Abstract
Fragile X mental retardation protein (FMRP) is an RNA binding protein with 842 target mRNAs in mammalian brain. Silencing of the fragile X mental retardation 1 (FMR1) gene leads to loss of expression of FMRP and upregulated metabotropic glutamate receptor 5 (mGluR5) signaling resulting in the multiple physical and cognitive deficits associated with fragile X syndrome (FXS). Reduced FMRP expression has been identified in subjects with autism, schizophrenia, bipolar disorder, and major depression who do not carry the mutation for FMR1. Our laboratory has recently demonstrated altered expression of four downstream targets of FMRP-mGluR5 signaling in brains of subjects with autism: homer 1, amyloid beta A4 precursor protein (APP), ras-related C3 botulinum toxin substrate 1 (RAC1), and striatal-enriched protein tyrosine phosphatase (STEP). In the current study we investigated the expression of the same four proteins in lateral cerebella of subjects with schizophrenia, bipolar disorder, and major depression and in frontal cortex of subjects with schizophrenia and bipolar disorder. In frontal cortex we observed: 1) reduced expression of 120 kDa form of APP in subjects with schizophrenia and bipolar disorder; 2) reduced expression of 61 kDa and 33 kDa forms of STEP in subjects with schizophrenia; 3) reduced expression of 88 kDa form of APP in subjects with bipolar disorder; and 3) trends for reduced expression of 88 kDa form of APP and homer 1 in subjects with schizophrenia and bipolar disorder, respectively. In lateral cerebella there was no group difference, however we observed increased expression of RAC1 in subjects with bipolar disorder, and trends for increased RAC1 in subjects with schizophrenia and major depression. Our results provide further evidence that proteins involved in the FMRP-mGluR5 signaling pathway are altered in schizophrenia and mood disorders.
Keywords: schizophrenia, bipolar disorder, RAC1, STEP, APP, homer 1
1. Introduction
Emerging evidence suggests that downregulation of fragile X mental retardation protein (FMRP) may be common to multiple psychiatric disorders including autism, schizophrenia, bipolar disorder, and major depression, rather than simply a hallmark of fragile X syndrome (FXS) (Fatemi et al., 2010a, 2011a, 2013a,b; Fatemi and Folsom, 2011, 2014; Fernandez et al., 2013; Kelemen et al., 2013; Kovács et al., 2013; Jacquemont et al., 2014). In FXS, reduced FMRP is the result of gene silencing of the Fragile X mental retardation 1 gene (FMR1). FMRP normally acts as a translational repressor and negative regulator of group I metabotropic glutamate receptors (mGluRs). In particular, the loss of FMRP regulation of metabotropic glutamate receptor 5 (mGluR5) is believed to result in enhanced glutamatergic signaling which ultimately results in the multiple physical and cognitive deficits associated with FXS (Bear et al., 2004; Dölen and Bear, 2008).
While there is a great deal of overlap between autism and FXS with regard to symptoms (Bailey et al., 1998; Irwin et al., 2001; Hatton et al., 2006; Gothelf et al., 2008; Hallahan et al., 2009; Hutsler and Zhang, 2010) and comorbidity (Bailey et al., 1998; Chudley et al., 1998; Wassink et al., 2001; Kaufman et al., 2004; Hatton et al., 2006), recent findings of reduced FMRP in brains of subjects with autism were from individuals who did not carry the mutation of FMR1 (Fatemi and Folsom, 2011; Fatemi et al., 2011a). Similarly, recent findings of reduced FMRP expression in brains and peripheral blood lymphocytes of subjects with schizophrenia were also from people who did not carry the FMR1 mutation (Fatemi et al., 2010a; Fatemi et al., 2013b; Kelemen et al., 2013; Kovács et al., 2013). Thus, a genetic mutation of FMR1 is not required to result in reduced FMRP expression.
Our laboratory has provided evidence of impairment of FMRP-mGluR5 signaling in patients with schizophrenia and mood disorders (Fatemi et al., 2010a, 2011b; Fatemi and Folsom, 2014). Western blotting studies have identified reduced expression of FMRP in lateral cerebellum from subjects with schizophrenia, bipolar disorder, and major depression (Fatemi et al., 2010a) and from superior frontal cortex [Brodmann Area 9 (BA9)] of subjects with schizophrenia and bipolar disorder (Fatemi et al., 2013b). Our results were recently verified by the finding of reduced FMRP in peripheral blood lymphocytes of people with schizophrenia (Kelemen et al., 2013; Kovács et al., 2013). Moreover, mGluR5 protein levels were significantly reduced in both brain sites in schizophrenia and bipolar disorder while mRNA levels for mGluR5 were significantly reduced in lateral cerebellum of subjects with schizophrenia and major depression and BA9 of subjects with bipolar disorder (Fatemi et al., 2013b).
These preliminary findings suggest that FMRP-mGluR5 signaling is altered in subjects with schizophrenia and mood disorders. FMRP is estimated to bind to approximately 5% of all transcripts in the mammalian brain (Darnell et al., 2001; Darnell and Klann, 2013). The next step in our investigation of the FMRP-mGluR5 signaling system is to identify changes in some of the specific downstream targets. Recently we identified changes in four such targets – homer 1, amyloid beta A4 precursor protein (APP), ras-related C3 botulinum toxin substrate 1 (RAC1), and striatal-enriched protein tyrosine phosphatase (STEP) - in cerebellar vermis and BA9 of adults and children with autism when compared to controls (Fatemi et al., 2013a). Brain volumetric studies and functional imaging studies have shown that both BA9 and the cerebellum show abnormalities in subjects with schizophrenia and mood disorders (Liotti et al., 2002; Krüger et al., 2003; Holmes et al., 2005; Crespo-Facorro et al., 2007; Baldaçara et al., 2008; Bonilha et al., 2008). The cerebellum and prefrontal cortex are connected through the cortico-pontocerebellar and cerebello-thalamocortical pathways (Schmahmann and Pandya, 1997). Disruptions of this circuitry have been hypothesized to contribute to cognitive dysfunction associated with schizophrenia (Andreasen et al., 1996). Due to the importance of these two regions in schizophrenia, we hypothesized that we would observe similar changes in expression of FMRP-mGluR5 signaling molecules in subjects with schizophrenia and mood disorders.
2. Materials and methods
2.1. Brain Procurement
The current study was approved by the Institutional Review Board of the University of Minnesota-School of Medicine. The Harvard Brain and Tissue Resource Center provided postmortem superior frontal cortex [Brodmann Area 9 (BA9)] from the McLean 74 Cohort. Postmortem lateral cerebella were provided by the Stanley Foundation Neuropathology Consortium under approved ethical guidelines. Psychiatrists established DSM-IV diagnoses of schizophrenia, bipolar disorder, major depression, or no disorder prior to death using information from family interviews and from all available medical records. The Harvard Brain and Tissue Resource Center and the Stanley Medical Research Foundation collected details regarding subject selection, demographics, diagnostic process, and tissue processing. The McLean 74 Cohort consists of 20 subjects with schizophrenia, 19 subjects with bipolar disorder, and 28 normal controls (Table 1). For the current study, the Stanley collection consisted of 9–13 subjects with schizophrenia, 7–12 subjects with bipolar disorder, 8–13 with major depression without psychotic features and 10–12 normal controls (Table 2). All groups were matched for a variety of demographic measures and analyzed statistically for the impact of all confounds on various protein values (Tables 1 and 2).
Table 1.
Demographic Information for the Three Diagnostic Groups from the McLean 74 Cohort in BA9.
| Sample Size | Bipolar
|
Control
|
Schizophrenia
|
F, t, or χ2
|
p
|
|---|---|---|---|---|---|
| N = 19 | N = 28 | N = 20 | NA | NA | |
| Age | 61.75 (19.20) | 56.62 (14.73) | 60.71 (12.07) | 0.36a | 0.70 |
| Sex | 4 M:15 F | 16 M:12 F | 14 M:6 F | 10.38b | 0.006 |
| PMI | 22.25 (5.39) | 21.63 (3.86) | 23.86 (7.20) | 0.99a | 0.38 |
| pH | 6.45 (0.76) | 6.45 (0.17) | 6.49 (0.30) | 0.07a | 0.92 |
| Side of Brain | 10 L:9R | 14 L:14R | 10 L:10R | 0.087b | 0.96 |
| Suicidal death | 4 (1 violent) | 0 | 0 | 10.96b | 0.027 |
| Drug/Alc hx | 0.42 (0.51) | 0.68 (0.67) | 0.30 (0.47) | 6.84b | 0.15 |
| Severity of Substance abuse | 0.58 (1.39) | 0.43 (0.96) | 0.70 (1.45) | 0.28b | 0.76 |
| Severity of Alcohol abuse | 0.95 (1.43) | 0.86 (1.76) | 0.60 (1.23) | 0.28b | 0.78 |
| Age of onset | 23.53 (7.67) | -- | 20.53 (3.52) | 2.11c | 0.16 |
| Duration of Illness | 39.69 (18.27) | -- | 39.94 (15.15) | 0.008c | 0.93 |
| Barbituate Use | -- | -- | 1 | 1.61b | 0.45 |
| Benzodiazepine Use | 1 | 1 | 1 | 7.04b | 0.03 |
| Opiate Use | -- | 4 | 3 | 0.37b | 0.83 |
| Amphetamine Use | -- | 4 | -- | 3.26b | 0.20 |
| Cocaine Use | -- | 1 | -- | 0.77b | 0.68 |
| Propoxyphene Use | -- | 2 | 1 | 0.21b | 0.90 |
| APD Use | 14 | -- | 17 | 39.10b | 0.0001 |
| CPZ Equivalent | 522 (287) | -- | 782 (546) | 1.42a | 0.25 |
| AD Use | 2 | -- | 1 | 2.95b | 0.23 |
| AC Use | 9 | -- | 6 | 15.57b | 0.0001 |
| MS Use | 9 | -- | 1 | 22.21b | 0.0001 |
ANOVA;
Chi Square Test;
t-test for bipolar vs. schizophrenia;
AC, anticonvulsant; AD, Antidepressant; APD, antipsychotic; CPZ, chlorpromazine; MS, mood stabilizer; NA, not applicable.
Table 2.
