Abstract
Research is a systematic process, which uses scientific methods to generate new knowledge that can be used to solve a query or improve on the existing system. Any research on human subjects is associated with varying degree of risk to the participating individual and it is important to safeguard the welfare and rights of the participants. This review focuses on various steps involved in methodology (in continuation with the previous section) before the data are submitted for publication.
Key words: Data collection, informed consent, reliability, research design, validity
INTRODUCTION
Research uses a systematic approach to generate new knowledge to answer questions based on needs of patient health and practice. The investigator identifies research question, examines the ethical implications, describes the research design and collects appropriate data[1,2,3] which is evaluated by statistical tests before it can be published.[4] Before putting this to use in clinical practice, the relevant data are critically appraised for validity and reliability.[1] This review covers these aspects of the research methodology, in continuation with the first part by Garg et al. published in this issue of Indian Journal of Anaesthesia (IJA).[5]
REGULATORY AND ETHICAL CONSIDERATIONS
The Indian Council of Medical Research (ICMR) is the apex body in India responsible for the formulation, coordination and promotion of biomedical research. The International Committee of Medical Journal Editors (ICMJE) makes it mandatory for clinical trials to be included in a clinical trials registry for acceptance for publication. Clinical Trials.gov, run by the United States National Library of Medicine, was the first online registry established in 2005 and is widely used today. All trials to be conducted in India should have mandatory prospective registration with the Clinical Trial Registry of India (CTRI- www.ctri.in). Good clinical practice (GCP) guidelines is a set of guidelines for biomedical studies which encompasses the design, conduct, termination, audit, analysis, reporting and documentation of the studies involving human subjects. It protects rights of human subjects and the authenticity of biomedical data. (www.cdsco.nic.in/html/GCP1.html). Table 1 lists the type of the research involved and their regulatory bodies.[6]
Table 1.
Research involved and their regulatory bodies
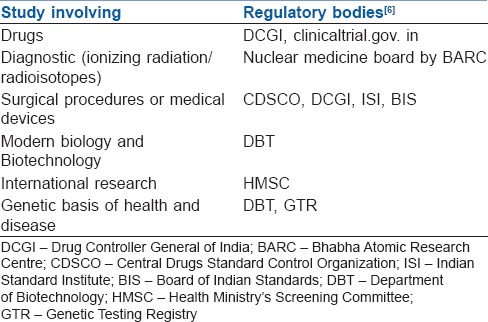
The International Standard Randomised Controlled Trial Number (ISRCTN) registry is a primary clinical trial registry recognised by the World Health Organization. The ICMJE provides content validation of all submitted studies (proposed, ongoing or completed). The study is assigned a unique identification number, and records of the study in the database can be easily accessed (www.isrctn.com).
To conduct a clinical trial in India, Institution Ethics Committee (IEC) approval is mandatory, and it must be registered with CTRI- www.ctri.nic.in.[2,6] When ‘off-label’ use of a drug (drug being used for a new indication/new dose/formulation/route) is tested for purely academic purposes and not for commercial use, currently there is no requirement of regulator approval.[2,6] However, the IEC has to consider the risks-benefits and ethical basis for approval of the research.
Drugs Controller General of India (DCGI) in India insists on registration and approval of clinical trials through CTRI and ensures scientific and safe conduct of the study. Most of the academic medical centres have Institutional Review Board (IRB) or IEC. They (‘internal’ Ethics Committees) can assess research proposals first and approve before submitting to national bodies. The approval may also go in parallel with DCGI approval. It is responsible for the supervision and protection of rights, safety and welfare of human subjects. During the progress of the trial, the IEC reviews safety reports, any significant violation/deviations in the protocol and for any amendments in the study protocol or informed consent.[2,7]
If IEC is not available in the institution, proposals can be sent independent ethics committee outside the institution (‘external’ Ethics Committees).[2] The ICMR suggests the establishment of registered Independent Ethics Committees (Ind EC) without institutional affiliation, functioning as per national guidelines. Proposals can also be sent to another institution, following established protocol, including providing a ‘no objection certificate’ and allow the external IEC necessary access.[2] When there is a large load of research, multiple ECs can function in the same institution as also subcommittees (e.g., subcommittees on adverse event, data safety monitoring, expedited review, etc.,).
