Abstract
Objectives
Interleukin 18 (IL-18) is a regulatory cytokine that degrades the disc matrix. Bone morphogenetic protein-2 (BMP-2) stimulates synthesis of the disc extracellular matrix. However, the combined effects of BMP-2 and IL-18 on human intervertebral disc degeneration have not previously been reported. The aim of this study was to investigate the effects of the anabolic cytokine BMP-2 and the catabolic cytokine IL-18 on human nucleus pulposus (NP) and annulus fibrosus (AF) cells and, therefore, to identify potential therapeutic and clinical benefits of recombinant human (rh)BMP-2 in intervertebral disc degeneration.
Methods
Levels of IL-18 were measured in the blood of patients with intervertebral disc degenerative disease and in control patients. Human NP and AF cells were cultured in a NP cell medium and treated with IL-18 or IL-18 plus BMP-2. mRNA levels of target genes were measured by real-time polymerase chain reaction, and protein levels of aggrecan, type II collagen, SOX6, and matrix metalloproteinase 13 (MMP13) were assessed by western blot analysis.
Results
The serum level of patients (IL-18) increased significantly with the grade of IVD degeneration. There was a dramatic alteration in IL-18 level between the advanced degeneration (Grade III to V) group and the normal group (p = 0.008) Furthermore, IL-18 induced upregulation of the catabolic regulator MMP13 and downregulation of the anabolic regulators aggrecan, type II collagen, and SOX6 at 24 hours, contributing to degradation of disc matrix enzymes. However, BMP-2 antagonised the IL-18 induced upregulation of aggrecan, type II collagen, and SOX6, resulting in reversal of IL-18 mediated disc degeneration.
Conclusions
BMP-2 is anti-catabolic in human NP and AF cells, and its effects are partially mediated through provocation of the catabolic effect of IL-18. These findings indicate that BMP-2 may be a unique therapeutic option for prevention and reversal of disc degeneration.
Cite this article: S. Ye, B. Ju, H. Wang, K-B. Lee. Bone morphogenetic protein-2 provokes interleukin-18-induced human intervertebral disc degeneration. Bone Joint Res 2016;5:412–418. DOI: 10.1302/2046-3758.59.BJR-2016-0032.R1.
Keywords: Bone morphogenetic protein-2 (BMP-2), Interleukin-18 (IL-18), Nucleus pulposus and annulus fibrosus cells, Intervertebral disc degeneration (IVD)
Article focus
This study investigated the effects of the anabolic cytokine BMP-2 and the catabolic cytokine IL-18 on human nucleus pulposus (NP) and annulus fibrosus (AF) cells.
Key messages
IL-18 induced upregulation of the catabolic regulator MMP13 and downregulation of the anabolic regulators aggrecan, type II collagen, SOX6, and SOX9 at 24 hours, contributing to degradation of disc matrix enzymes.
However, BMP-2 antagonised the IL-18 induced upregulation of aggrecan, type II collagen, and SOX6, resulting in a reversal of IL-18 mediated disc degeneration.
BMP-2 is anti-catabolic in human AF cells, and its effects are partially mediated through provocation of the catabolic effect of IL-18.
Strengths and limitations
These findings indicate that BMP-2 may be a unique therapeutic option for prevention and reversal of disc degeneration.
Introduction
Low back pain is a chronic, economically debilitating and common ailment. Approximately 80% of adults experience low back pain at some point during their lifetime, and it is one of the most common causes of limitation in activity amongst the younger age group.1 It results in lost productivity, which further increases the economic burden of this ailment.2 Intervertebral disc degeneration (IVD) and associated disorders are recognised as major common causes of low back pain.3 At present, most treatments focused on alleviating low back pain are directed towards degenerate IVDs (e.g., disc ablation, replacement), however, the pathobiological mechanisms of intervertebral disc degeneration are still not clear.
IVDs are composed mainly of cartilaginous vertebral endplates (EP), the fibrous annulus fibrosus (AF), and the nucleus pulposus (NP).4 Inflammation is one of the factors known to contribute to IVD. The pro-inflammatory cytokine IL-18 has been reported to amplify cartilage destruction associated with osteoarthritis (OA).5 However, to our knowledge the mechanism by which IL-18 contributes to disc degeneration has not been reported.
