. doi: 10.1038/83130. [DOI] [PubMed] [Google Scholar]
- 5.Bauer S, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. [PubMed] [Google Scholar]
- 6.Vivier E, Tomasello E, Paul P. Lymphocyte activation via NKG2D: towards a new paradigm in immune recognition? Curr. Opin. Immunol. 2002;14:306–311. doi: 10.1016/s0952-7915(02)00337-0. [DOI] [PubMed] [Google Scholar]
- 7.Cerwenka A, Lanier LL. NKG2D ligands: unconventional MHC class I-like molecules exploited by viruses and cancer. Tissue Antigens. 2003;61:335–343. doi: 10.1034/j.1399-0039.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- 8.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat. Rev. Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 9.Cosman D, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 10.Chalupny NJ, Sutherland CL, Lawrence WA, Rein-Weston A, Cosman D. ULBP4 is a novel ligand for human NKG2D. Biochem. Biophys. Res. Commun. 2003;305:129–135. doi: 10.1016/s0006-291x(03)00714-9. [DOI] [PubMed] [Google Scholar]
- 11.Conejo-Garcia JR, et al. A Tumor-Associated NKG2D Immunoreceptor ligand, induces activation and expansion of effector immune cells. Cancer Biol. Ther. 2003;2:446–451. doi: 10.4161/cbt.2.4.479. [DOI] [PubMed] [Google Scholar]
- 12.Groh V, et al. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat. Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 13.Conejo-Garcia JR, et al. Ovarian carcinoma expresses the NKG2D ligand Letal and promotes the survival and expansion of CD28- antitumor T cells. Cancer Res. 2004;64:2175–2182. doi: 10.1158/0008-5472.can-03-2194. [DOI] [PubMed] [Google Scholar]
- 14.Wu J, Groh V, Spies T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial gamma delta T cells. J. Immunol. 2002;169:1236–1240. doi: 10.4049/jimmunol.169.3.1236. [DOI] [PubMed] [Google Scholar]
- 15.Favier B, et al. Uncoupling between immunological synapse formation and functional outcome in human gamma delta T lymphocytes. J. Immunol. 2003;171:5027–5033. doi: 10.4049/jimmunol.171.10.5027. [DOI] [PubMed] [Google Scholar]
- 16.Groh V, et al. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vetter CS, et al. Expression of stress-induced MHC class I related chain molecules on human melanoma. J. Invest. Dermatol. 2002;118:600–605. doi: 10.1046/j.1523-1747.2002.01700.x. [DOI] [PubMed] [Google Scholar]
- 18.Jinushi M, et al. Expression and role of MICA and MICB in human hepatocellular carcinomas and their regulation by retinoic acid. Int. J. Cancer. 2003;104:354–361. doi: 10.1002/ijc.10966. [DOI] [PubMed] [Google Scholar]
- 19.Bauer S, Willie ST, Spies T, Strong RK. Expression, purification, crystallization and crystallographic characterization of the human MHC class I related protein MICA. Acta. Crystallogr. D Biol. Crystallogr. 1998;54:451–453. doi: 10.1107/s0907444997015229. [DOI] [PubMed] [Google Scholar]
- 20.Bahram S, Spies T. Nucleotide sequence of a human MHC class I MICB cDNA. Immunogenetics. 1996;43:230–233. doi: 10.1007/BF00587305. [DOI] [PubMed] [Google Scholar]
- 21.Bahram S, Spies T. The MIC gene family. Res. Immunol. 1996;147:328–333. doi: 10.1016/0923-2494(96)89646-5. [DOI] [PubMed] [Google Scholar]
- 22.Steinle A, Groh V, Spies T. Diversification, expression, and gamma delta T cell recognition of evolutionarily distant members of the MIC family of major histocompatibility complex class I-related molecules. Proc. Natl. Acad. Sci. U. S. A. 1998;95:12510–12515. doi: 10.1073/pnas.95.21.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerwenka A, et al. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 24.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat. Immunol. 2002;1:119–126. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 25.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165– 171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carayannopoulos LN, Naidenko OV, Fremont DH, Yokoyama WM. Cutting edge: murine UL16-binding protein-like transcript 1: a newly described transcript encoding a high-affinity ligand for murine NKG2D. J. Immunol. 2002;169:4079–4083. doi: 10.4049/jimmunol.169.8.4079. [DOI] [PubMed] [Google Scholar]
