Abstract
Human safety and well-being is threatened by microbes causing numerous infectious diseases resulting in a large number of deaths every year. Despite substantial progress in antimicrobial drugs, many infectious diseases remain difficult to treat. Antimicrobial polymers offer a promising antimicrobial strategy for fighting pathogens and have received considerable attention in both academic and industrial research. This mini-review presents the advances made in antimicrobial polymers since 2013. Antimicrobial mechanisms exhibiting either passive or active action and polymer material types containing bound or leaching antimicrobials are introduced. This article also addresses the applications of these antimicrobial polymers in the medical, food, and textile industries.
Keywords: antimicrobial, polymer, microbe, bacteria, review
1. Introduction
Microbes are living organisms, such as bacteria, fungi, and parasites, which are the critical sources of infections [1]. Infectious diseases result from pathogenic microbes and kill more people than any other single cause [2]. An antimicrobial is an agent used to kill microbes or inhibit their growth. Although numerous antimicrobial drugs have been developed to kill or inhibit microbes, many infectious diseases remain difficult to treat [3,4]. Antimicrobial polymers were discovered since 1965 [5] and have attracted considerable attention in both academic and industrial research. Table 1 shows recent review articles on antimicrobial polymers from various perspectives. These reviews focus on methods for producing antimicrobial polymers and various applications of antimicrobials. Increasing efforts develops learning from nature, green or nontoxic biocides. The medical, food, and textile industries are three major areas of applied antimicrobials. More than 27,845 patents for antimicrobial polymers have been filed in the Google Patent Search database since 2013. In addition, antimicrobial medical devices have attracted substantial attention in clinical trials [5]. Compared with their small molecular counterparts, antimicrobial polymers demonstrate superior efficacy, reduced toxicity, minimized environmental problems, and greater resistance [6].
Table 1.
Recent review articles of antimicrobial polymers.
| Subject | Topic | Reference |
|---|---|---|
| Application | Stimuli-responsive polymeric materials for human health applications | [7] |
| Antimicrobial polymers for anti-biofilm medical devices | [8] | |
| Antimicrobial peptides in the treatment of bacterial biofilm infections | [9] | |
| Overview | Antimicrobial peptides and enzymes | [10] |
| Anti-infectious surfaces achieved by polymer modification | [11] | |
| Antimicrobial polymers | [6] | |
| Antimicrobial polymers with metal nanoparticles | [12] | |
| Synthesis and characteristic | Antimicrobial N-halamine polymers and coatings | [13] |
| Antimicrobial modifications of polymers | [14] | |
| Antibacterial dental resin composites | [15] | |
| Novel formulations for antimicrobial peptides | [16] | |
| Coatings and surface modifications imparting antimicrobial activity to orthopedic implants | [17] | |
| Antimicrobial activity of chitosan derivatives containing n-quaternized moieties | [18] | |
| Cationic polymers and their self-assembly for antibacterial applications. | [19] | |
| Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts | [20] |
When microbes adhere to a substrate, they excrete biofilms to anchor themselves to the substrate. In biofilms, cells grow in multicellular aggregates and become embedded within a self-produced matrix of an extracellular polymeric substance. A biofilm extracellular polymeric substance is a polymeric conglomeration composed of polysaccharides and other components, such as proteins and DNA. Defective biofilms cannot offer an environment for microbes to grow. Therefore, antimicrobial applications entail strategies for preventing microbial viability or adhesion. For example, antimicrobial peptides act primarily by disrupting the bacterial cell membrane, and heparin exhibits anti-adhesive activity and hydrophilic characteristics that prevent the growth of microbes [21]. Several reviews have described antimicrobial management [22,23,24,25,26,27,28]. Biofilms are difficult to remove and resist many biocides. Therefore, to prevent the spread of diseases, inhibiting biofilm formation and reducing microbial attachment are a more promising antimicrobial strategy than killing microbes [17,29].
Many promising antimicrobial polymers have been reported, and the number of FDA-approved antimicrobial polymers has increased drastically in the past decade [5]. This review describes the new developments in antimicrobial polymers over the past three years. According to the mechanism of antimicrobial activity, the activity of antimicrobial polymers can be categorized as either passive or active (Section 2). Based on the polymer material type, antimicrobial polymers can be classified as bound or leaching antimicrobials (Section 3). These antimicrobial polymers are applied in the medical, food, and textile industries (Section 4). Finally, the conclusion and prospects for future research are addressed (Section 5).
2. Passive or Active Action
2.1. Passive Action
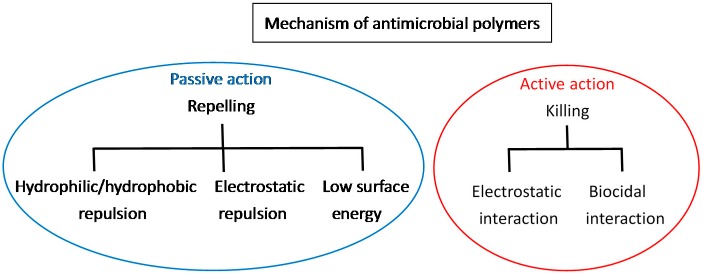
A passive polymer layer can reduce protein adsorption on its surface, thereby preventing the adhesion of bacteria. However, although passive surfaces repel bacteria, they do not actively interact with or kill bacteria. Due to the mainly hydrophobic and negatively-charged properties of microbes, passive polymers should be either (1) hydrophilic; (2) negatively-charged; or (3) have a low surface free energy (Figure 1) [8,30]. Typical passive polymers comprise (1) self-healing, slippery liquid-infused porous surface (SLIPS), such as poly(dimethyl siloxane); (2) uncharged polymers, such as poly(ethylene glycol) (PEG), poly(2-methyl-2-oxazoline), polypeptoid, polypoly(n-vinyl-pyrrolidone), and poly(dimethyl acrylamide); and (3) charged polyampholytes and zwitterionic polymers, such as phosphobetaine, sulfobetaine, and phospholipid polymers [31,32]. Table 2 lists selected passive polymers and their antimicrobial applications. Among these passive polymers, PEG has been extensively studied and has demonstrated excellent antimicrobial effects in drastically reducing protein adsorption and bacterial adhesion. Due to high chain mobility, large exclusion volume, and steric hindrance effect of highly hydrated layer [30], PEG has been the most commonly used passive antimicrobial material [33], and research has shown that it exhibits high antifouling ability to prevent protein and cell adhesion effectively, consequently preventing the growth of microbes.
Figure 1.
The schematic reaction mechanisms of passive and active action of the antimicrobial polymers.
Table 2.