Bone morphogenetic proteins (BMPs) are members of the transforming growth factor beta (TGF-β) superfamily, and function as potent regulators of bone and cartilage formation.6-9 Previous reports indicated that TGF-β1, BMP-2, and osteogenic protein-1 (OP-1) have the ability to stimulate proteoglycan synthesis and maintain joint integrity.10-14 However, to our knowledge, there are limited data available as to whether these growth factors antagonise interlukin-18 in IVD. In this study, we provide evidence that BMP-2 may antagonise the catabolic effects of IL-18, indicating that BMP-2 is a unique therapeutic option for prevention and reversal of disc degeneration.
Materials and Methods
Reagents and cell lines
Recombinant human BMP-2 was purchased from DaeWoong Pharmaceutical (Seoul, Korea). IL-18 was purchased from Santa Cruz Biotechnology (Santa Cruz, California), in addition to antibodies targeting MMP13, SOX6, aggrecan, and type II collagen. The human AF cell line was obtained from ScienCell Research Laboratories (Carlsbad, California). The NP cell medium for culturing human annulus fibrosus cells (HAFC) in vitro was purchased from ScienCell Research Laboratories (Carlsbad, California). The NP cell medium was supplemented with 2% fetal bovine serum, 1% NP cell growth supplement, 100 mg/ml streptomycin, and 100 IU/ml penicillin (Gibco). Cells were maintained under standard conditions at 37°C in a 5% CO2 humidified atmosphere.
Enzyme-linked immunosorbent assay (ELISA) study
We collected whole blood from a total of 40 patients with disc degeneration before surgery. We received permission from our Institution’s Review Board, as well as informed consent from patients. The gross morphology of the discs was graded by the Thompson grading scheme using MRI (grades I toV). We have divided as two groups according to the presence/absence of disc degeneration. The blood was collected from the patients (n = 26) with degenerative disc disease (grades II to V) underwent spine fusion surgery (posterior lumbar interbody fusion) due to disc degenerative disease (herniation of the NP, spinal stenosis, and spondylolisthesis). The mean age of the patients was 58 (sd 12.2; 46 to 72) and there were 16 men and ten women. Also, the blood was collected from the patients (n = 14) without degenerative disc disease (anterior corpectomy/discectomy and fusion/fixation in young trauma patients: the mean age was 25 (sd 5.2; 19 to 30) and there were nine men and five women. Cytokine detection was carried out using a commercially available human IL-18 ELISA kit. The assay was performed according to the manufacturer’s instructions and all samples were tested twice. The level of secretion of IL-18 in the whole blood of patients were determined using the human IL-18 ELISA kit by Invitrogen (Carlsbad, California). An Epoch microplate reader from Bio-Tek (Seoul, Korea) was used to measure the optical density at 450 nm. Cytokine concentration was calculated from the standard curve.
RNA isolation and real-time PCR
NP and AF cells were treated in a dose dependent manner of IL-18 (0 ng/ml to25 ng/ml) for 12 and 24 hours, after which total RNA was extracted from NP and AF cells using easy-BLUE (iNtRON, Seoul, Korea). One microgram of RNA from each sample was reverse transcribed in a 20 µl reaction volume using the PrimeScript RT reagent kit (TakaRa, Japan) according to the manufacturer’s instructions. Next, real-time PCR reactions were carried out in a 10 µl reaction mixture (1 µl cDNA, 5 µl 2x SYBR Premix Ex TaqII, 0.4 µl of 10 µmol/l forward and reverse primers, 0.2 µl Rox reference dye, and 3 µl H2O). Quantitative real-time PCR was carried out using an ABI Prism 7900 Sequence Detection System (Applied Biosystems, Foster City, California). The PCR programme comprised 30 seconds at 95oC, followed by 40 cycles of 15 seconds at 95oC and one minute at 60oC. The relative expression of target genes was calculated by using the comparative threshold method. Results are reported normalised to Glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Melting curves for each PCR reaction were generated to ensure the purity of the amplification product. Primer sequences are provided in Table I.
Table I.