The IRB consists of 7–15 members and at least five members are required to form the quorum to make a decision on the research [Table 2].[2]
Table 2.
Composition of Institution Ethics Committee

CONSENT
All the research involving human participants should follow four basic ethical principles;[2] (a) Respect for persons autonomy, (b) beneficence (balance the risks against benefits bearing in mind the welfare of the research participant[s]), (c) nonmaleficence (no harm or reduce exposure to greater harm) and (d) Justice (distribution of research subjects equitably in all groups, for example, social, economic demographic, etc).
Informed consent is a process by which a subject confirms his/her willingness to participate in a clinical study.[4] It protects the individual's freedom of choice and respect for individual's autonomy. It ensures proper regulations in clinical trials and assures patient safety by dealing with both legal and ethical basis.[7] The process of informed consent consists of providing relevant information, its comprehension and voluntariness.[2] The details of the clinical study are explained to the subject in a simple and easily understandable language. The ‘subject/participant information sheet’ should include research aspect of the study, sponsor of the study, purpose and procedure, side effects, risks and discomforts, benefits, compensation for any study-related injury, alternatives to participation, right to withdraw, confidentiality of records and contact information of the investigators and IRB.[2,6] The informed and written consent form is duly signed by the subject in a document called ‘informed consent form’.[1,2,3] The documents consisting of patient/participant information sheet and informed consent form should be reviewed and approved by the IEC before enrolment of the participants.
A legal authorised representative (LAR) should be involved in the decision-making of vulnerable subjects who lack the ability to consent. The consent is taken from parent/LAR (in kids <7 years) and consent of parent/LAR along with assent form (oral/written) in children aged 7–18 years.[2] Audio/audio-visual recording of the informed consent process may be required in case of certain regulatory, clinical trials.[2] After the completion/termination of the study, all records within the IEC must be archived for at least 3 years; those related to regulatory, clinical trials must be archived for 5 years as per CDSCO regulation. Longer preservation may be needed as required by the sponsors/regulatory bodies.
Many finer aspects of the legal and ethical issues in research are discussed by Yip et al in this issue of IJA.[8]
The ethical duty of confidentiality refers to the obligation of an individual or organisation to safeguard entrusted information of the research data. It is essential for the integrity of the research project and protects information from unauthorised access, use, disclosure, modification, loss or theft.[6,7]
Data related to any of the studies of individual participant can be disclosed only under the following circumstances:
(a) Threat to a person's life, (b) Communication with drug registration authority in cases of severe adverse reaction, (c) Communication to health authority whenever there is risk to public health, (d) In a court of law under the orders of the presiding judge and (e) As a requirement for government agencies or regulatory authorities.[2]
DATA COLLECTION
‘Data’ includes the information that is systematically collected by the investigator during the study. The primary data are those which are originally done for the first time. The secondary data are a compilation of information done by someone else and have already been passed through the statistical process. A Data Monitoring Committee or Data and Safety Monitoring Board may be appointed, independent of IEC for interim analysis; their report forms the basis for early termination of planned study when there is compelling evidence of beneficial effectiveness or harmful side effects or for major flaws in the study.
The two main types of data are qualitative and quantitative, and most studies will have a combination of both. While quantitative data are easy to analyse and fairly reliable, qualitative data provide more depth in the description of the sample.[9]
Data collection methods [Figure 1]:
Figure 1.
