Abstract
We aimed to examine the effect of Wisteria floribunda agglutinin-positive Mac-2-binding protein (WFA+-M2BP) level on survival comparing with other laboratory liver fibrosis markers in hepatitis C virus (HCV)-related compensated liver cirrhosis (LC) (n = 165). For assessing prognostic performance of continuous fibrosis markers, we adapted time-dependent receiver operating characteristics (ROC) curves for clinical outcome. In time-dependent ROC analysis, annual area under the ROCs (AUROCs) were plotted. We also calculated the total sum of AUROCs in all time-points (TAAT score) in each fibrosis marker. WFA+-M2BP value ranged from 0.66 cutoff index (COI) to 19.95 COI (median value, 5.29 COI). Using ROC analysis for survival, the optimal cutoff point for WFA+-M2BP was 6.15 COI (AUROC = 0.79348, sensitivity = 80.0%, specificity = 74.78%). The cumulative five-year survival rate in patients with WFA+-M2BP ≥ 6.15 COI (n = 69) was 43.99%, while that in patients with WFA+-M2BP < 6.15 COI (n = 96) was 88.40% (p < 0.0001). In the multivariate analysis, absence of hepatocellular carcinoma (p = 0.0008), WFA+-M2BP < 6.15 COI (p = 0.0132), achievement of sustained virological response (p < 0.0001) and des-γ-carboxy prothrombin < 41 mAU/mL (p = 0.0018) were significant favorable predictors linked to survival. In time-dependent ROC analysis in all cases, WFA+-M2BP had the highest TAAT score among liver fibrosis markers. In conclusion, WFA+-M2BP can be a useful predictor in HCV-related compensated LC.
Keywords: hepatitis C virus, liver cirrhosis, hepatocellular carcinoma, Wisteria floribunda agglutinin-positive Mac-2-binding protein (WFA+-M2BP), survival, time-dependent receiver operating characteristics (ROC) analysis
1. Introduction
Chronic hepatitis C (CHC) infection is a major cause of hepatocellular carcinoma (HCC) [1,2,3]. CHC also constitute the major etiologies of liver cirrhosis (LC) worldwide [1,2,3,4]. In our country, high annual HCC incidence rate in hepatitis C virus (HCV)-related LC patients had been reported (7%–8% per year) [5,6]. Clinical outcome in cirrhotic subjects is highly variable and can be influenced by several factors, such as liver disease etiology, severity of liver disease and presence of complications [4,7]. In decompensated LC patients with ascites or encephalopathy, survival decreases to one or two years [7,8,9].
On the other hand, the advent of direct-acting antivirals (DAAs) has spurred a revolution for CHC therapy with sustained viral response (SVR) rates exceeding 90% in the daily clinical practice [10]. The introduction of these highly effective DAAs for CHC patients is also expected to reduce the incidence of HCV-related HCC. However, the achievement of an SVR does not indicate that it completely eliminates the risk for HCC development, particularly when the subjects have already been complicated with LC [2,3,10,11,12].
Recently, Japanese investigators have established a novel liver fibrosis marker (Wisteria floribunda agglutinin-positive Mac-2-binding protein (WFA+-M2BP)), which is a glycobiomarker associated with CHC-related liver fibrosis with a unique fibrosis-related glycoalteration [13,14,15]. In addition, results for WFA+-M2BP can be obtained easily [13,14,15]. Thereafter, several investigators validated the usefulness of WFA+-M2BP on the liver fibrosis or clinical outcomes in various etiologies of chronic liver diseases (CLDs) [16,17,18,19,20,21,22,23,24,25]. Thus, clinical evidences with regard to WFA+-M2BP on liver fibrosis in CLDs have been accumulated recently.
However, to the best of our knowledge, there are no available data with regard to the effect of WFA+-M2BP level on survival in HCV-related compensated LC patients. As mentioned above, because clinical outcome in LC patients is highly variable, this investigation may be clinically important. The goal of our study is therefore to examine the effect of WFA+-M2BP level on survival comparing with other laboratory liver fibrosis markers in patients with HCV-related compensated LC. Moreover, in order to further assess prognostic performance of continuous liver fibrosis markers, we adapted time-dependent receiver operating characteristics (ROC) analysis for clinical outcome based on previous reports [26,27].
2. Results
2.1. Baseline Characteristics
The baseline characteristics in the current analysis are presented in Table 1 (n = 165). There are 93 males and 72 females with the median age of 67 years. For the entire cohort, the WFA+-M2BP value ranged from 0.66 cutoff index (COI) to 19.95 COI (median value, 5.29 COI). Sixty-eight out of 165 patients (41.2%) had HCC on radiological findings, which included stage I in 22, stage II in 26, stage III in 10 and stage IV in 10. The median observation period in the present analysis was 3.852 years (range: 0.219–9.241 years). As for HCV genotype and HCV viral load, genotype 1 (84.8% (140/165)) and high viral load as defined by HCV-RNA ≥ 5 log copies/mL (87.3% (144/165)) were in the majority at study entry. During follow-up period, SVR was achieved in 68 patients (41.2%) by antiviral therapies. Of these, 34 patients were treated with interferon-based antiviral therapies and the remaining 34 patients were treated with interferon-free antiviral therapies.
Table 1.
Baseline characteristics (n = 165).
| Variables | Number or Median Value (Range) |
|---|---|
| Age (years) | 67 (23–93) |
| Gender, male/female | 93/72 |
| HCC, None/stage I/II/III/IV | 97/22/26/10/10 |
| Total bilirubin (mg/dL) | 0.8 (0.3–2.0) |
| Serum albumin (g/dL) | 3.7 (2.9–4.9) |
| Prothrombin time (%) | 81 (58.8–115.6) |
| Platelet count (×104/mm3) | 10.8 (1.7–38.7) |
| Hyaluronic acid (ng/mL) | 201 (22–2270) |
| WFA+-M2BP (cutoff index) | 5.29 (0.66–19.95) |
| AST (IU/L) | 52 (17–343) |
| ALT (IU/L) | 46 (7–396) |
| ALP (IU/L) | 282 (130–985) |
| GGT (IU/L) | 44 (12–357) |
| AFP (ng/mL) | 10.6 (1.2–4867) |
| DCP (mAU/mL) # | 24 (6–96900) |
| HCV genotype 1/2/unknown | 140/23/2 |
| HCV viral load ≥ 5 log IU/mL, yes/no | 144/21 |
| MELD score | 3.7 (−5.0–23.7) |
Data are expressed as number or median (range). HCC, hepatocellular carcinoma; WFA+-M2BP, Wisteria floribunda agglutinin-positive Mac-2-binding protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma glutamyl transpeptidase; AFP, alpha-fetoprotein; DCP, des-γ-carboxy prothrombin; #, missing data (n = 8); HCV, hepatitis C virus; MELD, model for end-stage liver disease.