Demographic Information for the Four Diagnostic Groups from Stanley Medical Research Institute in Lateral Cerebellum.
| Sample Size | Bipolar
|
Control
|
Schizophrenia
|
Depression
|
F or χ2
|
p
|
|---|---|---|---|---|---|---|
| N = 12 | N = 12 | N = 13 | N = 13 | NA | NA | |
| Age | 43.58 (12.25) | 45.33 (9.58) | 45.31 (13.44) | 46.08 (9.87) | 0.11a | 0.96 |
| Sex | 6 F, 6 M | 4 F, 8 M | 5 F, 8 M | 5 F, 8 M | 0.75b | 0.86 |
| Race | 12 W, 1B | 11 W, 1B | 10 W, 3A | 13 W | 14.32b | 0.11 |
| PMI | 33.92 (16.48) | 24.58 (10.67) | 33.46 (15.73) | 26.62 (10.97) | 1.48a | 0.23 |
| pH | 6.17 (0.26) | 6.29 (0.26) | 6.18 (0.25) | 6.18 (0.23) | 0.66a | 0.58 |
| Side of Brain | 6 L, 6R | 7 L, 5R | 7 L, 6R | 9 L, 4R | 1.08b | 0.78 |
| Brain Wt | 1413.8 (182) | 1535.4 (166.1) | 1462.7 (108) | 1453.08 (127.5) | 1.42a | 0.25 |
| Family hx | 0.83 (0.83) | 0.17 (0.58) | 1.08 (0.86) | 0.69 (0.48) | 21.65b | 0.001 |
| Suicidal death | 7 (3 violent) | 0 | 6 (4 violent) | 8 (4 violent) | 13.05b | 0.042 |
| Drug/Alc hx | 0.67 (0.78) | 0.33 (0.78) | 0.62 (0.77) | 0.38 (0.65) | 5.64b | 0.46 |
| Age of onset | 21.83 (8.95) | -- | 23.85 (8.03) | 36.15 (12.4) | 7.66a | 0.001 |
| Duration of illness | 20.83 (10.69) | -- | 21.85 (12.05) | 10 (7.8) | 5.21a | 0.010 |
| Severity of Substance abuse | 1.67 (2.15) | 0.17 (0.58) | 1.38 (1.94) | 1.15 (2.03) | 13.89b | 0.53 |
| Drug Dependence | 1 | -- | 1 | -- | 2.09b | 0.55 |
| Drug Abuse | 3 | -- | 1 | 1 | 4.49b | 0.21 |
| Severity of Alcohol abuse | 2.25 (1.96) | 1.08 (0.29) | 1.62 (1.66) | 1.69 (1.89) | 10.11b | 0.81 |
| Alcohol Dependence | 1 | -- | -- | 2 | 3.74b | 0.29 |
| Alcohol Abuse | 1 | -- | 1 | -- | 2.09b | 0.55 |
| Fluphenazine (lifetime) | 25,433.33 (24,807.78) | -- | 41,076.92 (50,385.95) | -- | 6.21a | 0.019 |
| Antidepressant Use | 6 | -- | 5 | 8 | 11.14b | 0.011 |
ANOVA;
Chi Square Test;
NA, not applicable.
2.2. SDS-PAGE and Western Blotting
Brain tissue was prepared for SDS-PAGE and western blotting using previously established protocols (Fatemi et al., 2008, 2009a,b, 2010a,b, 2011a; Fatemi and Folsom, 2011). For both BA9 and lateral cerebellum, 30 μg of tissue was used as this amount of protein provided values within the linear range of optical density measurements. For APP 6% resolving gels were used; for homer 1, neuronal specific enolase (NSE), and beta-actin 10% resolving gels were used; and for STEP and RAC1 12% resolving gels were employed. For all experiments 5% stacking gels were used. Interblot variability was minimized by including samples from subjects of each group (control, schizophrenia, bipolar disorder, major depression) on each gel. Moreover, interblot variability was further minimized by expressing proteins of interest as ratios to β-actin and NSE, both of which varied minimally and nonsignificantly across gels (Tables 3 and 4). All samples were run in duplicate. Samples were electrophoresed for 15 minutes at 75 V followed by 60 minutes at 150 V. Following electrophoresis, samples were electroblotted onto nitrocellulose membranes for 2 h at 300 mAmp at 4 °C. Blots were then blocked with 0.2% I-Block (Tropix, Bedford, MA, USA) in PBS with 0.3% Tween 20 for one hour at room temperature (RT). Blots were incubated in the appropriate primary antibodies overnight at 4 ° C. The primary antibodies used were anti-RAC1 (1:500; BD Transduction, San Jose, CA, USA), anti-APP (1:500; Abcam Inc., Cambridge, MA, USA), anti-homer 1 (1:750; Abnova, Taipei, Taiwan), anti-STEP (1:400; Abgent, San Diego, CA, USA; this antibody has previously been used to determine levels of STEP by Carty et al., 2012), anti-NSE (1:2,000; Abcam Inc., Cambridge, MA, USA), and anti-β actin (A5441, Sigma Aldrich (St. Louis, MO, USA), 1:5 000). Blots were then washed for 30 minutes at RT in PBS supplemented with 0.3% Tween 20 (PBST). Following the wash step, blots were incubated in the proper secondary antibodies: either goat anti-mouse IgG (A9044, Sigma Aldrich, 1:80 000) or goat anti-rabbit IgG (A9169, Sigma Aldrich, 1:80 000) for one hour at RT. Blots were washed twice in PBST for 15 min., each. After the second wash, bands were visualized using the ECL-plus detection system (GE Healthcare, Little Chalfont, Buckinghamshire, UK) and exposed to CL-Xposure film (Thermo Scientific, Rockford, IL, USA). The molecular weights of approximately 120 kDa and 88 kDa (APP); 61 kDa, 33 kDa (STEP); 46 kDa (NSE); 45 kDa (homer 1); 42 kDa (β-actin); and 21 kDa (RAC1) immunoreactive bands were quantified with background subtraction using a Bio-Rad GS-800 Calibrated Densitometer (Bio-Rad, Hercules, CA, USA) and Quantity One 1-D Analysis software (Bio-Rad, Hercules, CA, USA). Sample densities were analyzed blind to nature of diagnosis. Results obtained are based on at least two independent experiments.
Table 3.
Western Blotting Results for RAC1, homer 1, APP, STEP. Values Expressed as Ratios to β-actin and Neuronal Specific Enolase in BA9.
| ANOVA
|
Control
|
Schizophrenia
|
Bipolar Disorder
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | P | Protein | P | Protein | P | Δ | Protein | P | Δ | |
| APP 120 kDa/β-actin | 6.37 | 0.003 | 0.41 ± 0.31 | RG | 0.21 ± 0.14 | 0.007 | ↓ | 0.21 ± 0.17 | 0.012 | ↓ |
| APP 88 kDa/β-actin | 5.10 | 0.009 | 0.13 ± 0.13 | RG | 0.057 ± 0.044 | 0.028 | ↓ | 0.052 ± 0.039 | 0.023 | ↓ |
| Homer 1/β-actin | 3.02 | 0.056 | 0.28 ± 0.31 | RG | 0.31 ± 0.31 | NC | -- | 0.10 ± 0.10 | 0.035 | ↓ |
| RAC1/β-actin | 1.94 | NC | 0.66 ± 0.70 | RG | 0.40 ± 0.36 | NC | -- | 0.39 ± 0.42 | NC | -- |
| STEP 61 kDa/β-actin | 4.19 | 0.019 | 1.16 ± 0.66 | RG | 0.66 ± 0.53 | 0.008 | ↓ | 0.87 ± 0.56 | NC | -- |
| STEP 33 kDa/β-actin | 2.90 | NC | 0.099 ± 0.10 | RG | 0.039 ± 0.027 | 0.012 | ↓ | 0.076 ± 0.10 | NC | -- |
| β-actin | 0.21 | NC | 7.04 ± 1.70 | RG | 7.31 ± 1.23 | NC | -- | 7.29 ± 1.82 | NC | -- |
| APP 120 kDa/NSE | 5.83 | 0.005 | 0.42 ± 0.27 | RG | 0.24 ± 0.15 | 0.009 | ↓ | 0.23 ± 0.20 | 0.012 | ↓ |
| APP 88 kDa/NSE | 5.15 | 0.009 | 0.14 ± 0.14 | RG | 0.068 ± 0.054 | 0.041 | ↓ | 0.052 ± 0.036 | 0.014 | ↓ |
| Homer 1/NSE | 2.52 | NC | 0.36 ± 0.43 | RG | 0.38 ± 0.39 | NC | -- | 0.13 ± 0.16 | NC | -- |
| RAC1/NSE | 1.09 | NC | 0.62 ± 0.54 | RG | 0.45 ± 0.43 | NC | -- | 0.43 ± 0.48 | NC | -- |
| STEP 61 kDa/NSE | 4.73 | 0.012 | 1.27 ± 0.75 | RG | 0.70 ± 0.53 | 0.005 | ↓ | 0.90 ± 0.61 | NC | -- |
| STEP 33 kDa/NSE | 3.51 | 0.036 | 0.088 ± 0.063 | RG | 0.045 ± 0.028 | 0.007 | ↓ | 0.063 ± 0.067 | NC | -- |
| NSE | 0.016 | NC | 6.43 ± 1.81 | RG | 6.54 ± 2.27 | NC | -- | 6.44 ± 2.61 | NC | -- |
NC, no change; RG, reference group; Δ, change; ↓, decrease; –, no change; Values for β-actin and NSE were reprinted from Fatemi et al. (2013b) with permission from Nature Publishing Group. P values in the Schizophrenia and Bipolar groups are for t-test comparisons with Controls. Values in bold are significant after adjustment for multiple comparisons.
Table 4.