Examples of passive polymers for antimicrobial applications.
| Polymer | Target | Remark | Reference |
|---|---|---|---|
| Poly(ethylene glycol) | Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa | Used as neutral polymer brush systems to prevent protein and cell adhesion | [33] |
| Poly(sulfobetaine methacrylate) | Pseudomonas aeruginosa, Staphylococcus epidermidis | Resist protein adsorption, cell attachment, and bacterial adhesion | [34] |
| Poly[3-dimethyl (methacryloyloxyethyl) ammonium propane sulfonate-b-2-(diisopropylamino)ethyl methacrylate] | Staphylococcus aureus | Zwitterionic coronae and pH-responsive cores can impart bacterial anti-adhesive properties | [35] |
| Poly(2-methyl-2-oxazoline) | Escherichia coli | Dual-functional antimicrobial surface of poly(l-lysine)-graft-poly(2-methyl-2-oxazoline)-quarternery ammonium | [36] |
| Albumin, whey | Bacillus subtilis, Escherichia coli | No bacterial growth was observed on albumin-glycerol and whey-glycerol after 24 h inoculation | [37] |
| Polyphenols | Streptococcus mitis, Fusobacterium nucleatu, Porphyromonas gingivalis | Effective against periodontal bacteria | [38] |
2.2. Active Action
Active polymers actively kill bacteria that adhere to the polymer surface. Polymers functionalized with active agents, such as cationic biocides, antimicrobial peptides, or antibiotics, can kill bacteria on contact. The mechanism of polymers killing microbes depends on the active agents (Figure 1). The most widely used active antimicrobial polymers are functionalized with positively-charged quaternary ammonium, which interacts with the cell wall and destroys the cytoplasmic membrane, resulting in the leakage of intracellular components and consequent cell death [20]. In addition, polyethylenimine, polyguanidine, and N-halamine are representative polymers that demonstrate active antimicrobial activity. Polyethylenimine brings about bacterial cell membrane rupture by the electrostatic interaction between polyethylenimine and the cell membrane. Polyguanidine has bacterial growth inhibition through adhesion and subsequent disruption of Ca2+ salt bridges or cell death. N-halamine makes cell inhibition or inactivation by action of the oxidative halogen targeted at thio or amino groups of cell receptors [6]. Table 3 lists active polymers and their antimicrobial applications, indicating that most of these new antimicrobials materials are based on quaternary ammonium salts.
Table 3.
Examples of active polymers for antimicrobial applications.
| Polymer | Target | Antimicrobial Substance | Remark | Reference |
|---|---|---|---|---|
| Nisin-immobilized organosilicon | Bacillus subtilis | Nisin | Superior antimicrobial activity, and resistant to several cleaning conditions | [39] |
| Polyurethane containing quaternary ammonium | Staphylococcus aureus, Escherichia coli | Quaternary ammonium | Good antimicrobial activities against even at low concentrations (5 wt %) | [40] |
| Poly(n,n-diethylethylendiamine-co-yrosol-based acrylic) | Staphylococcus epidermidis, Staphylococcus aureus | Tertiary amine | Combination of two active compounds provide a synergistic action against biofilms and suppress reactive species oxygen | [41] |
| Organosilicon quaternary ammonium chloride | Staphylococcus aureus | Quaternary ammonium | Exerted long-lasting antimicrobial activity for at least four hours | [42] |
| Poly(2-(dimethylamino)ethyl methacrylate) tethering quaternary ammonium | Bacillus subtilis, Escherichia coli | Quaternary ammonium | Higher C–N+ content and relatively smooth morphology would find potential antimicrobial activity | [43] |
| Acrylamide polymers with quaternary ammonium | Staphylococcus albus, Escherichia coli, Rhizoctonia solani, Fusarium oxysporum | Quaternary ammonium | Benzyl group attached to nitrogen atom showed better inhibitory effect on bacteria and phytopathogenic fungi | [44] |
3. Bound or Leaching Antimicrobials
Several recent comprehensive state-of-the-art reviews summarize the progress of and research on antimicrobial polymers [6,11]. Antimicrobial polymers can be divided into three types: polymeric biocides, biocidal polymers, and biocide-releasing polymers [5]. Recently, synergistic combination has been commonly used to provide multiple functional antimicrobials for fighting pathogens.
3.1. Polymeric Biocides
Polymeric biocides are polymers that covalently link bioactive repeating units with antimicrobial activity such as amino, carboxyl, or hydroxyl groups [8,14,18]. The polymerization process may either enhance or reduce the antimicrobial activity of bioactive functional groups. Table 4 lists examples of polymeric biocides synthesized from antimicrobial monomers.
Table 4.
Examples of polymeric biocides for antimicrobial applications.
| Monomer | Target | Antimicrobial Substance | Remark | Reference |
|---|---|---|---|---|
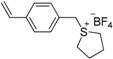 |
Staphylococcus aureus, Escherichia coli | Sulfonium salt | A high antibacterial activity against Gram-positive bacteria than Gram-negative bacteria | [5] |
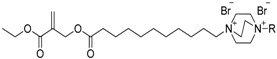 |
Staphylococcus aureus, Escherichia coli | Quaternary ammonium | Activity depends on the length of hydrophobic segments | [20] |
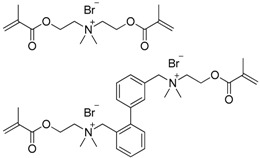 |
Escherichia coli | Quaternary Ammonium | Antimicrobial dental materials | [20] |
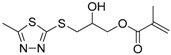 |
Micrococcus luteus, Staphylococcus aureus, Bacillus subtilis | Benzimidazole | Against Gram-positive bacterial strains MIC values 5.4–53.9 μM | [45] |
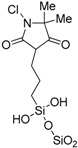 |
Staphylococcus aureus, Escherichia coli | Halogen | Inactivate 100% Staphylococcus aureus and Escherichia coli with a contact time of 10 and 30 min | [46] |
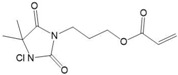 |
Staphylococcus aureus, Escherichia coli | N-halamine | Excellent biocidal efficacy by inactivating 100% of the bacteria with the contact times less than 10 min | [47] |
3.2. Biocidal Polymers
Requiring no bioactive repeating units, the antimicrobial site of biocidal polymers is embodied by the entire macromolecule. Many biocidal polymers contain cationic biocides, such as quaternary ammonium, phosphonium, tertiary sulfonium, and guanidinium. Microbes generally have a negative charge at the outer membrane of the cell. Cationic polymers can lead to the destabilization of the cell surface and the ultimately induction of bacterial death [19]. The antimicrobial activity of cationic polymers relate to the charge density of cationic groups.