2.2. Cumulative Overall Survival Stratified by WFA+-M2BP for the Entire Cohort (n = 165)
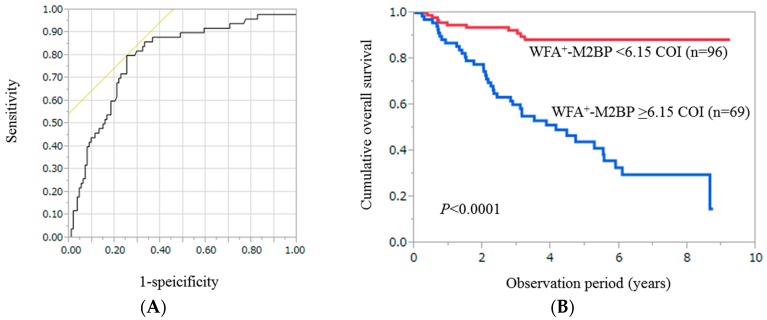
Using ROC analysis, the optimal cutoff point for WFA+-M2BP level was 6.15 COI (AUROC = 0.79348, sensitivity = 80.0%, specificity = 74.78%) (Figure 1A). For the entire cohort, the cumulative one-, three- and five-year survival rates in patients with WFA+-M2BP ≥ 6.15 COI (n = 69) were 86.96%, 60.14% and 43.99%, respectively, while those in patients with WFA+-M2BP < 6.15 COI (n = 96) were 94.76%, 92.41% and 88.40%, respectively (p < 0.0001) (Figure 1B).
Figure 1.
(A) Receiver operating characteristics (ROC) curves of Wisteria floribunda agglutinin-positive Mac-2-binding protein (WFA+-M2BP) for survival in all cases (n = 165). The optimal cutoff point for WFA+-M2BP level was 6.15 cutoff index (COI) (annual area under the ROC (AUROC) = 0.79348, sensitivity = 80.0%, specificity = 74.78%); (B) Cumulative overall survival stratified by WFA+-M2BP in all cases. The cumulative one-, three- and five-year survival rates were: 86.96%, 60.14% and 43.99%, respectively, in patients with WFA+-M2BP ≥ 6.15 COI (n = 69), while 94.76%, 92.41% and 88.40%, respectively, in patients with WFA+-M2BP < 6.15 COI (n = 96) (p < 0.0001).
2.3. Cumulative Overall Survival Stratified by WFA+-M2BP for Patients with HCC (n = 68)
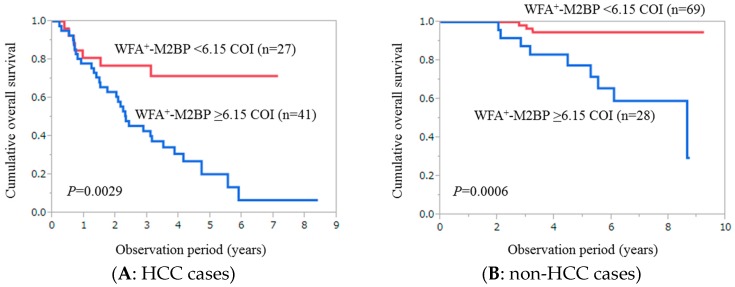
For HCC patients, the cumulative one-, three- and five-year survival rates in patients with WFA+-M2BP ≥ 6.15 COI (n = 41) were 78.05%, 42.8% and 20.27%, respectively, while those in patients with WFA+-M2BP < 6.15 COI (n = 27) were 81.02%, 76.97% and 71.47%, respectively (p = 0.0026) (Figure 2A).
Figure 2.
(A) Cumulative overall survival stratified by WFA+-M2BP for patients with hepatocellular carcinoma (HCC) (n = 68). The cumulative one-, three- and five-year survival rates were 78.05%, 42.8% and 20.27%, respectively, in patients with WFA+-M2BP ≥ 6.15 COI (n = 41), while 81.02%, 76.97% and 71.47%, respectively, in patients with WFA+-M2BP < 6.15 COI (n = 27) (p = 0.0026); (B) Cumulative overall survival stratified by WFA+-M2BP for patients without HCC (n = 97). The cumulative one-, three- and five-year survival rates were 100%, 87.5% and 77.58%, respectively, in patients with WFA+-M2BP ≥ 6.15 COI (n = 28), while 100%, 98.31% and 94.76%, respectively, in patients with WFA+-M2BP < 6.15 COI (n = 69) (p = 0.0006).
2.4. Cumulative Overall Survival Stratified by WFA+-M2BP for Patients without HCC (n = 97)
For patients without HCC, the cumulative one-, three- and five-year survival rates in patients with WFA+-M2BP ≥ 6.15 COI (n = 28) were 100%, 87.5% and 77.58%, respectively, while those in patients with WFA+-M2BP < 6.15 COI (n = 69) were 100%, 98.31% and 94.76%, respectively (p = 0.0006) (Figure 2B).
2.5. Comparison of WFA+-M2BP Level in Patients with and without HCC
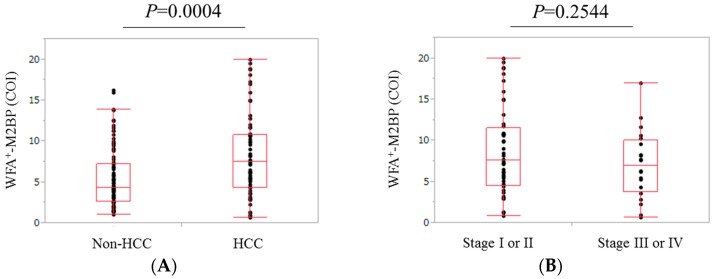
The median (range) WFA+-M2BP value in patients with HCC (7.50 COI (0.66–19.95 COI)) was significantly higher than that in patients without HCC (4.29 COI (1.06–16.24 COI)) (p = 0.0004) (Figure 3A).
Figure 3.
