Abstract
The percentage of elderly people with associated age-related health deterioration, including cancer, has been increasing for decades. Among age-related diseases, the incidence of cancer has grown substantially, in part because of the overlap of some molecular pathways between cancer and aging. Studies with model organisms suggest that aging and age-related conditions are manipulable processes that can be modified by both genetic and environmental factors, including dietary habits. Variations in genetic backgrounds likely lead to differential responses to dietary changes and account for some of the inconsistencies found in the literature. The intricacies of the aging process, coupled with the interrelational role of bioactive food components on gene expression, make this review a complex undertaking. Nevertheless, intriguing evidence suggests that dietary habits can manipulate the aging process and/or its consequences and potentially may have unprecedented health benefits. The present review focuses on 4 cellular events: telomerase activity, bioenergetics, DNA repair, and oxidative stress. These processes are linked to both aging and cancer risk, and their alteration in animal models by selected food components is evident.
Keywords: nutrigenomics, diet, aging, longevity, DNA repair, telomere, cancer, caloric restriction, oxidative stress
Introduction
Although cancer and aging have been studied as independent diseases, mounting evidence suggests that cancer is an aging-associated disease and that cancer and aging share many molecular pathways (1). The development of genomic technologies provides an exciting opportunity to examine the molecules controlling cellular networks common in aging and cancer. Investigations that use functional genomics argue that certain facets of the mammalian aging process can be the result of the accumulation, over time, of various forms of molecular and cellular damage. It is possible that some genetic programs that coordinate aspects of growth and development in early age may persist into adulthood, at which time they may become detrimental (2). The deterioration of cellular biology may lead to cellular abnormalities, tissue death, and senescence of the entire organism. Another consequence of these molecular events may be cellular aberrations, which may lay the foundation for many diseases, including cancer. DNA repair and gene expression, as described below, are highly dependent on adequate nourishment and, thus, dependent on the appropriate dietary supply of essential and nonessential dietary components. The emergence of nutrigenomics as a science offers possibilities to better define specific dietary needs and the effect of food and food components on gene expression, and to understand the nutrigenomics–disease interrelation (3). Nutrigenomics comprises variations in genetic profiles, along with the impact of epigenetic processes and transcriptomic homeostasis on the response to individual bioactive food components. The nutrigenomics concept builds on the assumption that 1) bioactive food components can influence the human genome directly or indirectly and thereby influence the expression of genes and gene products; 2) as a result of this influence, dietary patterns and/or specific dietary components may modify multiple cellular processes, including aging, as well as the onset, incidence, progression and/or severity of multiple diseases, including cancer; and 3) the health consequences of a diet are dependent on the balance states of health and disease on an individual’s genetic background. Advances in nutrigenomics and nutrient signaling possibly might lead to unraveling the link between aging and cancer. Other “-omics” (including, e.g., proteomics, lipidomics, metabolomics, and microbiomics) surely may influence the response to foods and supplements, and they are often referred to collectively as the “-omics of nutrition.” Nevertheless, other factors can influence the direction and/or magnitude of the response. The understanding of mechanistic controls in response to different diets and associated bioactive constituents may help to identify dietary changes that could delay aging and its related physiologic and pathologic changes, including cancer.
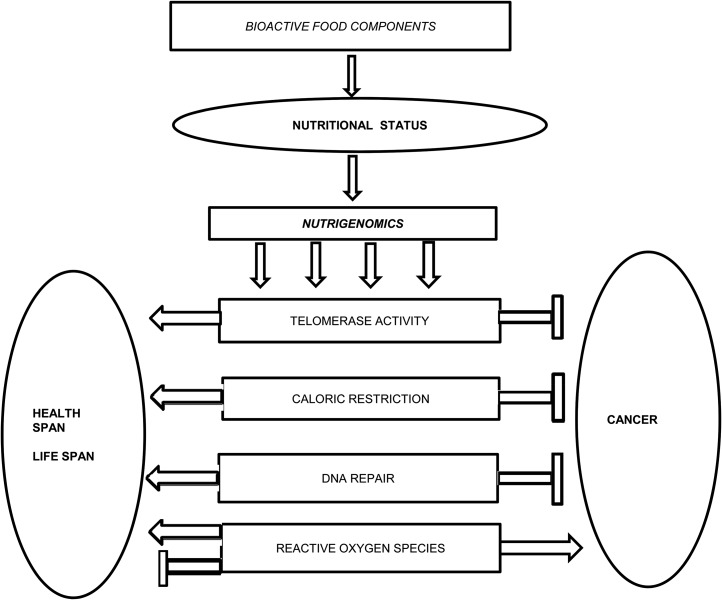
The 4 processes presented in this review—telomerase activation, calorie restriction, DNA repair, and oxidative stress—will be discussed from the standpoint of bioactive food components existent in the blood or tissue that contribute to general nutritional status. Through gene activation or suppression, food components or their metabolic products can block, initiate, or maintain specific reactions related to aging and cancer (Figure 1).
FIGURE 1.
Nutrigenomics, aging, and cancer interrelations. Bioactive food components will influence genes that are implicated in telomerase activities, the impact of caloric restriction, DNA repair, and the actions of reactive oxygen species.
Multiple other factors beyond the scope of this review might also influence the aging process and the associated risk of developing cancer and other diseases. One of these variables is the microbiome (4). Aging has been recognized as affecting the human gut microbiota composition, which may be related to the progression of diseases and frailty in the elderly population. Microbially produced metabolites may exhibit enhanced beneficial effects, or they may be degraded to inactive or toxic compounds (5). At least part of the impact of the microbiome likely relates to its influence on the inflammatory process, which is a common pathway for aging and cancer (Table 1).
TABLE 1.
