Abstract
Epidemiological studies have linked consumption of n-3 PUFAs with a variety of beneficial health benefits, particularly with respect to putative anti-inflammatory effects. Unfortunately, many of these results remain somewhat controversial because in most instances there has not been a linkage to specific molecular mechanisms. For instance, dietary exposure to low levels of mercury has been shown to be damaging to neural development, but concomitant ingestion of n-3 PUFAs as occurs during consumption of fish, has been shown to counteract the detrimental effects. As the mechanisms mediating the neurotoxicity of environmental mercury are not fully delineated, it is difficult to conceptualize a testable molecular mechanism explaining how n-3 PUFAs negate its neurotoxic effects. However, environmental exposure to mercury also has been linked to increased autoimmunty. By way of a molecular understanding of this immuno-toxic association, disruption of CD95 signaling is well established as a triggering factor for autoimmunity, and we have previously shown that environmentally relevant in vitro and dietary exposures to mercury interfere with CD95 signaling. In particular we have shown that activation of caspase 8, as well as downstream activation of caspase 3, in response to CD95 agonist stimulation is depressed by mercury. More recently we have shown in vitro that the n-3 PUFA docosahexaenoic acid counteracts the negative effect of mercury on CD95 signaling by restoring caspase activity. We hypothesized that concomitant ingestion of n-3 PUFAs with mercury might be protective from the immuno-toxic effects of mercury, as it is with mercury’s neuro-toxic effects, and in the case of immuno-toxicity this would be related to restoration of CD95 signal strength. We now show that dietary ingestion of n-3 PUFAs generally promotes CD95 signaling by upregulating caspase 8 activation. Apart from accounting for the ability of n-3 PUFAs to specifically counteract autoimmune sequelae of mercury exposure, this novel finding for the first time suggests a testable molecular mechanism explaining the overall anti-inflammatory properties of n-3 PUFAs.
Keywords: n-3 PUFA, T-cells, Autoimmunity, CD95, Caspase 8
Introduction
Generally, an immune response to self-antigens is held in check by processes collectively known as tolerance. Autoimmune disease arises when normal tolerogenic mechanisms are disrupted, either by genetic abnormalities, environmental insults, or a combination of both. Mercury (Hg) is a widespread environmental xenobiotic which at low to moderate exposures is a potent immunomodulator, contributing to increased autoimmune disease in animal models [Druet, 1995;Germolec et al., 2012;Pollard et al., 1997;Bagenstose et al., 1999;Rowley et al., 2005]. In humans epidemiological studies have found correlations between Hg blood levels and proinflammatory cytokine levels, antinuclear antibody titers, and alterations in Ig isotype profiles associated with autoimmunity [Nyland et al., 2011b;Nyland et al., 2011a;Somers et al., 2015;Gardner et al., 2010].
CD95 is a membrane receptor protein expressed on most lymphoid cells, and CD95-mediated apoptosis is a principle mechanism whereby inappropriate lymphocyte activation and proliferation is held in check. The physiological importance of CD95 signaling in maintaining tolerance is perhaps best exemplified by the phenotype of MRL mice homozygous for the lpr mutation. MRL strain mice are genetically prone to an autoimmune proliferative syndrome characterized by lymphadenopathy and a lupus-like immunopathology. However the emergence of this pathology is profoundly accelerated in MRL mice having a homozygous defect (the lpr mutation) in CD95. Mice having the lpr mutation, which constitutes a loss of function mutation in CD95, develop lymphadenopathy and splenomegaly and generate large quantities of autoantibodies [Takahashi et al., 1994;Watanabe-Fukunaga et al., 1997]. Similarly, CD95 loss of function mutations or CD95 deficient-conditions results in an lpr-like autoimmune lymphoproliferative syndrome (ALPS) in humans [Rieux-Laucat et al., 2003;Le Deist et al., 1996;Fisher et al., 1995;Puck et al., 1997].
In the normal functioning immune system CD95-mediated apoptosis is triggered subsequent to cell activation. Initially the process, referred to as Activation Induced Cell Death (AICD), primarily targets self-reactive lymphocytes (reviewed in [Baumann et al., 2002]) so as to limit the development of autoimmunity [Janssen et al., 2000 ,Defrance et al., 2002]. AICD is also necessary to eliminate lymphocytes reactive to foreign antigens. During any immune response, cellular or antibody mediated cytotoxicity and expression of inflammatory cytokines can be harmful to the host. Therefore following elimination of a pathogen, in order to limit bystander effects to healthy tissue, AICD is necessary to terminate inflammatory responses, and return the system to homeostasis [Strasser et al., 2004;Barreiro et al., 2004;Krueger et al., 2003]. In a series of papers we have utilized in vitro model systems to dissect the molecular mechanism underlying the ability of Hg to disrupt tolerance, and have shown that one way in which low-level mercury exposures may contribute to autoimmune disorders is through interference with the CD95 signaling cascade [Whitekus et al., 1999;McCabe, Jr. et al., 2003;Ziemba et al., 2005;McCabe, Jr. et al., 2005].
On the molecular level, the initial steps in the CD95 apoptotic program involve formation of the Death Inducing Signal Complex (DISC) [Algeciras-Schimnich et al., 2002]. Following its formation, recruitment of pro-caspase-8 to the DISC leads to its proteolytic activation, which in turn initiates the activation of caspase 3 [Scaffidi et al., 1999; Algeciras-Schimnich, Shen, Barnhart, Murmann, Burkhardt, and Peter, 2002]. Caspase-3 plays a central role as the key executioner during many forms of apoptotic cell death, and accordingly caspase 8 activation of caspase-3 is a central feature in the apoptotic signaling pathway stimulated by CD95 [Zhivotovsky et al., 1997; Thorburn, 2004]. We have shown that the mechanism of Hg interference with CD95 signaling and apoptosis is proximal to the CD95 receptor, in that upon initiation of CD95 signaling the DISC fails to properly form, such that caspase 8 activation is attenuated in Hg exposed cells [McCabe, Jr., Whitekus, Hyun, Eckles, McCollum, and Rosenspire, 2003].
More recently we have extended our observations to an in vivo system. Specifically, the Staphylococcal aureus Enterotoxin B (SEB) model system provides a useful tool for assessing early events in Hg-induced alterations in death receptor signaling in vivo. SEB is classified as a superantigen, as it binds to the T cell receptor (TCR) and activates most Vβ8 T-cells independent of antigen specificity [Kawabe et al., 1990;Marrack et al., 1990]. SEB activated cells are characterized by early expression of CD69, followed by proliferation and then AICD [Vasseur et al., 1999]. Although AICD does not occur for at least 48 hours after SEB challenge [Yang et al., 1998], we have shown that while death is mediated by CD95, that caspase 8 is maximally activated within 12 hours. Significantly, we demonstrated that in mice which were exposed to environmentally relevant levels of Hg in their drinking water prior to SEB challenge, that extrinsic (CD95-mediated) apoptotic signaling (as measured by activation of caspases 8 and 3) was attenuated, confirming our in vitro findings [Laiosa et al., 2007].