Western Blotting Results for RAC1, Homer 1, APP, and STEP. Values Expressed as Ratios to β-actin and Neuronal Specific Enolase in Lateral Cerebella.
| Control
|
Schizophrenia
|
Bipolar Disorder
|
Major Depression
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein | P | Protein | P | Δ | Protein | P | Δ | Protein | P | Δ | |
| APP 120 kDa/β-actin | 0.017 ± 0.018 | RG | 0.020 ± 0.012 | NC | -- | 0.039 ± 0.046 | NC | -- | 0.036 ± 0.037 | NC | -- |
| APP 88 kDa/β-actin | 0.060 ± 0.059 | RG | 0.051 ± 0.041 | NC | -- | 0.090 ± 0.110 | NC | -- | 0.11 ± 0.12 | NC | -- |
| Homer 1/β-actin | 0.075 ± 0.033 | RG | 0.083 ± 0.061 | NC | -- | 0.085 ± 0.035 | NC | -- | 0.058 ± 0.030 | NC | -- |
| RAC1/β-actin | 0.15 ± 0.17 | RG | 0.29 ± 0.16 | 0.046 | ↑ | 0.34 ± 0.18 | 0.015 | ↑ | 0.38 ± 0.28 | 0.024 | ↑ |
| STEP 61 kDa/β-actin | 0.094 ± 0.055 | RG | 0.090 ± 0.039 | NC | -- | 0.076 ± 0.050 | NC | -- | 0.084 ± 0.045 | NC | -- |
| STEP 33 kDa/β-actin | 0.51 ± 0.17 | RG | 0.52 ± 0.22 | NC | -- | 0.55 ± 0.16 | NC | -- | 0.43 ± 0.10 | NC | -- |
| β-actin | 22.3 ± 6.67 | RG | 20.8 ± 5.67 | NC | -- | 18.6 ± 2.64 | NC | -- | 22.7 ± 3.26 | NC | -- |
| APP 120 kDa/NSE | 0.016 ± 0.017 | RG | 0.020 ± 0.014 | NC | -- | 0.042 ± 0.024 | NC | -- | 0.040 ± 0.040 | NC | -- |
| APP 88 kDa/NSE | 0.13 ± 0.31 | RG | 0.052 ± 0.042 | NC | -- | 0.093 ± 0.120 | NC | -- | 0.120 ± 0.130 | NC | -- |
| Homer 1/NSE | 0.071 ± 0.029 | RG | 0.072 ± 0.044 | NC | -- | 0.076 ± 0.039 | NC | -- | 0.063 ± 0.020 | NC | -- |
| RAC1/NSE | 0.17 ± 0.22 | RG | 0.34 ± 0.19 | NC | -- | 0.34 ± 0.18 | NC | -- | 0.42 ± 0.31 | 0.038 | ↑ |
| STEP 61 kDa/NSE | 0.090 ± 0.042 | RG | 0.083 ± 0.037 | NC | -- | 0.076 ± 0.057 | NC | -- | 0.100 ± 0.072 | NC | -- |
| STEP 33 kDa/NSE | 0.50 ± 0.16 | RG | 0.48 ± 0.17 | NC | -- | 0.51 ± 0.17 | NC | -- | 0.48 ± 0.17 | NC | -- |
| NSE | 20.7 ± 2.73 | RG | 20.4 ± 1.52 | NC | -- | 19.5 ± 1.86 | NC | -- | 21.1 ± 2.69 | NC | -- |
NC, no change; RG, reference group, Δ, change; ↑, increase; –, no change. P values in the Schizophrenia, Bipolar, and Major Depression groups are for t-tests, values in bold are significant after adjustment for multiple comparisons.
2.3. Statistical Analysis
All protein measurements for each group were normalized against β-actin and neuronal specific enolase (NSE) and expressed as ratios of these two housekeeping proteins: RAC 1/β-actin, homer 1/β-actin, APP 120 kDa/β-actin, APP 88 kDa/β-actin, STEP 61 kDa/β-actin, STEP 33 kDa/β-actin, RAC 1/NSE, homer 1/NSE, APP 120 kDa/NSE, APP 88 kDa/NSE, STEP 61 kDa/NSE, and STEP 33 kDa/NSE. Statistical analysis was performed using previously described protocols (Fatemi et al., 2008, 2010a, 2011a,b). An initial MANOVA was performed for each brain region using all protein measurements in the dependent variable set with subsequent ANOVA’s on individual proteins if the initial MANOVA was significant. When ANOVA’s were significant, specific contrast t-tests were conducted on the relationships of interest. The level of significance was then adjusted for the number of specific contrasts [i.e. p < 0.025 for the McLean 74 cohort (Table 3) and p < 0.0167 for the Stanley Neuropathology Consortium (Table 4)]. Relationships between possible confounding variables and outcome measures were explored using analysis of variance (ANOVA) for categorical confounders and Pearson correlation coefficients for continuous confounders. When confounding variables showed significant relationships to outcomes, analysis of covariance was used to explore these effects on group differences. All analyses were conducted using SPSS v.19 (SPSS inc, Chicago, IL).
3. Results
3.1. Western Blotting Results for RAC1, homer 1, APP, and STEP in BA9
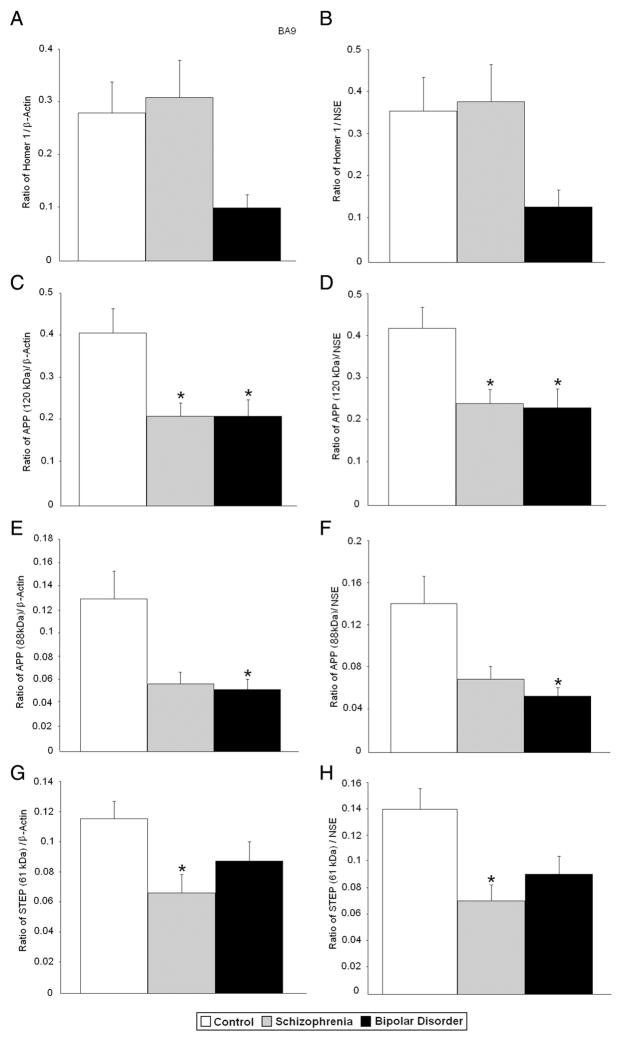
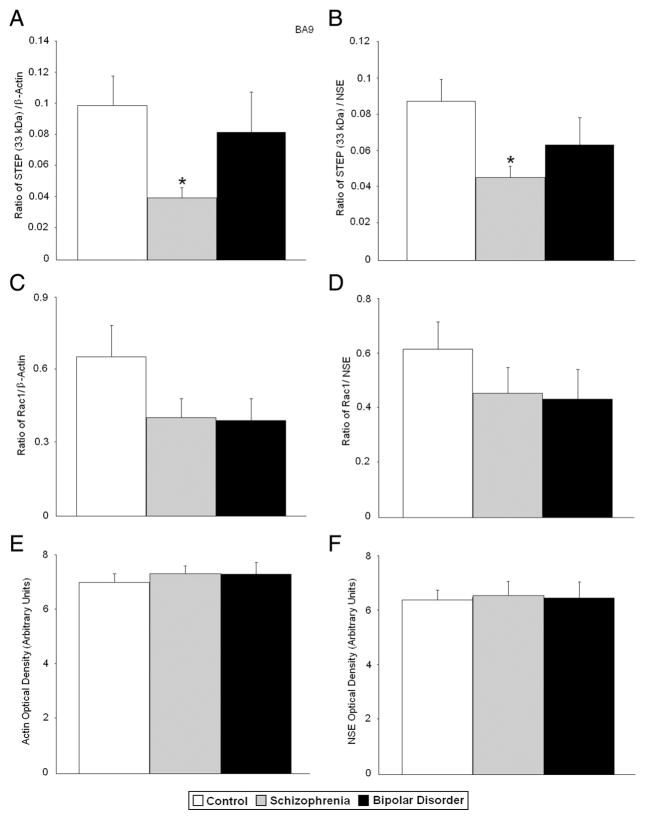
All protein measurements were normalized against β-actin or NSE. An initial MANOVA using all normalized protein measurements in the dependent variable set yielded a significant difference between diagnostic groups (Wilks’ Lambda F(20,98) =1.69, p = .047). In BA9, ANOVA identified group differences for APP 120 kDa/β-actin [F(2,64) = 6.37, p < 0.003], APP 120 kDa/NSE [F(2,64) = 5.83, p < 0.005], APP 88 kDa/β-actin [F(2,62) = 5.10, p < 0.009], APP 88 kDa/NSE [F(2,62) = 5.15, p < 0.009], STEP 61 kDa/β-actin [F(2,64) = 4.19, p < 0.019], STEP 61 kDa/NSE [F(2,64) = 4.73, p < 0.012], and STEP 33 kDa/NSE [F(2,62) = 3.51, p < 0.036] with a trend for homer 1/β-actin [F(2,61) = 3.02, p < 0.056] (Fig. 1; Table 3).
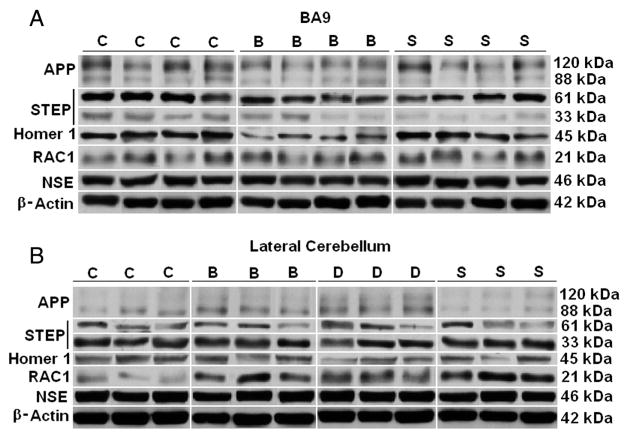
Fig. 1.