Summary of processes linked to aging and cancer risk1
| Cellular events and outcomes |
| Telomerase as a target |
| Telomeres shorten after each DNA replication |
| Telomere length may be considered as a biological marker (telomere clock) |
| There are interindividual telomere lengths at birth |
| When telomeres become critically short, this induces DNA damage–like responses paralleled by the silencing of telomerase |
| In cancer, because of a high level of telomerase activity, cancer cells continue to replicate |
| In the absence of telomeres, some immortalized mammalian cell lines are still able to maintain telomere lengths |
| One or more p53 functions in various metabolic pathways may be mediated by telomere dysfunction |
| Various nutrients potentially influence telomere lengths |
| Bioenergetics as a link between aging and cancer prevention |
| Calorie restriction without malnutrition has been associated with increased lifespan in lower species |
| Calorie restriction may decrease the risk of certain diseases |
| Calorie restriction may be beneficial only for subjects consuming a high-calorie diet |
| Commonly associated genetic variances in relation to bioenergetics are related to the melanocortin system, mTOR, sirtuins, FTO, and PPARγ |
| BMI may be too insensitive as a marker for predicting its impact on the aging process |
| Not all calorie-restricted diets may have the same impact; diet composition may be an important factor |
| DNA repair—a common link |
| MMR is a system that removes nucleotides resulting from errors in replication and recombination with the aim of maintaining DNA fidelity |
| MMR mutations may not have the same significance for all cancers |
| Cell damage may be promoted by deficiencies in several essential nutrients |
| The actions of nutrients may be influenced by the microbiome |
| Defects in MMR genes may also arise from epigenetic changes in DNA |
| Several bioactive food components may overcome the adverse consequences of MMR |
| Oxidative stress as a target for aging and cancer prevention |
| ROS and RNS and a basal level of oxidative stress are essential for cell survival |
| Severe oxidative stress often leads to oxidative damage and cell death |
| A new paradigm suggests that ROS production in aging may be the result of adaptive signaling rather than a detrimental by-product |
| There is evidence that some antioxidants are linked to an increased lifespan |
| NF-κB is upregulated in many cancers, and it is also an important regulator of ROS scavengers |
| The free radical theory of aging is under debate because of its inability to explain contradictory results of antioxidant activities |
FTO, fat mass obesity; MMR, DNA “mismatch repair” system; mTOR, mechanistic target of rapamycin; RNS, reactive nitrogen species; ROS, reactive oxygen species.
Telomerase as a Target
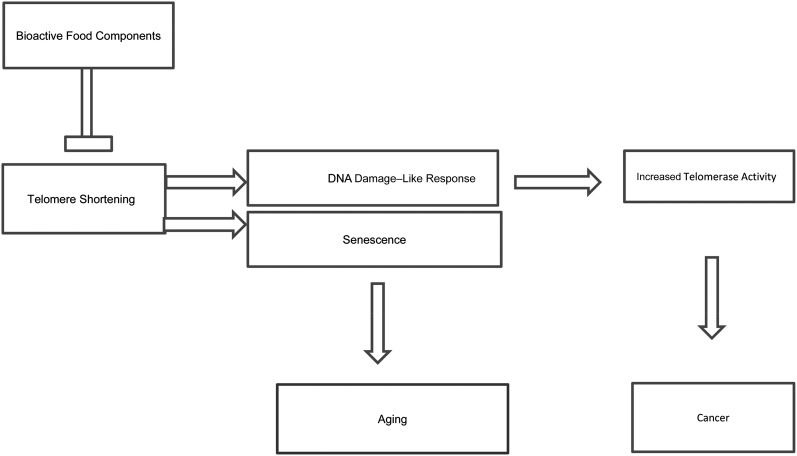
Telomeres are nucleoprotein complexes that are essential for genomic stability and are located at the end of eukaryotic chromosomes. Telomeres shorten after each DNA replication, so their length relates to the number of past replications, and they could be considered biological markers of aging in normal human cells (6, 7). In addition, telomere length may reflect the cumulative burden of oxidative stress and inflammation, proposed by some as a potential biomarker of biological age, or a telomeric clock of human aging (8). However, age-adjusted telomere length is highly variable because of interindividual differences in telomere length at birth, and most likely because of the rate of telomere attrition thereafter (9). Regardless, when telomeres become critically short, they lose their capping capacity and induce DNA damage–like responses (6). This process is paralleled by the silencing of telomerase, which is responsible for de novo telomere extension in most normal adult tissues, resulting in the loss of proliferative ability known as replicative senescence (1). In cancer, this property is lost, and because of great telomerase activity, cancer cells continue to replicate (Figure 2). Upregulation of telomerase activity has been observed to prolong the life of cells in culture in a phenotypical youthful state, whereas its downregulation may be used in cancer therapy (10), providing evidence that the aging and cancer processes can involve common genes, and their regulation can result in entirely different responses. However, telomerase downregulation prevents tissue renewal and repair, which contributes to senescence and aging (11). Telomerase activity is characterized by the expression of the telomerase reverse transcriptase (TERT)4 gene, suggesting that TERT serves as the major limiting agent for telomerase activity (12). Various nutrients potentially influence telomere length through mechanisms that reflect their role in cellular functions, including inflammation, oxidative stress, DNA integrity, DNA methylation, and activity of telomerase (13). Some dietary compounds, such as genistein, epigallocatechin gallate, and sulforaphane, show promise as pathways by which TERT is downregulated and cellular apoptosis is induced in human breast cancer cells (14, 15). However, in the absence of telomerase activity, some immortalized mammalian cell lines and tumors still maintain or even increase overall telomere length through alternative mechanisms that are telomerase-independent (16). Nevertheless, telomerase activity is not limited to telomere extension. Telomerase is recognized as upregulating genes known to be or suspected of being implicated in cancer by activating other different processes, including glycolysis, which enables cancer cells to use sugar rapidly and facilitates their growth (17).
FIGURE 2.
Telomerase as a target: bioactive food components may influence telomere length, inducing a DNA damage–like response and increasing telomerase activity and cancer risk or inducing senescence and aging.
The well-known tumor protein p53 (p53), a tumor suppressor gene also known as tumor protein (EC:2.7.1.37) and located on the seventeenth chromosome (17p 13.1), has an undisputed role in cancer. Its activity stems from its ability to respond to a variety of stressors to trigger cell-cycle arrest, apoptosis, or senescence (18). An increasing body of evidence suggests that p53 also drives aging, yet the specific role of p53 functions in various metabolic pathways remains to be further elucidated. It seems to mediate the connection between telomere and mitochondrial dysfunctions (19). Anticancer therapies that target p53 and reactivate or enhance its activity are considered to be good alternatives for treating various neoplasms. Increasing the amount of p53 may seem to be an adequate solution for the treatment of tumors or prevention of their spreading. This, however, is not a viable method of treatment, because it can cause premature aging (20). Therefore, it is important to examine whether clinical approaches compromise tissue homeostasis and contribute to aging and how the balance between both can be accomplished. A number of nutrients, such as isothiocyanates, found in cruciferous vegetables, spices such as ginger and curcumin, and many herbs, participate in regulating p53-dependent pathways. Components of a Western-style diet, such as high consumption of red meat and foods that increase glycemic load, are associated with p53 disease pathways (21). Besides p53, other genes and pathways appear to be involved in compromising mitochondrial function in the setting of telomere dysfunction, with p53 deficiency only partially rescuing PPARγ coactivator expression and mitochondrial dysfunction; the identification of these other pathways will expand our understanding of how critical short telomeres compromise cellular function and precipitate aging (22).
