Abstract
IMPORTANCE
Basal cell carcinoma (BCC) is the most common type of skin cancer and is usually nonpigmented. Shiny white structures (SWSs) are frequently present in BCC.
OBJECTIVE
To determine the diagnostic accuracy of various morphologies of SWSs for diagnosis of nonpigmented BCC.
DESIGN, SETTING, AND PARTICIPANTS
Nonpigmented skin tumors, determined clinically and dermoscopically, were identified from a database of lesions consecutively biopsied over a 3-year period (January 2, 2009, to December 31, 2012) from a single dermatology practice. Data analysis was conducted from October 9, 2014, to November 15, 2015. Investigators blinded to histopathologic diagnosis evaluated the polarized dermoscopic images for the presence of SWSs, which were categorized as blotches, strands, short white lines, and rosettes. Measures of diagnostic accuracy for BCC were estimated. Participants included 2375 patients from a dermatologic clinic in Plantation, Florida. Review of the medical records identified 2891 biopsied skin lesions; 457 of these were nonpigmented neoplasms.
MAIN OUTCOMES AND MEASURES
Diagnosis of BCC with dermoscopy compared with all other diagnoses combined was the primary outcome; the secondary outcome was diagnosis of BCC compared with amelanotic melanoma. We calculated diagnostic accuracy measured as odds ratios (ORs), sensitivity, and specificity of shiny white blotches and/or strands for the diagnosis of BCC.
RESULTS
Of the 457 nonpigmented neoplasms evaluated, 287 (62.8%) were BCCs, 106 (23.2%) were squamous cell carcinoma, 39 (8.5%) were lichen planus–like keratosis, 21 (4.6%) were melanomas, and 4 (0.9%) were nevi. The prevalence of SWSs was 49.0% (n = 224). In multivariate analysis (reported as OR [95% CI]) controlling for age, sex, and anatomical location, the presence of any SWS was associated with a diagnosis of BCC (2.3 [1.5–3.6]; P < .001). Blotches (6.3 [3.6–10.9]; P < .001), strands (4.9 [2.9–8.4]; P < .001), and blotches and strands together (6.1 [3.3–11.3]; P < .001) were positively associated with BCC. Shiny white blotches and strands together had a diagnostic sensitivity of 30% and specificity of 91%.
CONCLUSIONS AND RELEVANCE
The combined presence of shiny white blotches and strands is associated with high diagnostic specificity for nonpigmented BCC.
Basal cell carcinoma (BCC) is the most common malignant neoplasm in fair-skinned populations worldwide. 1–4 In the United States, age-adjusted BCC incidence rates have doubled over the past 2 decades, with recent estimates of 1019 cases per 100000 person-years for women and 1488 cases per 100000 person-years for men.5 Although it rarely metastasizes, BCC can cause significant local tissue destruction and cosmetic impairment, making treatment options challenging in advanced stages.6 Diagnosing BCC early has the greatest short-term potential to decrease patient morbidity and health care costs associated with treatment.
Dermoscopic features for pigmented BCCs were originally described by Menzies et al7 in 2000. These features include large blue-gray ovoid nests, multiple non aggregated bluegray dots, ulceration; arborizing “tree like” telangiectasia, spoke-wheel areas, and leaf like areas. These criteria were established using nonpolarized dermoscopy and were selected because they have high (>80%) diagnostic specificity.7,8 However, 4 of the 6 criteria are limited exclusively to pigmented BCC, which accounts for less than 10% of all BCCs in fair-skinned populations.7–9 Lallas et al10 recently found that approximately 30% of clinically amelanotic BCCs reveal pigment structures under dermoscopy; however, the vast majority of BCCs still have no pigment criteria dermoscopically.