(A) Comparison of WFA+-M2BP level in patients with and without HCC (p = 0.0004); (B) Comparison of WFA+-M2BP level in patients with stage I or II HCC and stage III or IV HCC (p = 0.2554).
2.6. Comparison of WFA+-M2BP Level in Patients with Stage I or II HCC and Those with Stage III or IV HCC
The difference of WFA+-M2BP levels in patients with stage I or II HCC (median: 7.57 COI, range: 0.8–17.95 COI) and those with stage III or IV HCC (median: 6.92 COI, range: 0.66–16.93 COI) did not reach significance (p = 0.2544) (Figure 3B).
2.7. Correlation between WFA+-M2BP Level and Tumor Markers for HCC Patients
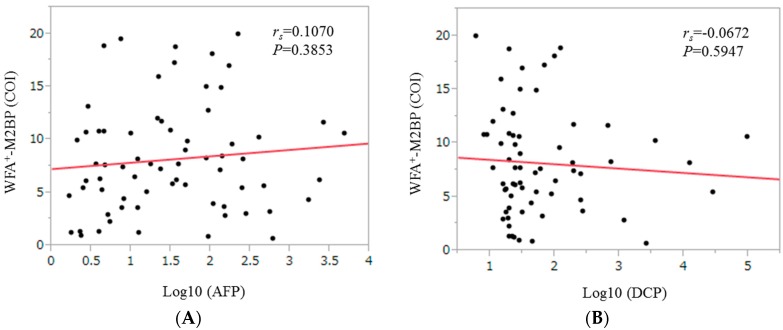
In patients with HCC, the WFA+-M2BP level did not significantly correlate with alpha-fetoprotein (AFP) (rs = 0.1070, p = 0.3853) and des-γ-carboxy prothrombin (DCP) (rs = −0.0672, p = 0.5947) (Figure 4).
Figure 4.
Correlation between WFA+-M2BP level and tumor markers (alpha-fetoprotein (AFP) (A) and des-γ-carboxy prothrombin (DCP) (B)) for HCC patients.
2.8. Univariate and Multivariate Analyses of Factors Associated with Overall Survival for the Entire Cohort
Univariate analysis identified the following factors as significantly linked to overall survival (OS): age ≥ 71 years (p < 0.0001); presence of HCC (p < 0.0001); serum albumin ≥ 3.5 g/dL (p < 0.0001); prothrombin time ≥ 76% (p < 0.0001); platelet count ≥ 9.6 × 104/mm3 (p = 0.0067); hyaluronic acid ≥ 265 ng/mL (p < 0.0001); WFA+-M2BP ≥ 6.15 COI (p < 0.0001); Fibrosis-4 (FIB-4) index ≥ 4.97755 (p = 0.0007); achievement of SVR during follow-up period (p < 0.0001); alkaline phosphatase ≥ 317 IU/L (p < 0.0001); AFP ≥ 10.8 ng/mL (p < 0.0001); and DCP ≥ 41 mAU/mL (p < 0.0001) (Table 2). The hazard ratios (HRs) and 95% confidence intervals calculated using multivariate analysis for the twelve factors with p value less than 0.05 in univariate analysis are shown in Table 2. Absence of HCC (p = 0.0008), WFA+-M2BP < 6.15 COI (p = 0.0132), achievement of SVR (p < 0.0001) and DCP < 41 mAU/mL (p = 0.0018) were revealed to be significant favorable predictors linked to OS.
Table 2.
Univariate and multivariate of factors associated with overall survival.
| Variables | Number | Univariate Analysis (p Value) | Multivariate Analysis | ||
|---|---|---|---|---|---|
| HR | 95% CI | p Value | |||
| Age ≥ 71 years, yes/no | 60/105 | <0.0001 | 2.110 | 0.919–4.950 | 0.0783 |
| Gender, male/female | 93/72 | 0.2684 | |||
| Presence of HCC, yes/no | 68/97 | <0.0001 | 4.527 | 1.833–12.320 | 0.0008 |
| Total bilirubin ≥ 0.8 mg/dL, yes/no | 97/68 | 0.3132 | |||
| Serum albumin ≥ 3.5 g/dL, yes/no | 122/43 | <0.0001 | 0.910 | 0.439–1.885 | 0.7975 |
| Prothrombin time ≥ 76%, yes/no | 112/53 | <0.0001 | 0.816 | 0.410–1.621 | 0.5611 |
| Platelet count ≥ 9.6 × 104/mm3, yes/no | 116/49 | 0.0067 | 0.697 | 0.313–1.554 | 0.3761 |
| Hyaluronic acid ≥ 265 ng/mL, yes/no | 59/96 | <0.0001 | 1.942 | 0.890–4.382 | 0.0964 |
| WFA+-M2BP ≥ 6.15 COI, yes/no | 69/96 | <0.0001 | 2.870 | 1.242–6.940 | 0.0132 |
| APRI ≥ 1.27615, yes/no | 92/73 | 0.0552 | |||
| FIB-4 index ≥ 4.97755, yes/no | 80/85 | 0.0007 | 1.649 | 0.681–4.336 | 0.2758 |
| Achievement of an SVR, yes/no | 68/97 | <0.0001 | 23.833 | 4.579–439.545 | <0.0001 |
| AST ≥ 55 IU/L, yes/no | 76/79 | 0.0595 | |||
| ALT ≥ 20 IU/L, yes/no | 148/17 | 0.3304 | |||
| ALP ≥ 317 IU/L, yes/no | 68/97 | <0.0001 | 1.161 | 0.545–2.553 | 0.7022 |
| GGT ≥ 58 IU/L, yes/no | 49/116 | 0.1018 | |||
| AFP ≥ 10.8 ng/mL, yes/no | 82/83 | <0.0001 | 1.426 | 0.659–3.318 | 0.3768 |
| DCP ≥ 41 mAU/mL, yes/no # | 37/120 | <0.0001 | 3.543 | 1.602–7.976 | 0.0018 |
| MELD score ≥ 5.1, yes/no | 55/110 | 0.0006 | 1.471 | 0.760–2.874 | 0.2521 |
HCC, hepatocellular carcinoma; WFA+-M2BP, Wisteria floribunda agglutinin-positive Mac-2-binding protein; COI, cutoff index; APRI, aspartate aminotransferase to platelet ration index; SVR, sustained virological response; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma glutamyl transpeptidase; AFP, alpha-fetoprotein; DCP, des-γ-carboxy prothrombin; MELD, model for end-stage liver disease; #, missing data (n = 8); HR, hazard ratio; CI, confidence interval.