Most environmental exposure to mercury occurs through the consumption of fish, and it has been suggested that high levels of dietary n-3 polyunsaturated fatty acids (n-3 PUFA) found in fish may serve to ameliorate deleterious effects of mercury on the nervous system [Clarkson et al., 2006;Strain et al., 2008]. We have recently shown in an in vitro system that docosahexaenoic acid (DHA), the primary n-3 PUFA found in fish counteracts the attenuation of CD95-induced caspase 8 activation and ensuing cell death by Hg in T cells [Gill et al., 2015]. In this report we now investigate the ability of dietary n-3 PUFA’s to counteract Hg-dependent attenuation of extrinsic apoptotic signaling and caspase 8 activation in SEB activated T-cells in vivo.
Materials and methods
Experimental animals
Seven week old female BALB/c mice were ordered from Jackson Laboratories (Bar Harbor, ME). Mice were allowed to acclimate for one week after arrival at Wayne State University. The animals were housed under conventional conditions and given water and rodent laboratory chow (Ralston Purina, St. Louis, MO) ad libitum. The Wayne State University animal care program is AAALAC accredited and all experimental procedures received Institutional Animal Care and Use Committee approval. All animal care and treatment procedures were in compliance with the “Guiding Principles in the Care and Use of Animals” (DHEW Publication, NIH80-23).
Experimental diets
After acclimation for one week on normal rodent chow mice were randomly placed into treatment groups. Mice were put on a control diet (AIN-93M) containing soybean oil, or a diet (D14122201) which was similar to AIN-93M, but where menhaden oil was mostly substituted for the soybean oil, so as to be enriched in omega-3 fatty acids (Research Diets, New Brunswick, NJ). Overall, the soybean diet has less Omega-3 fatty acid, as additional Omega-6 fatty acid was substituted in its place. Total Omega-3(%) of fatty acids was 7.8 and 28.6 for the soybean and fish oil diets respectively. Total Omega-6 (%) of fatty acids was 53.6 and 18.4 for the soybean and fish oil diets respectively (Table S1).
Reagents and treatment protocols
HgCl2 was obtained from Aldrich Chemicals (St. Louis, MO). A stock solution of 5 mg/ml was prepared by dissolving the HgCl2 in double distilled H2O. The stock HgCl2 was further diluted in distilled water to a final concentration of 10 mg/L. The working solution was autoclaved and given to mercury exposed groups of mice ad-lib in their drinking water for two weeks. Animals that were not exposed to mercury were given sterile drinking water. Staphylococcal enterotxin B (SEB) was obtained from Toxin Technology (Sarasota, FL). It was diluted to 1 mg/ml and dispensed into 100 µl aliquots and frozen until used. Just before use, an aliquot of SEB was thawed and diluted to 200µg/ml with sterile saline. Mice which were exposed to SEB were injected with 20 µg of the toxin via the tail vein.
Lymphocyte isolation
Mice were euthanized by CO2 narcosis followed by cervical dislocation 24 hrs after SEB injection. After euthanasia, spleens were removed, cleaned of fat and connective tissue and placed in cold RPMI buffered with 20 mM Hepes (ThermoFisher, Waltham MA). Cell suspensions were created by passing through a 70 um stainless steel screen and rinsing with additional RPMI. Splenocytes were pelleted at 900 × g for 6 minutes and suspended in cold RPMI. Splenocytes were then further purified by density gradient centrifugation utilizing Lymphocyte separation medium (ThermoFisher, Waltham MA).
Detection of caspase 8 activity
One to two million lymphocytes from each mouse was aliquoted into individual tubes. Cells were suspended in 80 µl of caspalux LlD2 caspase 8 fluorogenic substrate (Oncolmmunin, Gaithersburg, MD) and placed in in a humidified, 5% CO2, 37 ° C incubator. After 20 minutes antibodies were added for surface staining. After1 hour of total incubation, cells were gently pelleted, washed and prepared for flow cytometry. Active caspase 8 enzymes cleave the cell permeable substrate, resulting in ≥ 10 fold increase in fluorescence intensity detectable in the FITC channel of the Cyan (Beckman/ Coulter) flow cytometer.
Antibodies and Lymphocyte staining for flow cytometry
The following monoclonal fluorochrome conjugated antibodies were used: CD4 (clone GK1.5) conjugated to APC-CY7, CD8a (clone 53–6.7) conjugated to AF 647, Vβ8 (clone F23.1) conjugated to PE, Vβ6 (clone RR4-7) conjugated to biotin, CD69 (clone H1.2F3) conjugated to PE-CY7. All antibodies were obtained from BD, Franklin Lakes NJ. Biotin was detected by Pacific Orange conjugated streptavidin obtained from Invitrogen/Thermo Fisher, Waltham, MA. After incubation with the caspase 8 fluorogenic substrate, cells were stained with a cocktail of the above described labeled antibodies. Cells were then washed and stained with Pacific Orange conjugated streptavidin. Labeled cells were then examined on a Cyan (Beckman/Coulter) flow cytometer. Flow cytometry results were analyzed utilizing Summit software (Beckman/Coulter).
Statistics
For each experiment four to six mice were analyzed individually. Each point was analyzed on at least two separate independent experiments. Results are presented as the mean ± SEM with the appropriate number of mice indicated in the figure legend. Comparisons between means were performed using the two tailed Student’s T test and p ≤ 0.05 was considered statistically significant.
Results
Gating scheme and analysis protocol
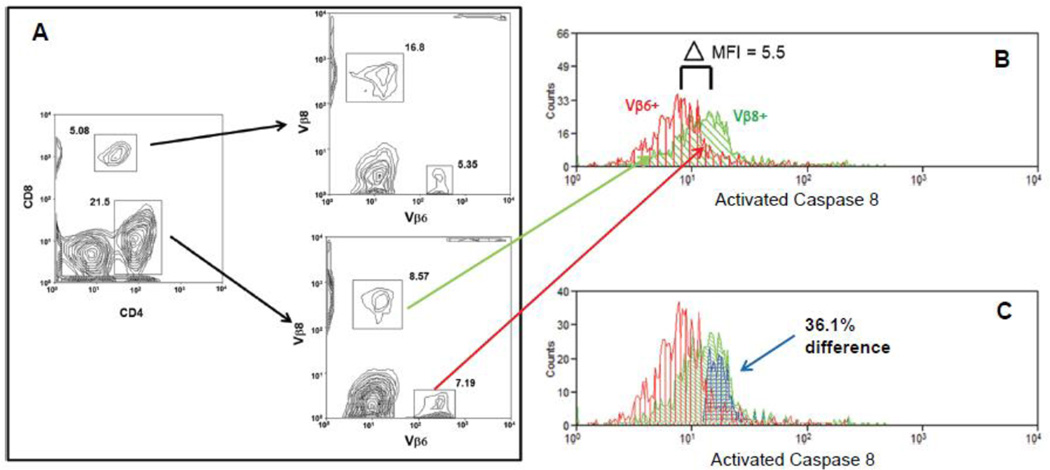
Figure 1 illustrates the gating scheme and protocol that was utilized to analyze flow cytometric data pertaining to SEB activated T cells throughout this manuscript. Specifically, figure 1 is a representative example (n=6) where a BALB/c mouse was inoculated IV with SEB and caspase 8 activation analyzed. We have previously shown that in BALB/c mice the peak of caspase 8 activation in response to SEB occurs 24 hours after IV inoculation [Laiosa, Eckles, Langdon, Rosenspire, and McCabe, Jr., 2007]. Accordingly the mouse was sacrificed at the 24 hour mark, and then the spleen removed and lymphocytes purified as described above. Cells were then treated with a cell permeable substrate which becomes fluorescent upon encountering active caspase 8 [Komoriya et al., 2000]. The cells were then stained with a cocktail of fluorescent antibodies specific for CD8, CD4, and T cell receptors of the Vβ8 and Vβ6 gene family lineages. Finally the cells were analyzed with a Cyan flow cytometer and Summit software. In these experiments we have stained for and examined T cells of the Vβ8 lineage, because SEB targets Vβ8 lineage TCRs [Kawabe and Ochi, 1990;Marrack, Blackman, Kushnir, and Kappler, 1990]. For control purposes we also have examined Vβ6 lineage T cells. Since SEB does not activate Vβ6+ T cells, these cells serve as a true internal control.