Polarized dermoscopy has emerged as the screening modality of choice because it does not require a liquid interface or skin contact and enhances the visualization of certain dermoscopic structures, including vessels, vascular blush, and shiny white structures (SWSs).11 Few studies have focused on the dermoscopic features present in clinically and dermoscopically nonpigmented BCCs, particularly using polarized dermoscopy. Of the 6 criteria for pigmented BCC identified by Menzies et al,7 only 2 (arborizing vessels and ulceration) may be helpful in identifying nonpigmented BCCs. However, Lallas et al12 demonstrated that both ulceration and arborizing vessels are features associated mainly with the nodular subtype of BCC. Additional proposed dermoscopic criteria for BCC include short fine telangiectasias (SFTs), multiple small erosions, concentric structures, and multiple in-focus blue-gray dots. However, the sensitivity and specificity of these individual criteria for BCC diagnosis have not been determined, and the interrater reliability of some criteria, such as SFT, has been shown to be poor.12–14 Hence, there is a need to identify additional features to aid in the detection of nonpigmented BCCs, including those lacking ulceration or arborizing vessels. Previous studies12–17 observed that many nonpigmented BCCs manifest SWSs when viewed with polarized light, but these dermoscopic features have not been formally and systematically evaluated for their diagnostic potential. The primary objective of this study was to determine measures of diagnostic accuracy for various morphologies of SWSs in the diagnosis of nonpigmented BCC.
Methods
This study was approved by the institutional review board of the University of Miami. All images originated from a deidentified database of lesions consecutively biopsied in a dermatology practice in Plantation, Florida. Standard procedures in this practice included capturing clinical and dermoscopic images of all lesions selected for biopsy. Images were captured with a Nikon 1 camera (Nikon USA, Inc) using Dermlite DL2 pro HR for polarized images and Dermlite fluid for nonpolarized images at 10-fold magnification (3 Gen, LLC). Only the individual lesion’s close-up clinical (cropped images without patient identifiers) and dermoscopic images were included in the study database. One of us (C.N.-D.) reviewed the clinical and dermoscopic images of all lesions biopsied over a 3-year period (January 2, 2009–December 31, 2012) and selected those without discernible pigment. Any tumors revealing pigmented structures clinically or dermoscopically were excluded.13 Collision tumors were also excluded. Dermatofibromas were excluded using the rationale that, although this tumor frequently manifests SWSs,18 they can typically be identified via clinical and dermoscopic evaluation without difficulty. Seborrheic keratoses were also excluded since they are rarely amelanotic, are easy to identity based on clinical and dermoscopic morphology, and are infrequently biopsied; as a result of these factors, data on seborrheic keratoses were not available for analysis. Anatomical site of the tumor and participants’ age and sex were recorded.
Image Assessment
Two of us (C.N.-D. and S.B.) initially trained in dermoscopic analysis by an expert dermoscopist (A.A.M.) were blinded to histopathologic diagnosis and reviewed the polarized and nonpolarized contact dermoscopic images of all lesions for consensus agreement on the presence of SWSs. A third reviewer (A.A.M) resolved disagreement when consensus could not be achieved.
If SWSs were present, they were classified as (1) blotches (also known as clods; discrete, small or large structureless areas); (2) strands (long thick or thin lines, randomly distributed or parallel, and not orthogonally oriented); (3) rosettes (cluster of 4 white dots in a 4-leaf clover–like arrangement); and (4) short white lines (also known as crystalline structures and chrysalis; fine lines that intersect or are oriented orthogonally to each other).19,20 Shiny white structures that could not be classified into one of these specific morphologies were categorized as nonspecified.
All lesions were evaluated for the presence or absence of any Menzies criteria. Lesions without Menzies criteria were considered featureless. Using the consensus method described above, featureless lesions were further evaluated for the presence of additional BCC criteria, including SFT; multiple in-focus, blue-gray dots; multiple small erosions; and concentric structures. To evaluate interrater accuracy in classifying the morphology of SWSs, we calculated the Cohen κ coefficient between the 2 reviewers (C.N.-D and S.B.) in a randomly selected subset of lesions (n = 28).
Statistical Analysis
Distribution of participant and lesion characteristics was evaluated by histologic diagnosis of the study lesions. Descriptive statistics and graphical methods were used to describe the study participants and the characteristics of the individual lesions. Based on bivariate cross-tabulations, relative frequencies for lesion characteristics for squamous cell carcinoma (SCC), lichen planus–like keratosis (LPLK), melanoma, and nevi were relatively consistent; therefore, a dichotomous variable for histopathologic diagnosis (BCC vs all other diagnoses combined) was created and used as the primary study outcome variable. As a secondary outcome, BCC vs amelanotic melanoma was evaluated. Univariate associations between lesion diagnosis and participant characteristics were assessed using unpaired, 2-tailed t tests and Pearson χ2 analysis for continuous and categorical variables, respectively.