Representative samples of APP, STEP, Homer 1, RAC1, NSE, and β-actin from BA9 (A) of subjects with schizophrenia (S), bipolar disorder (B), and matched controls (C) and from lateral cerebellum (B) from subjects with schizophrenia (S), bipolar disorder (B), major depression (D) and matched controls (C). β-actin images for BA9 and NSE images for BA9 and lateral cerebellum were reprinted from Fatemi et al. (2013b) with permission from Nature Publishing Group. β-actin images from lateral cerebellum were reprinted from Fatemi et al. (2010a) with permission from Elsevier.
Follow up t-tests (after adjustment for multiple comparisons) found significant reductions for APP 120 kDa/β-actin, STEP 61/β-actin, and STEP 33 kDa/β-actin (p < 0.007, p < 0.008, and p < 0.012, respectively) and APP 120 kDa/NSE, STEP 61 kDa/NSE, and STEP 33 kDa/β-actin (p < 0.009, p < 0.005, and p < 0.007, respectively) in BA9 of subjects with schizophrenia (Table 3, Figs. 2 and 3). Trends in the same direction were found for APP 88 kDa/β-actin (p < 0.028) and APP 88 kDa/NSE (p < 0.041). In subjects with bipolar disorder, follow up t-tests found significant reductions in APP 120 kDa/β-actin (p < 0.012) and APP 88 kDa/β-actin (p < 0.023), and APP 120 kDa/NSE, and APP 88 kDa/NSE (p < 0.012, and p < 0.014, respectively). A trend in the same direction was found for homer 1/β-actin (p < 0.035) in subjects with bipolar disorder (Table 3; Figs. 2 and 3). There were no significant changes in protein levels for RAC1 in BA9 in either disorder (Table 3, Fig. 3). There were no changes in levels of β-actin or NSE in BA9.
Fig. 2.
Expression of Homer 1/β-actin (A), Homer 1/NSE (B), APP 120 kDa/β-actin (C), APP 120 kDa/NSE (D), APP 88 kDa/β-actin (E), APP 88 kDa/NSE (F), STEP 61 kDa/β-actin (G), STEP 61 kDa/NSE (H), in BA9 of healthy controls vs. subjects with schizophrenia and bipolar disorder. Histogram bars shown as mean ± standard error, *, p < 0.025.
Fig. 3.
Expression of STEP 33 kDa/β-actin (A), STEP 33 kDa/NSE (B), RAC1/β-actin (C), RAC1/NSE (D), β-actin (E), and NSE (F) in BA9 of healthy controls vs. subjects with schizophrenia, and bipolar disorder. Histogram bars shown as mean ± standard error, *, p < 0.025. Histogram bars for β-actin and NSE were reprinted from Fatemi et al. (2013b) with permission from Nature Publishing Group.
3.2. Western Blotting Results for RAC1, homer 1, APP, and STEP in Lateral Cerebellum
The initial MANOVA using all normalized protein measurements in the dependent variable set failed to yield a significant difference between diagnostic groups (Wilks’ Lambda F(36,48) =0.61, p = 0.94). Table 4 summarizes our results.
3.3. Analysis of Confounds for Protein Data in BA9
No significant differences were found on the outcome measures as a function of hemisphere side, history of substance abuse, multiple substance abuse, severity of substance abuse, post mortem interval, or pH. Nor did we find significant differences on use of barbiturates, opiates, amphetamines, cocaine or propoxyphene. We also examined age of onset and antidepressant use and found no significant differences. While gender, suicide, and benzodiazepine use, anticonvulsant use, and mood stabilizer use were significantly different between diagnostic groups (p < 0.006, p < 0.027, p < 0.03, respectively), they were not associated with any of the outcome measures. When comparing bipolar subjects who committed suicide versus those who did not, we found that subjects who had committed suicide displayed increased expression of homer 1 (p < 0.035; data not shown). However, group differences showed that there was a trend towards reduced homer 1/NSE when compared with controls (Table 3). Thus, suicide could not lead to this change and its impact was not considered meaningful. Among possible confounds, severity of alcohol abuse was associated with significant differences on APP 120 kDa/β-actin [F(3,59) = 3.47, p < 0.021], however analysis of covariance with alcohol use severity as a covariate did not alter the results reported above. There were no differences between subjects with alcohol abuse vs. those without alcohol abuse in each group with regard to any of the protein measures. Use of antipsychotic medication was also associated with significantly lower levels on a number of outcome measures, however antipsychotic use was also almost completely confounded within schizophrenia and bipolar disorder groups. When examined within those with bipolar disorder there were no differences on the outcome measures as a function of antipsychotic medication use. Within the schizophrenia group, those on antipsychotic medications (17 out of 20) displayed significantly lower values on APP 120 kDa/β-actin [F(1,18) = 8.60, p < 0.009], APP 120 kDa/NSE [F(1,18) = 6.04, p = 0.024], and STEP 61 kDa/NSE [F(1,18) = 6.10, p < 0.024]. We further explored the potential effect of antipsychotic medications by examining the effect of chlorpromazine equivalent dose (CPZ) on these three outcomes within subjects with schizophrenia. We found no relationship between CPZ level and APP 120 kDa/β-actin (r = 0.04), APP 120 kDa/NSE (r = 0.01) or STEP 61 kDa/NSE (r =0.01). While there were significant differences between the groups for use of anticonvulsants and mood stabilizers (p < 0.0001 and p < 0.0001, respectively), the effect sizes were low as measured by eta squared (η2) values (η2 = 0.02 and η2 = 0.04, respectively) and there were no significant effects on any of the outcome measures. We did find a negative association between age and levels of APP 88 kDa/NSE and APP 88 kDa/β-actin and disease duration and levels of APP 120 kDa/NSE and STEP 61 kDa/NSE. Likewise, we re-ran the previous analyses with age as a covariate and found the results unaffected. We compared bipolar and schizophrenic subjects on disease duration and found no significant differences [F(1,31) = 0.008, p < 0.93].
3.4. Analysis of Confounds for Protein Data in Lateral Cerebellum
In lateral cerebella, there were no effects of age, sex, PMI, race, side of brain, brain weight, pH, history of substance abuse, substance abuse severity, alcohol abuse severity, alcohol abuse, alcohol dependence, on any of the outcome measures. Where there were significant between group differences in terms of family history of mental illness (p < 0.001), age of onset (p < 0.001), duration of illness (p < 0.010), lifetime use of fluphenazine (p < 0.019), and use of antidepressants (p < 0.011), they were not associated with any of the outcome measures. Although suicide was associated with higher RAC1/β-actin levels [F(2,47) = 5.83, p < 0.005], this was entirely confounded within diagnostic groups and could not be explored as a confound in comparisons with normal controls. Further analyses within the two diagnostic groups found no significant interaction of diagnosis and suicide on RAC1/β-actin levels. We further investigated suicide and alcohol severity in lateral cerebellum. With regards to suicide, the only significant data consisted of an increase in STEP 33 kDa (p < 0.01) in subjects with schizophrenia who had a history of suicide. However, STEP 33 kDa data was not changed significantly in the schizophrenia group (Table 4). Thus, by inference, suicide had no meaningful impact on protein values in cerebellum. By the same token, reanalysis of data based on history of alcohol abuse showed increases STEP 61 kDa β-actin and NSE ratios (p < 0.018, p < 0.007, respectively) in depressed subjects with alcohol abuse; increased homer 1 (p < 0.037) in controls with alcohol abuse and decreased STEP 33 kDa (p < 0.019) in subjects with schizophrenia who abused alcohol. Again, none of these proteins were changed significantly (Table 4) in the original cerebellar data. Thus, the impact of suicide or alcohol abuse was not meaningful in cerebellar data.
4. Discussion
Our results provide evidence that four proteins that are involved in FMRP-mGluR5 signaling display altered expression in BA9 of subjects with schizophrenia and mood disorders. The most important salient findings are: 1) APP 120 kDa/β-actin and APP 120 kDa/NSE were significantly reduced in BA9 of subjects with schizophrenia and bipolar disorder; 2) APP 88 kDa/β-actin and APP 88 kDa/NSE were significantly reduced in BA9 of subjects with bipolar disorder; 3) STEP 61 kDa/β-actin and STEP 61 kDa/NSE were significantly reduced in BA9 of subjects with schizophrenia; 4) STEP 33 kDa/β-actin and STEP 33 kDa/NSE were significantly reduced in BA9 of subjects with schizophrenia; 5) homer 1/β-actin showed a trend for reduction in BA9 of subjects with bipolar disorder; and 6) APP 88 kDa/β-actin and APP 88 kDa/NSE showed a trend towards reduction in BA9 of subjects with schizophrenia. It should be noted that those results trending but not achieving significance (APP 88 kDa/β-actin, APP 88 kDa/NSE, and homer 1//β-actin) still displayed effect size differences considered to be moderate to large (d = 0.71, d = 0.64, d = 0.73; respectively). In lateral cerebellum, despite a significant increase in RAC1/β-actin in subjects with bipolar disorder and a trend for increased RAC1/β-actin in subjects with schizophrenia and RAC1/β-actin and RAC1/NSE in subjects with major depression, absence of significance in the initial MANOVA between groups undermined the potential statistical meaning of these values.
The gene for APP is located at 21q21.3 (Goldgaber et al., 1987; Kang et al., 1987) and codes for a protein that is involved in synapse formation and neural plasticity (Priller et al., 2006; Turner et al., 2003). Westmark and Malter (2007) have demonstrated that FMRP binds to a guanine-rich, G-quartet-like sequence of the coding region of APP mRNA and that APP mRNA co-immunopercipitates with FMRP in resting synaptoneurosomes. Subsequent studies have also demonstrated binding of FMRP to APP mRNA (Ascano et al., 2012; Darnell et al., 2011). Furthermore, APP translation is increased when synaptoneurosomes are stimulated by the group 1 mGluR agonist (S)-3,5-dihydroxyphenylglycine (DHPG) demonstrating that APP translation is mGluR5 dependent and regulated by FMRP (Westmark and Malter, 2007). Finally, Westmark and Malter (2007) identified elevated levels of APP and its cleavage product beta amyloid (Aβ) in brains of Fmr1 KO mice.