Figure 1.
Gating scheme and analysis protocol. (A) Visualization and gates defining CD8 and CD4 positive T cells, followed by visualization and gates defining the Vβ6 and Vβ8 subpopulations. (B) Histograms of SEB stimulated caspase 8 activation for CD4+ Vβ8+ (green) and CD4+ Vβ6+ (red) T cells. Δ MFI represents the difference between the mean fluorescent indexes for the two histograms. (C) Overton subtraction of the CD4+ Vβ6+ caspase 8 activity histogram (red) from the CD4+ Vβ8+ caspase 8 activity histogram (green) yields the difference histogram (blue).
Figure 1A shows the initial bivariate plot identifying CD4+ and CD8+ T cells. After gating on either the CD4+ or CD8+ cells, we identify and gate on either Vβ8+ or Vβ6+ lineage cells in a sequential bivariate plot (figure 1B). In this case we have chosen to initially gate on the CD4+ cells, and in the sequential bivariate plot have identified Vβ8+ and Vβ6+ lineage CD4+ cells. In figure 1B we have sequentially gated on the Vβ8+ and Vβ6+ cells, and then plotted the histograms (green for Vβ8+ and red for Vβ6+) representing fluorescence intensity of the active caspase 8 probe in each population. Taking the median of each histogram as the mean fluorescent index (MFI) of the histogram, we find that the difference between the MFI of the two histograms is 5.5. The difference in MFI (5.5) is taken as a measure of the difference in caspase 8 activation between Vβ8+ and Vβ6+ CD4 T cells. In this case since it is known that only Vβ8+ cells respond to SEB [Kawabe et al., 1991;Marrack, Blackman, Kushnir, and Kappler, 1990], the Vβ6+ histogram can be regarded as background, or alternatively as an internal negative control.
Aside from a direct comparison of the MFI between two histograms, a complimentary method of analysis involves subtracting one histogram from the other. This usually involves utilization of the Overton algorithm [Overton, 1988]. In figure 1C we have utilized the Overton algorithm included in the Summit software package to subtract the Vβ6+ histogram from the Vβ8+ histogram. Again, the Vβ8+ histogram is plotted in green, while the Vβ6+ histogram is plotted in red. The difference is symbolized as a third histogram (plotted in blue), which represents 36.1% of the Vβ8+ histogram. In other words if we take the Vβ6+ histogram as a control, then after SEB stimulation, on a normalized basis, 36.1 % of the Vβ8+ cells express a greater active caspase 8 signal than the Vβ6+ cells.
Dietary fish oil interferes with the ability of inorganic mercury to attenuate extrinsic apoptotic signaling in SEB stimulated CD4 and CD8 T-cells
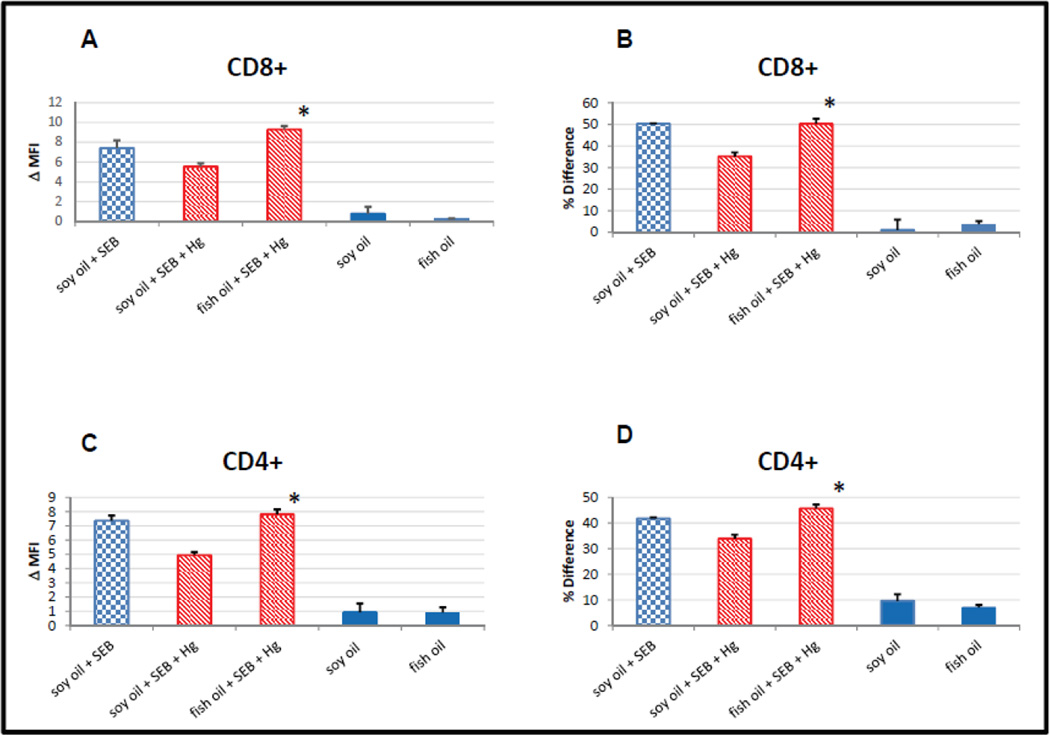
In figure 2 BALB/c mice were initially divided into two groups. One group was put on a diet enriched in Manhaden (fish) oil, while the other group was put on a control diet with soybean oil substituted for the fish oil. Both diets were essentially equivalent except in terms of their fatty acid composition. The typical fatty acid analysis for each diet as provided by the manufacturer is given in the supplemental material (Table 1). The major differences between the two are that the fish oil diet contained the n-3 PUFAs Eicosapentaenoic acid and Docosahexaenoic acid, while the soybean control diet did not.
Figure 2.