Interestingly, the rate of telomere shortening can be either increased or decreased by specific lifestyle factors, including the composition of the diet consumed. Dietary exposure to antioxidants has the capability of protecting DNA telomeres from oxidative damage, potentially reducing the risk of multiple diseases, including breast cancer, in certain groups of humans (23). Specifically, a multitude of nutrients have been shown to be associated with telomere length, including folate; vitamin B-12; nicotinamide; vitamins A, C, D, and E; magnesium; zinc; iron; n–3 FAs; polyphenols; and curcumin (17). In the Nurses’ Health Study, PUFA consumption was negatively associated, and dietary fiber—specifically cereal fiber—was positively associated with leukocyte telomere length in middle-aged and older women (24). The interrelation with fat consumption is complex. Although an inverse association between baseline ω-3 FA concentrations and subsequent rate of telomere shortening over time was found, no significant association was found between ω-3 FA concentrations and telomere length at baseline in subjects with stable coronary artery disease in the Heart and Soul study (25). A rat study found a relation between telomere length in the kidney and a protein-restricted diet early in life. These animals also displayed a long-term suppression of appetite, reduced growth rate, and increased lifespan, in addition to significantly longer telomeres in the kidney (26).
Although a low-protein diet showed benefits in some animal studies, a human study conducted in the United States showed that a low-protein diet may be hazardous for older adults (27). Contradictorily, high protein intake was associated with increased mortality for adults who were middle-aged at baseline (27); therefore, age is an important factor in establishing optimal protein intake. More uniform results were found in association with adherence to the Mediterranean diet, so an overall lifestyle dietary pattern would potentially be more beneficial than choosing individual food components (28). According to some studies, people living in Mediterranean countries and following a Mediterranean diet have longer and healthier lives than do people living in other industrialized countries (29). However, some other factors besides diet likely contribute to their health status. Regardless, it is clear that diet and dietary food components play an important role in telomere length and telomerase activity as it relates to aging and cancer.
It remains unclear whether the expanded intake of one dietary component might influence response to the others, but such interactions are likely. It would be rather simplistic and unwise at this point to make recommendations regarding the use of certain nutrients and/or diets until further research can add more knowledge and understanding of the complex process of aging (Figure 2).
Bioenergetics as a Link between Aging and Cancer Prevention
Historically, calorie restriction without malnutrition has been associated with an increased life span in a variety of lower species (30). Some studies in animal models concluded that caloric restriction not only increases life span, but also decreases the risk of certain diseases. A study initiated in 1989 at the National Primate Research Center at the University of Wisconsin, Madison, reported a delayed onset of diseases and decreased mortality in a calorie-restricted group of rhesus monkeys compared with a control group (31). Interestingly, a National Institute of Aging intramural study reported no significant difference in survival between control-fed and calorie-restricted monkeys (32). The 2 studies admittedly used different diets and cohort compositions, which may account for some of the differences. In addition, the body weights of the National Institute of Aging control animals consistently were lower than average for both sexes; thus, additional restrictions did not make a difference (33). Therefore, calorie restriction would not automatically lead to an increase in lifespan under all conditions, but calorie restriction may be beneficial for subjects consuming a high number of calories. Individuals exposed to an obesogenic lifestyle may experience different consequences. A few commonly associated genetic variances and their implications in relation to bioenergetics, diet, and aging are reviewed briefly below as applicable to the melanocortin system, mechanistic target of rapamycin (mTOR), sirtuins, and PPARγ.
The melanocortin system possibly exhibits regulatory control of energy homeostasis through influence on satiety and glucose metabolism. At the base of this theory is the fact that pro-opiomelanocortin (POMC) neurons located in the arcuate nucleus synthesize melanocortins and exhibit leptin receptors, so it is likely that leptin-induced reductions in food intake might be mediated by the melanocortin system (34). Few studies, to our knowledge, have examined both the melanocortin peptide and receptor expression in association with fat intake. Overall, these studies suggest that the introduction of a high-fat diet at an early age increases melanocortin signaling, whereas in adulthood, a high-fat diet decreases melanocortin signaling (35). Thus, the timing of a high-fat diet introduction and, presumably, differences in metabolic needs may account for some of these differences in response related to subjects’ ages. Variation in the melanocortin 4 receptor (MC4R) has also been reported, but because the overall occurrence is ∼6% in obese individuals, its physiologic significance remains unclear (36). In any event, individuals carrying the I251L common allele of MC4R were predisposed to better clinical outcomes of weight loss after gastric bypass, and had a reduced risk of type 2 diabetes and better weight loss during diet interventions than those without the variance (37).
It appears that MC4R activation promotes insulin-stimulated (mTOR) signaling (38). Because brain mTOR signaling is involved in regulating nutrient intake and energy metabolism (38), targeted strategies likely are the best approaches for dietary interventions.
However, mTOR has more complex actions, so understanding mTOR signaling in different metabolic organs may increase the knowledge necessary for delaying aging and age-related diseases (39). Target of rapamycin (TOR) is found in 2 structurally and functionally distinct complexes named TOR complex 1 (TORC1) and TOR complex 2 (TORC2). Genetic ablation alone of mTORC2 signaling was not studied in enough depth to conclude its potential to modulate lifespan, but sole reduction of mTORC1 signaling is apparently able to increase lifespan (39).
Another factor associated with obesity and premature death is PPARγ (40), which encodes a transcription factor (PPARγ2) that controls the expression of multiple genes involved in cellular differentiation, lipid storage, and insulin sensitization. Pro12Ala (also known as rs1801282), a common single nucleotide polymorphism in the PPARγ gene, and 16 rare missense and nonsense mutations have been identified and their functional significance examined. Ethnicity contributes to variation in the frequency of the Pro12Ala polymorphism, which can range from 2% to 25% (40) and thus significantly influence variation in response to dietary change in different populations. In addition, a wide range of dietary components (capsaicin, 6-gingerol, isohumulone, luteolin, naringenin, resveratrol, and n–3 FAs) have been reported to influence PPAR activity (41). Although it is assumed that there are interactions between these foods and the shift in PPARγ, more research is needed to understand these interrelations entirely in different ethnicities.
Sirtuins are a class of proteins that usually exhibit histone deacetylase or, sometimes, mono-ribosyltransferase activity, and are named after silent information regulator 2 (Sir2). The role of sirtuins in aging and longevity was described in Saccharomyces cerevisiae, Caenorhabditis elegans, Drosophila, and other lower organisms. Increasing evidence suggests that sirtuins play an important role in delaying the aging process and promote longevity in mammals as well (42). Sirtuins have been implicated in influencing a wide range of cellular processes, such as aging, transcription, apoptosis, inflammation, and stress resistance, as well as energy efficiency and alertness during low calorie intake (43, 44). A number of molecular links, including nicotinamide adenine dinucleotide, nicotinamide, biotin, and related metabolites, are suggested to be the most important conduits mediating calorie restriction–induced Sir2/silent information regulator T1 (SIRT) activation and lifespan extension (45). Energy metabolism and DNA repair may be commonplace between aging and cancer, in which sirtuins may play a role (46).