Because of APP’s roles in synapse formation and neural plasticity (Turner et al., 2003; Priller et al., 2006), it has been investigated as a candidate gene for schizophrenia. However, the results have been equivocal (Jones et al., 1992; Coon et al., 1993; Fukuda et al., 1993; Karayiorgou et al., 1994; Morris et al., 1994; Mortilla et al., 1994; Forsell and Lannfelt, 1995). Less is known about levels of APP expression in subjects with schizophrenia, while Aβ has been shown to be reduced in cerebrospinal fluid of elderly patients with schizophrenia, supporting our results (Frisoni et al., 2011; Albertini et al., 2012). Our findings of reduced APP in BA9 of subjects with schizophrenia and bipolar disorder are not similar to our previous findings in autism (Fatemi et al., 2013a), possibly suggesting differences in processing of APP in autism vs. schizophrenia and mood disorders. The lack of change in APP expression in lateral cerebella of subjects with schizophrenia and mood disorders could potentially be due to brain regional differences.
Analysis of impact of antipsychotics on APP levels showed absence of any significant relationships between CPZ equivalent dose and APP protein outcomes. Direct effects of antipsychotic drugs on APP expression are currently unclear, although it has been hypothesized that antipsychotic medications may have a neuroprotective effect (Palotás et al., 2003; Kállmán et al., 2012). While typical antipsychotics such as haloperidol or thioridazine have been shown to increase APP expression (Palotás et al., 2003; Shi et al., 2012), atypical antipsychotics such as quetiapine or risperidone either reduced Aβ levels or had no effect on APP levels, respectively (Palotás et al., 2003; Zhu et al., 2013). Furthermore, reserpine reduced cytosolic APP level while increasing membrane fractions of APP (Komachi et al., 1994). The above effects by antipsychotics also vary with brain location or duration of treatment. None of these reports indicated use of prefrontal or cerebellar areas of the brain. Thus, it is unlikely that our results in either subjects with schizophrenia or bipolar disorder are due to effects of antipsychotics (Table 5).
Table 5.
The Impact of Antipsychotics on FMRP Target Expression.
| Target | Reference | Animal | Drug | Duration of Treatment | Result |
|---|---|---|---|---|---|
| Homer 1 | De Bartolomeis et al. (2002) | Rat | Haloperidol | Acute | ↑ mRNA in CP; no change in PC or PFC |
| Olanzapine | Acute | ↑ mRNA in NA core; no change in PC or PFC | |||
| Homer 1a | Dell’aversano et al. (2009) | Rat | Haloperidol | Acute | ↑ mRNA in striatum; ↓ mRNA in PC |
| Homer 1 | Fatemi et al. (2006) | Rat | Olanzapine | Chronic | ↑ mRNA in frontal cortex |
| Homer 1a | Iasevoli et al. (2010a) | Rat | Haloperidol | Acute | ↑ mRNA in striatum; ↓ mRNA in ACC, motor cortex, and MAC |
| Homer 1a,b | Iasevoli et al. (2010a) | Rat | Haloperidol | Chronic | ↑ homer 1a mRNA in CP and cortex; ↑ homer 1b mRNA in striatum and ACC, MAC, motor cortex, and somatosensory cortex |
| Sertindole | Acute and Chronic | No change in any of the brain regions | |||
| Homer 1a | Iasevoli et al. (2010b) | Rat | Haloperidol | Acute | ↑ mRNA in striatal subregions |
| Olanzapine | Acute | ↑ mRNA in DL, VL, CAcc | |||
| Risperidone | Acute | ↑ mRNA in DL, VL CP | |||
| Sulpiride | Acute | ↑ mRNA in VL, CAcc | |||
| Homer 1a | Tomasetti et al. (2011) | Rat | Haloperidol | Acute and Chronic | ↑ mRNA in striatum (acute and chronic); ↓ mRNA in motor and premotor cortex (chronic) |
| Quetiapine | Acute and Chronic | ↑ mRNA in ACC, motor, and premotor cortices (acute); ↓ mRNA in motor, premotor, and somatosensory cortex (chronic) |
|||
| Homer 1 | Polese et al. (2002) | Rat | Haloperidol | Acute | ↑ mRNA in CP and NA |
| Clozapine | Acute | ↑ mRNA in NA | |||
| Rac1 | Hill et al. (2006) | Human | N/A | N/A | ↓ mRNA in white matter of DLPFC |
| Monkey | Haloperidol and olanzapine | Chronic | RAC1 not measured in monkeys, only Duo and Cdc42. | ||
| Rac1 | Eisenberg et al. (2008) | Rat PANC-1 cell line | Chlorpromazine | Acute | ↑ protein for GFP-RAC1 |
| APP | Kállmán et al. (2012) | Rat | Sertindole | N/A | ↓ mRNA in hippocampus; no change in cortex |
| Aβ (not APP) | Zhu et al. (2013) | Mouse | Quetiapine | Chronic | ↓ protein in cortex and CSF; no change in hippocampus |
| APP | Komachi et al. (1994) | Rat | Resperine | Acute | ↓ protein in hippocampus; no change in striatum |
| APP | Palotás et al. (2003) | Rat | Haloperidol | Acute and Chronic | ↑ protein in temporal cortex, acutely; no chronic effect |
| Risperidone | Acute and Chronic | No effect | |||
| APP | Shi et al. (2012) | Rat | Thioridazine | Subchronic | ↑ mRNA and protein in cerebral cortex |
| STEP | Carty et al. (2012) | Rat | Haloperidol, clozapine, risperidone | Chronic | ↑ phosphorylated STEP 61 kDa in frontal cortex; no changes in protein levels |
ACC, anterior cingulate cortex; CAcc, core of the nucleus accumbens; CP, caudate and putamen; DLPFC, dorsolateral prefrontal cortex; DL, dorsolateral caudate putamen; HUVEC, human umbilical vein endothelial cells; IC, internal capsule; MAC, medial agranular cortex; NA, nucleus accumbens; PANC-1, pancreas cell line 1; PFC, prefrontal cortex; PC, parietal cortex; SS, somatosensory cortex; VL, ventrolateral caudate putamen.
The gene that codes for STEP - also known as protein tyrosine phosphatase, non-receptor type 5 (PTPN5) – is localized to 11p15.1 (Li et al., 1995). STEP is present in the postsynaptic density (PSD), where it is an important regulator of N-methyl-D-aspartate (NMDA) receptor function (Goebel-Goody et al., 2012a,b). Darnell et al (2011) have identified a highly significant association between STEP mRNA and FMRP. Basal levels of STEP are elevated in Fmr1 KO mice, suggesting control of its expression (Goebel-Goody et al., 2012a,b). Similar to findings for APP, STEP mRNA is increased following treatment with DHPG in wild-type but not in Fmr1 KO mice (Zhang et al., 2008; Goebel-Goody et al., 2012a,b). Moreover, the DHPG-induced increase in STEP translation required mGluR5 activation (Zhang et al., 2008).
We examined two isoforms of STEP: STEP 61 kDa, which is membrane bound and has phosphatase activity (Goebel-Goody et al., 2012a,b), and STEP 33 kDa, which is a cleavage product of STEP 61 kDa (Fitzpatrick and Lombroso, 2011). We further investigated STEP expression using a mono-clonal antibody against STEP (clone 23E5) and obtained similar results (data not shown). Our results of reduced STEP 61 kDa in BA9 are in contrast to findings of increased expression of STEP 61 kDa in anterior cingulate cortex and dorsolateral prefrontal cortex of subjects with schizophrenia (Carty et al., 2012). However, those findings have not been independently verified by other laboratories. Moreover, the difference in results may be due to regional differences (superior frontal cortex vs. dorsolateral prefrontal cortex). Indeed, brain regional differences in expression of STEP isoforms have previously been identified (Boulanger et al., 1995). This may also explain why the STEP 61 kDa isoform predominates in BA9 and STEP 33 kDa isoform predominates in lateral cerebellum. Administration of MK-801 resulted in increased expression of STEP 61 kDa in primary cortical cultures and in synaptosomal fractions of mouse cortex (Carty et al., 2012). STEP KO mice were less likely to experience cognitive impairments following subchronic administration of PCP (Carty et al., 2012). Haplotype analysis has found a nominal association between the single nucleotide polymorphism (SNP) rs4075664 of PTPN5 and schizophrenia in an Israeli population sample (Pelov et al., 2012), with three SNPs displaying a stronger association with schizophrenia in males: rs4075664, rs2278732, and rs4757710 (Pelov et al., 2012).
We observed reduced expression of STEP 61 kDa/NSE in subjects with schizophrenia who were taking antipsychotic drugs when compared to those who were not. As with APP 120 kDa/NSE and APP 120 kDa/β-actin, there was no significant relationship between CPZ equivalent dose and STEP 61 kDa/NSE in subjects with schizophrenia. Carty et al. (2012) found that chronic (three-week) treatment with haloperidol, clozapine, or risperidone increased phosphorylation of STEP 61 kDa, which leads to its inactivation. However, they found no impact of antipsychotics on STEP 61 kDa mRNA or protein levels (Carty et al., 2012). Thus, it is unlikely that our reported changes in schizophrenia are due to antipsychotic treatment. In BA9 of children with autism we observed significant reduction in expression of STEP 61 kDa with no significant differences in BA9 or cerebellar vermis of adults with autism (Fatemi et al., 2013a). We found significant reduction of STEP 33 kDa in BA9 of adults with autism, consistent with our current findings in schizophrenia (Fatemi et al., 2013a).
It has previously been reported that STEP mRNA is not expressed in cerebellum (Lombroso et al., 1991). However, not only have we observed the presence of major STEP bands 61 kDa, and 33 kDa in both frontal and cerebellar tissues from three different US brain banks (Fatemi et al., 2013a, current report, and unpublished results), we have also observed the same isoforms in mouse cerebellar tissues (data not shown). Additionally, previously published data has identified presence of mRNA for STEP in cerebellum (Hodges et al., 2006) and protein expression has also been demonstrated in human cerebellum (Human Protein Atlas) and mouse cerebellum (GENSAT).
Homer proteins are components of the postsynaptic density (PSD) that have multiple roles in synaptogenesis, receptor trafficking, and involvement in dopaminergic and glutamatergic signaling (Szumlinski et al., 2006). Homer proteins directly interact with mGluR5 and help regulate mGluR signaling (Ango et al., 2002; Sergé et al., 2002; Kammermeier, 2008; Ronesi and Huber, 2008). In wild type mice, but not Fmr1 KO mice, disruption of homer 1-mGluR5 interactions has been shown to inhibit mGluR5-induced long-term depression and protein synthesis (Ronesi and Huber, 2008).