Diets supplemented with fish oil, but not soybean oil prevents mercury from interfering with SEB dependent activation of caspase 8 in CD8 and CD4 positive T cells. Blue checkered bars indicate results from mice maintained on the soy oil enriched diet that were inoculated with SEB (positive controls). Red cross-hatched bars represent results from mice on either the soy oil or fish oil diets that were stimulated with SEB and exposed to Hg. Solid blue bars represent results from mice on either the soy or fish oil diets that were not stimulated with SEB and not exposed to Hg (negative controls). Error bars represent SEM; * indicates statistically significant difference in SEB dependent caspase 8 activation in T cells from mercury exposed mice maintained on fish oil enriched diets vs diets enriched with soybean oil. (A) Difference (MFI) between SEB dependent caspase 8 activation in CD8+, Vβ8+ and Vβ6+ T cells. * p < .003. (B) Difference (Overton subtraction) between SEB dependent caspase 8 activation in CD8+, Vβ8+ and Vβ6+ T cells. *p < .030 (C) Difference (MFI) between SEB dependent caspase 8 activation in CD4+, Vβ8+ and Vβ6+ T cells. *p < .006 (D) Difference (MFI) between SEB dependent caspase 8 activation in CD8+, Vβ8+ and Vβ6+ T cells. *p < .007
After two weeks of being on either the n-3 PUFA supplemented or control soybean enriched diet, each initial group was subdivided into two, creating a total of four groups. HgCl2 (10 mg/L) was added to the drinking water of one group of mice who were on the n-3 PUFA supplemented diet, and one group of mice who were on the control diet. All four groups of mice were then maintained on their original diets (n-3 PUFA enriched or control) for an additional two weeks. At this point mice from the two groups which had mercury in their drinking water were inoculated with SEB to stimulate extrinsic apoptotic signaling as previously described [Laiosa, Eckles, Langdon, Rosenspire, and McCabe, Jr., 2007]. As a positive control for extrinsic apoptotic signaling in the absence of fish oil or mercury, 4 mice which were on the control diet, and which were not exposed to mercury, were also inoculated with SEB, yielding 5 groups total. In a similar vein mice from the groups which were not exposed to mercury and were not treated with SEB served as negative controls for the effect of diet alone on extrinsic apoptotic signaling. In any event, 24 hours after SEB inoculations all mice, including controls which were not inoculated with SEB, were sacrificed. After sacrifice, spleens were removed, spenocytes purified, and as in figure 1 stained for CD4, CD8, Vβ8, Vβ6 and active caspase 8.
After staining, for each mouse in each of the experimental groups, flow cytometric analysis was conducted as outlined in figure 1 by first gating on either CD8+ or CD4+ cells, and then determining the profile of the active caspase 8 signal associated with Vβ8 and Vβ6 cells within each of the CD8 or CD4 populations. In figure 2A, the difference between the MFI determined for the active caspase 8 signal associated with Vβ6+ CD8+ cells was subtracted from that of the Vβ8+ CD8+ cells in each mouse, and then averaged over all mice within each experimental group, and plotted. The results for the positive control (soy oil + SEB) are potted as the blue checkered bar, while the results for mice which were exposed to Hg + SEB (with or without Hg) are plotted as red hatched bars. Negative controls which were not inoculated with SEB (and which were not exposed to Hg) are plotted as solid blue bars.
In figure 2B, instead of subtracting the active caspase 8 MFI associated with the Vβ6+ CD8+ cells from that of the MFI associated with the Vβ8+ CD8+ cells, the Overton algorithm was utilized to subtract the entire active caspase 8 histogram of the Vβ6+ CD8+ cells from that of the Vβ8+ CD8+ cells for each mouse. Again, the results were averaged over all of the mice within each of the experimental groups and plotted as in figure 2A. The results for CD4 cells were obtained and plotted in exactly the same manner as for the CD8 cells, with the obvious exception that the initial gate was for CD4+ cells. The differences in MFI and Overton subtractions are plotted in figures 2C and 2D respectively.
Independent of whether differences between Vβ8 and Vβ6 active caspase 8 histograms are analyzed in terms of differences in MFI or by utilization of the Overton subtraction algorithm, we arrive at the same findings. Figures 2A and 2B are completely self- consistent, as are figures 2C and 2D. Overall, figure 2 confirms our previous results showing that in the absence of SEB stimulation caspase 8 is minimally activated, independent of whether the mice are exposed to Hg or not. However, in mice fed control soybean oil enriched diets, low levels of dietary inorganic mercury attenuates apoptotic signaling in SEB stimulated CD8, as well as CD4 cells [Laiosa, Eckles, Langdon, Rosenspire, and McCabe, Jr., 2007]. But figure 2 also clearly shows that when mice are maintained on fish oil enriched diets; mercury no longer has the ability to attenuate extrinsic apoptotic signaling triggered by SEB.
Dietary fish oil augments extrinsic apoptotic signaling in SEB stimulated CD4 and CD8 T-cells
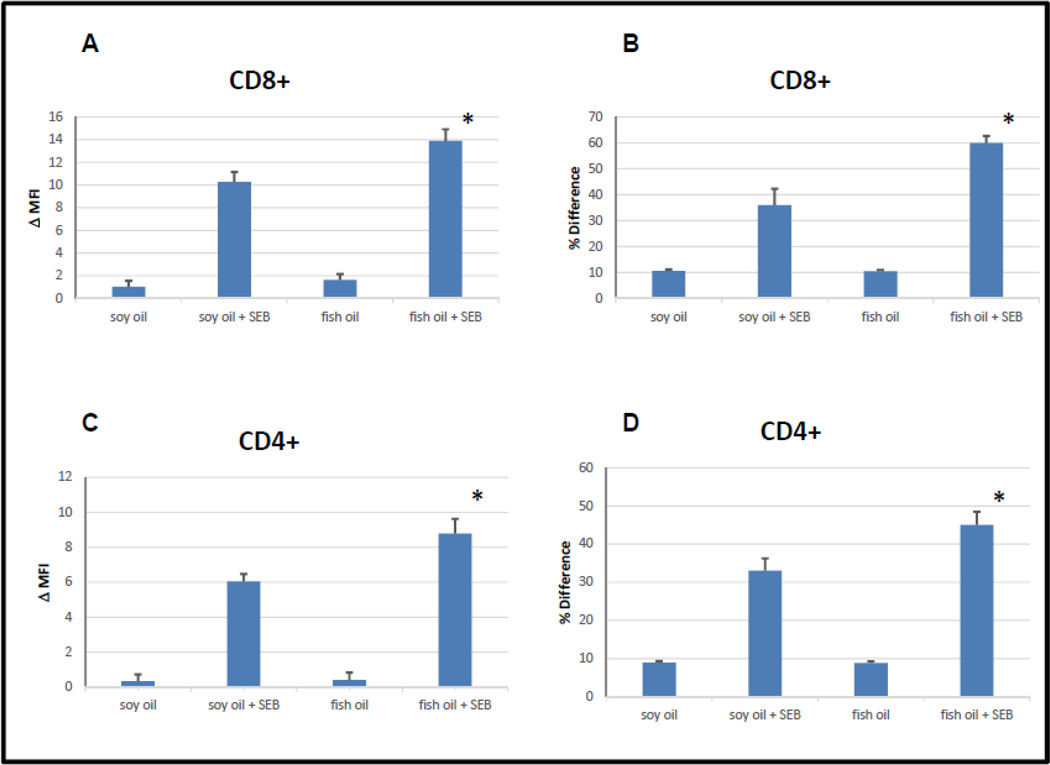
In figures 3 and 4 we looked more closely at the effect of fish oil enhanced diets on SEB stimulated apoptotic signaling in T cells which were not exposed to mercury. As in figure 2, BALB/c mice were maintained on either a fish oil or a soybean oil enhanced control diet for two weeks, and then were inoculated or not with SEB. Mice were sacrificed 24 hours later, spleens were removed, spenocytes purified, and cells stained for CD4, CD8, CD69, Vβ8, Vβ6, and active caspase 8, and then analyzed by flow cytometry.