Table 5 lists examples of biocidal polymers in relevant literature. Due to its properties of nontoxicity, biodegradability, and biocompatibility, chitosan is the most representative natural material exhibiting inherent antimicrobial activity. The antimicrobial activity of chitosan depends heavily on pH value. With a pH value of less than pKa, electrostatic interaction occurs between protonated amino groups and the cell wall. When the pH is higher than the pKa value, the antimicrobial activity of chitosan derives from hydrophobic interaction and chelation effects. Other natural antimicrobial polymers include heparin, poly-ε-lysine, and gramicidin A [6]. Antimicrobial peptides have been recognized as promising candidates for the new generation of antibacterial surfaces [10,58,59]. Thus far, more than 1000 antimicrobial peptides have been found. In addition to directly killing microbes by disrupting cell membranes and inhibiting cellular processes, the antimicrobial mechanisms of peptides can also exert immunomodulatory effects resulting in microbial clearance through stimulation of the noninflammatory host immune response [9]. The challenges of microbial peptides for therapeutic use include unwanted side effects, high production costs, deficient stability, and adaptive antimicrobial resistance [9].
Table 5.
Examples of biocidal polymers for antimicrobial applications.
| Polymer | Target | Remark | Reference |
|---|---|---|---|
| Quaternary ammonium polyethyleneimine | Gram-positive and Gram-negative bacteria | n-alkylated polyethyleneimine has effective antimicrobial activity, dependent on the hydrophobic and positively charged immobilized long polymeric chains | [15] |
| Quaternary phosphonium modified epoxidized natural rubber | Staphylococcus aureus, Escherichia coli | Moderate growth inhibition of microbes | [48] |
| Arginine–tryptophan-rich peptide | Gram-positive and Gram-negative bacteria | Retain antimicrobial functionality for at least 21 days, showing negligible cytotoxicity | [49,50] |
| Guanylated polymethacrylate | Staphylococcus epidermidis, Candida albicans | Guanidine copolymers were much more active compared to the amine analogues | [51] |
| Chitosan | Bacteria, yeast, fungi | Widely-used antimicrobial agent either alone or blended with other compounds | [52,53,54] |
| Ammonium ethyl methacrylate homopolymers | Methicillin-resistant Staphylococcus aureus, Escherichia coli | Very little or no hemolytic activity and higher inhibitory effects against Gram-positive bacteria than Gram-negative bacteria | [55] |
| Metallo-terpyridine carboxymethyl cellulose | Staphylococcus aureus, Streptococcus thermophilus, Escherichia coli, Saccharomyces cervisiae | Minimum inhibitory concentration ranged from 6 to 8 mg/L to achieve ≥90% inhibition | [56] |
| Poly(n-vinylimidazole) modified silicone rubber | Pseudomonas aeruginosa, Staphylococcus aureus | More antibacterial activity against Pseudomonas aeruginosa than Staphylococcus aureus | [57] |
3.3. Biocide-Releasing Polymers
Biocide-releasing polymers can be realized by (1) polymerization of biocide-releasing molecules to polymeric backbone; or (2) polymer/biocide-releasing molecules composites. The polymer in biocide-releasing systems is used as a carrier for biocides. Polymers exhibit antibacterial properties through the incorporation of antibiotic and/or antiseptic compounds. The controlled release system of biocide-release polymers has numerous advantages such as maintaining a high local biocide concentration close to microbes and facilitating the delivery of biocides with short in vivo half-lives. This type of antimicrobial polymer demonstrates great potential for use in the medical industry. Numerous biodegradable polymer devices have been developed as antibiotic carriers for various applications [60,61,62,63]. Table 6 shows various biocide-releasing polymer systems, including new polymer composites that exhibit antimicrobial activity.
Table 6.
Examples of biocide-releasing polymers for antimicrobial applications.
| Polymer | Target | Antimicrobial Substances | Remark | Reference |
|---|---|---|---|---|
| Dextrans | Staphylococcus aureus | Gentamicin | Enhance gentamicin stability over time and prolong drug release for six days | [60] |
| Poly-l-lysine, polyethylene glycol | Staphylococcus aureus | Staphylolytic LysK enzyme | LysK can lyse bacteria | [68] |
| Poly(octanediol-co-citrate) | Staphylococcus aureus, Escherichia coli | Choline chloride, tetraethylammonium bromide, hexadecyltrimethylammonium bromide, methyltriphenylphosphonium bromide | Preserve cytocompatibility while showing elastic properties advantageous for wound dressings | [69] |
| Cyclodextrin | Staphylococcus aureus, Escherichia coli | Triclosan | Reduce drug amount to inhibit pathogen growth and toxic impact on environmental strains | [70] |
| Poly(methyl methacrylate) | Pseudomonas aeruginosa, Staphylococcus aureus | Silver | Light-activated antimicrobial materials doped with porphyrin and sliver | [71] |
| Poly(methyl methacrylate) | Staphylococcus epidermidis, Escherichia coli | Silver, nanoparticles, and imidazole complex | Time-dependent antimicrobial activities | [72] |
| Cyclodextrin | Staphylococcus aureus, Escherichia coli | Silver, chitosan | Cyclodextrin stabilized Ag-chitosan and provided higher antimicrobial activity | [73] |
| Acrylic bone cements | Enterococcus faecalis V583 | Chlorhexidrina | Retain both mechanical and antimicrobial properties | [74] |
| Polycaprolactone | Staphylococcus aureus, Pseudomonas aeruginosa | Silver | A strong antimicrobial and anti-biofilm properties | [75] |
A chitosan-agarose hybrid material and nanocomposite ionogels decorated with silver were produced using an ionic liquid, 1-butyl-3-methylimidazolium chloride. The prepared antimicrobial composite ionogels were biocompatible and demonstrated favorable electrical conductivity, as well as thermal and conformational stability [64]. A resorbable antibiotic-eluting polymer composite bone void filler was developed to exhibit both osteoconductive and antimicrobial properties for reducing the rates of orthopedic device-related infections [65]. Synergistic combinations of iron-sequestering polymers and conventional antibiotics may drastically reduce the minimum inhibitory concentrations of antibiotics and offer a promising early intervention or adjuvant to antibiotics [66]. Incorporating crystal violet and di(octyl)phosphinic-acid-capped zinc oxide nanoparticles into medical-grade silicone can provide a dual-mechanism antimicrobial polymer as a strategy for reducing the risk of infection [67].
In addition, antimicrobial polymers can be classified as surface-bound or solution-based polymers. Surface-bound polymers have direct antimicrobial activity on the polymer surface. However, solution-based polymers need to be used in solutions to have antimicrobial activity. In general, biocidal polymers are surface-bound polymers, while biocide-releasing polymers are solution-based polymers to release biocides in solutions. Depending on the property of bioactive repeating units, polymeric biocides can be surface-bound or solution-based polymers.
4. Applications
The major areas of applied antimicrobial polymers are the medical, food, and textile industries. The recent advances in these three areas are addressed as follows.