A mutation screening of homer gene family with schizophrenia suggested that it was unlikely that homer 1 contributed to the etiology of schizophrenia (Norton et al., 2003). However, recently Spellman et al. (Spellmann et al., 2011) found that two single nucleotide polymorphisms (SNPs) of homer 1 (rs2290639 and rs4704560) were associated both with positive and global baseline subscale scores of the positive and negative syndrome scale (PANSS). The rs2290639 SNP was also associated with improvement on global subscale scores following four weeks of antipsychotic treatment (Spellmann et al., 2011). Homer 1 KO mice exhibit a number of defects similar to schizophrenia including impaired PPI, increased anxiety, and enhanced MK-801-stimulated motor activity (Szumlinski et al., 2005). Our observation of a near significant reduction in homer 1 expression in BA9 of subjects with bipolar disorder is analogous to our previous report of a significant reduction in homer 1 expression in BA9 of adults with autism (Fatemi et al., 2013a).
A review of the literature indicates that antipsychotic effect on homer expression varies dependent on its class (typical vs. atypical), brain region, and duration of treatment (acute, subacute, or choric). Thus, in rat, haloperidol mostly increased mRNA for homer 1 in basal ganglia and other brain regions but not in prefrontal cortex (De Bartolomeis et al., 2002; Polese et al., 2002; Iasevoli et al., 2010a; Tomasetti et al., 2011) both acutely and chronically. Olanzapine increased mRNA in frontal cortex chronically (Fatemi et al., 2006) as well as in other brain regions (Iasevoli et al., 2010b). Other atypical agents like risperidone, quetiapine, clozapine, or sulpride varied in their effects depending on duration of treatment or brain regions examined (Polese et al., 2002; Iasevoli et al., 2010b; Tomasetti et al., 2011). However, none of these effects were in frontal or cerebellar cortices. More importantly, some antipsychotics like sertindole were without an effect either acutely or chronically (Iasevoli et al., 2010a). Additionally, none of the above reports evaluated the impact of antipsychotics on protein expression as changes in mRNA do not always lead to concordant effects in protein expression. Thus, while our analysis did not show an impact of antipsychotics on homer 1 expression in BA9 in bipolar subjects, a general effect of antipsychotics on this protein cannot be ruled out because of the complex array of data presented above (Table 5).
RAC1 is involved in functions that are relevant to neurotransmission, specifically the modulation of dendritic spine morphology and density (Luo et al., 1996; Threadgill et al., 1997; Nakayama et al., 2000). Dominant-negative mutants of RAC1 have been shown to reduce the number of dendrites of neurons (Threadgill et al., 1997; Nakayama et al., 2000), while constitutively active forms of RAC1 increase the number of small immature spines (Luo et al., 1996; Threadgill et al., 1997). Altered dendritic spine morphology or density may contribute to cognitive impairments of schizophrenia (Hayashi-Takagi et al., 2010). Indeed, inhibition of RAC1 activity in the hippocampus of mice inhibited the induction of long term potentiation (LTP) (Martinez and Tejada-Simon, 2011). RAC1 interacts with FMRP via cytoplasmic FMRP interacting proteins 1 and 2 (CYFIP1/2; Schenck et al., 2001, 2003). In Fmr1 KO mice, there is increased expression of RAC1 mRNA, suggesting that FMRP acts as a negative regulator of RAC1 (Bongmba et al., 2011). While MANOVA analysis found no significant group differences for lateral cerebellum, there were near significant increases in RAC1 for subjects with schizophrenia and mood disorders. We have previously observed upregulation of RAC1 in BA9 of children and adults with autism and in cerebellar vermis of adults with autism (Fatemi et al., 2013a; Fatemi and Folsom, 2014). Thus, there appears concordance in our data both in autism and in schizophrenia and mood disorders.
Treatment of a rat pancreas cell line (PANC-1) with chlorpromazine resulted in increased GFP-RAC1 expression (Table 5; Eisenberg et al., 2008). Hill et al. (2006) reported on a significant decrease in RAC1 mRNA expression in white matter of prefrontal cortex in subjects with schizophrenia (Hill et al., 2006). However, these authors did not report on any change in RAC1 expression in PFC of monkeys treated chronically with either haloperidol or olanzapine (Table 5; Hill et al., 2006). Thus, it appears unlikely that our results with RAC1 are due to antipsychotic treatment.
In conclusion, investigation of the FMRP regulon in BA9 and lateral cerebellum, shows evidence for abnormalities in several targets of FMRP signaling in schizophrenia and mood disorders. Because these four proteins are involved in a number of signaling pathways, further research needs to be done to determine if these changes are the result of dysregulated FMRP-mGluR5 signaling or whether other pathways contribute to their altered expression. Our results are also supported by recent discovery of mutations in several targets of FMRP in schizophrenia (Fromer et al., 2014; Purcell et al., 2014) pointing to new molecular targets for etiological studies and avenues for treatment in this disorder.
Acknowledgments
Grant support by the National Institutes of Mental Health (Grant #1R01MH086000-01A2), and the Ewald Bipolar Disease Research Fund to SHF is gratefully acknowledged. S.H. Fatemi is also supported by the Bernstein Endowed Chair in Adult Psychiatry. Tissue samples from the Stanley Medical Research Institute and assistance with demographic information from Dr. Edwin Fuller-Torrey and Dr. Maree J. Webster, to SHF is gratefully acknowledged. Tissue samples from the Harvard Brain Tissue Resource Center, which is supported in part by PHS grant number R24 MH068855 is gratefully acknowledged.
Footnotes
Contributors
Author SHF designed the study and wrote the protocol. Author TDF performed the experiments and was involved in data analysis. Author PDT performed statistical analysis. Both SHF and TDF wrote and edited this manuscript and approve of the final version of the manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest.
Role of the Funding Source
Grant support by the National Institute of Mental Health (Grant #1R01MH086000-01A2), and the Ewald Bipolar Disease Research Fund to SHF is gratefully acknowledged. (SHF). NIMH, and the Ewald Bipolar Disease Research Fund had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Contributor Information
Timothy D. Folsom, Email: folso013@umn.edu.
Paul D. Thuras, Email: pthuras@gmail.com.
S. Hossein Fatemi, Email: fatem002@umn.edu.
References
- Albertini V, Benussi L, Paterlini A, Glionna M, Prestia A, Bocchio-Chiavetto L, Amicucci G, Galluzzi S, Adorni A, Geroldi C, Binetti G, Frisoni GB, Ghidoni R. Distinct cerebrospinal fluid amyloid-beta peptide signatures in cognitive decline associated with Alzheimer’s disease and schizophrenia. Electrophoresis. 2012;33(24):3738–3744. doi: 10.1002/elps.201200307. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, Watkins GL, Hichwa RD. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci U S A. 1996;93(18):9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ango F, Robbe D, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L. Homer-dependent cell surface expression of metabotropic glutamate receptor type 5 in neurons. Mol Cell Neurosci. 2002;20(2):323–329. doi: 10.1006/mcne.2002.1100. [DOI] [PubMed] [Google Scholar]
- Ascano M, Jr, Mukherjee N, Bandaru P, Miller JB, Nusbaum JD, Corcoran DL, Langlois C, Munschauer M, Dewell S, Hafner M, Williams Z, Ohler U, Tuschl T. FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature. 2012;492(7429):382–386. doi: 10.1038/nature11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DB, Mesibov GB, Hatton DD, Clark RD, Roberts JE, Mayhew L. Autistic behavior in young boys with fragile X syndrome. J Autism Dev Disord. 1998;28(6):499–507. doi: 10.1023/a:1026048027397. [DOI] [PubMed] [Google Scholar]
- Baldaçara L, Borgio JG, Lacerda AL, Jackowski AP. Cerebellum and psychiatric disorders. Rev Bras Psiquiatr. 2008;30(3):281–289. doi: 10.1590/s1516-44462008000300016. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27(7):370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bongmba OY, Martinez LA, Elhardt ME, Butler K, Tejada-Simon MV. Modulation of dendritic spines and synaptic function by Rac1: A possible link to fragile X syndrome pathology. Brain Res. 2011;1399:79–95. doi: 10.1016/j.brainres.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Molnar C, Horner MD, Anderson B, Forster L, George MS, Nahas Z. Neurocognitive deficits and prefrontal cortical atrophy in patients with schizophrenia. Schizophr Res. 2008;101(1–3):142–151. doi: 10.1016/j.schres.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger LM, Lombroso PJ, Raghunathan A, During MJ, Wahle P, Naegele JR. Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci. 1995;15(2):1532–1544. doi: 10.1523/JNEUROSCI.15-02-01532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty NC, Xu J, Kurup P, Brouillette J, Goebel-Goody SM, Austin DR, Yuan P, Chen G, Correa PR, Haroutunian V, Pittenger C, Lombroso PJ. The tyrosine phosphatase STEP: implications in schizophrenia and the molecular mechanism underlying antipsychotic medications. Transl Psychiatry. 2012;2:e137. doi: 10.1038/tp.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudley AE, Gutierrez E, Jocelyn LJ, Chodirker BN. Outcomes of genetic evaluation in children with pervasive developmental disorder. J Dev Behav Pediatr. 1998;19(5):321–325. doi: 10.1097/00004703-199810000-00001. [DOI] [PubMed] [Google Scholar]