Figure 3.
SEB activation of caspase 8 in CD8+ and CD4+ T cells is enhanced in diet supplemented with fish oil. Error bars represent SEM; * indicates statistically significant difference between SEB dependent caspase 8 stimulation in T cells derived from mice maintained on fish oil enriched diets vs T cells derived from mice maintained on soybean oil enriched diets. (A) Difference (MFI) between SEB dependent caspase 8 activation in CD8+, Vβ8+ and Vβ6+ T cells. *p < .026 (B) Difference (Overton subtraction) between SEB dependent caspase 8 activation in CD8+, Vβ8+ and Vβ6+ T cells. *p < .027 (C) Difference (MFI) between SEB dependent caspase 8 activation in CD4+, Vβ8+ and Vβ6+ T cells. *p < .016 (D) Difference (Overton subtraction) between SEB dependent caspase 8 activation in CD8+, Vβ8+ and Vβ6+ T cells. *p < .042
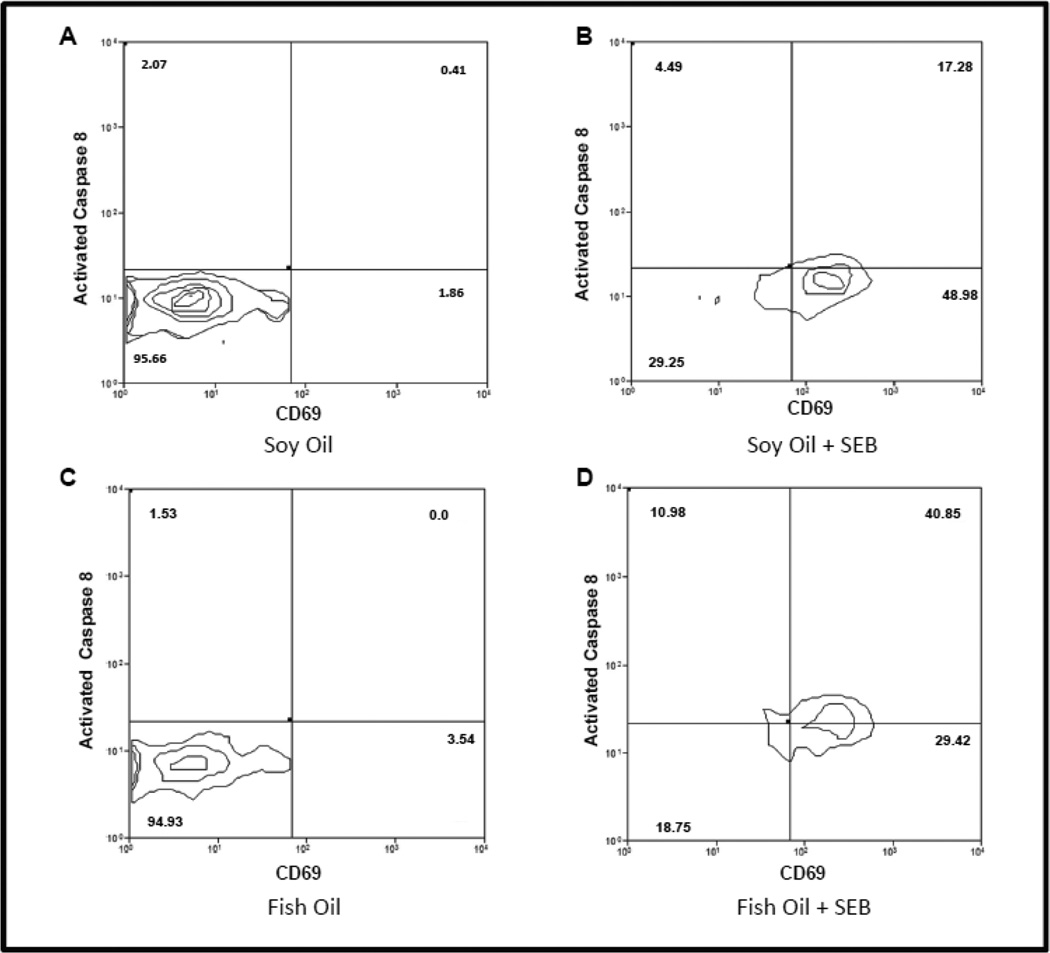
Figure 4.
Fish oil enriched diets augment SEB induced caspase 8 activation but not SEB dependent T cell activation. (A) Representative example (n =4) of activated caspase 8 vs CD69 expression in CD4+ T cells obtained from mice maintained on a soybean enriched diet. (B) Representative example (n=4) of activated caspase 8 vs CD69 expression in CD4+ T cells obtained from mice maintained on a soybean enriched diet and stimulated with SEB. (C) Representative example (n=4) of activated caspase 8 vs CD69 expression in CD4+ T cells obtained from mice maintained on fish oil enriched diet. (D) Representative example of activated caspase 8 vs CD69 expression in CD4+ T cells obtained from a mouse maintained on fish oil enriched diet and stimulated with SEB.
Figures 3A and 3B summarize the results for CD8 T cells, while Figures 3C and 3D summarize the results for CD4 T cells. Following the protocol in figure 2, figures 3A and 3C plot the differences between the MFI associated with the active caspase 8 signals arising from Vβ8+ and Vβ6+ cells, averaged over all mice within each experimental group. Figures 3B and 3D plot the results (averaged over all mice within each experimental group) after the Overton subtraction algorithm has been used to determine the differences between the active caspase 8 histograms associated with Vβ8+ and Vβ6+ cells. As in figure 2, whether differences between Vβ8 and Vβ6 active caspase 8 histograms are analyzed in terms of differences in MFI or by utilization of the Overton subtraction algorithm, we arrive at the same findings. In total figure 3 demonstrates that compared to soybean oil enriched control diets, fish oil enhanced diets significantly enhance the efficacy of SEB stimulated apoptotic signaling in CD8+ (figure 3A and 3B) as well as in CD4+ cells (figure 3C and 3D).
Fish oil enhanced diets augment SEB induced apoptotic signaling but not SEB dependent T cell activation
In figure 3 we demonstrated that on a population basis, SEB stimulates a statistically significant greater activation of caspase 8 in Vβ8+ T cells when mice were maintained on fish oil enhanced diets as opposed to maintenance on control diets. In figure 4 we simultaneously look at SEB induced apoptotic signaling (as measured by activated caspase 8) and cell activation (as measured by CD69 expression) in individual Vβ8+ CD4 cells from four representative mice examined in figure 3 which were or were not inoculated with SEB, and which were maintained on either fish oil or control soybean oil enriched diets. Specifically, figure 4A is a representative (n =4) bivariate plot displaying the measures of activated CD69 on the “x” axis, and activated caspase 8 on the “y” axis, obtained from Vβ8+ CD4 T cells derived from a control mouse which was not inoculated with SEB, and which was maintained on a soybean oil diet. Figure 4B is a similar representative (n =4) bivariate plot obtained from Vβ8+ CD4 T cells obtained from a mouse which were also maintained on a control soybean oil diet, but which was inoculated with SEB. Comparison of figure 4B with figure 4A clearly shows that in mice maintained on control diets that after inoculation with SEB there is a substantial increase in CD69 expression, and a modest increase in caspase 8 activation in Vβ8+ CD4 T cells.