The fat mass obesity (FTO)–associated gene also appears to have an effect on bioenergetics and, thereby, the aging process, because FTO gene variants increase preference for a high-fat diet and decrease satiety (47). Although the complete biological effects of FTO remain to be determined, it is known that it encodes a nuclear 2-oxoglutarate–dependent nucleic acid demethylase. The investigation of the obesity-predisposing a single nucleotide polymorphism in the fat mass and obesity associated FTO gene, allele T vs. A (rs9939609 T/A) variant of the FTO gene revealed differences in brain structure and function in the human posterior fusiform gyrus, helping with the understanding of how FTO is a predisposing factor for obesity through visual food perception and is linked to a greater risk of developing diabetes secondarily to an association with increased BMI and associated health and lifespan consequences (48).
The adrenal steroid dehydroepiandrosterone sulfate (DHEAS) is generally regarded to be an endocrine indicator of aging, because its concentration diminishes markedly with age. Interestingly, calorie restriction reportedly did little to prevent or delay the decline of DHEAS concentrations in aging rhesus macaques. Urbanski et al. (49) suggested that dietary manipulation may affect liver enzymes involved in the metabolism of this and other steroid hormones and thus impact their usefulness as biomarkers of aging. Nevertheless, DHEAS concentrations coupled with knowledge of genetic polymorphisms still may help identify those who might obtain the greatest benefits from calorie restriction.
BMI may be too insensitive a marker for predicting its impact on the aging process; body composition or metabolic syndrome may be better indicators. The aging process is characterized metabolically by insulin resistance, changes in body composition, and physiologic decline in growth hormone, insulin-like growth factor I, and sex steroids (50). Lifestyle interventions consistently were found to improve inflammatory and metabolic profiles, which interestingly are largely independent of obesity. These studies suggest that alterations in dietary composition, such as polyphenols, isothiocyanates, and the intake of several other bioactive food components, may be the most effective lifestyle changes to reduce disease risk independent of calorie intake; therefore, diet composition could be as important as calorie intake and deserves special attention. For example, dietary resveratrol at doses achievable in humans can possibly reduce many of the negative consequences of excess calorie intake by providing endothelial cell protection, partly through signaling of transglutaminase 2 (TGM2), an arterial calcification–related protein that is positively associated with hypertension and atherosclerosis (51).
Overall, although bioenergetics may be a factor in aging and cancer risk, the effect appears to be dependent on BMI only at the extremes, and it may relate to adiposity. Genetic and epigenetic processes also may be keys to the variation in response to foods in modifying some complications associated with aging processes. Although dietary restriction extends the lifespan across a variety of taxa, a prolongevity effect may not be a foregone conclusion for many genotypes, as shown when marked genetic variation between mice strains led to responses ranging from life extension to life shortening (52). We should be cautious because severe calorie restriction actually may have detrimental effects. The age at which calorie restriction begins and the amount and content of macronutrient compositions in the diet are very important. It would be interesting to know whether the restriction of certain nutrients would be able to help achieve the same benefits as caloric restriction does in certain circumstances.
DNA Repair: A Common Link
The accumulation of DNA damage and resulting genomic instability appears to be an important initiating event in aging and cancer (53). DNA “mismatch repair” system (MMR) is a system that removes nucleotides resulting from errors in replication and recombination with the aim of maintaining DNA fidelity. If not corrected, DNA sequence errors may lead to cancer and/or other diseases (54). Among the cancers associated with MMR deficiency, colorectal cancer and ovarian cancer are the most investigated. Although the incidence of MMR mutation in hereditary nonpolyposis colorectal cancer is very high (53), in ovarian cancer the incidence of germline MMR gene mutations is only 2%. Therefore, MMR mutations may not have the same significance for all cancers. In ovarian cancer, some other mechanisms of gene inactivation count for the loss of expression of 1 of the 7 main genes [MutatorS (Muts) Homologue (MSH)2, MSH3, MSH6, MutatorL (MutL) Homologue (MLH)1, MLH3, Post-Meiotic Segregation-1 gene (PMS)1, and PMS2] in up to 29% of cases (55). Mutations in several of these genes have been reported to lead to DNA instability. To better understand the role of individual MMR genes and functions in cancer, susceptibility studies that used mice deficient in the murine homologs of the human genes MLH1, PMS1, and PMS2 were conducted (56). These mice had different tumor susceptibilities, most notably to intestinal adenomas and adenocarcinoma, suggesting that a general increase in replication errors may not be sufficient for intestinal tumor formation and that these genes share overlapping, but not identical functions (56).
Cell damage may be promoted by deficiencies of several essential nutrients, e.g., choline, folate, and curcumin, and are discussed below in relation to DNA damage and repair.
Human choline deficiency is associated with increased DNA strand breaks and tissue (liver and muscle) dysfunction (57). Choline and its metabolites are recognized as having multiple roles in maintaining cell membrane structural integrity, nerve impulse transmission, cell signaling and lipid transport, and metabolism. In the state of choline deficiency, the leakage of reactive oxygen species (ROS) from mitochondria is increased due to mitochondrial membrane damage. Choline deficiency leads to an alteration in DNA methylation, which consequently alters gene expressions for genes critically involved in MMR and thereby contributes to an increased cancer risk (57). Male Fischer rats fed a choline- and methionine-deficient diet without carcinogen treatment developed preneoplastic hepatocyte nodules in 100% of cases and a 51% incidence of hepatocellular carcinoma (58). Plasma trimethylamine N-oxide, an oxidative derivative of choline produced by intestinal bacteria, was positively associated with rectal cancer and major adverse cardiovascular events. The positive association between plasma trimethylamine N-oxide and colorectal cancer risk is consistent with an involvement of the gut microbiome in colorectal cancer pathogenesis. These data suggest that choline actions in cancer and aging are strongly influenced by the microbiome, a fact that adds to the complexity of its action (59). Because fish is an important source of choline, it seems that the connection between fish intake and the risk of cancer and cardiovascular diseases has multiple facets that are not understood entirely yet.
Defects in the MMR genes may also arise from epigenetic changes in DNA methylation or by deregulation of microRNAs. Folate deficiency has been shown to induce DNA damage normally repaired via the base excision repair pathway. In the case of folate deficiency and base excision repair deficiencies, potential interaction may accelerate colonic aberrant crypt foci formation and tumor development. Folate-deficient wild-type mice have a significantly increased aberrant crypt foci formation when exposed to 1,2-dimethylhydrazine, a known colon and liver carcinogen; however, folate deficiency reduced development of these foci in β-polhaplo–insufficient mice, suggesting a protective effect in mutant models when the nutrient is absent (60). This response likely resulted from a reduction in cell proliferation, proapoptotic genes, and apoptosis. Thus, folate may have a dual role in protecting normal cells but causing decreased cell division and/or apoptosis in abnormal or mutant cells (61).