Preliminary estimates of the diagnostic accuracy of lesion characteristics were made by dichotomizing the study sample (BCCvs all other diagnoses combined)with each of the dermoscopic features evaluated. Regression models for binary outcomes were created using the general estimating equations approach with a log link and an exchangeable correlation structure. Because significant associations were observed between sex, age, and lesion diagnosis, these variables were included in all of the regression models to control for potential confounding. Estimates for sensitivity and specificity are presented with their associated 95%CIs. Crude and adjusted odds ratios (ORs) for the association between lesion diagnosis (BCC vs all other diagnoses combined) and dermoscopic features were performed using logistic regression. Adjusted models included age, sex, and anatomical location (head and neck vs other area). Data analysis was conducted from October 9, 2014, to November 15, 2015. All analyses were performed with Stata, version 12.1 (StataCorp).
Results
A review of records on 2375 patients identified 2891 skin lesions; of these, 457 were nonpigmented neoplasms, including 287 (62.8%) BCCs, 106 (23.2%) SCCs, 39 (8.5%) LPLKs, 21 (4.6%) melanomas, and 4 (0.9%) nevi. Demographics and anatomical location of the BCC neoplasms are reported in Table 1. Basal cell carcinoma lesions were more likely than other diagnoses to be located on the head and neck, to occur in younger individuals, and to occur inmen (P < .05 for all comparisons).
Table 1.
Demographics and Tumor Location Characteristics
| Characteristic | No. (%) | P Value | ||
|---|---|---|---|---|
| Overall (N = 457) | BCC (n = 287) | Other Diagnoses (n = 170) | ||
| Age, mean (SD), y | 64.3 (14.1) | 62.5 (14.7) | 67.5 (12.6) | <.001a |
| Sex | ||||
| Male | 282 (61.7) | 190 (66.2) | 92 (54.1) | .01b |
| Female | 175 (38.3) | 97 (33.8) | 78 (45.9) | |
| Anatomical location | ||||
| Head and neck | 134 (29.3) | 110 (38.3) | 24 (14.1) | <.001b |
| Trunk | 124 (27.1) | 86 (30.0) | 38 (22.3) | |
| Extremity | ||||
| Upper | 84 (18.4) | 49 (17.1) | 35 (20.6) | |
| Lower | 113 (24.7) | 42 (14.6) | 71 (41.8) | |
| Genitalia | 1 (0.2) | 0 | 1 (0.6) | |
| Missing | 1 (0.2) | 0 | 1 (0.6) | |
Abbreviation: BCC, basal cell carcinoma.
Determined by unpaired, 2-tailed t test.
Determined by Pearson χ2 analysis.
Basal cell carcinoma subtype distribution was nodular for 223 lesions (77.7%), superficial for 25 (8.7%), and morpheaform for 36 (12.5%). Histologic subtype was unavailable for 3 BCCs (1.0%).
The prevalence of SWSs in the entire study sample was 49.0% (n = 224): 54.0% (n = 155) of BCCs, 41.5% (n = 44) of SCCs, 41.0% (n = 16) of LPLKs, 42.9% (n = 9) of melanomas, and 0% of nevi (Table 2). The prevalence of SWSs did not differ by BCC subtype (P = .83, analyzed only for nodular vs superficial BCC). When stratified by morphology, of the 457 nonpigmented neoplasms, strands (29.5% [135 of 457]) were the most prevalent SWSs identified, followed by blotches (28.9% [132]), short white lines (9.0% [41]), rosettes (8.8% [40]), and nonspecified (4.6% [21]).
Table 2.