2.9. Causes of Death
During the observation period, 50 patients (30.3%) died. The causes of death were HCC progression in 24 patients, liver failure in 19 patients and miscellaneous causes in seven patients.
2.10. Time-Dependent ROC Analyses for OS in all Cases
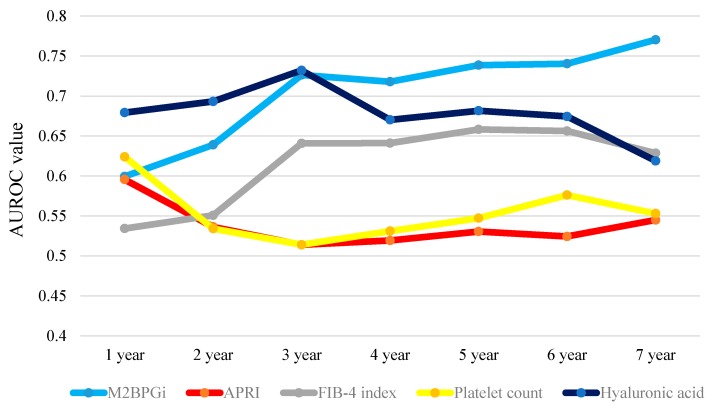
Results for time-dependent ROC analyses at one-, two-, three-, four-, five-, six- and seven-year of WFA+-M2BP, APRI, FIB-4 index, platelet count and hyaluronic acid in all cases are presented in Table 3. The plots of annual AUROCs of five liver fibrosis markers as shown in Figure 5. The total sum of AUROCs in all time-points (TAAT score) in WFA+-M2BP was the highest among five liver fibrosis markers (TAAT score = 4.93226), followed by hyaluronic acid (TAAT score = 4.74996).
Table 3.
Results for time-dependent receiver operating characteristics analysis.
| All Cases | 1-Year | 2-Year | 3-Year | 4-Year | 5-Year | 6-Year | 7-Year | Total Value |
| WFA+-M2BP | 0.59942 | 0.63898 | 0.72643 | 0.71793 | 0.73864 | 0.74045 | 0.77041 | 4.93226 |
| APRI | 0.59556 | 0.53636 | 0.51389 | 0.51932 | 0.53050 | 0.52431 | 0.54490 | 3.76484 |
| FIB-4 index | 0.53427 | 0.55076 | 0.64085 | 0.64111 | 0.65829 | 0.65625 | 0.62857 | 4.31010 |
| Platelet count | 0.62403 | 0.53415 | 0.51403 | 0.53104 | 0.54725 | 0.57610 | 0.55306 | 3.87966 |
| Hyaluronic acid | 0.6793 | 0.69324 | 0.73218 | 0.67026 | 0.68162 | 0.67448 | 0.61888 | 4.74996 |
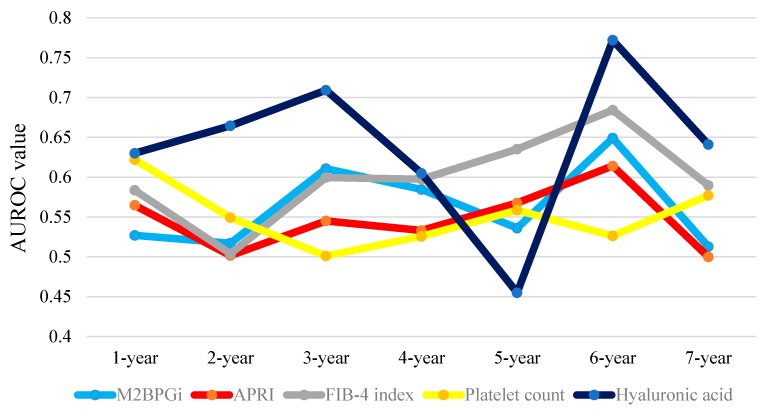
| HCC Cases | 1-Year | 2-Year | 3-Year | 4-Year | 5-Year | 6-Year | 7-Year | Total Value |
| WFA+-M2BP | 0.52695 | 0.51717 | 0.61068 | 0.58456 | 0.53604 | 0.64912 | 0.51282 | 3.93734 |
| APRI | 0.56469 | 0.50166 | 0.54505 | 0.53309 | 0.56757 | 0.61404 | 0.50000 | 3.82610 |
| FIB-4 index | 0.58356 | 0.50388 | 0.59956 | 0.59743 | 0.63514 | 0.68421 | 0.58974 | 4.19352 |
| Platelet count | 0.62197 | 0.54928 | 0.50111 | 0.52574 | 0.55856 | 0.52632 | 0.57692 | 3.85990 |
| Hyaluronic acid | 0.63005 | 0.66445 | 0.70912 | 0.60478 | 0.45495 | 0.77193 | 0.64103 | 4.47631 |
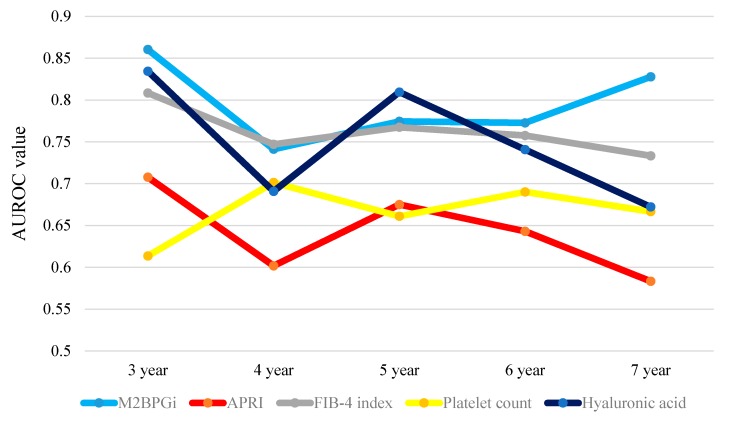
| Non-HCC | 1-Year | 2-Year | 3-Year | 4-Year | 5-Year | 6-Year | 7-Year | Total Value |
| WFA+-M2BP | NA | NA | 0.86039 | 0.74122 | 0.77451 | 0.77273 | 0.82778 | 3.97663 |
| APRI | NA | NA | 0.70779 | 0.60187 | 0.67507 | 0.64310 | 0.58333 | 3.21116 |
| FIB-4 index | NA | NA | 0.80844 | 0.74707 | 0.76751 | 0.75758 | 0.73333 | 3.81393 |
| Platelet count | NA | NA | 0.61364 | 0.70141 | 0.66106 | 0.69024 | 0.66667 | 3.33302 |
| Hyaluronic acid | NA | NA | 0.83442 | 0.69087 | 0.80952 | 0.74074 | 0.67222 | 3.74777 |
WFA+-M2BP, Wisteria floribunda agglutinin-positive Mac-2-binding protein; APRI, aspartate aminotransferase to platelet ration index; HCC, hepatocellular carcinoma; NA, not available.