4.1. Medical Industry
The surfaces of all medical devices provide an environment for microbial growth, and are susceptible to microbial infection. Despite continual improvements in materials and techniques, most hospital-acquired infections originate from medical devices. An innovative antimicrobial copolymer of 4-vinyl-n-hexylpyridinium bromide (VP) and dimethyl(2-methacryloyloxyethyl) phosphonate (DMMEP) was developed to reduce biofilm formation and to improve the long-term use of medical devices. Coating a copolymer (VP:DMMEP 30:70) on titanium drastically reduces the adhesion of various pathogenic bacteria (e.g., Streptococcus sanguinis, Escherichia coli, Staphylococcus aureus, Staphylococcus epidermidis). Furthermore, soft tissue cells (human gingival or dermis fibroblasts) are minimally affected by such a coating [76].
Antimicrobial peptides and synthetic mimics of antimicrobial peptides are a new generation of antimicrobial agents with high antimicrobial, broad spectrum activity against a variety of pathogens and modulation of the immune response [77,78,79,80,81,82]. Antimicrobial wound-dressings composed of cotton gauze containing antimicrobial peptides incorporated with polycation (chitosan) and polyanion (alginic acid sodium salt) exert a high antimicrobial effect (in the range of 4–6 log reduction) for Staphylococcus aureus and Klebsiella pneumonia. These dressings have also been proven to be noncytotoxic to normal human dermal fibroblasts [83].
A novel controlled release zinc oxide/gentamicin-chitosan composite gel was developed. By slowly releasing the antibiotic, this composite gel demonstrated highly effective antimicrobial properties, inhibiting Staphylococcus aureus and Pseudomonas aeruginosa growth under both planktonic and surface-attached conditions. When used in a wound dressing, it maintained a moist environment at the wound interface and provided a cooling sensation and soothing effect. Moreover, this system is fully scalable to any other soluble drug because the entire solution remains trapped in the ZnO-chitosan composite gel [84].
The infection of catheter-associated urinary tract is the commonest hospital-acquired infection. Impregnation of urinary catheters with a combination of rifampicin, sparfloxacin and triclosan was developed. The release of the drugs from the silicone catheter segments were more than one month. The antimicrobial catheters could prevent colonization of Proteus mirabilis, Staphylococcus aureus, and Escherichia coli for 7–12 weeks. The impregnated catheters might reduce catheter-associated urinary tract infection in both short-term and long-term urinary catheter use [85].
4.2. Food Industry
Food safety and quality have attracted increasing attention because of concerns about consumer health. The advent of new technologies has been addressed by the food industry to reduce the risks to consumer health. In particular, substantial progress in food packaging has been achieved using antimicrobial polymers.
Nisin is the only bacteriocin approved as a food preservative because of its favorable properties of negligible toxicity and antibacterial effectiveness [86]. Nisin-loaded chitosan/poly(l-lactic acid) antimicrobial films were developed for applications in food packaging. The diffusion process of antimicrobial nisin from the manufactured film is spontaneous and endothermic. The well-controlled release of nisin from the film demonstrates high antimicrobial activity against Staphylococcus aureus [87].
Antimicrobial packing films were developed by compounding low-density polyethylene and its blend of ethylene vinyl acetate with potassium sorbate. A new approach to incorporating preservatives into a polyolefin matrix by using glycerol mono-oleate as a dispersant was reported to obtain uniform dispersions of the preservatives in the packing films, thereby significantly improving the thermal stability with no viscosity reduction [88].
A cellulose acetate film incorporated with a solution of bacteriophages was developed for food packaging. This film showed antimicrobial activity against Salmonella Typhimurium ATCC 14028, and the bacteriophages could remain viable for 14 days [89].
4.3. Textile Industry
Textiles are favorable substrates for microbial growth under appropriate conditions of temperature and moisture. Antimicrobial agents have yielded new opportunities for additional applications involving textile fibers. The market for antimicrobial textiles has grown dramatically over the past two decades.
Ag:ZnO/chitosan nanocomposite coatings were developed using a modified sol-gel method with 3-glycidyloxypropyltrimethoxysilane and tetraethoxysilane as functionalization agents and were applied to antimicrobial fabrics. This prepared hybrid nanocomposite demonstrated high antimicrobial activity and exhibited higher thermal stability than that of chitosan. Composite coatings on the textile blend of cotton/polyester (50%/50%) exerted the most advanced effect [90].
Natural fibers contributing to the reduction of environmental pollution as well as the burden of waste disposal have recently received considerable attention and become highly valuable materials. A new type of textile consisting of mulberry fibers uniformly laden with titania nanorods was prepared using sol-gel electrospinning and facile dip-coating methods. The mulberry fiber-TiO2 composite textile exhibited improved antimicrobial activity compared with pure mulberry textile. Furthermore, the advantages of this unique natural-synthetic composite textile are its anti-yellowing and self-cleaning properties, which are due to the scattering effect of UV radiation by titania nanorods [91].
For fabricating ecofriendly antimicrobial textile material, the impregnation of polypropylene (PP) and corona-modified PP nonwoven material with thymol by super-critical solvent impregnation with carbon dioxide as a working fluid was proposed. The thymol impregnation yield was approximately 7% for both PP and corona-modified PP nonwoven fabrics, providing antimicrobial activity against S. aureus, E. coli, and Candida albicans. Nevertheless, the higher rate of thymol release from the corona-modified material was due to the higher fiber surface hydrophilicity [92].
5. Conclusions
Recently, antimicrobial polymers have received considerable attention in both academic and industrial research. This mini-review summarizes the advances made in antimicrobial polymers since 2013. Passive antimicrobial polymers preventing bacterial adhesion and growth provide a more promising strategy than that of killing microbes directly by using active antimicrobial polymers. Among three bound or leaching polymers–polymeric biocides, biocidal polymers, and biocide-releasing polymers–the biocide-releasing system demonstrates the most potential because of the controlled release characteristics. Despite the substantial progress of antimicrobial polymers, the precise mechanisms underlying antimicrobial interaction with microbes necessitates further clarification. In particular, biofilm-associated mechanisms will require an intensive effort to design a promising antimicrobial agent. Combining diverse antimicrobial mechanisms may contribute to a more effective antimicrobial polymer. Further challenges are developments of long-acting or reusable antimicrobial polymers, a broad range of antimicrobial activity, and an activity-controlled system on demand sites.
Acknowledgments
This work was supported by a grant from the Ministry of Science and Technology, Taiwan, under grant number MOST 105-2622-E-239-001-CC3.