Figure 4C is the representative (n =4) bivariate plot for activated caspase 8 and CD69 derived from VB8+ CD4 T cells obtained from a mouse which was maintained on a fish oil enriched diet, and which was not inoculated with SEB. Comparison of figures 4A and 4C demonstrates that maintenance on fish oil enriched diets in the absence of SEB has little effect on either CD69 expression or activation of caspase 8 compared to controls. Figure 4D is the representative (n =4) bivariate plot for activated caspase 8 and CD69 derived from Vβ8+ CD4 T cells obtained from a mouse which were maintained on a fish oil enriched diet, but which was also inoculated with SEB. Comparison of figures 4C and 4D demonstrates that in mice maintained on fish oil enriched diets that SEB stimulates a substantial increase in CD69 expression, as well as an increase in activation of caspase 8. Comparison of figures 4B and 4D demonstrates that while SEB stimulates similar increases in CD69 expression in both fish oil enhanced and control diets, in agreement with figure 3 it stimulates a much greater increase in caspase 8 activation in mice which have been maintained on a fish oil enhanced diet. We found similar results for CD8 T cells (not shown).
Discussion
As previously reviewed, inorganic mercury (Hg2+) induced failure of CD95- mediated activation of caspase 8 may in part explain the connection between Hg2+ exposure and inflammation and autoimmunity. In these experiments we have shown that dietary n-3 PUFAs ameliorate the ability of Hg2+ to interfere with CD95-mediated activation of caspase 8 in vivo, implying that dietary n-3 PUFAs may reverse the autoimmune and inflammatory sequelae of environmental mercury exposures. These results support our previous findings in an in vitro system where we showed that DHA counteracts Hg2+ dependent attenuation of CD95-mediated activation of caspase 8 and subsequent cell death in T cells [Gill, Lanni, Jen, McCabe, Jr., and Rosenspire, 2015]. Furthermore, these new results are relevant with respect to contemporary human diets and mercury exposures, and provide molecular support for nutritional interventions to counteract environmental mercury exposure.
The reasons for this are although exposures to high levels of (inorganic or organic) mercury are unquestionably lethal, exposure to high levels of mercury are now uncommon. Currently most environmental exposure to mercury is to low level organic mercury through diet. The principle contemporary dietary source of organic mercury being fish [Clarkson and Magos, 2006], but fish also contain high levels of n-3 PUFAs [Tolan et al., 1972;van Gent et al., 1979;Gebauer et al., 2006]. However regardless of the source, ingested organic Hg is rapidly metabolized to Hg2+. In fact even for animals exposed exclusively to organic Hg, within a short period the mercury burden in the spleen is almost entirely Hg2+. Most significantly, in animals exposed exclusively to organic mercury, it appears that while organic Hg is initially associated with immunosuppression, the Hg2+ metabolite is the active species interacting with the immune system that is responsible for autoimmune phenomena [Havarinasab et al., 2005b;Havarinasab et al., 2005a].
As we were concerned with the autoimmune consequences of mercury exposures, in these experiments BALB/c mice were directly exposed to Hg2+ in their drinking water. We have previously shown that following the exposure protocol used in these experiments that BALB/c mice demonstrate splenic Hg burdens of the order of 1 part per million [Laiosa, Eckles, Langdon, Rosenspire, and McCabe, Jr., 2007]. This is well in line with normal human Hg splenic burdens obtained from autopsy data [Kevorkian et al., 1972]. The n-3 PUFA exposures we utilized were also practical. The Adequate Intake (the amount based on observation of intake of a group of healthy individuals) for omega-3 fatty acid (alpha-linolenic acid) is 0.6% to 1.2% of energy (Psota et al., 2006]). The omega-3 fatty acid contained in the current diet is about 2.4% of total energy. Thus the intake of the omega-3 fatty acid in these mice is twice of the amount recommended for humans to prevent deficiency and maintain health.
It has previously been suggested that n-3 PUFAs found in fish may serve to reverse deleterious effects of mercury on the nervous system, which would otherwise be manifest due to fish consumption [Clarkson and Magos, 2006;Strain, Davidson, Bonham, Duffy, Stokes-Riner, Thurston, Wallace, Robson, Shamlaye, Georger, Sloane-Reeves, Cernichiari, Canfield, Cox, Huang, Janciuras, Myers, and Clarkson, 2008]. This conjecture has been supported by a recent epidemiological investigation which directly demonstrated a statistically significant correlation between exposure to mercury and poorer performance on school-age assessment of IQ. However if exposure to mercury was accompanied by co-exposure to the n-3 PUFA docosahexaenoic acid (DHA), IQ performance was similar to the non-exposed group [Jacobson et al., 2015]. Whether these statistical associations are causal are difficult to determine. The problem is that the mechanisms by which low environmental exposures to mercury disrupts the nervous system such that cognitive function (i.e., IQ) is diminished are, for the most part, unknown [Clarkson and Magos, 2006]. Consequently it is challenging to arrive at a testable mechanism by which n-3 PUFAs may negate deleterious effects of mercury on the nervous system.
The situation is different for the immune system where environmental exposure to mercury is associated with disruption of tolerance and autoimmunity. As outlined above, the CD95 signaling pathway is crucial to maintain tolerance, and we have previously shown that environmentally relevant levels of mercury disrupt the CD95 signaling pathway, both in vitro and in vivo. We have found that the mechanism of Hg interference with CD95 signaling and apoptosis is proximal to the CD95 receptor, in that upon initiation of CD95 signaling the Death Inducing Signaling Complex (DISC) fails to properly form, and as a result caspase 8 is inefficiently activated in Hg exposed cells. We found that this was related to the failure of CD95 to properly associate with other components of the DISC, and that this in turn is related to the fact that in mercury intoxicated T cells proper pre-ligand clustering of CD95 is compromised [McCabe, Jr., Whitekus, Hyun, Eckles, McCollum, and Rosenspire, 2003;Ziemba, McCabe, and Rosenspire, 2005].
Pre-ligand clustering of CD95, which in most cases is a prerequisite for CD95 signaling, has been shown to be dependent upon lipid rafts [Muppidi et al., 2004;Siegel et al., 2004], and lipid rafts have been directly shown to modulate CD95-mediated apoptosis [Legembre et al., 2006]. Of interest here is the fact it is now also quite clear that n-3 PUFAs profoundly influence the structure and function of lipid rafts [Shaikh et al., 2009;Chapkin et al., 2008;Kim et al., 2010]. We suggest that on the plasma membranes of Hg intoxicated lymphocytes, n-3 PUFA augments pre-ligand clustering of CD95 in lipid rafts, effectively countering the effect of Hg to disrupt pre-ligand clustered CD95. However at this point it can’t be ruled out that either a resolvin or protectin, (two classes of molecules recently identified as anti-inflammatory metabolites of n-3 PUFAs [Kohli et al., 2009]), may be the active agent interacting with lipid rafts.