Figure 5.
Time-dependent ROC analyses of five liver fibrosis markers (WFA+-M2BP, aspartate aminotransferase to platelet ration index (APRI), Fibrosis-4 (FIB-4) index, platelet count and hyaluronic acid) for overall survival (OS) in all cases. This figure presents the plots of annual AUROCs of five liver fibrosis markers. The total sum of AUROCs in all time-points (TAAT) score indicates total sum of AUROCs in all time-points in each liver fibrosis marker. TAAT score: WFA+-M2BP: 4.93226; APRI: 3.76484; FIB-4 index: 4.31010; platelet count: 3.87966 and hyaluronic acid: 4.74996.
2.11. Time-Dependent ROC Analyses for OS in HCC Patients
Results for time-dependent ROC analyses at one-, two-, three-, four-, five-, six- and seven-year of WFA+-M2BP, APRI, FIB-4 index, platelet count and hyaluronic acid in HCC patients are shown in Table 3. The plots of annual AUROCs of five liver fibrosis markers are shown in Figure 6. The TAAT score in hyaluronic acid was the highest among five liver fibrosis markers (TAAT score = 4.47631), followed by FIB-4 index (TAAT score = 4.19352).
Figure 6.
Time-dependent ROC analyses of five liver fibrosis markers (WFA+-M2BP, APRI, FIB-4 index, platelet count and hyaluronic acid) for OS in HCC cases. This figure presents the plots of annual AUROCs of five liver fibrosis markers. The TAAT score indicates total sum of AUROCs in all time-points in each liver fibrosis marker. TAAT score: WFA+-M2BP: 3.93734; APRI: 3.82610; FIB-4 index: 4.19352; platelet count: 3.85990 and hyaluronic acid: 4.47631.
2.12. Time-Dependent ROC Analyses for OS in Non-HCC Patients
Results for time-dependent ROC analyses at three-, four-, five-, six- and seven-year of WFA+-M2BP, aspartate aminotransferase to platelet ration index (APRI), FIB-4 index, platelet count and hyaluronic acid in non-HCC patients are shown in Table 3. The plots of annual AUROCs of five liver fibrosis markers are shown in Figure 7. The one- and two- year cumulative survival rate were both 100% in non-HCC patients and thus AUROCs in one- and two-year were not available. The TAAT score in WFA+-M2BP was the highest among five liver fibrosis markers (TAAT score = 3.97663), followed by FIB-4 index (TAAT score = 3.81393).
Figure 7.
Time-dependent ROC analyses of five liver fibrosis markers (WFA+-M2BP, APRI, FIB-4 index, platelet count and hyaluronic acid) for OS in non-HCC cases. This figure presents the plots of annual AUROCs of five liver fibrosis markers. The TAAT score indicates total sum of AUROCs in all time-points in each liver fibrosis marker. TAAT score: WFA+-M2BP: 3.97663; APRI: 3.21116; FIB-4 index: 3.81393, platelet count: 3.33302 and hyaluronic acid: 3.74777.
2.13. Comparison of the Proportion of A3 for Patients in Whom LC Was Diagnosed by Liver Biopsy According to WFA+-M2BP (n = 113)
As for liver inflammation stage (A stage) in histological findings, in patients in whom LC was diagnosed by liver biopsy (n = 113), 21 patients had A1 stage, 79 had A2 stage and 13 had A3 stage. The proportion of A3 in patients with WFA+-M2BP ≥ 6.15 COI was significantly higher than that in patients with WFA+-M2BP < 6.15 COI (20.51% (8/39) vs. 6.76% (5/74), p = 0.0293).
3. Discussion
HCV-related LC involves a highly heterogeneous condition with a broad spectrum of clinical characteristics, ranging from compensated stage to decompensated stage, each of which is characterized by a different prognosis [7,8,9]. It is obvious that decompensated LC patients had worse prognosis. We therefore conducted this study in limited patients with compensated LC. To our knowledge, this is the first report regarding the effect of WFA+-M2BP on clinical outcomes in HCV-related compensated LC patients. Furthermore, outcomes for CLDs are usually time-dependent. However, few studies have analyzed clinical data using time-dependent ROC analysis [26,27]. Thus, for detailed examination of the effect of WFA+-M2BP on clinical outcomes, we used both multivariate analysis and time-dependent ROC analysis, which took time-dependence into account [26,27].
In our results, WFA+-M2BP level ≥ 6.15 COI as determined by ROC analysis for survival was an independent adverse predictor linked to OS and for the entire cohort and non-HCC patients, it had the highest TAAT scores among liver fibrosis markers. In addition, cumulative survival rates were well stratified by WFA+-M2BP level irrespective of presence or absence of HCC. These results demonstrated that WFA+-M2BP can be helpful for predicting clinical outcome in HCV-related compensated LC. Our baseline data of WFA+-M2BP (range: 0.66–19.95 COI) may reflect the fact that HCV-related compensated LC patients had heterogeneous patients population. The insignificant correlation between WFA+-M2BP level and AFP or DCP values indicate that WFA+-M2BP could not be an alternative tumor marker to AFP or DCP.
In our previous study, we reported that WFA+-M2BP correlated with not only the degree of liver fibrosis but also the degree of liver inflammation activity [20,21,25]. Indeed, the proportion of A3 in patients with WFA+-M2BP ≥ 6.15 COI was significantly higher than that in patients with WFA+-M2BP < 6.15 COI in this study (p = 0.0293), and this may be associated with worse clinical outcome in patients with higher WFA+-M2BP level [7,28]. The statistical significance between non-HCC and HCC patients in terms of WFA+-M2BP level can be also explained by liver inflammation, although the difference of the proportion of A3 in HCC and non-HCC patients with available liver biopsy data did not reach significance (p = 0.2299, data not shown).