- Coon H, Hoff M, Holik J, Delisi LE, Crowe T, Freedman R, Shields G, Boccio AM, Lerman M, Gershon ES. C to T nucleotide substitution in codon 713 of amyloid precursor protein gene not found in 86 unrelated schizophrenics from multiplex families. Am J Med Genet. 1993;48(1):36–39. doi: 10.1002/ajmg.1320480109. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Barbadillo L, Pelayo-Terán JM, Rodríguez-Sánchez JM. Neuropsychological functioning and brain structure in schizophrenia. Int Rev Psychiatry. 2007;19(4):325–336. doi: 10.1080/09540260701486647. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Klann E. The translation of translational control by FMRP: therapeutic targets for FXS. Nat Neurosci. 2013;16(11):1530–1536. doi: 10.1038/nn.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Jensen K, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107(4):489–499. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD, Darnell RB. FMRP stalls translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146(2):247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bartolomeis A, Aloj L, Ambesi-Impiombato A, Bravi D, Caracò C, Muscettola G, Barone P. Acute administration of antipsychotics modulates Homer striatal gene expression differentially. Brain Res Mol Brain Res. 2002;98(1–2):124–129. doi: 10.1016/s0169-328x(01)00327-8. [DOI] [PubMed] [Google Scholar]
- Dell’aversano C, Tomasetti C, Iasevoli F, de Bartolomeis A. Antipsychotic and antidepressant co-treatment: effects on transcripts of inducible postsynaptic density genes possibly implicated in behavioural disorders. Brain Res Bull. 2009;79(2):123–129. doi: 10.1016/j.brainresbull.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Dölen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol. 2008;586(6):1503–1508. doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S, Giehl K, Henis YI, Ehrlich M. Differential interference of chlorpromazine with the membrane interactions of oncogenic K-Ras and its effects on cell growth. J Biol Chem. 2008;283(40):27279–27288. doi: 10.1074/jbc.M804589200. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. Dysregulation of fragile X mental retardation protein and metabotropic glutamate receptor 5 in superior frontal cortex of individuals with autism: a postmortem brain study. Mol Autism. 2011;2:6–16. doi: 10.1186/2040-2392-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. GABA receptor subunit distribution and FMRP-mGluR5 signaling abnormalities in the cerebellum of subjects with schizophrenia, mood disorders, and autism. Schizophr Res. 2014 doi: 10.1016/j.schres.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Bell C, Nos L, Fried P, Pearce DA, Singh S, Siderovski DP, Willard FS, Fukuda M. Chronic olanzapine treatment causes differential expression of genes in frontal cortex of rats as revealed by DNA microarray technique. Neuropsychopharmacology. 2006;31(9):1888–1899. doi: 10.1038/sj.npp.1301002. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, King DP, Reutiman TJ, Folsom TD, Laurence JA, Lee S, Fan YT, Paciga SA, Conti M, Menniti FS. PDE4B polymorphisms and decreased PDE4B expression are associated with schizophrenia. Schizophr Res. 2008;101(1–3):36–49. doi: 10.1016/j.schres.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD. GABA(A) Receptor Downregulation in Brains of Subjects with Autism. J Autism Dev Disord. 2009a;39(2):223–230. doi: 10.1007/s10803-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Thuras PD. Expression of GABA(B) Receptors Is Altered in Brains of Subjects with Autism. Cerebellum. 2009b;8(1):64–69. doi: 10.1007/s12311-008-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Kneeland RE, Liesch SB, Folsom TD. Fragile X mental retardation protein levels are decreased in major psychiatric disorders. Schizophr Res. 2010a;124(1–3):246–247. doi: 10.1016/j.schres.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Rooney RJ, Patel DH, Thuras PD. mRNA and protein levels for GABAAalpha4, alpha5, beta1, and GABABR1 receptors are altered in brains of subjects with autism. J Autism Dev Disord. 2010b;40(6):743–750. doi: 10.1007/s10803-009-0924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Thuras PD. Deficits in GABA(B) receptor system in schizophrenia and mood disorders: a postmortem study. Schizophr Res. 2011a;128(1–3):37–43. doi: 10.1016/j.schres.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Kneeland RE, Liesch SB. Metabotropic glutamate receptor 5 upregulation in children with autism is associated with underexpression of both fragile X mental retardation protein and GABAA receptor beta 3 in adults with autism. Anat Rec (Hoboken) 2011b;294(10):1635–1645. doi: 10.1002/ar.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Kneeland RE, Yousefi MK, Liesch SB, Thuras PD. Impairment of fragile X mental retardation protein-metabotropic glutamate receptor 5 signaling and its downstream cognates ras-related C3 botulinum toxin substrate 1, amyloid beta A4 precursor protein, striatal-enriched protein tyrosine phosphatase, and homer 1, in autism: a postmortem study in cerebellar vermis and superior frontal cortex. Mol Autism. 2013a;4(1):21. doi: 10.1186/2040-2392-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Rooney RJ, Thuras PD. mRNA and protein expression for novel GABAA receptors θ and ρ2 are altered in schizophrenia and mood disorders; relevance to FMRP-mGluR5 signaling pathway. Transl Psychiatry. 2013b;3:e271. doi: 10.1038/tp.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez E, Rajan N, Bagni C. The FMRP regulon: from targets to disease convergence. Front Neurosci. 2013;7:191. doi: 10.3389/fnins.2013.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick CJ, Lombroso P. The Role of Striatal-Enriched Protein Tyrosine Phosphatase (STEP) in Cognition. Front Neuroanat. 2011;5:47. doi: 10.3389/fnana.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsell C, Lannfelt L. Amyloid precursor protein mutation at codon 713 (Ala–>Val) does not cause schizophrenia: non-pathogenic variant found at codon 705 (silent) Neurosci Lett. 1995;184(2):90–93. doi: 10.1016/0304-3940(94)11176-j. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Prestia A, Geroldi C, Adorni A, Ghidoni R, Amicucci G, Bonetti M, Soricelli A, Rasser PE, Thompson PM, Giannakopoulos P. Alzheimer’s CSF markers in older schizophrenia patients. Int J Geriatr Psychiatry. 2011;26(6):640–648. doi: 10.1002/gps.2575. [DOI] [PubMed] [Google Scholar]
- Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, Georgieva L, Rees E, Palta P, Ruderfer DM, Carrera N, Humphreys I, Johnson JS, Roussos P, Barker DD, Banks E, Milanova V, Grant SG, Hannon E, Rose SA, Chambert K, Mahajan M, Scolnick EM, Moran JL, Kirov G, Palotie A, McCarroll SA, Holmans P, Sklar P, Owen MJ, Purcell SM, O’Donovan MC. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506(7487):179–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R, Hattori M, Sasaki T, Kazamatsuri H, Kuwata S, Shibata Y, Nanko S. No evidence for a point mutation at codon 713 and 717 of amyloid precursor protein gene in Japanese schizophrenics. Jpn J Hum Genet. 1993;38(4):407–411. doi: 10.1007/BF01907987. [DOI] [PubMed] [Google Scholar]
- GENSAT, d. Mouse Brain Atlas. http://www.gensat.org/index.html.
- Goebel-Goody SM, Baum M, Paspalas CD, Fernandez SM, Carty NC, Kurup P, Lombroso PJ. Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol Rev. 2012a;64(1):65–87. doi: 10.1124/pr.110.003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel-Goody SM, Wilson-Wallis ED, Royston S, Tagliatela SM, Naegele JR, Lombroso PJ. Genetic manipulation of STEP reverses behavioral abnormalities in a fragile X syndrome mouse model. Genes Brain Behav. 2012b;11(5):586–600. doi: 10.1111/j.1601-183X.2012.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgaber D, Lerman MI, McBride OW, Saffiotti U, Gajdusek DC. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer’s disease. Science. 1987;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Furfaro JA, Hoeft F, Eckert MA, Hall SS, O’Hara R, Erba HW, Ringel J, Hayashi KM, Patnaik S, Golianu B, Kraemer HC, Thompson PM, Piven J, Reiss AL. Neuroanatomy of Fragile X syndrome is associated with aberrant behavior and the Fragile X mental retardation protein (FMRP) Ann Neurol. 2008;63(1):40–51. doi: 10.1002/ana.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan B, Daly EM, McAlonan G, Loth E, Toal F, O’Brien F, Robertson D, Hales S, Murphy C, Murphy KC, Murphy DG. Brain morphometry volume in autistic spectrum disorder: a magnetic resonance imaging study of adults. Psychol Med. 2009;39(2):337–346. doi: 10.1017/S0033291708003383. [DOI] [PubMed] [Google Scholar]
- Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Jr, Roberts J, Mirrett P. Autistic behavior in children with Fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A. 2006;140A(17):1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, Makino Y, Seshadri AJ, Ishizuka K, Srivastava DP, Xie Z, Baraban JM, Houslay MD, Tomoda T, Brandon NJ, Kamiya A, Yan Z, Penzes P, Sawa A. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13(3):327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11(6):557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- Hodges A, Strand AD, Aragaki AK, Kuhn A, Sengstag T, Hughes G, Elliston LA, Hartog C, Goldstein DR, Thu D, Hollingsworth TR, Collin F, Synek B, Holmans PA, Young AB, Wexler NS, Delorenzi M, Kooperberg C, Augood SJ, Faull RL, Olson JM, Jones L, Luthi-Carter R. Regional and cellular gene expression changes in human Huntington’s disease brain. Hum Mol Genet. 2006;15(6):965–977. doi: 10.1093/hmg/ddl013. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, MacDonald A, III, Carter CS, Barch DM, Andrew Stenger V, Cohen JD. Prefrontal functioning during context processing in schizophrenia and major depression: an event-related fMRI study. Schizophr Res. 2005;76(2–3):199–206. doi: 10.1016/j.schres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Human Protein Atlas. http://www.proteinatlas.org/
- Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- Iasevoli F, Tomasetti C, Marmo F, Bravi D, Arnt J, de Bartolomeis A. Divergent acute and chronic modulation of glutamatergic postsynaptic density genes expression by the antipsychotics haloperidol and sertindole. Psychopharmacology (Berl) 2010a;212(3):329–344. doi: 10.1007/s00213-010-1954-0. [DOI] [PubMed] [Google Scholar]
- Iasevoli F, Fiore G, Cicale M, Muscettola G, de Bartolomeis A. Haloperidol induces higher Homer1a expression than risperidone, olanzapine and sulpiride in striatal sub-regions. Psychiatry Res. 2010b;177(1–2):255–260. doi: 10.1016/j.psychres.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB, Swain RA, Weiler IJ, Greenough WT. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet. 2001;98(2):161–171. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Berry-Kravis E, Hagerman R, von Raison F, Gasparini F, Apostol G, Ufer M, Des Portes V, Gomez-Mancilla B. The challenges of clinical trials in fragile X syndrome. Psychopharmacology (Berl) 2014;231(6):1237–1250. doi: 10.1007/s00213-013-3289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CT, Morris S, Yates CM, Moffoot A, Sharpe C, Brock DJ, St Clair D. Mutation in codon 713 of the beta amyloid precursor protein gene presenting with schizophrenia. Nat Genet. 1992;1(4):306–309. doi: 10.1038/ng0792-306. [DOI] [PubMed] [Google Scholar]