In summary, we have now shown that diets enriched in n-3 PUFAs will in a mercury independent manner enhance the ability of CD95 to trigger caspase 8 activation. As CD95-mediated caspase 8 activation precedes activation-induced cell death, these results are consistent with previous findings where it was shown that n-3 PUFAs promote activation-induced cell death in murine T cells, and thus limit detrimental inflammatory immune responses by selective deletion of activated Th1-like cells [Switzer et al., 2003]. We suggest that n-3 PUFAs promote AICD by augmenting CD95 pre-ligand clustering, independent of whether or not the cells have been exposed to mercury.
Supplementary Material
HIGHLIGHTS.
Dietary n-3 PUFAs counter Hg2+ immunotoxicity
Hg2+ interference with SEB-mediated signal transduction is ameliorated by n-3 PUFA rich diets
Dietary n-3 PUFAs augment SEB-mediated activation of caspase 8 in vivo
Acknowledgments
This work was supported by the National Institute of Environmental Health Sciences grants ES021285 and ES020957.
ABBREVIATIONS
- n-PUFA
n-polyunsaturated fatty acid, Autoimmunity
- CD95
Caspase 8
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest
References
- 1.Algeciras-Schimnich A, Shen L, Barnhart BC, Murmann AE, Burkhardt JK, Peter ME. Molecular ordering of the initial signaling events of CD95. Mol. Cell Biol. 2002;22:207–220. doi: 10.1128/MCB.22.1.207-220.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagenstose LM, Salgame P, Monestier M. Murine mercury-induced autoimmunity: a model of chemically related autoimmunity in humans. Immunol Res. 1999;20:67–78. doi: 10.1007/BF02786508. [DOI] [PubMed] [Google Scholar]
- 3.Barreiro R, Luker G, Herndon J, Ferguson TA. Termination of antigen-specific immunity by CD95 ligand (Fas ligand) and IL-10. J. Immunol. 2004;173:1519–1525. doi: 10.4049/jimmunol.173.3.1519. [DOI] [PubMed] [Google Scholar]
- 4.Baumann S, Krueger A, Kirchhoff S, Krammer PH. Regulation of T cell apoptosis during the immune response. Curr. Mol. Med. 2002;2:257–272. doi: 10.2174/1566524024605671. [DOI] [PubMed] [Google Scholar]
- 5.Chapkin RS, Wang N, Fan YY, Lupton JR, Prior IA. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochim. Biophys. Acta. 2008;1778:466–471. doi: 10.1016/j.bbamem.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev.Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 7.Defrance T, Casamayor-Palleja M, Krammer PH. The life and death of a B cell. Adv. Cancer Res. 2002;86:195–225. doi: 10.1016/s0065-230x(02)86006-7. [DOI] [PubMed] [Google Scholar]
- 8.Druet P. Metal-induced autoimmunity. Hum. Exp. Toxicol. 1995;14:120–121. doi: 10.1177/096032719501400129. [DOI] [PubMed] [Google Scholar]
- 9.Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, Strober W, Lenardo MJ, Puck JM. Dominant interfering Fas gene mutations impair apoptosis in a human autoimune lymphoproliferative syndrome. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 10.Gardner RM, Nyland JF, Silva IA, Ventura AM, de Souza JM, Silbergeld EK. Mercury exposure, serum antinuclear/antinucleolar antibodies, and serum cytokine levels in mining populations in Amazonian Brazil: a cross-sectional study. Environ. Res. 2010;110:345–354. doi: 10.1016/j.envres.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebauer SK, Psota TL, Harris WS, Kris-Etherton PM. n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am. J. Clin. Nutr. 2006;83:1526S–1535S. doi: 10.1093/ajcn/83.6.1526S. [DOI] [PubMed] [Google Scholar]
- 12.Germolec D, Kono DH, Pfau JC, Pollard KM. Animal models used to examine the role of the environment in the development of autoimmune disease: findings from an NIEHS Expert Panel Workshop. J. Autoimmun. 2012;39:285–293. doi: 10.1016/j.jaut.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill R, Lanni L, Jen KL, McCabe MJ, Jr, Rosenspire A. Docosahexaenoic acid counteracts attenuation of CD95-induced cell death by inorganic mercury. Toxicol. Appl. Pharmacol. 2015;282:61–67. doi: 10.1016/j.taap.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Havarinasab S, Haggqvist B, Bjorn E, Pollard KM, Hultman P. Immunosuppressive and autoimmune effects of thimerosal in mice. Toxicol. Appl. Pharmacol. 2005a;204:109–121. doi: 10.1016/j.taap.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Havarinasab S, Hultman P. Organic mercury compounds and autoimmunity. Autoimmun. Rev. 2005b;4:270–275. doi: 10.1016/j.autrev.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson JL, Muckle G, Ayotte P, Dewailly E, Jacobson SW. Relation of Prenatal Methylmercury Exposure from Environmental Sources to Childhood IQ. Environ. Health Perspect. 2015;123:827–833. doi: 10.1289/ehp.1408554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssen O, Sanzenbacher R, Kabelitz D. Regulation of activation-induced cell death of mature T-lymphocyte populations. Cell Tissue Res. 2000;301:85–99. doi: 10.1007/s004419900155. [DOI] [PubMed] [Google Scholar]
- 18.Kawabe Y, Ochi A. Selective anergy of V beta 8+,CD4+ T cells in Staphylococcus enterotoxin B-primed mice. J. Exp. Med. 1990;172:1065–1070. doi: 10.1084/jem.172.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawabe Y, Ochi A. Programmed cell death and extrathymic reduction of Vbeta8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature. 1991;349:245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- 20.Kim W, Khan NA, McMurray DN, Prior IA, Wang N, Chapkin RS. Regulatory activity of polyunsaturated fatty acids in T-cell signaling. Prog. Lipid Res. 2010;49:250–261. doi: 10.1016/j.plipres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komoriya A, Packard BZ, Brown MJ, Wu ML, Henkart PA. Assessment of caspase activities in intact apoptotic thymocytes using cell-permeable fluorogenic caspase substrates. J. Exp. Med. 2000;191:1819–1828. doi: 10.1084/jem.191.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krueger A, Fas SC, Baumann S, Krammer PH. The role of CD95 in the regulation of peripheral T-cell apoptosis. Immunol. Rev. 2003;193:58–69. doi: 10.1034/j.1600-065x.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 23.Laiosa MD, Eckles KG, Langdon M, Rosenspire AJ, McCabe MJ., Jr Exposure to inorganic mercury in vivo attenuates extrinsic apoptotic signaling in Staphylococcal aureus enterotoxin B stimulated T-cells. Toxicol. Appl. Pharmacol. 2007 doi: 10.1016/j.taap.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Deist F, Emile J-F, Rieux-Laucat F, Benkerrou M, Roberts I, Brousse N, Fischer A. Clinical, immunological, and pathological consequences of Fas-deficient conditions. Lancet. 1996;348:719–723. doi: 10.1016/S0140-6736(96)02293-3. [DOI] [PubMed] [Google Scholar]