In HCC patients, hyaluronic acid had the highest TAAT score among five liver fibrosis markers. In this population, the presence of HCC itself can affect prognosis. On the other hand, in our previous study, we reported that serum hyaluronic acid could predict protein-energy malnutrition (PEM) in HCV-related liver disease [29]. Especially in this patient population, sufficient management for PEM may be essential for ameliorating outcome since HCC therapy can accelerate progression of PEM.
Notably, an achievement of SVR during follow-up period was the strongest favorable predictor linked to OS with the HR of 23.833 (p < 0.0001) in our multivariate analysis. A recent meta-analysis demonstrated that CHC subjects with SVR have a substantially reduced risk for HCC incidence (HR: 0.1–0.25), liver-related mortality (HR: 0.03–0.2) and all-cause mortality (HR: 0.1–0.3) as compared with no antiviral treatment or treatment failure, which are in agreement with our current results [30]. In compensated LC patients, antiviral therapy can be strongly recommended for improving clinical outcomes.
We acknowledge several limitations in this study. First, since this longitudinal study had a retrospective nature, our results should be cautiously interpreted; Second, in 52 patients (31.5%), LC was diagnosed not by liver biopsy but radiological findings alone, leading to bias; Third, liver biopsy involves restriction being prone to errors in a statistical analysis arising from the unrepresentativeness of the sample taken for assessing the degree of liver fibrosis, also leading to bias; Fourth, there were several missing values in our analysis; Fifth, calculating TAAT score is not a well established assessment method for predicting survival and this is only a proposal. However, our current results demonstrated that WFA+-M2BP performed well as a prognostic marker in HCV-related compensated LC patients.
Finally, we concluded that WFA+-M2BP can be a useful predictor in patients with HCV-related compensated LC.
4. Patients and Methods
4.1. Patients
Between March 2007 and June 2015, a total of 165 individuals with HCV-related compensated LC with available stored serum samples were admitted to the Division of Hepatobiliary and Pancreatic disease, Department of Internal Medicine, Hyogo College of Medicine, Hyogo, Japan and they were subjected to this analysis. Compensated LC was defined as Child-Pugh A LC. Subjects with HCV-related liver disease are defined as those with HCV antibody positive and hepatitis B surface antigen negative. In patients who did not receive liver biopsy (n = 52), LC was diagnosed through radiological findings: clinical features suggestive of portal hypertension such as splenomegaly, varices, ascites and a shrunken or deformed and nodular liver as identified on computed tomography (CT) or ultrasonography (US) findings [7]. The primary endpoint in our study is overall survival (OS). Firstly, we examined the relationship between WFA+-M2BP level and baseline characteristics or clinical outcomes. Subsequently, we investigated variables linked to OS using univariate and multivariate analyses. Finally, we compared the effect of WFA+-M2BP level on survival with those of other liver fibrosis markers including APRI, FIB-4 index, platelet count and hyaluronic acid using time-dependent ROC analysis. In principle, in patients with serum albumin level ≤3.5 g/dL, branched chain amino acids were administered [7]. In patients without HCC or those with curative treatment for HCC, antiviral therapies including interferon-based therapies or interferon-free therapies were also considered [7].
The ethics committee of Hyogo college of medicine approved the current study protocol (approval number: 1831; date: 1 March 2016) and this study protocol adhered to all provisions of the Declaration of Helsinki.
4.2. Measurement of WFA+-M2BP and Calculation of ARPI and FIB-4 Index
We tested WFA+-M2BP level using stored serum sample and it was measured based on a lectin-Ab sandwich immunoassay using the fully automatic immunoanalyzer, HISCL-2000i (Sysmex Co., Hyogo, Japan) as reported previously [31,32]. We calculated APRI score as described elsewhere: aspartate aminotransferase (AST/upper limit of normal)/platelet count (expressed as platelets × 109/L) × 100 [33]. We calculated FIB-4 index as described elsewhere: age (years) × AST (IU/L)/platelet count (×109/L) ×√alanine aminotransferase (ALT) (IU/L) [34].
4.3. HCC Surveillance and HCC Therapy
Follow-up consisted of periodical evaluation by imaging studies including US, CT and/or magnetic resonance imaging for HCC incidence or HCC recurrence and periodical blood tests such as tumor markers. For cases with HCC, most adequate therapy for each case was selected according to Japanese guidelines through discussion with radiologists, surgeons and hepatologists [35,36,37].
4.4. Statistical Analysis
In continuous parameters, groups were compared by using Student’s t-test or Mann–Whitney U test, or Spearman’s rank correlation coefficient rs, as applicable. In categorical parameters, groups were compared by using Fisher’s exact tests or Pearson χ2 test, as applicable. In continuous parameters, we performed ROC curve analysis of survival for selection of the optimal cutoff value that is associated with maximal total value of specificity and sensitivity and we classified them into two groups using these cutoff values and treated them as nominal covariates. Kaplan–Meier curves were generated and compared by using the log-rank test. Parameters with p value less than 0.05 in the univariate analysis were entered into the multivariate analyses (Cox proportional hazard model). Furthermore, we analyzed time-dependent ROC curves of WFA+-M2BP, APRI, FIB-4 index, platelet count and hyaluronic acid for survival and compared between area under the ROCs (AUROCs) for these liver fibrosis markers in each time point (one-, two-, three-, four-, five-, six-, and seven-year) [26,27]. We also calculated the total sum of AUROCs in all time-points (TAAT score) in each liver fibrosis marker.
OS was calculated as the time interval from the date at which we obtained stored serum sample during hospitalization with the purpose of liver biopsy, HCC therapy or others until death (due to any cause) or the last follow-up visit. Data are presented as median (range) unless otherwise stated. We regarded variables with p value less than 0.05 as statistically significant variables. Statistical analysis was performed using JMP 11 (SAS Institute Inc., Cary, NC, USA).
Acknowledgments
The authors would like to thank Nozomi Kanazawa, Yoko Matsushita and Sayaka Fujii for data collection. Financial support: none.