Author Contributions
Yung-Sheng Lin designed the main parts and led the development of the paper. Keng-Shiang Huang, Chih-Hui Yang, Shu-Ling Huang, Cheng-You Chen, Yuan-Yi Lu and Yung-Sheng Lin performed the discussion and wrote the paper. All authors reviewed the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ross A.G.P., Olds G.R., Cripps A.W., Farrar J.J., McManus D.P. Enteropathogens and chronic illness in returning travelers. N. Engl. J. Med. 2013;368:1817–1825. doi: 10.1056/NEJMra1207777. [DOI] [PubMed] [Google Scholar]
- 2.Lin Y.S., Lee M.Y., Yang C.H., Huang K.S. Biomedical devices for pathogen detection using microfluidic chips. Curr. Proteom. 2014;11:116–120. doi: 10.2174/157016461102140917122234. [DOI] [Google Scholar]
- 3.Chan C.F., Huang K.S., Lee M.Y., Yang C.H., Wang C.Y., Lin Y.S. Applications of nanoparticles for antimicrobial activity and drug delivery. Curr. Org. Chem. 2014;18:204–215. doi: 10.2174/13852728113176660144. [DOI] [Google Scholar]
- 4.Sun D., Shahzad M.B., Li M., Wang G., Xu D. Antimicrobial materials with medical applications. Mater. Technol. 2015;30:B90–B95. doi: 10.1179/1753555714Y.0000000239. [DOI] [Google Scholar]
- 5.Siedenbiedel F., Tiller J.C. Antimicrobial polymers in solution and on surfaces: Overview and functional principles. Polymers. 2012;4:46–71. doi: 10.3390/polym4010046. [DOI] [Google Scholar]
- 6.Jain A., Duvvuri L.S., Farah S., Beyth N., Domb A.J., Khan W. Antimicrobial polymers. Adv. Healthc. Mater. 2014;3:1969–1985. doi: 10.1002/adhm.201400418. [DOI] [PubMed] [Google Scholar]
- 7.Islam M.R., Gao Y.F., Li X., Zhang Q.M., Wei M., Serpe M.J. Stimuli-responsive polymeric materials for human health applications. Chin. Sci. Bull. 2014;59:4237–4255. doi: 10.1007/s11434-014-0545-6. [DOI] [Google Scholar]
- 8.Francolini I., Donelli G., Crisante F., Taresco V., Piozzi A. Antimicrobial polymers for anti-biofilm medical devices: State-of-art and perspectives. Adv. Exp. Med. Biol. 2015;831:93–117. doi: 10.1007/978-3-319-09782-4_7. [DOI] [PubMed] [Google Scholar]
- 9.Strempel N., Strehmel J., Overhage J. Potential application of antimicrobial peptides in the treatment of bacterial biofilm infections. Curr. Pharm. Des. 2015;21:67–84. doi: 10.2174/1381612820666140905124312. [DOI] [PubMed] [Google Scholar]
- 10.Alves D., Olívia Pereira M. Mini-review: Antimicrobial peptides and enzymes as promising candidates to functionalize biomaterial surfaces. Biofouling. 2014;30:483–499. doi: 10.1080/08927014.2014.889120. [DOI] [PubMed] [Google Scholar]
- 11.Gour N., Ngo K.X., Vebert-Nardin C. Anti-infectious surfaces achieved by polymer modification. Macromol. Mater. Eng. 2014;299:648–668. doi: 10.1002/mame.201300285. [DOI] [Google Scholar]
- 12.Palza H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015;16:2099–2116. doi: 10.3390/ijms16012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hui F., Debiemme-Chouvy C. Antimicrobial N-halamine polymers and coatings: A review of their Synthesis, characterization, and applications. Biomacromolecules. 2013;14:585–601. doi: 10.1021/bm301980q. [DOI] [PubMed] [Google Scholar]
- 14.Sedlarik V. Antimicrobial modifications of polymers. In: Chamy R., Rosenkranz F., editors. Biodegradation—Life of Science. InTech; Rijeka, Croatia: 2013. pp. 187–204. [Google Scholar]
- 15.Beyth N., Farah S., Domb A.J., Weiss E.I. Antibacterial dental resin composites. React. Funct. Polym. 2014;75:81–88. doi: 10.1016/j.reactfunctpolym.2013.11.011. [DOI] [Google Scholar]
- 16.Carmona-Ribeiro A.M., de Melo Carrasco L.D. Novel formulations for antimicrobial peptides. Int. J. Mol. Sci. 2014;15:18040–18083. doi: 10.3390/ijms151018040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kargupta R., Bok S., Darr C.M., Crist B.D., Gangopadhyay K., Gangopadhyay S., Sengupta S. Coatings and surface modifications imparting antimicrobial activity to orthopedic implants. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014;6:475–495. doi: 10.1002/wnan.1273. [DOI] [PubMed] [Google Scholar]
- 18.Martins A.F., Facchi S.P., Follmann H.D., Pereira A.G., Rubira A.F., Muniz E.C. Antimicrobial activity of chitosan derivatives containing n-quaternized moieties in its backbone: A review. Int. J. Mol. Sci. 2014;15:20800–20832. doi: 10.3390/ijms151120800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deka S.R., Sharma A.K., Kumar P. Cationic polymers and their self-assembly for antibacterial applications. Curr. Top. Med. Chem. 2015;15:1179–1195. doi: 10.2174/1568026615666150330110602. [DOI] [PubMed] [Google Scholar]
- 20.Xue Y., Xiao H., Zhang Y. Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. Int. J. Mol. Sci. 2015;16:3626–3655. doi: 10.3390/ijms16023626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desrousseaux C., Sautou V., Descamps S., Traore O. Modification of the surfaces of medical devices to prevent microbial adhesion and biofilm formation. J. Hosp. Infect. 2013;85:87–93. doi: 10.1016/j.jhin.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Veerachamy S., Yarlagadda T., Manivasagam G., Yarlagadda P.K. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014;228:1083–1099. doi: 10.1177/0954411914556137. [DOI] [PubMed] [Google Scholar]