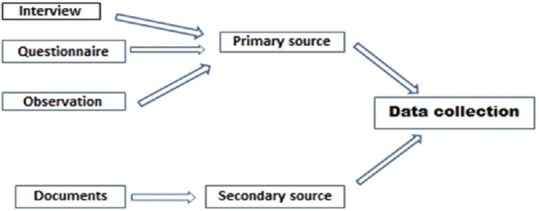
Methods of data collection
Interview: This method allows face to face contact with respondents, exploring the topic in depth. It allows the interviewer to explain or help to clarify questions increasing the usefulness of a response. It can be of different types-structured, unstructured (informal, conversational approach), semi-structured, focused and standardised.[9,10,11] There can be disadvantages-interviewer clarifications can lead to inconsistencies and influence the responses; the subject may distort information through recall error, selective perceptions and in the desire to please the interviewer.[10] Sometimes, the data may be too voluminous to record or reduce it
Observation: This method provides direct information about the behaviour of individuals and groups. It allows the investigator to understand the situation and context. It could be ‘Participant’ observation: The observer takes part in the situation he or she observes or ‘Nonparticipant’ observation: The observer watches the situation, openly or concealed, but does not participate[9,10,11]
Questionnaire: It is a simple and inexpensive method not even requiring any research assistants. More honest responses may be available when anonymity is provided. Written questions are presented that are to be answered by the respondents. A written questionnaire can be administered in different ways, such as by sending questionnaires by mail with clear instructions on how to answer the questions and asking for mailed responses; gathering all or part of the respondents in one place at one time, giving oral or written instructions, and letting the respondents fill out the questionnaires; or hand-delivering questionnaires to respondents and collecting them later.[10,11] The disadvantage of this method are observer bias and breach in confidentiality; also, this cannot be used on illiterate subjects. As with other types of outcome measurements, questionnaires and interviews are to be assessed for validity (accuracy) and for reproducibility (precision)-using ‘face validity, content validity and construct validity’
Documents: It is an inexpensive and unobtrusive method of data collection from locally available records or documents (existing research, hospital records, databases, videotapes, etc.).[9,10,11] There is disadvantage of accuracy, authenticity and availability (missing data/omission of needed data). Anaesthesia information management systems used in modern practice have the ability to collect data automatically, in large volumes, which can be converted for specific, focused outcome assessments for research purposes.
Compilation of data includes systematic arrangement of data to facilitate the presentation and analysis.[12] The data collected are entered in a database where the information about subjects and variables are stored. Simple study database can be maintained in a spreadsheet (MS Excel©) or statistical software (e.g., Statistical Analysis System (SAS®) (NC, USA), IBM SPSS (Statistical Package for the Social Sciences) Statistics® (IBM Inc., NY, USA). More complex database require integrated database management software (e.g., Access© (Windows) and Filemaker© Pro (Apple Inc.,).[13] Database ‘queries’ sort and filter the data as well as calculate values based on the raw data fields.[12,13] Queries are used to monitor data entry, report on study progress and format the results for analysis. Data must be stored in ‘secure servers’ so that confidentiality is maintained.[13] Backup files and off-site storage may be necessary to prevent any data loss. Common methods of summarising and presenting data are tables, pie charts, bar charts, histograms, frequency and cumulative frequency curves, dot plots and x-y scatterplots.[13,14,15]
RESEARCH TOOLS: DEVELOPMENT AND VALIDATION
‘Research tool’ is the means of collecting information for the purpose of a study. Observation forms, interview schedules, questionnaires are all classified as research tools. The first practical step in doing a research process is to construct a research tool. Four stage process is involved in developing a research tool.[9,10,11,12]
Concept development: The researcher should understand the basic knowledge pertaining to the study
Specification of concept dimensions: The researcher should be able to build in a dimension based on the concept of the study
Selection of indicators: Once the concept and its dimensions are developed, each concept element is measured by indicators (respondent's knowledge, opinion, expectation, etc., are measured with scales, devices). More than one indicator increases the score and validity of the study
Formation of index: Dimension of a concept or different measurements of a dimension are then put into an overall index.
The error may occur at any stage of research, i.e., from selection to interpretation of data to conclusion. Two types of error can occur – random and systematic error. The random error must be reduced as far as possible, and the systemic error should be eliminated. Errors can occur from three sources:[16,17,18,19]
Investigator: Due to ignorance, incompetence and bias
Instrument: Due to variability, calibration, problems and malfunctioning
Subject: Due to bias, noncompliance and biological variation in response.
Any research can be affected by factors that can invalidate the findings. A good research tool should meet the tests of validity, reliability and practicality.
Validity refers to the extent to which a test measures what we actually wish to measure. Reliability refers to accuracy and precision of a measurement procedure.
The practicality characteristic of a measuring instrument can be judged in terms of economy, convenience and interpretability.
Determining validity can be viewed as constructing an evidence-based argument regarding how well a tool measures what it is supposed to do.
USES OF VALIDITY IN SCIENTIFIC METHODS
External validity refers to generalising the study results to other population groups with similar risk factors, settings, measurement and treatment variables.
