Abstract
Several years ago it was proposed that the AMP-activated protein kinase cascade might protect cells against stresses that deplete cellular ATP. Young et al. have now directly tested this by studying the effects of ischemia and reperfusion in perfused hearts from mice expressing a dominant-negative mutant that suppresses the kinase activity in cardiac muscle. Compared with control hearts, the mutant hearts showed clear evidence for increased necrotic damage and increased apoptosis. These findings may have implications for the treatment of ischemic heart disease.
AMP-activated protein kinase (AMPK) is the downstream component of a protein kinase cascade that is highly conserved in all eukaryotic cells (1). AMPK is activated by the rising cellular AMP that (due to the action of adenylate kinase) always accompanies a fall in the cellular ATP/ADP ratio, and this activation is antagonized by high concentrations of ATP (Figure 1). Downstream targets and processes regulated by the kinase are being identified on a regular basis (Figure 2). In general, AMPK switches off ATP-consuming processes such as biosynthetic pathways, while switching on catabolic processes that generate ATP, including cellular uptake of glucose (2) and fatty acids (3) and increased fatty acid oxidation (4) in the heart.
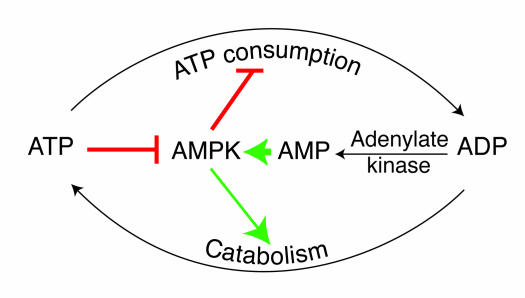
Figure 1.
Role of AMPK in regulating energy balance at the single-cell level. The way in which the AMPK system controls the balance between ATP consumption (e.g., by biosynthesis, cell growth, or muscle contraction) and ATP production via catabolism is illustrated. If the rate of ATP consumption exceeds its rate of production, ADP will tend to rise and be converted to AMP by the enzyme adenylate kinase. The rise in level of the activating ligand AMP, coupled with the fall in level of the inhibitory nucleotide ATP, activates AMPK, which then switches off ATP-consuming processes and switches on catabolism in an attempt to redress the balance.
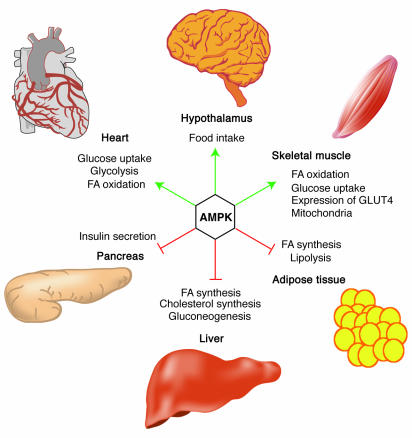
Figure 2.
Role of AMPK in regulating energy balance at the whole-body level. Green arrows indicate positive effects, and red lines with bars indicate negative effects. In the hypothalamus, activation of AMPK in response to low glucose or leptin levels increases food intake (18, 19); references for other effects of AMPK activation can be found in recent reviews (1). FA, fatty acid.
The first evidence that AMPK was activated by metabolic stresses appeared 13 years ago, when my group found that AMPK was activated by ATP depletion caused by incubation of isolated rat hepatocytes with high fructose (5), while Witters et al. (6) reported that it was activated by various metabolic poisons in hepatoma cells. We proposed at the time that the AMPK cascade was a system that might protect cells against stresses that compromise their energy status. Ischemia was one obvious such stress, and Lopaschuk et al. later reported that the kinase was activated by ischemia in perfused rat hearts and that this was associated with high rates of fatty acid oxidation during reperfusion (4). Subsequently, Young et al. provided evidence that AMPK activation in the heart increased glucose uptake via translocation of the transporter GLUT4 to the plasma membrane (2), while Hue’s group found that this activation caused phosphorylation and activation of the cardiac isoform of 6-phosphofructo-2-kinase, leading to an increase in fructose-2,6-bisphosphate, an activator of glycolysis (7). By these mechanisms, activation of AMPK during periods when the blood supply was compromised during ischemic heart disease would stimulate both glucose uptake and glycolysis and thus generate more ATP via anaerobic metabolism, while high rates of fatty acid oxidation would replenish ATP during subsequent reperfusion. Taken together, these findings suggested that AMPK might provide protection during ischemia and reperfusion. This remained, however, only an attractive hypothesis until now, with a new study by Young and coworkers that is reported in this issue of the JCI (8).