Abbreviations
CHC, chronic hepatitis C; HCC, hepatocellular carcinoma; LC, liver cirrhosis; HCV, hepatitis C virus; DAAs, direct acting antivirals; SVR, sustained virological response; WFA+-M2BP, Wisteria floribunda agglutinin-positive Mac-2-binding protein; CLDs, chronic liver diseases; ROC, receiver operating characteristics; CT, computed tomography; US, ultrasonography; OS, overall survival; APRI, aspartate aminotransferase to platelet ration index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AUROCs, area under the ROCs; TAAT, total sum of AUROCs in all time-points; COI, cutoff index; AFP, alpha-fetoprotein; DCP, des-γ-carboxy prothrombin; HR, hazard ratio.
Author Contributions
Kunihiro Hasegawa, Ryo Takata and Hiroki Nishikawa analysed data and wrote the paper and other remaining authors collected data. Kunihiro Hasegawa and Hiroki Nishikawa equally contributed to this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Van der Meer A.J., Veldt B.J., Feld J.J., Wedemeyer H., Dufour J.F., Lammert F., Duarte-Rojo A., Heathcote E.J., Manns M.P., Kuske L., et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. J. Am. Med. Assoc. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 3.Mandorfer M., Kozbial K., Schwabl P., Freissmuth C., Schwarzer R., Stern R., Chromy D., Stättermayer A.F., Reiberger T., Beinhardt S., et al. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J. Hepatol. 2016 doi: 10.1016/j.jhep.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Romanelli R.G., Stasi C. Recent advancements in diagnosis and therapy of liver cirrhosis. Curr. Drug Targets. 2016 doi: 10.2174/1389450117666160613101413. in press. [DOI] [PubMed] [Google Scholar]
- 5.Van Meer S., de Man R.A., Siersema P.D., van Erpecum K.J. Surveillance for hepatocellular carcinoma in chronic liver disease: Evidence and controversies. World J. Gastroenterol. 2013;19:6744–6756. doi: 10.3748/wjg.v19.i40.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osaki Y., Nishikawa H. Treatment for hepatocellular carcinoma in Japan over the last three decades: Our experience and published work review. Hepatol. Res. 2015;45:59–74. doi: 10.1111/hepr.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukui H., Saito H., Ueno Y., Uto H., Obara K., Sakaida I., Shibuya A., Seike M., Nagoshi S., Segawa M., et al. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J. Gastroenterol. 2016;51:629–650. doi: 10.1007/s00535-016-1216-y. [DOI] [PubMed] [Google Scholar]
- 8.Bunchorntavakul C., Reddy K.R. Treat chronic hepatitis C virus infection in decompensated cirrhosis—Pre- or post-liver transplantation? J. Viral. Hepat. 2016;23:408–418. doi: 10.1111/jvh.12534. [DOI] [PubMed] [Google Scholar]
- 9.Bruno S., Boccaccio V., Russo M.L., Maisonneuve P. Is the benefit of treating patients with cirrhosis proven? Liver Int. 2016;36:21–27. doi: 10.1111/liv.13013. [DOI] [PubMed] [Google Scholar]
- 10.Wirth T.C., Manns M.P. The impact of the revolution in hepatitis C treatment on hepatocellular carcinoma. Ann. Oncol. 2016 doi: 10.1093/annonc/mdw219. [DOI] [PubMed] [Google Scholar]
- 11.Webster D.P., Klenerman P., Dusheiko G.M. Hepatitis C. Lancet. 2015;385:1124–1135. doi: 10.1016/S0140-6736(14)62401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li D.K., Chung R.T. Impact of hepatitis C virus eradication on hepatocellular carcinogenesis. Cancer. 2015;121:2874–2882. doi: 10.1002/cncr.29528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujiyoshi M., Kuno A., Gotoh M., Fukai M., Yokoo H., Kamachi H., Kamiyama T., Korenaga M., Mizokami M., Narimatsu H., et al. Clinicopathological characteristics and diagnostic performance of Wisteria floribunda agglutinin positive Mac-2-binding protein as a preoperative serum marker of liver fibrosis in hepatocellular carcinoma. J. Gastroenterol. 2015;50:1134–1144. doi: 10.1007/s00535-015-1063-2. [DOI] [PubMed] [Google Scholar]
- 14.Toshima T., Shirabe K., Ikegami T., Yoshizumi T., Kuno A., Togayachi A., Gotoh M., Narimatsu H., Korenaga M., Mizokami M., et al. A novel serum marker, glycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA(+)-M2BP), for assessing liver fibrosis. J. Gastroenterol. 2015;50:76–84. doi: 10.1007/s00535-014-0946-y. [DOI] [PubMed] [Google Scholar]
- 15.Yamasaki K., Tateyama M., Abiru S., Komori A., Nagaoka S., Saeki A., Hashimoto S., Sasaki R., Bekki S., Kugiyama Y., et al. Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology. 2014;60:1563–1570. doi: 10.1002/hep.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamaki N., Kurosaki M., Kuno A., Korenaga M., Togayachi A., Gotoh M., Nakakuki N., Takada H., Matsuda S., Hattori N., et al. Wisteria floribunda agglutinin positive human Mac-2-binding protein as a predictor of hepatocellular carcinoma development in chronic hepatitis C patients. Hepatol. Res. 2015;45:E82–E88. doi: 10.1111/hepr.12466. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki R., Yamasaki K., Abiru S., Komori A., Nagaoka S., Saeki A., Hashimoto S., Bekki S., Kugiyama Y., Kuno A., et al. Serum Wisteria floribunda agglutinin-positive Mac-2 binding protein values predict the development of hepatocellular carcinoma among patients with chronic hepatitis c after sustained virological response. PLoS ONE. 2015;10:1500. doi: 10.1371/journal.pone.0129053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toyoda H., Kumada T., Tada T., Kaneoka Y., Maeda A., Korenaga M., Mizokami M., Narimatsu H. Serum WFA+-M2BP levels as a prognostic factor in patients with early hepatocellular carcinoma undergoing curative resection. Liver Int. 2016;36:293–301. doi: 10.1111/liv.12907. [DOI] [PubMed] [Google Scholar]