- 23.Brooks B.D., Brooks A.E. Therapeutic strategies to combat antibiotic resistance. Adv. Drug Deliv. Rev. 2014;78:14–27. doi: 10.1016/j.addr.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Draenert R., Seybold U., Grutzner E., Bogner J.R. Novel antibiotics: Are we still in the pre–post-antibiotic era? Infection. 2015;43:145–151. doi: 10.1007/s15010-015-0749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dryden M.S. Alternative clinical indications for novel antibiotics licensed for skin and soft tissue infection? Curr. Opin. Infect. Dis. 2015;28:117–124. doi: 10.1097/QCO.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 26.Lee B., Boucher H.W. Targeting antimicrobial-resistant bacterial respiratory tract pathogens: It is time to “get smart”. Curr. Opin. Pulm. Med. 2015;21:293–303. doi: 10.1097/MCP.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 27.Roca I., Akova M., Baquero F., Carlet J., Cavaleri M., Coenen S., Cohen J., Findlay D., Gyssens I., Heuer O.E., et al. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015;6:22–29. doi: 10.1016/j.nmni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Q., Wu Z., Chen H. Dual-function antibacterial surfaces for biomedical applications. Acta Biomater. 2015;16:1–13. doi: 10.1016/j.actbio.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Holban A.M., Iordanskii A., Grumezescu A.M., Bychkova A., Andronescu E., Mogoantă L., Mogoșanu G.D., Iordache F. Prosthetic devices with nanostructurated surfaces for increased resistance to microbial colonization. Curr. Pharm. Biotechnol. 2015;16:112–120. doi: 10.2174/138920101602150112150303. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H., Chiao M. Anti-fouling coatings of poly(dimethylsiloxane) devices for biological and biomedical applications. J. Med. Biol. Eng. 2015;35:143–155. doi: 10.1007/s40846-015-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L., Li W., Liu Q. Recent development of antifouling polymers: Structure, evaluation, and biomedical applications in nano/micro-structures. WIREs Nanomed. Nanobiotechnol. 2014;6:599–614. doi: 10.1002/wnan.1278. [DOI] [PubMed] [Google Scholar]
- 32.Ye Q., Zhou F. Antifouling surfaces based on polymer brushes. In: Zhou F., editor. Antifouling Surfaces and Materials. Springer; Berlin, Germany: 2015. pp. 55–81. [Google Scholar]
- 33.Yu K., Mei Y., Hadjesfandiari N., Kizhakkedathu J.N. Engineering biomaterials surfaces to modulate the host response. Colloids Surf. B Biointerfaces. 2014;124:69–79. doi: 10.1016/j.colsurfb.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Zhou R., Ren P.F., Yang H.C., Xu Z.K. Fabrication of antifouling membrane surface by poly(sulfobetaine methacrylate)/polydopamine co-deposition. J. Membr. Sci. 2014;466:18–25. doi: 10.1016/j.memsci.2014.04.032. [DOI] [Google Scholar]
- 35.Hippiusa C., Bütünb V., Erel-Goktepec I. Bacterial anti-adhesive properties of a monolayer of zwitterionic block copolymer micelles. Mater. Sci. Eng. C. 2014;41:354–362. doi: 10.1016/j.msec.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 36.Pidhatika B., Rakhmatullina E. The synthesis of polymeric dual-functional antimicrobial surface based on poly(2-methyl-2-oxazoline) Indones. J. Biotechnol. 2014;19:12–22. [Google Scholar]
- 37.Jones A., Abhyuday M., Sharma S. Protein-based bioplastics and their antibacterial potential. J. Appl. Polym. Sci. 2015;132 doi: 10.1002/app.41931. [DOI] [Google Scholar]
- 38.Shahzad M., Millhouse E., Culshaw S., Edwards C.A., Ramage G., Combet E. Selected dietary (poly)phenols inhibit periodontal pathogen growth and biofilm formation. Food Funct. 2015;6:719–729. doi: 10.1039/C4FO01087F. [DOI] [PubMed] [Google Scholar]
- 39.Duday D., Vreuls C., Moreno M., Frache G., Boscher N.D., Zocchi G., Archambeau C., van de Weerdt C., Martial J., Choquet P. Atmospheric pressure plasma modified surfaces for immobilization of antimicrobial nisin peptides. Surf. Coat. Technol. 2013;218:152–161. doi: 10.1016/j.surfcoat.2012.12.045. [DOI] [Google Scholar]
- 40.Liu G., Wu G., Jin C., Kong Z. Preparation and antimicrobial activity of terpene-based polyurethane coatings with carbamate group-containing quaternary ammonium salts. Prog. Org. Coat. 2015;80:150–155. doi: 10.1016/j.porgcoat.2014.12.005. [DOI] [Google Scholar]
- 41.Taresco V., Crisante F., Francolini I., Martinelli A., D’Ilario L., Ricci-Vitiani L., Buccarelli M., Pietrelli L., Piozzi A. Antimicrobial and antioxidant amphiphilic random copolymers to address medical device-centered infections. Acta Biomater. 2015;22:131–140. doi: 10.1016/j.actbio.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 42.Yuen J.W., Chung T.W., Loke A.Y. Methicillin-resistant Staphylococcus aureus (MRSA) contamination in bedside surfaces of a hospital ward and the potential effectiveness of enhanced disinfection with an antimicrobial polymer surfactant. Int. J. Environ. Res. Public Health. 2015;12:3026–3041. doi: 10.3390/ijerph120303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou F., Qin X., Li Y., Ren L., Zhao Y., Yuan X. Fluorosilicone multi-block copolymers tethering quaternary ammonium salt groups for antimicrobial purpose. Appl. Surf. Sci. 2015;347:231–241. doi: 10.1016/j.apsusc.2015.04.079. [DOI] [Google Scholar]
- 44.Zhang A.Q., Liu Q.Q., Lei Y.F., Hong S.H., Lin Y.L. Synthesis and antimicrobial activities of acrylamide polymers containing quaternary ammonium salts on bacteria and phytopathogenic fungi. React. Funct. Polym. 2015;88:39–46. doi: 10.1016/j.reactfunctpolym.2015.02.005. [DOI] [Google Scholar]
- 45.Babu P.N.K., Ramadevi B., Poornachandra Y., Ganesh Kumar C. Synthesis, antimicrobial, and anticancer evaluation of novel 2-(3-methylindolyl) benzimidazole derivatives. Med. Chem. Res. 2014;23:3970–3978. doi: 10.1007/s00044-014-0974-4. [DOI] [Google Scholar]
- 46.Cheng X., Li R., Du J., Sheng J., Ma K., Ren X., Huang T.S. Antimicrobial activity of hydrophobic cotton coated with N-halamine. Polym. Adv. Technol. 2015;26:99–103. doi: 10.1002/pat.3426. [DOI] [Google Scholar]
- 47.Li X., Liu Y., Jiang Z., Li R., Ren X., Huang T.S. Synthesis of an N-halamine monomer and its application in antimicrobial cellulose via an electron beam irradiation process. Cellulose. 2015;22:3609–3617. [Google Scholar]
- 48.Li C., Liu Y., Zeng Q.Y., Ao N.J. Preparation and antimicrobial activity of quaternary phosphonium modified epoxidized natural rubber. Mater. Lett. 2013;93:145–148. doi: 10.1016/j.matlet.2012.11.045. [DOI] [Google Scholar]
- 49.Lim K., Chua R.R., Saravanan R., Basu A., Mishra B., Tambyah P.A., Ho B., Leong S.S. Immobilization studies of an engineered arginine–tryptophan-rich peptide on a silicone surface with antimicrobial and antibiofilm activity. ACS Appl. Mater. Interfaces. 2013;5:6412–6422. doi: 10.1021/am401629p. [DOI] [PubMed] [Google Scholar]