- Kállmán J, Pákáski M, Szucs S, Kálmán S, Fazekas O, Santha P, Szabó G, Janka Z, Kálmán J. The role of immobilization stress and sertindole on the expression of APP, MAPK-1, and beta-actin genes in rat brain. Ideggyogy Sz. 2012;65(11–12):394–400. [PubMed] [Google Scholar]
- Kammermeier PJ. Endogenous homer proteins regulate metabotropic glutamate receptor signaling in neurons. J Neurosci. 2008;28(34):8560–8567. doi: 10.1523/JNEUROSCI.1830-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Müller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Kasch L, Lasseter VK, Hwang J, Elango R, Bernardini DJ, Kimberland M, Babb R, Francomano CA, Wolyniec PS. Report from the Maryland Epidemiology Schizophrenia Linkage Study: no evidence for linkage between schizophrenia and a number of candidate and other genomic regions using a complex dominant model. Am J Med Genet. 1994;54(4):345–353. doi: 10.1002/ajmg.1320540413. [DOI] [PubMed] [Google Scholar]
- Kaufman WE, Cortell R, Kau A, Bukelis I, Tierney E, Gray R, Cox C, Capone GT, Stanard P. Autism spectrum disorder in fragile X syndrome: Communication, social interaction, and specific behaviors. Am J Med Genet A. 2004;129A(3):225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Kelemen O, Kovács T, Kéri S. Contrast, motion, perceptual integration, and neurocognition in schizophrenia: the role of fragile-X related mechanisms. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:92–97. doi: 10.1016/j.pnpbp.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Komachi H, Shirasaki Y, Miyatake T, Yanagisawa K. Processing of beta/A4 amyloid precursor protein is altered in the hippocampus of reserpinized rat brain. Biochem Biophys Res Commun. 1994;202(1):293–298. doi: 10.1006/bbrc.1994.1926. [DOI] [PubMed] [Google Scholar]
- Kovács T, Keleman O, Kéri S. Decreased fragile X mental retardation protein (FMRP) is associated with lower IQ and earlier illness onset in patients with schizophrenia. Psychiatry Res. 2013;210(3):690–693. doi: 10.1016/j.psychres.2012.12.022. [DOI] [PubMed] [Google Scholar]
- Krüger S, Seminowicz D, Goldapple K, Kennedy SH, Mayberg HS. State and trait influences on mood regulation in bipolar disorder: blood flow differences with an acute mood challenge. Biol Psychiatry. 2003;54(11):1274–1283. doi: 10.1016/s0006-3223(03)00691-7. [DOI] [PubMed] [Google Scholar]
- Li X, Luna J, Lombroso PJ, Francke U. Molecular cloning of the human homolog of a striatum-enriched phosphatase (STEP) gene and chromosomal mapping of the human and murine loci. Genomics. 1995;28(3):442–449. doi: 10.1006/geno.1995.1173. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am J Psychiatry. 2002;159(11):1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- Lombroso PJ, Murdoch G, Lerner M. Molecular characterization of a protein-tyrosine-phosphatase enriched in striatum. Proc Natl Acad Sci U S A. 1991;88(16):7242–7246. doi: 10.1073/pnas.88.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Hensch TK, Ackerman L, Barbel S, Jan LY, Jan YN. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature. 1996;379(6568):837–840. doi: 10.1038/379837a0. [DOI] [PubMed] [Google Scholar]
- Martinez LA, Tejada-Simon MV. Pharmacological inactivation of the small GTPase Rac1 impairs long-term plasticity in the mouse hippocampus. Neuropharmacology. 2011;61(1–2):305–312. doi: 10.1016/j.neuropharm.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S, Leung J, Sharp C, Blackwood D, Muir W, St Clair D. Screening schizophrenic patients for mutations in the amyloid precursor protein gene. Psychiatr Genet. 1994;4(1):23–27. doi: 10.1097/00041444-199421000-00004. [DOI] [PubMed] [Google Scholar]
- Mortilla M, Amaducci L, Bruni A, Montesi MP, Trubnikov A, De Cataldo S, Pallanti S, Pazzagli A, Grecu L, Servi P. Absence of APP713 mutation in Italian and Russian families with schizophrenia. Neurosci Lett. 1994;165(1–2):45–47. doi: 10.1016/0304-3940(94)90705-6. [DOI] [PubMed] [Google Scholar]
- Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20(14):5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton N, Williams HJ, Williams NM, Spurlock G, Zammit S, Jones G, Jones S, Owen R, O’Donovan MC, Owen MJ. Mutation screening of the Homer gene family and association analysis in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2003;120B(1):18–21. doi: 10.1002/ajmg.b.20032. [DOI] [PubMed] [Google Scholar]
- Palotás A, Pákáski M, Palotás M, Hugyecz M, Molnár J, Penke B, Janka Z, Kálmán J. Effect of haloperidol and risperidone on amyloid precursor protein levels in vivo. Brain Res Bull. 2003;62(2):93–99. doi: 10.1016/j.brainresbull.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Pelov I, Teltsh O, Greenbaum L, Rigbi A, Kanyas-Sarner K, Lerer B, Lombroso P, Kohn Y. Involvement of PTPN5, the gene encoding the striatal-enriched protein tyrosine phosphatase, in schizophrenia and cognition. Psychiatr Genet. 2012;22(4):168–176. doi: 10.1097/YPG.0b013e3283518586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polese D, de Serpis AA, Ambesi-Impiombato A, Muscettola G, de Bartolomeis A. Homer 1a gene expression modulation by antipsychotic drugs: involvement of the glutamate metabotropic system and effects of D-cycloserine. Neuropsychopharmacology. 2002;27(6):906–913. doi: 10.1016/S0893-133X(02)00371-8. [DOI] [PubMed] [Google Scholar]
- Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J. Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci. 2006;26(27):7212–7221. doi: 10.1523/JNEUROSCI.1450-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, O’Dushlaine C, Chambert K, Bergen SE, Kähler A, Duncan L, Stahl E, Genovese G, Fernández E, Collins MO, Komiyama NH, Choudhary JS, Magnusson PK, Banks E, Shakir K, Garimella K, Fennell T, DePristo M, Grant SG, Haggarty SJ, Gabriel S, Scolnick EM, Lander ES, Hultman CM, Sullivan PF, McCarroll SA, Sklar P. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506(7487):185–190. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci. 2008;28(2):543–547. doi: 10.1523/JNEUROSCI.5019-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck A, Bardoni B, Moro A, Bagni C, Mandel JL. A highly conserved protein family interacting with the fragile X mental retardation protein (FMRP) and displaying selective interactions with FMRP-related proteins FXR1P and FXR2P. Proc Natl Acad Sci U S A. 2001;98(15):8844–8849. doi: 10.1073/pnas.151231598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck A, Bardoni B, Langmann C, Harden N, Mandel JL, Giangrande A. CYFIP/Sra-1 controls neuronal connectivity in Drosophila and links the Rac1 GTPase pathway to the fragile X protein. Neuron. 2003;38(6):887–898. doi: 10.1016/s0896-6273(03)00354-4. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J Neurosci. 1997;17(1):438–458. doi: 10.1523/JNEUROSCI.17-01-00438.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergé A, Fourgeaud L, Hémar A, Choquet D. Receptor activation and Homer differentially control the lateral mobility of metabotropic glutamate receptor 5 in the neuronal membrane. J Neurosci. 2002;22(10):3910–3920. doi: 10.1523/JNEUROSCI.22-10-03910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R, Zhang Y, Shi Y, Shi S, Jiang L. Inhibition of peroxisomal β–oxidation by thioridazine increases the amount of VLCFAs and Aβ generation in the rat brain. Neurosci Lett. 2012;528(1):6–10. doi: 10.1016/j.neulet.2012.08.086. [DOI] [PubMed] [Google Scholar]
- Spellmann I, Rujescu D, Musil R, Mayr A, Giegling I, Genius J, Zill P, Dehning S, Opgen-Rhein M, Cerovecki A, Hartmann AM, Schäfer M, Bondy B, Müller N, Möller HJ, Riedel M. Homer-1 polymorphisms are associated with psychopathology and response to treatment in schizophrenic patients. J Psychiatr Res. 2011;45(2):234–241. doi: 10.1016/j.jpsychires.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Kleschen MJ, Oleson EB, Dehoff MH, Schwarz MK, Seeburg PH, Worley PF, Kalivas PW. Behavioral and neurochemical phenotyping of Homer1 mutant mice: possible relevance to schizophrenia. Genes Brain Behav. 2005;4(5):237–288. doi: 10.1111/j.1601-183X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Kalivas PW, Worley PF. Homer proteins: implications for neuropsychiatric disorders. Curr Opin Neurobiol. 2006;16(3):251–257. doi: 10.1016/j.conb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Threadgill R, Bobb K, Ghosh A. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron. 1997;19(3):625–634. doi: 10.1016/s0896-6273(00)80376-1. [DOI] [PubMed] [Google Scholar]
- Tomasetti C, Dell’Aversano C, Iasevoli F, Marmo F, de Bartolomeis A. The acute and chronic effects of combined antipsychotic-mood stabilizing treatment on the expression of cortical and striatal postsynaptic density genes. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):184–197. doi: 10.1016/j.pnpbp.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Turner PR, O’Connor K, Tate WP, Abraham WC. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol. 2003;70(1):1–32. doi: 10.1016/s0301-0082(03)00089-3. [DOI] [PubMed] [Google Scholar]
- Wassink TH, Piven J, Patil SR. Chromosomal abnormalities in a clinic sample of individuals with autistic disorder. Psychiatr Genet. 2001;11(2):57–63. doi: 10.1097/00041444-200106000-00001. [DOI] [PubMed] [Google Scholar]
- Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol. 2007;5(3):e52. doi: 10.1371/journal.pbio.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Venkitaramani DV, Gladding CM, Zhang Y, Kurup P, Molnar E, Collingridge GL, Lombroso PJ. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008;28(42):10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, He J, Zhang R, Kong L, Tempier A, Kong J, Li XM. Therapeutic effects on memory deficit and brain β-amyloid plaque pathology in a transgenic mouse model of Alzheimer’s disease. Curr Alzheimer Res. 2013;10(3):270–278. doi: 10.2174/1567205011310030006. [DOI] [PubMed] [Google Scholar]