Internal validity implies that the differences observed between the treatment groups, apart from random error, are only due to the treatments under investigation.[9]
Validity assessment can be performed in three ways:
Content validity is the extent to which a measuring tool provides adequate coverage of all the aspects of the topic under study. (e.g., quality of pain relief to include measurement of analgesia, haemodynamics, sedation, etc.). ‘Face validity’ assesses whether the measurements appear reasonable; a measure of how representative a research project is ‘at face value’, and whether it appears to be a good project
Construct validity refers to the degree to which a measurement conforms to theoretical constructs. Convergent validity tests whether and how well those ‘constructs’ that are expected to be related are, in fact, related. Discriminant validity or divergent validity tests those ‘constructs’ that should have no relationship do, in fact, not have any relationship
Criterion validity assesses the degree to which a new measurement correlates with well-accepted existing measures. Predictive validity is a strong variety of criterion validity, representing the ability of the measurement to predict an outcome.
Other Types: Concurrent validity refers to the degree of correlation of two measures of the same concept administered at the same time. Consensual validity is a process by which a panel of experts judge the validity.[1,16,17,18,19]
RELIABILTY
A measuring instrument is reliable if it provides consistent results.[1,11]
The stability aspect refers to securing consistent results with repeated measurements of the same person and with the same instrument. Determination of the degree of stability by comparing the results of repeated measurements.
The equivalence aspect considers how much error may get introduced by different investigators or different samples of the items being studied.
PRACTICALITY
Measuring instrument practicality is tested in terms of economy, convenience and interpretability.
Economy consideration suggests that some trade-off is needed between the ideal research project and that which the budget can afford.
Convenience test suggests that the measuring instrument should be easy to administer. Interpretability consideration is especially important when persons other than the designers of the test are to interpret the results.
ANALYSIS PLAN: QUALITY AND APPROPRIATENESS OF ANALYSIS
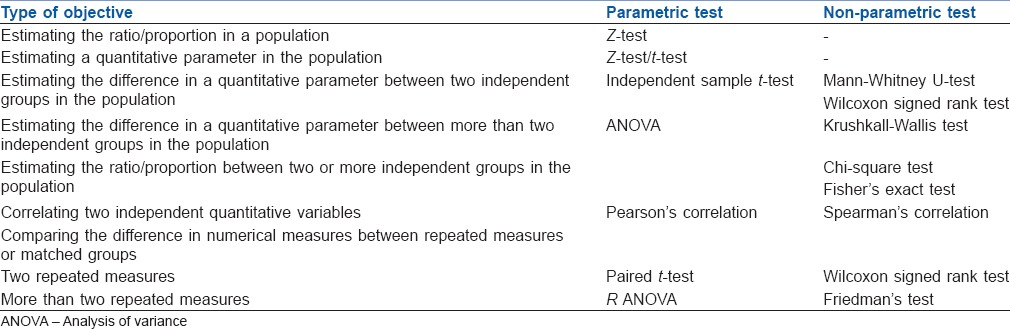
The statistics in research functions as a tool in designing research, analysing its data and drawing conclusions from it.[20,21] Descriptive statistics are the development of certain indices from the raw data, summarised in tables, charts or numerical forms. The inferential analysis is undertaken to apply various tests of significance to test hypotheses of a research question so as to validate conclusions. An essential part of presenting any type of inferential data is by probability (P value) which reassures the reader that the outcome was secondary to the effect of the studied variable and has not occurred purely by chance.[22] P < 5% is considered statistically significant. Statistical tests are used for testing the significance. Various parametric tests (variable normally distributed) and nonparametric tests (variables are not normally distributed) are used to meet the objective of the study [Table 3].[19,20] ‘Basic Statistical Tools in Research and Data analysis’ in this issue of IJA by Zulfiqar Ali describe these tests in detail.[23]
Table 3.
Tests of significance
SUMMARY
The ‘methodology’ in a research strategy outlines the steps involved in research process. The research problem is identified, aims and objectives are formulated, sample size is calculated; Ethics Committee approval and informed consent from the subject are taken; data collected are summarised. The research design is planned, and the collected data are then analysed using appropriate statistical tests. The derived evidence is put into clinical practice once the reader is convinced that the clinical study is valid and reliable.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Omi S. Health Research Methodology: A Guide for Training in Research Methods. 2nd ed. Manila: World Health Organization; 2001. [Google Scholar]
- 2.Draft “National Ethical Guidelines for Biomedical and Health Research involving Human Participants” 2016. [Last accessed on 2016 Aug 06]. Available from: http://www.icmr.nic.in/icmrnews/draft_ethical/draft_guidelines .