Real-time PCR primers
| Target | Primer sequence (forward/reverse)(5’-3’) |
|---|---|
| Glyceraldehyde 3-phosphate dehydrogenase | GGT ATC GTG GAA CTC AT |
| ACC ACC TGG TGC TCA GTG TA | |
| MMP-13 | GGC TCC GAG AAA TGC AGT CTT TCT T |
| ATC AAA TGG GTA GAA GTC GCC ATG C | |
| Aggrecan | TCC ACA AGG GAG AGA GGG TA |
| GTA GGT GGT GGC TAG GAC GA | |
| Sox6 | CTT ACT CGG CCA GAA GAT GC |
| ATT GGT CGC TTA ATG TGT GG | |
| Collagen type 1 | CAG CCG CTT CAC CTA CAG C |
| TTT TGT ATT CAA TCA CTG TCT TGC C 3 | |
| Collagen type 2 | GGC AAT AGC AGG TTC ACG TAC A |
| CGA TAA CAG TCT TGC CCC ACT T | |
| Interleukin-18R1 | ACG CCG AGT TTG AAG ATC AGG GGT |
| CCC TGG GCA AAA TCT CCA CAG CA |
Western blot analysis
NP and AF cells were seeded in poly-L-lysine-coated 6-well plates at a density of 3x105 cells per well and allowed to attach for 24 hours. Cells were then starved for four hours by placing them in a serum-free medium. IL-18 and recombinant human (rh)BMP-2 were added to cell cultures at various concentrations for 24 hours. Cells were then washed with PBS and harvested. Cell pellets were lysed in ice-cold PRO-PREPTM (Intron Biotechnology, Seongnam, Korea). Proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes (GE Healthcare Life Sciences, Buckinghamshire, United Kingdom). Blots were probed with antibodies against MMP13, aggrecan, type II collagen, or SOX6 overnight, followed by further incubation with secondary antibodies labelled with horseradish peroxidase. Immunoreactivity was detected using the Fusion FX7 detection system (Vilber, France).
Statistical analysis
Data are presented as mean values and standard errors of the mean (sem). Student’s t-test was used to compare results between control and experimental groups. A p-value < 0.05 was considered to be statistically significant.
Results
ELISA results for IL-18
ELISA assays were performed to determine the level of IL-18 in IVD patients. The degree of IVD degeneration was determined using MRI images according to Thompson’s classification system.15-17 Normal and Grade I represent healthy discs. Grades II and III indicate IVD degeneration. Grades IV to V indicate advanced IVD degeneration. The concentration of IL-18 increased significantly with the grade of IVD degeneration. Normal subjects had a mean IL-18 level of 173.01 pg/ml (sem 43.47), whereas Grade II patients had 231.99 pg/ml (sem 47.26), Grade III patients 640.25 pg/ml (sem 218.25), and Grade IV to V patients 2287.93 pg/ml (sem 300.11). Specifically, there was a significant difference in IL-18 level between the advanced degeneration group and the normal group (p = 0.008) as shown in Figure 1.
Fig. 1.
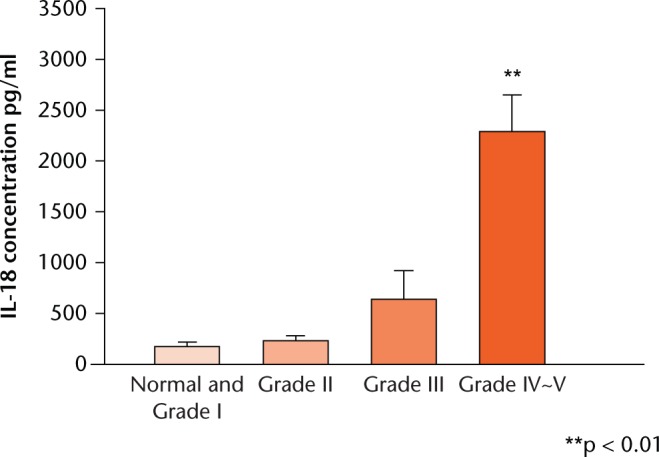
Graph showing whole blood from healthy individuals and disc degeneration patients before surgery was collected and analysed by enzyme-linked immunosorbent assay to determine the Interleuklin(IL)-18 level. Each value represents the mean and standard error of the mean of at least three independent experiments. ** p < 0.01 vs normal group.