Several other bioactive food components may overcome the adverse consequences of MMR by inducing other cellular processes. For example, the inhibitory effect of genistein on the induction of extra centrosomes is proposed as occurring through the inactivation of cyclin dependent kinase 2 (CDK2)/Cyclin-A/E via p21 upregulation; this hypothesis is supported by the observation that p21 knockdown with small interfering RNA reduced the activity of CDK2/Cyclin-A/E and restored the enhanced effect of a combined treatment with genistein and ionizing radiation in Human U2OS cells and mouse NIH 3T3 cells (62). Lee et al. (63) demonstrated genistein-increased stress tolerance through enhanced expression of stress resistance proteins, such as superoxide dismutase (SOD-3) and heat shock protein 16.2 (HSP-16.2); consequently, genistein affects health span and lifespan in nematodes and possibly in other animal models.
Another example is curcumin (the active ingredient in turmeric), a spice widely used in India and Southeast Asian countries. Curcumin is reported to inhibit the growth of colonic cells that have a defective DNA repair capacity because of a lack of functional MMR enzymes (64), possibly by overcoming abnormal repair processes by downregulating cell division and inducing apoptosis. Curcumin also has antioxidant properties that may increase a healthy lifespan, as discussed below (65).
MMR is as well implicated in increased colorectal cancer risk in association with red meat consumption. The investigation of polymorphisms in the nucleotide excision repair and MMR showed that modified pathways associated with red meat and poultry intake lead to modified colorectal cancer risk only in subjects who carried the XPD codon (XPD) 751 (66). Additionally, red meat subtypes differed from lamb and pork consumption in their association with the risk of colorectal cancer and its subsites (67). Such data point to individuality in response to foods and their impact on processes associated with aging and cancer.
Oxidative Stress as a Target for Aging and Cancer Prevention
It is now well established that ROS, reactive nitrogen species (RNS), and a basal level of oxidative stress are essential for cell survival; it is also well known that although severe oxidative stress often leads to widespread oxidative damage and cell death, a moderate level of oxidative stress induced by a variety of stressors can yield great beneficial effects on adaptive cellular responses to pathologic challenges in aging and aging-associated disease (68). An elevated oxidative status, accentuated during the aging process, is likely due to imbalanced pro-oxidant and antioxidant activities, and could cause DNA damage. Various bioactive food components, including isoflavone, indole-3-carbinol, curcumin, and resveratrol, have been reported to reduce oxidative stress. The role of oxidative stress in modifying the aging and cancer processes per se remains very controversial. An array of studies reveal that overexpression of the major antioxidant enzymes that regulate oxygen metabolism in the cell, either by themselves or in combination, does not have an impact on the lifespan of mice (69). However, when transgenic or knockout mice are tested with the use of models that develop various types of age-related pathologic changes, they show alterations in the progression and/or severity of these changes, as predicted by the oxidative stress theory: increased oxidative stress accelerates pathologic changes and reduced oxidative stress retards pathologic changes (70). It appears that either oxidative stress plays a very limited role, if any, in aging but a major role in health span, or the role that oxidative stress plays in aging depends on environmental factors (70).
To our knowledge, new evidence suggests that increased ROS production in aging may be the result of adaptive signaling, rather than a detrimental by-product of normal respiration that drives aging (71). Nevertheless, there is evidence in cell cultures of rat skeletal muscle that some antioxidants, such as curcumin, are linked to an increase in lifespan through a reduction in intracellular reactive species and lipofuscin accumulation. Recently, curcumin and its metabolite, tetrahydrocurcumin, were reported to increase the mean lifespan of ≥3 model organisms: nematode roundworms, Drosophila fruit flies, and mice (72). Although the positive effect of curcumin on longevity may be due to a reduction in oxidative stress and its effect on age-related genes, other mechanisms also are possible. Curcumin is recognized as upregulating the expression of SOD-3 genes, but downregulating the expression of several age-related genes, such as InR-insulin-like-receptor in Drosophila melanogaster (dInR), attacin–D (ATTD), Defensin (Def), Cecropin B (CecB), and Diptericin B (DptB). In addition, tetrahydrocurcumin extended lifespan in Drosophila in a dose–response relation and inhibited the oxidative stress response (73). The response to stress involves regulating O-type Forkhead domain transcription factor (FOXO) and Sir2; therefore, tetrahydrocurcumin may also regulate other cellular processes, suggesting that the response likely is not limited to its antioxidant properties (74). Thus, again, the response to prolonged life may be very dependent on genetic, and possibly epigenetic, backgrounds rather than on a broad-based general population phenomena.
Another interesting link between aging and cancer is NF-κB, which has been found to be constitutively upregulated in many cancers, in various aged tissues, and in resting fibroblasts from older donors. NF-κB also has been suggested as an important regulator of ROS scavengers. It has been shown that a specific nutrient combination (ascorbate, lysine, proline, arginine, N-acetylcysteine, selenium, copper, manganese, and calcium), as well as some nutrients alone, may suppress NF-kB activity in cancer cells (75).
Many phytochemicals are known to act as antioxidants and have been shown to help prevent or reduce the risk of cancer, inflammation, and cardiovascular disease by the alteration of several signal transduction pathways in cell culture and/or in some animal models. The relation between antioxidant vitamin intake and all-cause mortality in older adults also was examined with the use of data from the Leisure World Cohort Study, a prospective study of residents of the Leisure World retirement community in Laguna Hills, California. Neither dietary nor supplemental intake of vitamin A or vitamin C nor supplemental intake of vitamin E was significantly associated with mortality after multivariate adjustment (76). However, data on vitamin intake were self-reported with the use of mailed questionnaires that did not comprise all food sources for vitamin E, and data were not adjusted for energy and fat intake. Because the basal amount of vitamins was not measured, and intake was actually estimated, it is unknown whether a deficit existed and was addressed, or whether subjects enrolled had an optimal concentration or intake of vitamins to start with. As a result, it is hard to draw a conclusion regarding the effect of supplemental intake if by chance it happens to be on a nondeficit status, as some American subpopulations are known to be.
At this time, the free radical theory of aging, initially developed by Harman in 1956, is under debate (77). Increasing evidence indicates that ROS, consisting of superoxide, hydrogen peroxide, and multiple others, do not only cause oxidative stress, but rather may function as signaling molecules that promote health by preventing or delaying a number of chronic diseases and ultimately extend the lifespan (78). Because of the inability to explain many contradictory results of the role of antioxidants in cancer and aging, correlated with a growing body of emerging data on mitochondrial DNA damage by ROS, this theory is being revised (70). However, it is entirely possible for the management of oxidative stress to play a role in life-history evolution and that the need to manage, minimize, or repair oxidative damage might vary between different kinds of tissues and organisms (79). To better understand the relation between nutrient metabolism, aging, and cancer, it is critical to identify genetic and epigenetic factors that lead to variability in response and the role of the microbiome toward this variability.