Mouse hearts with reduced AMPK are more susceptible to damage during ischemia and reperfusion
Previously, Birnbaum’s group had constructed transgenic mice that expressed an inactive mutant of the α2 isoform of the AMPK catalytic subunit from a muscle creatine kinase promoter, which provided evidence that AMPK regulated glucose uptake in skeletal muscle in vivo (9). AMPK is a heterotrimer containing, in addition to the catalytic α subunit, a β subunit with a glycogen-binding domain (10, 11) and a γ subunit with two regulatory sites that bind AMP and ATP antagonistically (12). Birnbaum (9) had shown that expression of inactive α2 was very effective at suppressing endogenous AMPK activity and thus behaved as a dominant-negative mutant. This probably works because the inactive α2 competes with the active, endogenous α1 and α2 subunits for binding to the available β and γ subunits and (because the muscle creatine kinase promoter provides very high expression levels) completely displaces them from cellular AMPK complexes (Figure 3).
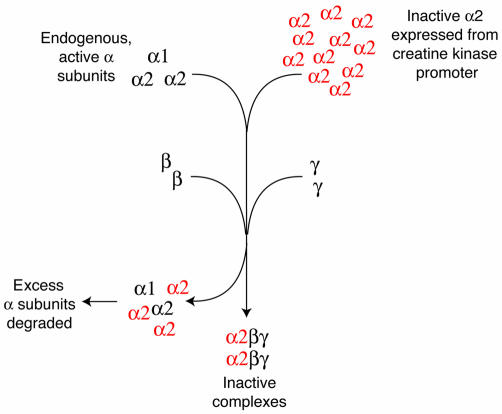
Figure 3.
How the dominant-negative mutant works. AMPK is an αβγ heterotrimer, and the catalytic α subunit is not functional unless it is complexed with β and γ. Expression in cardiac myocytes of inactive, mutant α2 subunit (red) from a strong promoter (muscle creatine kinase) that gives high expression levels means that expression of the inactive α2 is much higher than that of the endogenous, active α1 and α2 subunits. Because the availability of the β and γ subunits is limiting, most of the heterotrimers formed will contain the inactive, mutant α2. The excess α subunits (not complexed with β and γ) are probably recognized by cellular machinery as misfolded proteins and degraded.
Because the creatine kinase promoter is also active in cardiac muscle, Young and coworkers were able to use Birnbaum’s mouse model to study the role of AMPK in the heart (8). They confirmed that α2 activity was almost totally absent in the heart, although, surprisingly, the basal activity of the α1 isoform was not reduced. This would have been expected, because the α1 isoform also needs to bind to the β and γ subunits, but the authors suggest that the retention of α1 activity might result from the fact that much of the α1 isoform being measured is actually expressed in cells other than cardiac myocytes, such as endothelial cells. The hearts from the transgenic mice did not display any gross structural abnormalities, although they were marginally smaller than hearts from wild-type littermates, had a lower glycogen content, and exhibited a modest impairment in some parameters of contractile function. Of course, these mice also have abnormalities in skeletal muscle and have been found to take less voluntary exercise (9), so some of the changes in the heart could be secondary to defects in skeletal muscle. A previous study, which did not examine cell survival after ischemia and reperfusion, got around this problem by expressing the inactive α2 from a cardiac-specific promoter (13). However, Young et al. observed the most interesting changes in cardiac function, which are not likely to be secondary effects, using isolated perfused hearts. During low-flow ischemia in hearts of wild-type littermates, the activities of the α1 and α2 isoforms of AMPK increased markedly, as expected, and both remained elevated during reperfusion. However, in the transgenic hearts, α2 activity was negligible under all conditions, while the α1 activity did not increase above basal values. Satisfyingly, the altered metabolic responses of the hearts from the transgenic animals were completely consistent with the proposed roles of AMPK in controlling cardiac metabolism. Thus, the normal increases in glucose uptake and lactate production during ischemia and reperfusion, and the increase in fatty acid oxidation during reperfusion, were all abolished. The ATP and phosphocreatine contents were also lower in the transgenic hearts during ischemia and did not show any signs of recovery during reperfusion. In addition, left-ventricular contractile function was poorer in the transgenic hearts during ischemia, and recovery during reperfusion was impaired. Most interesting of all, this was accompanied by evidence of both increased necrotic tissue damage and increased apoptosis. Thus, AMPK protects against injury caused by ischemia and reperfusion, most likely by helping to preserve the levels of cellular ATP.
Could AMPK activation be used to treat ischemic heart disease?