Effect of IL-18 on disc degeneration
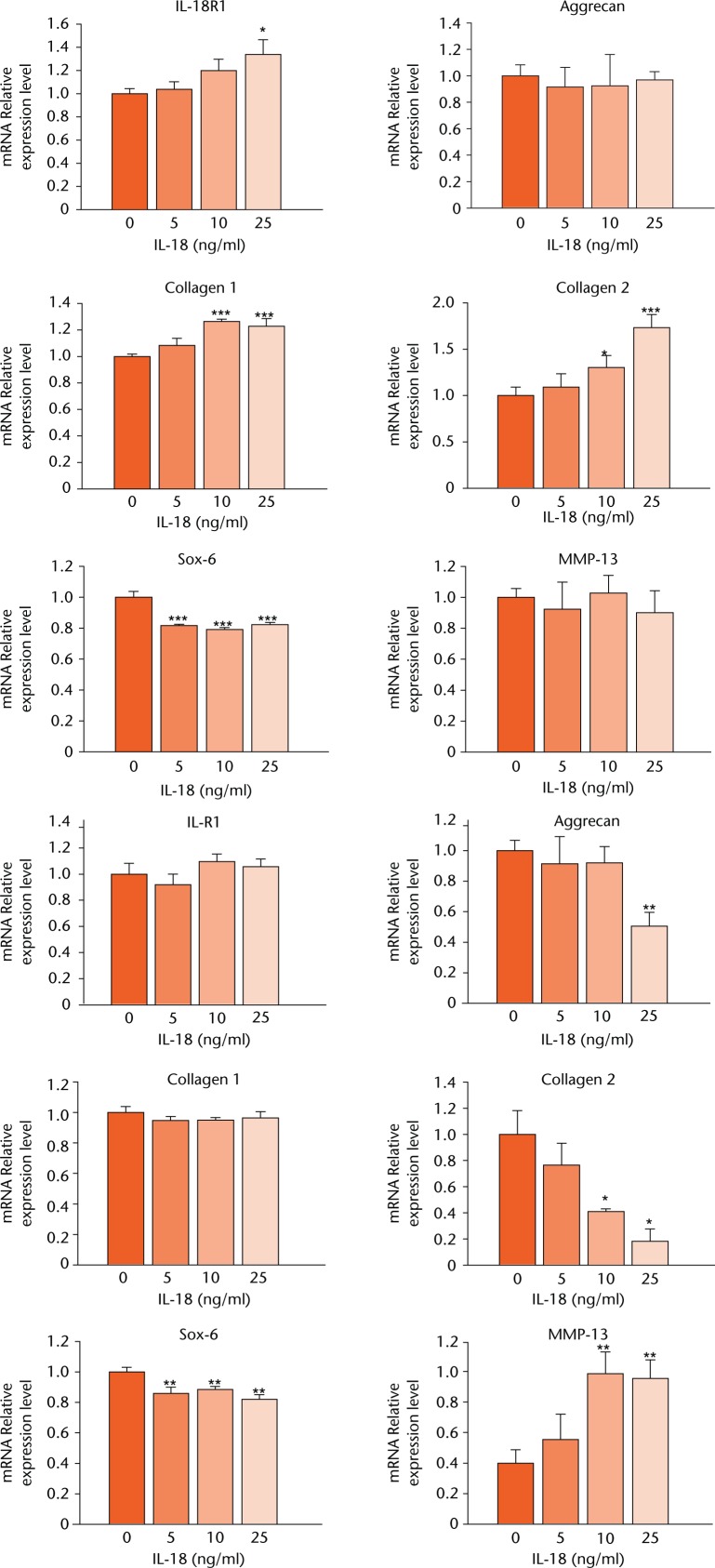
NP and AF cells were treated with different concentrations of IL-18 (0 ng/ml, 5 ng/ml, 10 ng/ml and 25 ng/ml) for 12 hours. Transcript levels of IL-18R1, type I collagen, and type II collagen increased by 1.32-fold (34%), 1.23-fold (23%), and 1.73-fold (73%), respectively, at an IL-18 concentration of 25 ng/ml compared with the control group with corresponding p-values of 0.019, 0.000631, and 0.000452, respectively. SOX6 was inhibited by 0.82-fold (18%; p = 0.006) at 25 ng/ml IL-18 compared with the control group. mRNA levels of aggrecan and MMP13 did not change in response to treatment with IL-18, as shown in Figure 2. Importantly, after treatment with 25 ng/ml IL-18 for 24 hours, mRNA expression levels of aggrecan, type II collagen, and SOX6 were significantly inhibited – 0.51-fold (49%), 0.18-fold (82%), 0.82-fold (18%), 0.78 fold (22%), respectively, in HAFCs compared with IL-18 untreated cells. The associated p-values were 0.002, 0.004, 0.000427, and 0.045, respectively. The MMP13 level increased by approximately 2.386-fold (138%) compared with the control group, with a p-value of 0.003. However, mRNA expression levels of IL-18R1 and type I collagen were not altered. These results suggest that treatment with IL-18 effectively induces gene expression of catabolic cytokines and reduces that of anabolic cytokines in human AF cells.