Conclusions
Aging and cancer processes are largely influenced by dietary factors. However, many studies have not been reproduced and, consequently, subpopulations could not be stratified properly to account for responders (both positive and negative) and nonresponders. It is rather simplistic to believe that all individuals will respond identically to any treatment, whether a food component or a drug. Thus, the use of nutrigenomics has the potential to revolutionize the understanding of nutrient needs for subpopulations for healthy living because it takes into account one’s genetic profile. As the discipline matures, there is hope that it will fill the void in the current understanding about nutrient-genome interactions and thus help to identify those individuals who likely will obtain the greatest benefit from dietary changes. Nevertheless, the complexity of discovering these interrelations is evident by the presence of ∼22,000 genes, 8–10 million polymorphisms, and daily exposures to >25,000 bioactive food components. The interrelations between telomeres, telomerase, aging, and cancer have opened a new and exciting research avenue. Whether accelerated telomere shortening is a cause or consequence of aging and disease conditions remains to be resolved. Nevertheless, a variety of food components appear to be capable of either decreasing or increasing telomere length. The larger issue is whether early telomerase promotion will increase mutations in cells and thus the risk of multiple diseases, including cancer. More probing studies are needed to resolve this controversy.
It is apparent that calorie restriction does not always increase longevity, but it may add healthy years by decreasing morbidity. It should be noted that there is not one single widely used calorie-restricted diet. There are many low-calorie diets that vary in key macro- or micronutrients; therefore, results vary as well. A question has been raised as to whether the benefit is due to, or can be obtained by, manipulation of a single macronutrient or multiple micronutrients. The most critical research need is to define and characterize the optimal nutritional status that would promote health and longevity. Because of different genetic and microbiome backgrounds, the optimal nutritional status may vary from individual to individual. For example, variation in the melanocortin receptor and associated signaling pathways, including PPAR, sirtuins, and FTO, is one of the potential modifiers of the response to foods and their biological outcomes. The challenge will be to identify biomarkers predictive of positive or negative outcomes. Although oxidative stress is commonplace during aging, and likely reflects an imbalance in dietary pro-oxidants and antioxidants, it is unclear whether it is a cause or result of prolonged cellular life. Although a variety of bioactive food components are recognized to reduce oxidative stress, it remains unclear whether this is the mechanism by which both aging and disease risks could be modified. Preclinical studies provide some of the most compelling evidence that modification of the expressions of antioxidant enzymes does not have a major impact on aging. Overall, the effectiveness of these antiaging and cancer prevention agents may be functioning through the modification of a molecular target that is independent of its antioxidant properties.
Some fundamental questions remain: Is it possible to change dietary habits to increase longevity while decreasing disease risk, given that there are common elements that influence both processes? Also, what is the point of no return resulting from the accumulated “-omic” changes that have occurred? At this time, it is challenging to draw firm conclusions, because the scientific literature is filled with inconsistent findings about the relation between foods or their components and aging or age-related diseases. Continued research with specific subpopulations on the basis of nutrigenomics technologies, the microbiome, and nutritional status could provide the needed evidence for the evaluation of the true effectiveness of dietary interventions. To maximize the utility of nutritional interventions, it is critical to identify not only the factors underlying chronic diseases modulated by nutrients, but also the nutritional status to be achieved to provide the longest health span and lifespan for a specific genetic background.
Acknowledgments
I thank John Milner, deceased, former director of the USDA Human Nutrition Center, for his insightful contribution at the beginning of writing this manuscript and his commitment for promoting nutritional science research. I also thank Cindy Clark, NIH Library Writing Center, for manuscript editing assistance. The sole author had responsibility for all parts of the manuscript.
Footnotes
Abbreviations used: ATTD, attacin–D; CDK2, cyclin dependent kinase 2; CecB, Cecropin B; Def, Defensin; DHEAS, dehydroepiandrosterone sulfate; dInR, InR- insulin-like-receptor in Drosophila melanogaster; DptB, Diptericin B; FOXO, O-type forkhead domain transcription factor; FTO, fat mass obesity; HSP-16.2, heat shock protein 16.2; MC4R, melanocorin 4 receptor; MLH, MutatorL (MutL) Homologue; MMR, DNA “mismatch repair” system; MSH, MutatorS (MutS) Homologue; mTOR, mechanistic target of rapamycin; p53, tumor protein p53; PMS, Post-Meiotic Segregation-1 gene; POMC, propiomelanocortin; RNS, reactive nitrogen species; ROS, reactive oxygen species; rs9939609 T/A, a single nucleotide polymorphism in the fat mass and obesity associated FTO gene, allele T vs. A; Sir2, silent information regulator 2; SIRT1, silent information regulator T1; SOD-3, superoxide dismutase; TERT, telomerase reverse transcriptase; TGM2, transglutaminase 2; TOR, target of rapamycin; TORC1, TOR complex 1; TORC2, TOR complex 2; XPD, XPD codon.