- 27.Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11521–11526. doi: 10.1073/pnas.201238598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 29.Doubrovina ES, et al. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J. Immunol. 2003;171:6891–6899. doi: 10.4049/jimmunol.171.12.6891. [DOI] [PubMed] [Google Scholar]
- 30.Pende D, et al. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62:6178–6186. [PubMed] [Google Scholar]
- 31.Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J. Immunol. 2002;169:4098–4102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 32.Sutherland CL, et al. UL16-binding proteins, novel MHC class I-related proteins, bind to NKG2D and activate multiple signaling pathways in primary NK cells. J. Immunol. 2002;168:671–679. doi: 10.4049/jimmunol.168.2.671. [DOI] [PubMed] [Google Scholar]
- 33.Herr HW. Suppressor cells in immunodepressed bladder and prostate cancer patients. J. Urol. 1980;123:635–639. [PubMed] [Google Scholar]
- 34.Ablin RJ, Bhatti RA, Bush IM, Guinan PD. Suppression of tumor-associated immunity by human seminal plasma and its possible role in the natural history of prostatic cancer. Eur. Urol. 1980;6:225–228. doi: 10.1159/000473337. [DOI] [PubMed] [Google Scholar]
- 35.Ablin RJ, Bhatti RA, Bush IM, Guinan PD. Immunosuppression of cell- and serum-medicated tumour-associated immunity in prostatic cancer by human seminal plasma. Eur. J. Cancer. 1980;16:775–780. doi: 10.1016/0014-2964(80)90130-9. [DOI] [PubMed] [Google Scholar]
- 36.Healy CG, et al. Impaired expression and function of signal-transducing zeta chains in peripheral T cells and natural killer cells in patients with prostate cancer. Cytometry. 1998;32:109–119. doi: 10.1002/(sici)1097-0320(19980601)32:2<109::aid-cyto6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 37.Roberts AI, et al. NKG2D receptors induced by IL-15 costimulate CD28-negative effector CTL in the tissue microenvironment. J. Immunol. 2001;167:5527–5530. doi: 10.4049/jimmunol.167.10.5527. [DOI] [PubMed] [Google Scholar]
- 38.Verneris MR, Karami M, Baker J, Jayaswal A, Negrin RS. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood. 2004;103:3065–3072. doi: 10.1182/blood-2003-06-2125. [DOI] [PubMed] [Google Scholar]
- 39.Troy A, Davidson P, Atkinson C, Hart D. Phenotypic characterization of the dendritic cell infiltrate in prostate cancer. J. Urol. 1998;160:214–219. [PubMed] [Google Scholar]
- 40.Lee C, et al. Transforming growth factor-beta in benign and malignant prostate. Prostate. 1999;39:285–290. doi: 10.1002/(sici)1097-0045(19990601)39:4<285::aid-pros9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 41.Kennedy-Smith AG, et al. Prostate specific antigen inhibits immune responses in vitro: A potential role in prostate cancer. J. Urol. 2002;168:741–747. [PubMed] [Google Scholar]
- 42.Wang J, Torbenson M, Wang Q, Ro JY, Becich M. Expression of inducible nitric oxide synthase in paired neoplastic and non-neoplastic primary prostate cell cultures and prostatectomy specimen. Urol. Oncol. 2003;21:117–122. doi: 10.1016/s1078-1439(02)00208-9. [DOI] [PubMed] [Google Scholar]
- 43.Castriconi R, et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4120–4125. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friese MA, et al. MICA/NKG2D-mediated immunogene therapy of experimental gliomas. Cancer Res. 2003;63:8996–9006. [PubMed] [Google Scholar]
- 45.Curran S, Murray GI. Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur. J. Cancer. 2000;36:1621–1630. doi: 10.1016/s0959-8049(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 46.Lokeshwar BL. MMP inhibition in prostate cancer. Ann. N. Y. Acad. Sci. 1999;878:271–289. doi: 10.1111/j.1749-6632.1999.tb07690.x. [DOI] [PubMed] [Google Scholar]
- 47.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 48.Molinero LL, Fuertes MB, Fainboim L, Rabinovich GA, Zwirner NW. Up-regulated expression of MICA on activated T lymphocytes involves Lck and Fyn kinases and signaling through MEK1/ERK, p38 MAP kinase, and calcineurin. J. Leukoc. Biol. 2003;73:815–822. doi: 10.1189/jlb.0602329. [DOI] [PubMed] [Google Scholar]
- 49.Bae VL, et al. Metastatic sublines of an SV40 large T antigen immortalized human prostate epithelial cell line. Prostate. 1998;34:275–282. doi: 10.1002/(sici)1097-0045(19980301)34:4<275::aid-pros5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]