- 50.Lim K., Chua R.R., Bow H., Tambyah P.A., Hadinoto K., Leong S.S. Development of a catheter functionalized by a polydopamine peptide coating with antimicrobial and antibiofilm properties. Acta Biomater. 2015;15:127–138. doi: 10.1016/j.actbio.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 51.Locock K.E., Michl T.D., Valentin J.D., Vasilev K., Hayball J.D., Qu Y., Traven A., Griesser H.J., Meagher L., Haeussler M. Guanylated polymethacrylates: A class of potent antimicrobial polymers with low hemolytic activity. Biomacromolecules. 2013;14:4021–4031. doi: 10.1021/bm401128r. [DOI] [PubMed] [Google Scholar]
- 52.Mogosanua G.D., Grumezescu A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014;463:127–136. doi: 10.1016/j.ijpharm.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 53.Radulescu M., Ficai D., Oprea O., Ficai A., Andronescu E., Holban A.M. Antimicrobial chitosan based formulations with impact on different biomedical applications. Curr. Pharm. Biotechnol. 2015;16:128–136. doi: 10.2174/138920101602150112151157. [DOI] [PubMed] [Google Scholar]
- 54.Xing K., Zhu X., Peng X., Qin S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015;35:569–588. doi: 10.1007/s13593-014-0252-3. [DOI] [Google Scholar]
- 55.Thoma L.M., Boles B.R., Kuroda K. Cationic methacrylate polymers as topical antimicrobial agents against Staphylococcus aureus nasal colonization. Biomacromolecules. 2014;15:2933–2943. doi: 10.1021/bm500557d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hassan E.A., Hassan M.L., Moorefield C.N., Newkome G.R. New supramolecular metallo-terpyridine carboxymethyl cellulose derivatives with antimicrobial properties. Carbohydr. Polym. 2015;116:2–8. doi: 10.1016/j.carbpol.2014.06.056. [DOI] [PubMed] [Google Scholar]
- 57.Melendez-Ortiz H.I., Alvarez-Lorenzo C., Burillo G., Magariños B., Concheiro A., Bucio E. Radiation-grafting of n-vinylimidazole onto silicone rubber for antimicrobial properties. Radiat. Phys. Chem. 2015;110:59–66. doi: 10.1016/j.radphyschem.2015.01.025. [DOI] [Google Scholar]
- 58.Mohamed M.F., Hammac G.K., Guptill L., Seleem M.N. Antibacterial activity of novel cationic peptides against clinical isolates of multi-drug resistant Staphylococcus pseudintermedius from infected dogs. PLoS ONE. 2014;9:1578. doi: 10.1371/journal.pone.0116259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lopez-Meza J.E., Ochoa-Zarzosa A., Barboza-Corona J.E., Bideshi D.K. Antimicrobial peptides: Current and potential applications in biomedical therapies. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/367243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aquino R.P., Auriemma G., Mencherini T., Russo P., Porta A., Adami R., Liparoti S., Della Porta G., Reverchon E., del Gaudio P. Design and production of gentamicin/dextrans microparticles by supercritical assisted atomisation for the treatment of wound bacterial infections. Int. J. Pharm. 2013;440:188–194. doi: 10.1016/j.ijpharm.2012.07.074. [DOI] [PubMed] [Google Scholar]
- 61.Hornyák I., Madácsi E., Kalugyer P., Vácz G., Horváthy D.B., Szendrői M., Han W., Lacza Z. Increased release time of antibiotics from bone allografts through a novel biodegradable coating. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/459867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Su L.C., Xie Z., Zhang Y., Nguyen K.T., Yang J. Study on the antimicrobial properties of citrate-based biodegradable polymers. Front. Bioeng. Biotechnol. 2014;2 doi: 10.3389/fbioe.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Macha I.J., Cazalbou S., Ben-Nissan B., Harvey K.L., Milthorpe B. Marine structure derived calcium phosphate-polymer biocomposites for local antibiotic delivery. Mar. Drugs. 2015;13:666–680. doi: 10.3390/md13010666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trivedi T.J., Rao K.S., Kumar A. Facile preparation of agarose-chitosan hybrid materials and nanocomposite ionogels using an ionic liquid via dissolution, regeneration and sol-gel transition. Green Chem. 2014;16:320–330. doi: 10.1039/C3GC41317A. [DOI] [Google Scholar]
- 65.Brooks B.D., Sinclair K.D., Grainger D.W., Brooks A.E. A resorbable antibiotic-eluting polymer composite bone void filler for perioperative infection prevention in a rabbit radial defect model. PLoS ONE. 2015;10:1578. doi: 10.1371/journal.pone.0118696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.El-Gendy N., Qian J., Eshelman K., Rivera M., Berkland C. Antibiotic activity of iron-sequestering polymers. Biomacromolecules. 2015;16:1480–1488. doi: 10.1021/bm5016392. [DOI] [PubMed] [Google Scholar]
- 67.Noimark S., Weiner J., Noor N., Allan E., Williams C.K., Shaffer M.S., Parkin I.P. Dual-mechanism antimicrobial polymer–ZnO nanoparticle and crystal violet-encapsulated silicone. Adv. Funct. Mater. 2015;25:1367–1373. doi: 10.1002/adfm.201402980. [DOI] [Google Scholar]
- 68.Filatova L.Y., Donovan D.M., Becker S.C., Lebedev D.N., Priyma A.D., Koudriachova H.V., Kabanov A.V., Klyachko N.L. Physicochemical characterization of the staphylolytic LysK enzyme in complexes with polycationic polymers as a potent antimicrobial. Biochimie. 2013;95:1689–1696. doi: 10.1016/j.biochi.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 69.Garcia-Arguelles S., Serrano M.C., Gutierrez M.C., Ferrer M.L., Yuste L., Rojo F., del Monte F. Deep eutectic solvent-assisted synthesis of biodegradable polyesters with antibacterial properties. Langmuir. 2013;29:9525–9534. doi: 10.1021/la401353r. [DOI] [PubMed] [Google Scholar]
- 70.Ramos A.I., Braga T.M., Fernandes J.A., Silva P., Ribeiro-Claro P.J., Paz F.A., Lopes M.D., Braga S.S. Analysis of the microcrystalline inclusion compounds of triclosan with β-cyclodextrin and its tris-o-methylated derivative. J. Pharm. Biomed. Anal. 2013;80:34–43. doi: 10.1016/j.jpba.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 71.Lyutakov O., Hejna O., Solovyev A., Kalachyova Y., Svorcik V. Polymethylmethacrylate doped with porphyrin and silver nanoparticles as light-activated antimicrobial material. RSC Adv. 2014;4:50624–50630. doi: 10.1039/C4RA08385G. [DOI] [Google Scholar]