References
- 1.Bernardes de Jesus B, Blasco M. Telomerase at the intersection of cancer and aging. Trends Genet 2013;29:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Magalhães JP. Programmatic features of aging originating in development: aging mechanisms beyond molecular damage? FASEB J 2012;26:4821–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenech M, Sohemy AE, Cahill L, Ferguson LR, French TC, Tai ES, Milner J, Koh WP, Xie L, Zucker M, et al. Nutrigenetics and nutrigenomics: viewpoints on the current status and applications in nutrition research and practice. J Nutrigenet Nutrigenomics 2011;4:69–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zapata HJ, Quagliarello VJ. The microbiota and microbiome in aging: potential implications in health and age-related diseases. J Am Geriatr Soc 2015;63:776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duda-Chodak A, Tarko T, Satora P, Sroka P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: a review. Eur J Nutr 2015;54:325–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaetzlein S, Rudolph KL. Telomere length regulation during cloning, embryogenesis and ageing. Reprod Fertil Dev 2005;17:85–96. [DOI] [PubMed] [Google Scholar]
- 7.Rizvi S, Raza ST, Mahdi F. Telomere length variations in aging and age-related diseases. Curr Aging Sci 2014;7:161–7. [DOI] [PubMed] [Google Scholar]
- 8.Aviv A, Kark JD, Susser E. Telomeres, atherosclerosis, and human longevity: a causal hypothesis. Epidemiology 2015;26:295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aviv A. Telomeres and human aging: facts and fibs. Sci Aging Knowledge Environ 2004;2004:pe43. [DOI] [PubMed] [Google Scholar]
- 10.Aragona M, Maisano R, Panetta S, Giudice A, Morelli M, La Torre I, La Torre F. Telomere length maintenance in aging and carcinogenesis. Int J Oncol 2000;17:981–9. [DOI] [PubMed] [Google Scholar]
- 11.Chang HY, Lawless C, Addinall SG, Oexle S, Taschuk M, Wipat A, Wilkinson DJ, Lydall D. Genome-wide analysis to identify pathways affecting telomere-initiated senescence in budding yeast. G3 (Bethesda) 2011;1:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniel M, Peek GW, Tollefsbol TO. Regulation of the human catalytic subunit of telomerase (hTERT). Gene 2012;498:135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ligi P. Nutrition and telomere length. J Nutr Biochem 2011;22:895–901. [DOI] [PubMed] [Google Scholar]
- 14.Meeran SM, Ahmed A, Tollefsbol TO. Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clin Epigenetics 2010;1:101–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meeran SM, Katiyar S, Katiyar SK. Berberine-induced apoptosis in human prostate cancer cells is initiated by reactive oxygen species generation. Toxicol Appl Pharmacol 2008;229:33–43. [DOI] [PubMed] [Google Scholar]
- 16.Fallet E, Jolivet P, Soudet J, Lisby M, Gilson E, Teixeira MT. Length-dependent processing of telomeres in the absence of telomerase. Nucleic Acids Res 2014;42:3648–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Semir D, Nosrati M, Li S, Kashani-Sabet M. Telomerase: going beyond the ends. Cell Cycle 2007;6:546–9. [DOI] [PubMed] [Google Scholar]
- 18.Nicolai S, Rossi A, Di Daniele N, Melino G, Annicchiarico-Petruzzelli M, Raschellà G. DNA repair and aging: the impact of the p53 family. Aging (Albany NY) 2015;7:1050–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahin E, DePinho RA. Axis of ageing: telomeres, p53 and mitochondria. Nat Rev Mol Cell Biol 2012;13:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joruiz SM, Bourdon JC. p53 Isoforms: key regulators of the cell fate decision. Cold Spring Harb Perspect Med 2016. Jan 22 (Epub ahead of print; DOI:10.1101/cshperspect.a026039). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slattery ML, Curtin K, Ma K, Edwards S, Schaffer D, Anderson K, Samowitz W. Diet activity, and lifestyle associations with p53 mutations in colon tumors. Cancer Epidemiol Biomarkers Prev 2002;11:541–8. [PubMed] [Google Scholar]
- 22.Moslehi J, DePinho RA, Sahin E. Telomeres and mitochondria in the aging heart. Circ Res 2012;110:1226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen J, Gammon MD, Terry MB, Wang Q, Bradshaw P, Teitelbaum SL, Neugut AI, Santella RM. Telomere length, oxidative damage, antioxidants and breast cancer risk. Int J Cancer 2009;124:1637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cassidy A, De Vivo I, Liu Y, Han J, Prescott J, Hunter DJ, Rimm EB. Associations between diet, lifestyle factors, and telomere length in women. Am J Clin Nutr 2010;91:1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farzaneh-Far R, Lin J, Epel E, Harris WS, Blackburn E, Whooley M. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA 2010;303:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jennings BJ, Ozanne SE, Dorling MW, Hales CN. Early growth determines longevity in male rats and may be related to telomere shortening in the kidney. FEBS Lett 1999;448:4–8. [DOI] [PubMed] [Google Scholar]
- 27.Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 2014;19:407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crous-Bou M, Fung TT, Prescott J, Julin B, Du M, Sun Q, Rexrode KM, Hu FB, De Vivo I. Mediterranean diet and telomere length in Nurses’ Health Study: population based cohort study. BMJ 2014;349:g6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boccardi V, Paolisso G, Mecocci P. Nutrition and lifestyle in healthy aging: the telomerase challenge. Aging; Albany (NY) 2016;8:12–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redman LM, Ravussin E. Caloric restriction in humans: impact on physiological, psychological, and behavioral outcomes. Antioxid Redox Signal 2011;14:275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009;325:201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 2012;489:318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun 2014;5:3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G, Baskin DG, Schwartz MW. Melanocortin receptors in leptin effects. Nature 1997;390:349. [DOI] [PubMed] [Google Scholar]
- 35.van den Heuvel JK, van Rozen AJ, Adan RA, la Fleur SE. An overview on how components of the melanocortin system respond to different high energy diets. Eur J Pharmacol 2011;660:207–12. [DOI] [PubMed] [Google Scholar]
- 36.Jackson D, Ramachandrappa S, Clark AJ, and Chan L. Melanocortin receptor accessory proteins in adrenal disease and obesity. Front Neurosci 2015;9:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirshahi UL, Still CD, Masker KK, Gerhard GS, Carey DJ, Mirshahi T. The MC4R(I251L) allele is associated with better metabolic status and more weight loss after gastric bypass surgery. J Clin Endocrinol Metab 2011;96:E2088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chai B, Li JY, Zhang W, Wu X, Zhang C, Mulholland MW. . Melanocortin-4 receptor activation promotes insulin-stimulated mTor signaling. Peptides 2010;31:1888–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Curr Opin Genet Dev 2013;23:53–62. [DOI] [PubMed] [Google Scholar]
- 40.Chuang LM, Hsiung CA, Chen YD, Ho LT, Sheu WH, Pei D, Nakatsuka CH, Cox D, Pratt RE, Lei HH, et al. Sibling-based association study of the PPARgamma2 Pro12Ala polymorphism and metabolic variables in Chinese and Japanese hypertension families: a SAPPHIRe study. Stanford Asian-Pacific Program in Hypertension and Insulin Resistance. J Mol Med (Berl) 2001;79:656–64. [DOI] [PubMed] [Google Scholar]
- 41.Hirai S, Takahashi N, Goto T, Lin S, Uemura T, Yu R, Kawada T. Functional food targeting the regulation of obesity-induced inflammatory responses and pathologies. Mediators Inflamm 2010;2010:367838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horio Y, Hayashi T, Kuno A, Kunimoto R. Cellular and molecular effects of sirtuins in health and disease. Clin Sci (Lond) 2011;121:191–203. [DOI] [PubMed] [Google Scholar]