Fig. 2.
Graphs showing mRNA expression levels of disc degeneration-related genes induced by interleukin (IL)-18 treatment in human annulus fibrosus cells (HAFCs). mRNA expression levels of IL-18R1, aggrecan, type I collagen, type II collagen, SOX6, and MMP13 were determined by real-time PCR after treatment with various concentrations of IL-18 for 12 or 24 hours. The expression of target genes was normalised by the expression of Glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Ratios of the normalised expression of target genes at each IL-18 dose relative to that of untreated cells are shown. Results are expressed as means and standard error.
Effect of BMP-2 and IL-18 on disc degeneration
NP and AF cells were treated with different doses of rhBMP-2 (0 ng/ml, 50 ng/ml, 250 ng/ml and 1000 ng/ml) with or without IL-18 (25 ng/ml) for 24 hours. Combination treatment with rhBMP-2 (250 ng/ml) and IL-18 (25 ng/ml) increased the mRNA expression levels of aggrecan by 31.97-fold, type II collagen by 3.01-fold, and SOX6 by 1.41-fold compared with IL-18 (25 ng/ml) alone, as shown in Figure 3. The associated p-values were 0.0000022, 0.001, and 0.000047, respectively. Meanwhile, treatment with both rhBMP-2 (1000 ng/ml) and IL-18 (25 ng/ml) increased the expression of aggrecan by 198.57-fold, type II collagen by 3.70-fold, and SOX6 by 1.42-fold compared with treatment with IL-18 only (25 ng/ml). The associated p-values were 0.0000015, 0.003, and 0.001, respectively.
Fig. 3.
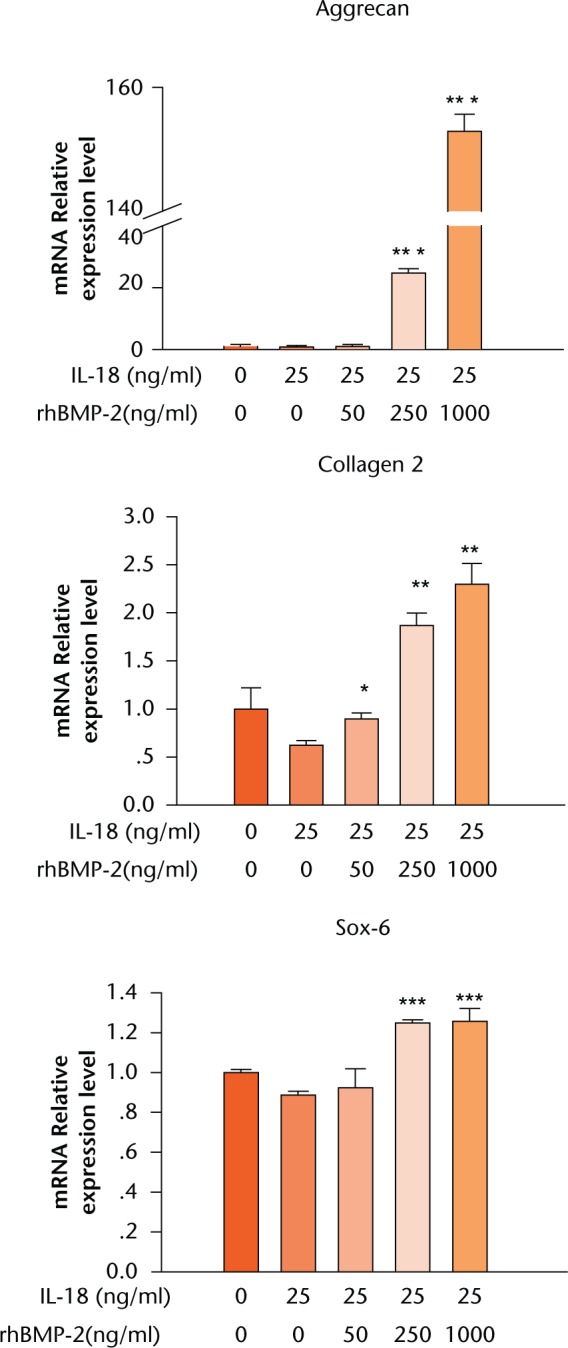
Graph showing mRNA levels after treatment of human annulus fibrosus cells (HAFCs) with interleukin (IL)-18 and recombinant human (rh)BMP-2 as determined by real-time PCR. HAFCs were treated with or without IL-18 and various concentrations of rhBMP-2 for 24 hours. The expression of target genes was normalised by the expression of Glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Results are expressed as means and standard error of the mean (*p < 0.05, **p < 0.01, ***p < 0.001 compared with untreated cells).