Cross-Classification of Dermoscopic Characteristics by Lesion Diagnoses
| Characteristic | No. (%) | P Valuea | |||||
|---|---|---|---|---|---|---|---|
| BCC (n = 287) | Other Diagnoses | ||||||
| Combined (n = 170) | SCC (n = 106) | LPLK (n = 39) | Melanoma (n = 21) | Nevus (n = 4) | |||
| Blotches | 110 (38.3) | 22 (12.9) | 16 (15.1) | 4 (10.3) | 2 (9.5) | 0 | <.001 |
| Strands | 108 (37.6) | 27 (15.9) | 18 (17.0) | 7 (18.0) | 2 (9.5) | 0 | <.001 |
| Blotches and strands | 86 (30.0) | 16 (9.4) | 13 (12.3) | 2 (5.1) | 1 (4.8) | 0 | <.001 |
| Short white lines | 18 (6.3) | 23 (13.5) | 14 (13.2) | 4 (10.3) | 5 (23.8) | 0 | .009 |
| Rosettes | 20 (7.0) | 20 (11.8) | 13 (12.3) | 4 (10.3) | 3 (14.3) | 0 | .08 |
| Nonspecified SWSs | 8 (2.8) | 13 (7.6) | 7 (6.6) | 5 (12.8) | 1 (4.8) | 0 | .02 |
| SWSs | |||||||
| Any | 155 (54.0) | 69 (40.6) | 44 (41.5) | 16 (41.0) | 9 (42.9) | 0 | .006 |
| None | 132 (46.0) | 101 (59.4) | 62 (58.5) | 23 (59.0) | 12 (57.1) | 4 (100) | |
Abbreviations: BCC, basal cell carcinoma; LPLK, lichen planus–like keratosis; SCC, squamous cell carcinoma; SWSs, shiny white structures.
P value based on Pearson χ2 for the association between dermoscopic features and diagnosis (BCC vs other diagnoses combined).
In multivariate analysis (reported as OR [95% CI]) controlling for age, sex, and anatomical location, the presence of any SWS was associated with a diagnosis of BCC (2.3 [1.5–3.6]; P < .001) (Table 3). Blotches (6.3 [3.6–10.9]; P < .001), strands (4.9 [2.9–8.4]; P < .001), and blotches and strands together (6.1 [3.3–11.3]; P < .001) (Figure) were all positively associated with a diagnosis of BCC. Short white lines (0.4 [0.2–0.9]; P = .02) and nonspecified SWSs (0.3 [0.1–0.8]; P = .02) were inversely associated with a diagnosis of BCC. Rosettes were not associated with a diagnosis of BCC (0.6 [0.3–1.3]; P = .22).
Table 3.
Estimates for the Association Between BCC and Other Diagnosis and Dermoscopic Characteristics
| Characteristic | Crude OR (95% CI) | P Value | Adjusted OR (95% CI)a | P Value |
|---|---|---|---|---|
| Blotches | 4.2 (2.5–6.9) | <.001 | 6.3 (3.6–10.9) | <.001 |
| Strands | 3.2 (2.0–5.1) | <.001 | 4.9 (2.9–8.4) | <.001 |
| Short white lines | 0.4 (0.2–0.8) | .01 | 0.4 (0.2–0.9) | .02 |
| Rosettes | 0.6 (0.3–1.1) | .08 | 0.6 (0.3–1.3) | .22 |
| Blotches and strands | 4.1 (2.3–7.3) | <.001 | 6.1 (3.3–11.3) | <.001 |
| Nonspecified SWSs | 0.3 (0.1–0.9) | .02 | 0.3 (0.1–0.8) | .02 |
| Any SWSs | 1.7 (1.2–2.5) | .006 | 2.3 (1.5–3.6) | <.001 |
Abbreviations: BCC, basal cell carcinoma; OR, odds ratio; SWSs, shiny white structures.
Adjusted for age, sex, and anatomical location (head and neck vs other).
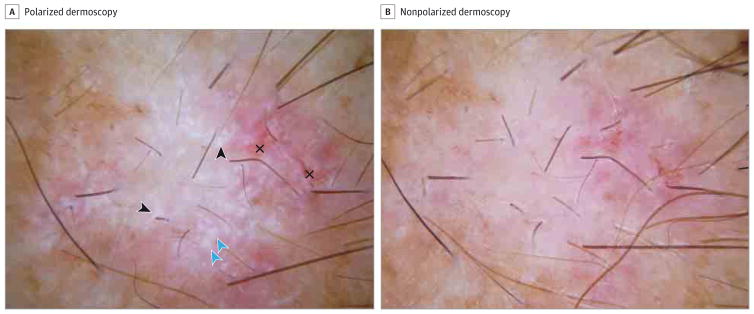
Figure. Dermoscopic Features of Basal Cell Carcinoma.
A, Pink lesion displaying numerous shiny white blotches (blue arrowheads) and strands (black arrowheads) with polarized dermoscopy. In addition, small erosions (crosses) are displayed (original magnification, ×10). B, Blotches and strands cannot be visualized with nonpolarized dermoscopy (original magnification, ×10).