- 3.Kumar R. Research Methodology: A Step by Step Guide for Beginners. 3rd ed. London: Sage Publications; 2011. [Google Scholar]
- 4.Field A, editor. Discovering Statistics Using SPSS. 3rd ed. London: Sage Publications Ltd; 2009. Why is my evil teacher forcing me to learn statistics; pp. 1–30. [Google Scholar]
- 5.Garg R. Methodology for research I. Indian J Anaesth. 2016;60:640–5. doi: 10.4103/0019-5049.190619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ethical Guidelines for Biomedical Research on Human Participants – Indian Council of Medical Research. New Delhi: 2006. [Google Scholar]
- 7.Bernard LO. Addressing ethical issues. In: Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TW, editors. Designing Clinical Research. 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 2007. pp. 225–39. [Google Scholar]
- 8.Yip C, Reena Han NL, Sng LB. Legal and ethical issues in research. Indian J Anaesth. 2016;60:684–8. doi: 10.4103/0019-5049.190627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow SC, Liu JP, editors. Design and Analysis of Clinical Trials: Concepts and Methodologies. 2nd ed. New Jersey: John Wiley and Sons; 2004. Clinical data management; pp. 628–82. [Google Scholar]
- 10.Cummings SR, Hulley SB. Designing questionnaires and interviews. In: Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TW, editors. Designing Clinical Research. 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 2007. pp. 241–55. [Google Scholar]
- 11.Kothari CR. Research Methodology: Methods and Techniques. 2nd ed. New Delhi: New Age International (P) Ltd; 2004. Tests of sound measurement. [Google Scholar]
- 12.Chow SC, Liu JP, editors. Design and Analysis of Clinical Trials: Concepts and Methodologies. 2nd ed. New Jersey: John Wiley and Sons; 2004. Basic statistical concepts; pp. 43–87. [Google Scholar]
- 13.Rastogi VB, editor. Biostatistics. 3rd ed. New Delhi: Medtech Publications; 2015. Statistical data; pp. 17–31. [Google Scholar]
- 14.McCluskey A, Lalkhen AG. Statistics I: Data and correlations. Contin Educ Anaesth Crit Care Pain. 2007;7:95–9. [Google Scholar]
- 15.McCluskey A, Lalkhen AG. Statistics II: Central tendency and spread of data. Contin Educ Anaesth Crit Care Pain. 2007;7:127–30. [Google Scholar]
- 16.Bowers D. Medical Statistics from Scratch: An Introduction for Health Professionals. 2nd ed. UK: John Wiley and Sons Ltd; 2008. [Google Scholar]
- 17.Downing SM. Validity: On meaningful interpretation of assessment data. Med Educ. 2003;37:830–7. doi: 10.1046/j.1365-2923.2003.01594.x. [DOI] [PubMed] [Google Scholar]
- 18.Downing SM, Haladyna TM. Validity threats: Overcoming interference with proposed interpretations of assessment data. Med Educ. 2004;38:327–33. doi: 10.1046/j.1365-2923.2004.01777.x. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan GM. A primer on the validity of assessment instruments. J Grad Med Educ. 2011;3:119–20. doi: 10.4300/JGME-D-11-00075.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Field A, editor. Discovering Statistics Using SPSS. 3rd ed. London: Sage Publications Ltd; 2009. Everything you ever wanted to know about statistics; pp. 31–60. [Google Scholar]
- 21.Rastogi VB, editor. Biostatistics. 3rd ed. New Delhi: MedTech Publications; 2015. Inferential statistics and hypothesis testing; pp. 237–372. [Google Scholar]
- 22.McCluskey A, Lalkhen AG. Statistics III: Probability and statistical tests. Contin Educ Anaesth Crit Care Pain. 2007;7:167–70. [Google Scholar]
- 23.Ali Z, Bhaskar SB. Basic statistical tools in research and data analysis. Indian J Anaesth. 2016;60:662–9. doi: 10.4103/0019-5049.190623. [DOI] [PMC free article] [PubMed] [Google Scholar]