- 25.Legembre P, Daburon S, Moreau P, Moreau JF, Taupin JL. Modulation of Fas-mediated apoptosis by lipid rafts in T lymphocytes. J. Immunol. 2006;176:716–720. doi: 10.4049/jimmunol.176.2.716. [DOI] [PubMed] [Google Scholar]
- 26.Marrack P, Blackman M, Kushnir E, Kappler J. The toxicity of staphylococcal enterotoxin B in mice is mediated by T cells. J. Exp. Med. 1990;171:455–464. doi: 10.1084/jem.171.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCabe MJ, Jr, Eckles KG, Langdon M, Clarkson TW, Whitekus MJ, Rosenspire AJ. Attenuation of CD95-induced apoptosis by inorganic mercury: caspase-3 is not a direct target of low levels of Hg2+ Toxicol. Lett. 2005;155:161–170. doi: 10.1016/j.toxlet.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 28.McCabe MJ, Jr, Whitekus MJ, Hyun J, Eckles KG, McCollum G, Rosenspire AJ. Inorganic mercury attenuates CD95-mediated apoptosis by interfering with formation of the death inducing signaling complex. Toxicol. Appl. Pharmacol. 2003;190:146–156. doi: 10.1016/s0041-008x(03)00159-5. [DOI] [PubMed] [Google Scholar]
- 29.Muppidi JR, Siegel RM. Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death. Nat. Immunol. 2004;5:182–189. doi: 10.1038/ni1024. [DOI] [PubMed] [Google Scholar]
- 30.Nyland JF, Fillion M, Barbosa F, Jr, Shirley DL, Chine C, Lemire M, Mergler D, Silbergeld EK. Biomarkers of methylmercury exposure immunotoxicity among fish consumers in Amazonian Brazil. Environ. Health Perspect. 2011a;119:1733–1738. doi: 10.1289/ehp.1103741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyland JF, Wang SB, Shirley DL, Santos EO, Ventura AM, de Souza JM, Silbergeld EK. Fetal and maternal immune responses to methylmercury exposure: a cross-sectional study. Environ. Res. 2011b;111:584–589. doi: 10.1016/j.envres.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Overton WR. Modified histogram subtraction technique for analysis of flow cytometry data. Cytometry. 1988;9:619–626. doi: 10.1002/cyto.990090617. [DOI] [PubMed] [Google Scholar]
- 33.Pollard KM, Hultman P. Effects of mercury on the immune system. Met. Ions. Biol. Syst. 1997;34:421–440. [PubMed] [Google Scholar]
- 34.Puck JM, Sneller MC. ALPS: an autoimmune human lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Sem. Immunol. 1997;9:77–84. doi: 10.1006/smim.1996.0056. [DOI] [PubMed] [Google Scholar]
- 35.Rieux-Laucat F, Le Deist F, Fischer A. Autoimmune lymphoproliferative syndromes: genetic defects of apoptosis pathways. Cell Death. Differ. 2003;10:124–133. doi: 10.1038/sj.cdd.4401190. [DOI] [PubMed] [Google Scholar]
- 36.Rowley B, Monestier M. Mechanisms of heavy metal-induced autoimmunity. Mol. Immunol. 2005;42:833–838. doi: 10.1016/j.molimm.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 37.Scaffidi C, Kirchhoff S, Krammer PH, Peter ME. Apoptosis signaling in lymphocytes. Curr Opin Immunol. 1999;11:277–285. doi: 10.1016/s0952-7915(99)80045-4. [DOI] [PubMed] [Google Scholar]
- 38.Shaikh SR, Rockett BD, Salameh M, Carraway K. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. J. Nutr. 2009;139:1632–1639. doi: 10.3945/jn.109.108720. [DOI] [PubMed] [Google Scholar]
- 39.Siegel RM, Muppidi JR, Sarker M, Lobito A, Jen M, Martin D, Straus SE, Lenardo MJ. SPOTS: signaling protein oligomeric transduction structures are early mediators of death receptor-induced apoptosis at the plasma membrane. J. Cell Biol. 2004;167:735–744. doi: 10.1083/jcb.200406101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Somers EC, Ganser MA, Warren JS, Basu N, Wang L, Zick SM, Park SK. Mercury Exposure and Antinuclear Antibodies among Females of Reproductive Age in the United States: NHANES. Environ. Health Perspect. 2015;123:792–798. doi: 10.1289/ehp.1408751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strain JJ, Davidson PW, Bonham MP, Duffy EM, Stokes-Riner A, Thurston SW, Wallace JM, Robson PJ, Shamlaye CF, Georger LA, Sloane-Reeves J, Cernichiari E, Canfield RL, Cox C, Huang LS, Janciuras J, Myers GJ, Clarkson TW. Associations of maternal long-chain polyunsaturated fatty acids, methyl mercury, and infant development in the Seychelles Child Development Nutrition Study. Neurotoxicology. 2008;29:776–782. doi: 10.1016/j.neuro.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strasser A, Pellegrini M. T-lymphocyte death during shutdown of an immune response. Trends Immunol. 2004;25:610–615. doi: 10.1016/j.it.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Switzer KC, McMurray DN, Morris JS, Chapkin RS. (n-3) Polyunsaturated fatty acids promote activation-induced cell death in murine T lymphocytes. J. Nutr. 2003;133:496–503. doi: 10.1093/jn/133.2.496. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 45.Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 46.Tolan A, Elton GA. Mercury in food. Biochem. J. 1972;130:69P–70P. doi: 10.1042/bj1300069p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Gent CM, Luten JB, Bronsgeest-Schoute HC, Ruiter A. Effect, on serum lipid levels of omega-3 fatty acids, of ingesting fish-oil concentrate. Lancet. 1979;2:1249–1250. doi: 10.1016/s0140-6736(79)92374-2. [DOI] [PubMed] [Google Scholar]
- 48.Vasseur F, Le CA, Pavlovitch JH, Penit C. Distribution of cycling T lymphocytes in blood and lymphoid organs during immune responses. J. Immunol. 1999;162:5164–5172. [PubMed] [Google Scholar]
- 49.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferative disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1997;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 50.Whitekus MJ, Santini RP, Rosenspire AJ, McCabe MJJ. Protection against CD95-mediated apoptosis by inorganic mercury in jurkat T cells. J Immunol. 1999;162:7162–7170. [PubMed] [Google Scholar]
- 51.Yang Y, Kim D, Fathman CG. Regulation of programmed cell death following T cell activation in vivo. Int. Immunol. 1998;10:175–183. doi: 10.1093/intimm/10.2.175. [DOI] [PubMed] [Google Scholar]
- 52.Zhivotovsky B, Burgess DH, Schlegel J, Porn MI, Vanags D, Orrenius S. Proteases in Fas-mediated apoptosis. J. Cell Biochem. 1997;64:43–49. [PubMed] [Google Scholar]
- 53.Ziemba SE, McCabe MJ, Jr, Rosenspire AJ. Inorganic mercury dissociated preassembled Fas/CD95 receptor oligomers in T lymphocytes. Toxicol Appl Pharmacol. 2005;206:333–342. doi: 10.1016/j.taap.2004.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.