- 43.Satoh A, Imai S. Systemic regulation of mammalian ageing and longevity by brain sirtuins. Nat Commun 2014;5:4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Satoh A, Brace C, Ben-Josef G, West T, Wozniak DF, Holtzman DM, Herzog ED, Imai S. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J Neurosci 2010;30:10220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y. Molecular links between caloric restriction and Sir2/SIRT1 activation. Diabetes Metab J 2014;38:321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez-Pastor B, Mostoslavsky R. Sirtuins, metabolism, and cancer. Front Pharmacol 2012;3: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corella D, Arnett DK, Tucker K, Kabagambe EK, Tsai M, Parnell L, Lai CQ, Lee YC, Warodomwichit D, Hopkins PN, et al. A high intake of saturated fatty acids strengthens the association between the fat mass and obesity-associated gene and BMI. J Nutr 2011;141:2219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kühn AB, Feis DL, Schilbach L, Kracht L, Hess ME, Mauer J, Brüning JC, Tittgemeyer M. FTO gene variant modulates the neural correlates of visual food perception. Neuroimage 2016;128:21–31. [DOI] [PubMed] [Google Scholar]
- 49.Urbanski HF, Mattison JA, Roth GS, Ingram DK. Dehydroepiandrosterone sulfate (DHEAS) as an endocrine marker of aging in calorie restriction studies. Exp Gerontol 2013;48:1136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beavers KM, Nicklas BJ. Effects of lifestyle interventions on inflammatory markers in the metabolic syndrome. Front Biosci (Schol Ed) 2011;3:168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mattison JA, Wang M, Bernier M, Zhang J, Park SS, Maudsley S, Steven S. An SS, Santhanam L, Martin B, Faulkner S, et al. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab 2014;20:183–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell 2010;9:92–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edifizi D, Schumacher B. Genome instability in development and aging: insights from nucleotide excision repair in humans, mice, and worms. Biomolecules 2015;5:1855–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torgovnick A, Schumacher B. DNA repair mechanisms in cancer development and therapy. Front Genet 2015;6:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao X, Melton DW, Gourley C. Mismatch repair deficiency in ovarian cancer–molecular characteristics and clinical implications. Gynecol Oncol 2014;132:506–12. [DOI] [PubMed] [Google Scholar]
- 56.Prolla TA, Baker SM, Harris AC, Tsao JL, Yao X, Bronner CE, Zheng B, Gordon M, Reneker J, Arnheim N, et al. Tumour susceptibility and spontaneous mutation in mice deficient in Mlh1, Pms1 and Pms2 DNA mismatch repair. Nat Genet 1998;18:276–9. [DOI] [PubMed] [Google Scholar]
- 57.Zeisel SH. Dietary choline deficiency causes DNA strand breaks and alters epigenetic marks on DNA and histones. Mutat Res 2012;733:34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghoshal AK, Farber E. The induction of liver cancer by dietary deficiency of choline and methionine without added carcinogens. Carcinogenesis 1984;5:1367–70. [DOI] [PubMed] [Google Scholar]
- 59.Bae S, Ulrich CM, Neuhouser ML, Malysheva O, Bailey LB, Xiao L, Brown EC, Cushing-Haugen KL, Zheng Y, Cheng TY, et al. Plasma choline metabolites and colorectal cancer risk in the Women’s Health Initiative Observational Study. Cancer Res 2014;74:7442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ventrella-Lucente LF, Unnikrishnan A, Pilling AB, Patel HV, Kushwaha D, Dombkowski AA, Schmelz EM, Cabelof DC, Heydari AR. Folate deficiency provides protection against colon carcinogenesis in DNA polymerase beta haploinsufficient mice. J Biol Chem 2010;285:19246–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chiang FF, Huang SC, Wang HM, Chen FP, Huang YC. High serum folate might have a potential dual effect on risk of colorectal cancer. Clin Nutr 2015;34:986–90. [DOI] [PubMed] [Google Scholar]
- 62.Shimada M, Kato A, Habu T, Komatsu K. Genistein, isoflavonoids in soybeans, prevents the formation of excess radiation-induced centrosomes via p21 up-regulation. Mutat Res 2011;716:27–32. [DOI] [PubMed] [Google Scholar]
- 63.Lee EB, Ahn D, Kim BJ, Lee SY, Seo HW, Cha YS, Jeon H, Eun JS, Cha DS, Kim DK. Genistein from Vigna angularis extends lifespan in Caernorhabditis elegans. Biomol Ther (Seoul) 2015;23:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chauhan DP. Chemotherapeutic potential of curcumin for colorectal cancer. Curr Pharm Des 2002;8:1695–706. [DOI] [PubMed] [Google Scholar]
- 65.Naick H, Jin S, Baskaran R. GADD45α modulates curcumin sensitivity through c-Abl- and JNK-dependent signaling pathways in a mismatch repair-dependent manner. Mol Cell Biochem 2016;414:13–22. [DOI] [PubMed] [Google Scholar]
- 66.Joshi AD, Kim A, Lewinger JP, Ulrich CM, Potter JD, Cotterchio M, Le Marchand L, Stern MC. Meat intake, cooking methods, dietary carcinogens, and colorectal cancer risk: findings from the Colorectal Cancer Family Registry. Cancer Med 2015;4:936–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carr PR, Walter V, Brenner H, Hoffmeister M. Meat subtypes and their association with colorectal cancer: Systematic review and meta-analysis. Int J Cancer 2016;138:293–302. [DOI] [PubMed] [Google Scholar]
- 68.Yan LJ. Positive oxidative stress in aging and aging-related disease tolerance. Redox Biol 2014;2:165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pérez VI, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell 2009;8:73–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salmon AB, Richardson A, Pérez VI. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med 2010;48:642–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shokolenko IN, Wilson GL, Alexeyev MF. Aging: a mitochondrial DNA perspective, critical analysis and an update. World J Exp Med 2014;4:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen LR, Parnell LD, Ordovas JM, Lai CQ. Curcumin and aging. Biofactors 2013;39:133. [DOI] [PubMed] [Google Scholar]
- 73.Siddique YH, Naz F, Jyoti S. Effect of curcumin on lifespan, activity pattern, oxidative stress and apoptosis in the brains of transgenic drosophilla model of Parkinson’s Disease. BioMed Res Int 2014;2014:606928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiang L, Nakamura Y, Lim YM, Yamasaki Y, Kurokawa-Nose Y, Maruyama W, Osawa T, Matsuura A, Motoyama N, Tsuda L. Tetrahydrocurcumin extends lifespan and inhibits the oxidative stress by regulating the FOXO forkhead transcription factor. Aging (Albany NY) 2011;3:1098–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harakeh S, Azar R, Azhar E, Damanhouri GA, Assidi M, Abu-Elmagd M, Alqahtani HM, Kumosani T, Niedzwiecki A, Rath M, Al-Hejin A, Barbour E, Diab-Assaf M. Specific nutrient combination effects on tax, NF-κB and MMP-9 in human T-cell lymphotropic virus -1 positive malignant T-lymphocytes. BMC Cancer 2015;15 Suppl 1:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paganini-Hill A, Kawas CH, Corrada MM. Antioxidant vitamin intake and mortality: the Leisure World Cohort Study. Am J Epidemiol 2015;181:120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol 1956;11:298–300. [DOI] [PubMed] [Google Scholar]
- 78.Ristow M, Schmeisser K. Mitohormesis: promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose response. 2014;12:288–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Speakman JR, Blount JD, Bronikowski AM, Buffenstein R, Isaksson C, Kirkwood TB, Monaghan P, Ozanne S, Beaulieu M, Briga M, et al. Oxidative stress and life histories: unresolved issues and current needs. col Evol. 2015;5:5745–57. [DOI] [PMC free article] [PubMed] [Google Scholar]