The overall sensitivity, specificity, and area under the receiver operating characteristic curve for blotches, strands, and blotches and strands together were similar. For all participants, the presence of blotches alone had the highest area under the receiver operating characteristic curve (0.63); sensitivity was 0.38 (95% CI, 0.33–0.44) and specificity was 0.84 (95%CI, 0.77–0.89). The use of blotches and strands together as a diagnostic criterion resulted in a lower sensitivity (30%) but higher specificity (91%) compared with the use of each structure (blotches or strands) independently. The positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio for blotches and strands together for the diagnosis of BCC was 84.3% (95% CI, 75.8%–90.8%), 43.3% (95% CI, 38.2%–48.7%), 3.2 (95% CI, 1.9–5.2), and 0.8 (95%CI, 0.7–0.8), respectively. When the presence of blotches and strands was compared for BCC against melanomaas the only diagnosis, the specificity rose to 95.2%(95% CI, 76.2%–99.9%).
Finally, of the 54 BCCs lacking Menzies criteria, 24 (44.4%) displayed additional BCC criteria: SFTs in 10 lesions (41.7%); multiple in-focus, blue-gray dots in 10 (41.7%); multiple small erosions in 4 (16.7%); and concentric structures in 1 (4.2%). Twenty-six of the 54 BCCs (48.1%) without Menzies criteria included both blotches and strands. Of these 26 BCCs, 3 (11.5%) were superficial, 1 (3.8%) was morpheaform, and 22 (84.6%) were nodular. Short fine telangiectasias were present in 5 BCCs (19.2%); multiple in-focus, blue-gray dots in 3 (11.5%); and concentric structures in 1 (3.8%). In all, 17 of the 26 BCCs (65.4%) lacking Menzies criteria but displaying both blotches and strands could be identified only by the presence of SWSs. Of note, 4 of the 287 BCCs (1.4%) did not display any Menzies criteria, nonclassic criteria and/or blotches, or strands.
The interrater accuracy for differentiating the various SWS morphologies from each other was determined. The Cohen κ coefficient values were 0.96, 0.86, 0.89, and 0.93 for blotches, strands, short lines, and rosettes, respectively.
Discussion
In this study, we evaluated the diagnostic accuracy of various morphologies of SWSs for the diagnosis of BCC among clinically and dermoscopically nonpigmented neoplasms using polarized dermoscopy. We identified the criterion of blotches and strands together to be significantly associated with BCC, having sensitivity and specificity of 30% and 91%, respectively. These measures of diagnostic accuracy are comparable to the original criteria identified for pigmented BCC (Table 4).7 In addition, the new criteria of SWSs may help us to detect a subset of nonpigmented BCCs that are otherwise unrecognizable using the current Menzies criteria.
Table 4.
Proposed Updated Criteria for Basal Cell Carcinoma
| Criterion | Sensitivity, % | Specificity, %a |
|---|---|---|
| Large blue-gray ovoid nests | 55 | 97/99 |
| Arborizing telangiectasia | 52 | 77/92 |
| Multiple blue-gray globules | 27 | 87/97 |
| Ulceration | 27 | 87/97 |
| Leaflike structures | 17 | 100/100 |
| Spoke-wheel–like structures | 10 | 100/100 |
| Blotches and strands | 30 | 91/95 |
The number on the left represents the specificity for a specific dermoscopic feature compared with a subset of melanocytic and nonmelanocytic benign lesions; the number on the right represents the specificity evaluated only against melanoma. Data obtained from Menzies et al.7
Shiny white structures are visible only with polarized dermoscopy and can exhibit a variety of morphologies.21 Some of these structures (blotches, strands, and short white lines) have been correlated with collagen alterations, such as fibrosis, in the underlying stroma.22 For this subset of SWSs, it is thought that collagen bundles have birefringent properties that cause rapid randomization of polarized light, which explains why they can be seen only with polarized dermoscopy.23
However, rosettes are thought to be an optical property resulting from the interaction between polarized light and keratin-filled adnexal openings.24 This optical effect is likely similar to the appearance of Maltese crosses found in lipid-filled fluids (as in the urine of patients with nephrotic syndrome).20,24
Although SWSs can be found in a variety of benign and malignant skin tumors, their presence should increase suspicion for malignant neoplasms, including BCC, SCC, and melanoma.25,26 Although the probable management of any lesion displaying SWSs would be the same (ie, biopsy), the morphology of SWSs may help to further delineate between the different malignant tumors; with rosettes, blotches and strands, and short fine lines increasing the likelihood for SCC, BCC, and melanoma, respectively.19,27 On the benign spectrum, dermatofibromas and LPLKs also commonly manifest SWSs. However, in most cases, clinical and dermoscopic evaluation with palpation should allow for accurate identification of dermatofibromas without biopsy. For this reason, we chose to exclude dermatofibromas from this investigation. In contrast, LPLKs remain a challenging lesion to identify clinically and are commonly biopsied; therefore, LPLKs were included in the study.28,29
The prevalence of SWSs in BCC and other skin tumors has been investigated. One study19 identified SWSs in 122 BCCs (69.1%) and in 71 melanomas (28.5%). Shiny white blotches (previously referred to as shiny white areas)were present in a higher percentage of BCCs than melanomas (39 [28.5%] vs 8 [3.2%]),19 which is similar to the prevalence of shiny white blotches in the study herein (38.3% of BCC and 9.5% of melanomas, respectively). In a second study21 restricted to BCC, shiny white areas were found in 38 (25.5%) of the lesions, shiny white lines and strands together in 103 (69.1%) of the lesions, and rosettes in 17 (11.4%) of the lesions.