- 19.Ura K., Furusyo N., Ogawa E., Hayashi T., Mukae H., Shimizu M., Toyoda K., Murata M., Hayashi J. Serum WFA(+)-M2BP is a non-invasive liver fibrosis marker that can predict the efficacy of direct-acting anti-viral-based triple therapy for chronic hepatitis C. Aliment. Pharmacol. Ther. 2016;43:114–124. doi: 10.1111/apt.13431. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa H., Enomoto H., Iwata Y., Hasegawa K., Nakano C., Takata R., Nishimura T., Yoh K., Aizawa N., Sakai Y., et al. Impact of serum Wisteria floribunda agglutinin positive Mac-2-binding protein and serum interferon-γ-inducible protein-10 in primary biliary cirrhosis. Hepatol. Res. 2016;46:575–583. doi: 10.1111/hepr.12595. [DOI] [PubMed] [Google Scholar]
- 21.Nishikawa H., Enomoto H., Iwata Y., Hasegawa K., Nakano C., Takata R., Nishimura T., Yoh K., Aizawa N., Sakai Y., et al. Clinical significance of serum Wisteria floribunda agglutinin positive Mac-2-binding protein level and high-sensitivity C-reactive protein concentration in autoimmune hepatitis. Hepatol. Res. 2016;46:613–621. doi: 10.1111/hepr.12596. [DOI] [PubMed] [Google Scholar]
- 22.Ichikawa Y., Joshita S., Umemura T., Shobugawa Y., Usami Y., Shibata S., Yamazaki T., Fujimori N., Komatsu M., Matsumoto A., et al. Serum Wisteria floribunda agglutinin-positive human Mac-2 binding protein may predict liver fibrosis and progression to hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Hepatol. Res. 2016 doi: 10.1111/hepr.12712. [DOI] [PubMed] [Google Scholar]
- 23.Nishikawa H., Enomoto H., Iwata Y., Kishino K., Shimono Y., Hasegawa K., Nakano C., Takata R., Yoh K., Nishimura T., et al. Clinical significance of serum Wisteria floribunda agglutinin-positive Mac-2-binding protein level in non-alcoholic steatohepatitis. Hepatol. Res. 2016 doi: 10.1111/hepr.12662. [DOI] [PubMed] [Google Scholar]
- 24.Zou X., Zhu M.Y., Yu D.M., Li W., Zhang D.H., Lu F.J., Gong Q.M., Liu F., Jiang J.H., Zheng M.H., et al. Serum WFA+-M2BP levels for evaluation of early stages of liver fibrosis in patients with chronic hepatitis B virus infection. Liver Int. 2016 doi: 10.1111/liv.13188. [DOI] [PubMed] [Google Scholar]
- 25.Ishii A., Nishikawa H., Enomoto H., Iwata Y., Kishino K., Shimono Y., Hasegawa K., Nakano C., Takata R., Nishimura T., et al. Clinical implication of serum Wisteria floribunda agglutinin-positive Mac-2-binding protein in treatment naïve chronic hepatitis B. Hepatol. Res. 2016 doi: 10.1111/hepr.12703. [DOI] [PubMed] [Google Scholar]
- 26.Tada T., Kumada T., Toyoda H., Tsuji K., Hiraoka A., Tanaka J. Impact of FIB-4 index on HCC incidence during nucleos(t)ide analogue therapy in CHB patients: an analysis using time-dependent ROC. J. Gastroenterol. Hepatol. 2016 doi: 10.1111/jgh.13473. [DOI] [PubMed] [Google Scholar]
- 27.Tada T., Kumada T., Toyoda H., Kiriyama S., Tanikawa M., Hisanaga Y., Kanamori A., Kitabatake S., Yama T., Tanaka J. HBcrAg predicts hepatocellular carcinoma development: An analysis using time-dependent receiver operating characteristics. J. Hepatol. 2016;65:48–56. doi: 10.1016/j.jhep.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Freeman A.J., Law M.G., Kaldor J.M., Dore G.J. Predicting progression to cirrhosis in chronic hepatitis C virus infection. J. Viral. Hepat. 2003;10:285–293. doi: 10.1046/j.1365-2893.2003.00436.x. [DOI] [PubMed] [Google Scholar]
- 29.Nishikawa H., Enomoto H., Yoh K., Iwata Y., Hasegawa K., Nakano C., Takata R., Kishino K., Shimono Y., Sakai Y., et al. Serum hyaluronic acid predicts protein-energy malnutrition in chronic hepatitis C. Medicine. 2016;95:e3920. doi: 10.1097/MD.0000000000003920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith-Palmer J., Cerri K., Valentine W. Achieving sustained virologic response in hepatitis C: A systematic review of the clinical, economic and quality of life benefits. BMC Infect. Dis. 2015;15:1500. doi: 10.1186/s12879-015-0748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuno A., Ikehara Y., Tanaka Y., Ito K., Matsuda A., Sekiya S., Hige S., Sakamoto M., Kage M., Mizokami M., et al. A serum “sweet-doughnut” 272 protein facilitates fibrosis evaluation and therapy assessment in patients 273 with viral hepatitis. Sci. Rep. 2013;3:1065. doi: 10.1038/srep01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuno A., Sato T., Shimazaki H., Unno S., Saitou K., Kiyohara K., Sogabe M., Tsuruno C., Takahama Y., Ikehara Y., et al. Reconstruction of a robust glycodiagnostic agent supported by multiple lectin-assisted glycan profiling. Proteom. Clin. Appl. 2013;7:642–647. doi: 10.1002/prca.201300010. [DOI] [PubMed] [Google Scholar]
- 33.Lin Z.H., Xin Y.N., Dong Q.J., Wang Q., Jiang X.J., Zhan S.H., Sun Y., Xuan S.Y. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–736. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 34.Vallet-Pichard A., Mallet V., Nalpas B., Verkarre V., Nalpas A., Dhalluin-Venier V., Fontaine H., Pol S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 35.Kaneko S., Furuse J., Kudo M., Ikeda K., Honda M., Nakamoto Y., Onchi M., Shiota G., Yokosuka O., Sakaida I., et al. Guideline on the use of new anticancer drugs for the treatment of hepatocellular carcinoma 2010 update. Hepatol. Res. 2012;42:523–542. doi: 10.1111/j.1872-034X.2012.00981.x. [DOI] [PubMed] [Google Scholar]
- 36.Yamakado K., Kudo M. Treatment strategies of intermediate-stage hepatocellular carcinomas in Japan (Barcelona Clinic Liver Cancer stage B) Oncology. 2014;87:78–81. doi: 10.1159/000368149. [DOI] [PubMed] [Google Scholar]
- 37.Kudo M., Izumi N., Kokudo N., Matsui O., Sakamoto M., Nakashima O., Kojiro M., Makuuchi M., HCC Expert Panel of Japan Society of Hepatology Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan society of hepatology (JSH) 2010 updated version. Dig. Dis. 2011;29:339–364. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]