While many in the AMPK field have long held the view, almost as an article of faith, that the kinase protects cells against the damaging effects of metabolic insults such as ischemia, the article by Young and colleagues (8) is important because it provides the first direct evidence that this is indeed the case. Could drugs that activate AMPK provide protection against tissue damage caused by ischemia and reperfusion? Evidence from trials using the drug acadesine (5-aminoimidazole-4-carboxamide riboside, or AICAR) suggests that this is at least a possibility (14). Acadesine is an adenosine analog that is taken up into cells via adenosine transporters (15) and converted inside the cell by adenosine kinase to 5-aminoimidazole-4-carboxamide riboside monophosphate (ZMP), an intermediate in the synthesis of GTP and ATP. It was originally proposed that acadesine might have beneficial effects by replacing, via de novo synthesis, the depleted levels of these nucleotides caused by ischemia and reperfusion. However, what was not realized at that time was that ZMP also mimics the effects of AMP to activate the AMPK system (16). In studies using animal models, acadesine has been shown to provide protection against injury caused by ischemia and reperfusion, and results in trials during human coronary artery bypass graft surgery have shown modest benefits (14). The rather limited efficacy of acadesine in these trials could either have been because the drug is not a very potent activator of AMPK (especially in cardiac myocytes; ref. 17), or because the AMPK system would have been rapidly activated during ischemia anyway and did not need a helping hand. However, further studies with novel, more potent AMPK activators are clearly warranted, and with the help of new insights such as those provided by the article in this issue (8), it is possible that this approach might produce new treatments for ischemic heart disease.
Acknowledgments
Work in the author’s laboratory is supported by the Wellcome Trust and the European Commission (contract no. QLG1-CT-2001-01488).
Footnotes
See the related article beginning on page 495.
Nonstandard abbreviations used: 5-aminoimidazole-4-carboxamide riboside monophosphate (ZMP); AMP-activated protein kinase (AMPK).
Conflict of interest: The author has declared that no conflict of interest exists.
References
- 1.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–120. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- 2.Russell RR, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am. J. Physiol. 1999;277:H643–H649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- 3.Luiken JJ, et al. Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes. 2003;52:1627–1634. doi: 10.2337/diabetes.52.7.1627. [DOI] [PubMed] [Google Scholar]
- 4.Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J. Biol. Chem. 1995;270:17513–17520. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- 5.Moore F, Weekes J, Hardie DG. AMP triggers phosphorylation as well as direct allosteric activation of rat liver AMP-activated protein kinase. A sensitive mechanism to protect the cell against ATP depletion. Eur. J. Biochem. 1991;199:691–697. doi: 10.1111/j.1432-1033.1991.tb16172.x. [DOI] [PubMed] [Google Scholar]
- 6.Witters LA, Nordlund AC, Marshall L. Regulation of intracellular acetyl-CoA carboxylase by ATP depletors mimics the action of the 5′-AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 1991;181:1486–1492. doi: 10.1016/0006-291x(91)92107-u. [DOI] [PubMed] [Google Scholar]
- 7.Marsin AS, et al. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr. Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 8.Russell RR, III, et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J. Clin. Invest. 2004;114:495–503. doi:10.1172/JCI200419297. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mu J, Brozinick JT, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol. Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 10.Hudson ER, et al. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Curr. Biol. 2003;13:861–866. doi: 10.1016/s0960-9822(03)00249-5. [DOI] [PubMed] [Google Scholar]
- 11.Polekhina G, et al. AMPK b-Subunit targets metabolic stress-sensing to glycogen. Curr. Biol. 2003;13:867–871. doi: 10.1016/s0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- 12.Scott JW, et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J. Clin. Invest. 2004;113:274–284. doi:10.1172/JCI200419874. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xing Y, et al. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative alpha2 subunit of AMP-activated protein kinase. J. Biol. Chem. 2003;278:28372–28377. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- 14.Mangano DT. Effects of acadesine on myocardial infarction, stroke, and death following surgery. A meta-analysis of the 5 international randomized trials. The Multicenter Study of Perioperative Ischemia (McSPI) Research Group. JAMA. 1997;277:325–332. doi: 10.1001/jama.277.4.325. [DOI] [PubMed] [Google Scholar]
- 15.Gadalla AE, et al. Distinct mechanisms underlie the activation of rat brain AMP-activated protein kinase and the inhibition of excitatory synaptic transmission by AICA riboside (Acadesine) in area CA1 of rat hippocampus. J. Neurochem. 2004;88:1272–1282. doi: 10.1046/j.1471-4159.2003.02253.x. [DOI] [PubMed] [Google Scholar]
- 16.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside: a specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 17.Javaux F, Vincent MF, Wagner DR, van den Berghe G. Cell-type specificity of inhibition of glycolysis by 5-amino-4-imidazolecarboxamide riboside. Lack of effect in rabbit cardiomyocytes and human erythrocytes, and inhibition in FTO-2B rat hepatoma cells. Biochem. J. 1995;305:913–919. doi: 10.1042/bj3050913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersson U, et al. AMP-activated protein kinase plays a role in the control of food intake. J. Biol. Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- 19.Minokoshi Y, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]