Another study30 examined 538 lesions, including BCC, SCC, actinic keratosis, LPLK, and melanoma, and found that SWSs were observed in 208 (38.7%), which is comparable to the overall prevalence in our study (224 [49.0%]). Basal cell carcinomas were more likely than other diagnoses to display a combination of white shiny areas and lines or strands (61 of 191 [31.9%]; P < .001) and to have white shiny lines distributed without any organized pattern (data not specified; P < .001). Finally, Popadić15 recently reported a prevalence of 51.7% (78 of 151 BCCs) for large shiny white areas in BCC, which we believe is the same structure as the blotches reported herein.
The diagnosis of nonpigmented BCC, particularly the superficial histologic subtype, remains challenging in clinical practice since they often lack any of the Menzies BCC criteria originally described for pigmented BCC.12,16,17 A plethora of case reports and case series have evaluated additional dermoscopic criteria for BCC, including SFTs, multiple small erosions, and multiple in-focus, blue-gray dots and concentric structures, among others.13,14,16 These features may be observed in up to 26.1% of BCCs13 and may be more common in superficial BCCs. Furthermore, Altamura et al13 showed that approximately 14% to 16% of nonpigmented and lightly pigmented BCCs have short, fine superficial telangiectasias, and approximately 8% to 11% of these tumors may have small erosions that could aid in their diagnosis. However, none of these features has been formally evaluated for measures of validity.
Twenty-six of 54 nonpigmented BCCs (48.1%) that did not have Menzies criteria could be identified using blotches and strands as a diagnostic criterion. Moreover, 65.4% of these BCCs did not display any other BCC criteria. This finding has a significant potential effect given the high burden of disease of BCC. Furthermore, our high interobserver reliability, which ranged from 0.86 to 0.96 for the 4 morphologies of SWS, strengthens our results.
Limitations of this study include its retrospective design, use of images from a single dermatology practice, and relatively small sample size, particularly the number of melanomas included. We also were unable to stratify the prevalence or diagnostic accuracy of SWSs by additional criteria, such as anatomical location, skin type, skin color, or presence of other dermoscopic features.
Conclusions
Shiny white blotches and/or strands identified with polarized light on dermoscopy had a diagnostic specificity of 91% for nonpigmented BCC. With this high level of specificity, these features should be added as another criterion that can be relied on for the detection of BCC.
Acknowledgments
Funding/Support: This study was supported in part by support grant/core grant P30 CA008748 from the Memorial Sloan Kettering Cancer Center.
Footnotes
Conflict of Interest Disclosures: Dr Rabinovitz reported receiving financial compensation with equipment as a speaker and for testing dermatoscopes for 3-Gen, Canfield, and Heine. No other disclosures were reported.
Role of the Funder/Sponsor: Memorial Sloan Kettering Cancer Center had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Previous Presentation: This work was described in an oral presentation at the International Dermoscopy Society Meeting conducted during the 73rd American Academy of Dermatology meeting; March 21, 2015; San Francisco, California.
Author Contributions: Drs Navarrete-Dechent and Marghoob had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Navarrete-Dechent, Marchetti, Marghoob.