- 72.Lyutakov O., Goncharova I., Rimpelova S., Kolarova K., Svanda J., Svorcik V. Silver release and antimicrobial properties of PMMA films doped with silver ions, nano-particles and complexes. Mater. Sci. Eng. C Mater. Biol. Appl. 2015;49:534–540. doi: 10.1016/j.msec.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 73.Punitha N., Ramesh P.S., Geetha D. Spectral, morphological and antibacterial studies of β-cyclodextrin stabilized silver-Chitosan nanocomposites. Spectrochim. Acta Part A Mol. Biomol. Spectr. 2015;136:1710–1717. doi: 10.1016/j.saa.2014.10.071. [DOI] [PubMed] [Google Scholar]
- 74.Rodriguez L.C., Palmer K., Montagner F., Rodrigues D.C. A novel chlorhexidine-releasing composite bone cement: Characterization of antimicrobial effectiveness and cement strength. J. Bioact. Compat. Polym. 2015;30:34–47. doi: 10.1177/0883911514566130. [DOI] [Google Scholar]
- 75.Tran P.A., Hocking D.M., O’Connor A.J. In situ formation of antimicrobial silver nanoparticles and the impregnation of hydrophobic polycaprolactone matrix for antimicrobial medical device applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015;47:63–69. doi: 10.1016/j.msec.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 76.Winkel A., Dempwolf W., Gellermann E., Sluszniak M., Grade S., Heuer W., Eisenburger M., Menzel H., Stiesch M. Introducing a semi-coated model to investigate antibacterial effects of biocompatible polymers on titanium surfaces. Int. J. Mol. Sci. 2015;16:4327–4342. doi: 10.3390/ijms16024327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fu T., Li Y., Thaker H.D., Scott R.W., Tew G.N. Expedient synthesis of SMAMPs via click chemistry. ACS Med. Chem. Lett. 2013;4:841–845. doi: 10.1021/ml400155a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuroda K., Caputo G.A. Antimicrobial polymers as synthetic mimics of host-defense peptides. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013;5:49–66. doi: 10.1002/wnan.1199. [DOI] [PubMed] [Google Scholar]
- 79.Thaker H.D., Cankaya A., Scott R.W., Tew G.N. Role of amphiphilicity in the design of synthetic mimics of antimicrobial peptides with Gram-negative activity. ACS Med. Chem. Lett. 2013;4:481–485. doi: 10.1021/ml300307b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takahashi H., Palermo E.F., Yasuhara K., Caputo G.A., Kuroda K. Molecular design, structures, and activity of antimircobial peptide-mimetic polymers. Marcomol. Biosci. 2013;13:1285–1299. doi: 10.1002/mabi.201300126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu R., Chen X., Chakraborty S., Lemke J.J., Hayouka Z., Chow C., Welch R.A., Weisblum B., Masters K.S., Gellman S.H. Tuning the biological activity profile of antibacterial polymers via subunit substitution pattern. J. Am. Chem. Soc. 2014;136:4410–4418. doi: 10.1021/ja500367u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choi H., Chakraborty S., Liu R., Gellman S.H., Weisshaar J.C. Single-cell, time-resolved antimicrobial effects of a highly cationic, random nylon-3 copolymer on live Escherichia coli. ACS Chem. Biol. 2016;11:113–120. doi: 10.1021/acschembio.5b00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gomes A.P., Mano J.F., Queiroz J.A., Gouveia I.C. Incorporation of antimicrobial peptides on functionalized cotton gauzes for medical applications. Carbohydr. Polym. 2015;127:451–461. doi: 10.1016/j.carbpol.2015.03.089. [DOI] [PubMed] [Google Scholar]
- 84.Vasile B.S., Oprea O., Voicu G., Ficai A., Andronescu E., Teodorescu A., Holban A. Synthesis and characterization of a novel controlled release zinc oxide/gentamicin-chitosan composite with potential applications in wounds care. Int. J. Pharm. 2014;463:161–169. doi: 10.1016/j.ijpharm.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 85.Fisher L.E., Hook A.L., Ashraf W., Yousef A., Barrett D.A., Scurr D.J., Chen X., Smith E.F., Fay M., Parmenter C.D., et al. Biomaterial modification of urinary catheters with antimicrobials to give long-term broadspectrum antibiofilm activity. J. Control. Release. 2015;202:57–64. doi: 10.1016/j.jconrel.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 86.Gharsallaoui A., Joly C., Oulahal N., Degraeve P. Nisin as a food preservative: Part 2: Antimicrobial polymer materials containing nisin. Crit. Rev. Food Sci. Nutr. 2016;56:1275–1289. doi: 10.1080/10408398.2013.763766. [DOI] [PubMed] [Google Scholar]
- 87.Wang H., Liu H., Chu C., She Y., Jiang S., Zhai L., Jiang S., Li X. Diffusion and antibacterial properties of nisin-loaded chitosan/poly (l-lactic acid) towards development of active food packaging film. Food Bioprocess Technol. 2015;8:1657–1667. doi: 10.1007/s11947-015-1522-z. [DOI] [Google Scholar]
- 88.Kuplennik N., Tchoudakov R., Zelas Z.B., Sadovski A., Fishman A., Narkis M. Antimicrobial packaging based on linear low-density polyethylene compounded with potassium sorbate. LWT Food Sci. Technol. 2015;62:278–286. doi: 10.1016/j.lwt.2015.01.002. [DOI] [Google Scholar]
- 89.Gouvêa D.M., Mendonça R.C., Soto M.L., Cruz R.S. Acetate cellulose film with bacteriophages for potential antimicrobial use in food packaging. LWT Food Sci. Technol. 2015;63:85–91. doi: 10.1016/j.lwt.2015.03.014. [DOI] [Google Scholar]
- 90.Busila M., Musat V., Textorbc T., Mahltigd B. Synthesis and characterization of antimicrobial textile finishing based on Ag:ZnO nanoparticles/chitosan biocomposites. RSC Adv. 2015;5:21562–21571. doi: 10.1039/C4RA13918F. [DOI] [Google Scholar]
- 91.Jang Y.S., Amna T., Hassan M.S. Nanotitania/mulberry fibers as novel textile with anti-yellowing and intrinsic antimicrobial properties. Ceram. Int. 2015;41:6274–6280. doi: 10.1016/j.ceramint.2015.01.050. [DOI] [Google Scholar]
- 92.Markovic D., Milovanovic S., Radeti M., Jokic B., Zizovic I. Impregnation of corona modified polypropylene non-woven materialwith thymol in supercritical carbon dioxide for antimicrobialapplication. J. Supercrit. Fluids. 2015;101:215–221. doi: 10.1016/j.supflu.2015.03.022. [DOI] [Google Scholar]