Acquisition, analysis, or interpretation of data: Navarrete-Dechent, Bajaj, Marchetti, Rabinovitz, Dusza, Marghoob.
Drafting of the manuscript: Navarrete-Dechent, Bajaj, Marchetti, Dusza.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Navarrete-Dechent, Bajaj, Dusza.
Obtained funding: Marghoob.
Administrative, technical, or material support: Navarrete-Dechent, Bajaj, Dusza, Marghoob.
Study supervision: Navarrete-Dechent, Marchetti, Dusza, Marghoob.
References
- 1.Reinau D, Surber C, Jick SS, Meier CR. Epidemiology of basal cell carcinoma in the United Kingdom: incidence, lifestyle factors, and comorbidities. Br J Cancer. 2014;111(1):203–206. doi: 10.1038/bjc.2014.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 3.Chinem VP, Miot HA. Epidemiology of basal cell carcinoma. An Bras Dermatol. 2011;86(2):292–305. doi: 10.1590/s0365-05962011000200013. [DOI] [PubMed] [Google Scholar]
- 4.Koh D, Wang H, Lee J, Chia KS, Lee HP, Goh CL. Basal cell carcinoma, squamous cell carcinoma and melanoma of the skin: analysis of the Singapore Cancer Registry data 1968–97. Br J Dermatol. 2003;148(6):1161–1166. doi: 10.1046/j.1365-2133.2003.05223.x. [DOI] [PubMed] [Google Scholar]
- 5.Wu S, Han J, Li WQ, Li T, Qureshi AA. Basal-cell carcinoma incidence and associated risk factors in US women and men. Am J Epidemiol. 2013;178(6):890–897. doi: 10.1093/aje/kwt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohan SV, Chang AL. Advanced basal cell carcinoma: epidemiology and therapeutic innovations. Curr Dermatol Rep. 2014;3:40–45. doi: 10.1007/s13671-014-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menzies SW, Westerhoff K, Rabinovitz H, Kopf AW, McCarthy WH, Katz B. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136(8):1012–1016. doi: 10.1001/archderm.136.8.1012. [DOI] [PubMed] [Google Scholar]
- 8.Menzies SW. Dermoscopy of pigmented basal cell carcinoma. Clin Dermatol. 2002;20(3):268–269. doi: 10.1016/s0738-081x(02)00229-8. [DOI] [PubMed] [Google Scholar]
- 9.Tan WP, Tan AW, Ee HL, Kumarasinghe P, Tan SH. Melanization in basal cell carcinomas:microscopic characterization of clinically pigmented and non-pigmented tumours. Australas J Dermatol. 2008;49(4):202–206. doi: 10.1111/j.1440-0960.2008.00469.x. [DOI] [PubMed] [Google Scholar]
- 10.Lallas A, Argenziano G, Kyrgidis A, et al. Dermoscopy uncovers clinically undetectable pigmentation in basal cell carcinoma. Br J Dermatol. 2014;170(1):192–195. doi: 10.1111/bjd.12634. [DOI] [PubMed] [Google Scholar]
- 11.Benvenuto-Andrade C, Dusza SW, Agero AL, et al. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol. 2007;143(3):329–338. doi: 10.1001/archderm.143.3.329. [DOI] [PubMed] [Google Scholar]
- 12.Lallas A, Tzellos T, Kyrgidis A, et al. Accuracy of dermoscopic criteria for discriminating superficial from other subtypes of basal cell carcinoma. J Am Acad Dermatol. 2014;70(2):303–311. doi: 10.1016/j.jaad.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Altamura D, Menzies SW, Argenziano G, et al. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62(1):67–75. doi: 10.1016/j.jaad.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 14.Lallas A, Apalla Z, Argenziano G, et al. The dermatoscopic universe of basal cell carcinoma. Dermatol Pract Concept. 2014;4(3):11–24. doi: 10.5826/dpc.0403a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popadić M. Statistical evaluation of dermoscopic features in basal cell carcinomas. Dermatol Surg. 2014;40(7):718–724. doi: 10.1111/dsu.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 16.Scalvenzi M, Lembo S, Francia MG, Balato A. Dermoscopic patterns of superficial basal cell carcinoma. Int J Dermatol. 2008;47(10):1015–1018. doi: 10.1111/j.1365-4632.2008.03731.x. [DOI] [PubMed] [Google Scholar]
- 17.Giacomel J, Zalaudek I. Dermoscopy of superficial basal cell carcinoma. Dermatol Surg. 2005;31(12):1710–1713. doi: 10.2310/6350.2005.31314. [DOI] [PubMed] [Google Scholar]
- 18.Agero AL, Taliercio S, Dusza SW, Salaro C, Chu P, Marghoob AA. Conventional and polarized dermoscopy features of dermatofibroma. Arch Dermatol. 2006;142(11):1431–1437. doi: 10.1001/archderm.142.11.1431. [DOI] [PubMed] [Google Scholar]
- 19.Liebman TN, Rabinovitz HS, Balagula Y, Jaimes-Lopez N, Marghoob AA. White shiny structures in melanoma and BCC. Arch Dermatol. 2012;148(1):146. doi: 10.1001/archdermatol.2011.618. [DOI] [PubMed] [Google Scholar]
- 20.Liebman TN, Scope A, Rabinovitz H, Braun RP, Marghoob AA. Rosettes may be observed in a range of conditions. Arch Dermatol. 2011;147(12):1468. doi: 10.1001/archdermatol.2011.312. [DOI] [PubMed] [Google Scholar]
- 21.Liebman TN, Jaimes-Lopez N, Balagula Y, et al. Dermoscopic features of basal cell carcinomas: differences in appearance under non-polarized and polarized light. Dermatol Surg. 2012;38(3):392–399. doi: 10.1111/j.1524-4725.2011.02205.x. [DOI] [PubMed] [Google Scholar]
- 22.Botella-Estrada R, Requena C, Traves V, Nagore E, Guillen C. Chrysalis and negative pigment network in Spitz nevi. Am J Dermatopathol. 2012;34(2):188–191. doi: 10.1097/DAD.0b013e3182222ac1. [DOI] [PubMed] [Google Scholar]
- 23.Marghoob AA, Cowell L, Kopf AW, Scope A. Observation of chrysalis structures with polarized dermoscopy. Arch Dermatol. 2009;145(5):618. doi: 10.1001/archdermatol.2009.28. [DOI] [PubMed] [Google Scholar]
- 24.Rubegni P, Tataranno DR, Nami N, Fimiani M. Rosettes: optical effects and not dermoscopic patterns related to skin neoplasms. Australas J Dermatol. 2013;54(4):271–272. doi: 10.1111/ajd.12024. [DOI] [PubMed] [Google Scholar]
- 25.Shitara D, Ishioka P, Alonso-Pinedo Y, et al. Shiny white streaks: a sign of malignancy at dermoscopy of pigmented skin lesions. Acta Derm Venereol. 2014;94(2):132–137. doi: 10.2340/00015555-1683. [DOI] [PubMed] [Google Scholar]
- 26.Balagula Y, Braun RP, Rabinovitz HS, et al. The significance of crystalline/chrysalis structures in the diagnosis of melanocytic and nonmelanocytic lesions. J Am Acad Dermatol. 2012;67(2):194.e1–194.e8. doi: 10.1016/j.jaad.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 27.Cuellar F, Vilalta A, Puig S, Palou J, Salerni G, Malvehy J. New dermoscopic pattern in actinic keratosis and related conditions. Arch Dermatol. 2009;145(6):732. doi: 10.1001/archdermatol.2009.86. [DOI] [PubMed] [Google Scholar]
- 28.Chen LL, Dusza SW, Jaimes N, Marghoob AA. Performance of the first step of the 2-step dermoscopy algorithm. JAMA Dermatol. 2015;151(7):715–721. doi: 10.1001/jamadermatol.2014.4642. [DOI] [PubMed] [Google Scholar]
- 29.Bassoli S, Rabinovitz HS, Pellacani G, et al. Reflectance confocal microscopy criteria of lichen planus–like keratosis. J Eur Acad Dermatol Venereol. 2012;26(5):578–590. doi: 10.1111/j.1468-3083.2011.04121.x. [DOI] [PubMed] [Google Scholar]
- 30.Liebman TN, Rabinovitz HS, Dusza SW, Marghoob AA. White shiny structures: dermoscopic features revealed under polarized light. J Eur Acad Dermatol Venereol. 2012;26(12):1493–1497. doi: 10.1111/j.1468-3083.2011.04317.x. [DOI] [PubMed] [Google Scholar]