Western blot analysis on disc degeneration
To examine the effect of BMP-2 in the regulation of IL-18-induced disc degeneration, levels of aggrecan, type II collagen, SOX6, MMP13, and GAPDH were measured by western blot analysis, as shown in Figure 4. IL-18 (25 ng/ml) decreased protein levels of aggrecan and type II collagen. However, levels of these proteins increased significantly in response to treatment with rhBMP-2 (1000 ng/ml). Protein levels of SOX6 and MMP13 did not change in response to IL-18 treatment alone, or combination treatment with rhBMP-2.
Fig. 4.
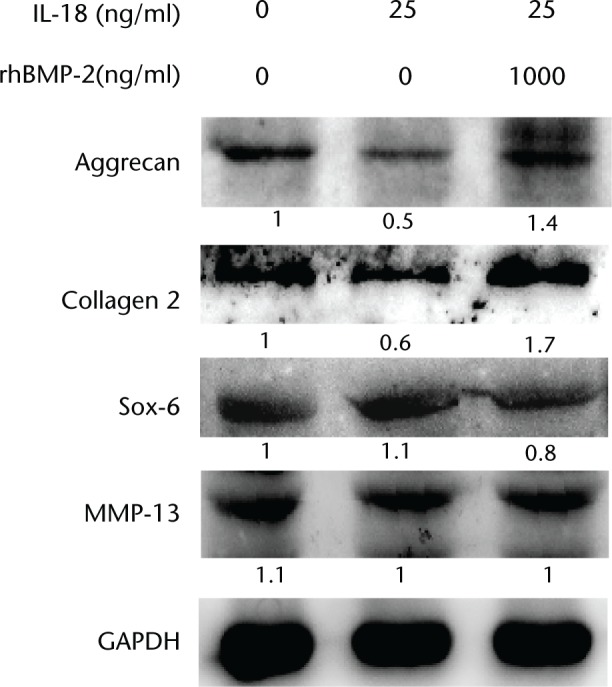
Western blot analysis of protein expression of aggrecan, type II collagen, MMP13, and SOX6 in response to combined treatment with interleukin (IL)-18 and recombinant human (rh)BMP-2. Cells were harvested at 24 hours and immunoblotted with specific antibodies. The relative densities (RD) of proteins were quantified and plotted against the signal obtained from the control after normalisation to Glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Discussion
To date, the pathogenesis of intervertebral disc degeneration is still not clear. It is believed that enzymes, growth factors, and cytokines play an important role in degenerated disc tissues.18-22 TGF-β has been shown to stimulate proteoglycan production by disc cells23,24 and attenuate the expression of active MMP-2 in NP cells.25 However, the role of rhBMP-2 as a member of the TGF-β family in IL-18-induced IVD has, to our knowledge, not previously been evaluated.
In our study, we observed significantly higher IL-18 serum levels in the IVD group of patients than the normal group. The more severe the grade of disc degeneration, the higher the level of IL-18 expression. IL-18, therefore, appears to have a close association with the progression of degenerative disease and low back pain.
In addition, transcript levels of SOX6 decreased significantly in NP and AF cells after treatment with IL-18 for 12 hours. In contrast, transcript levels of IL-18R1, type I collagen, and type II collagen increased, and there were no changes in the levels of aggrecan or MMP13. However, after 24 hours of treatment with IL-18, transcript levels of aggrecan, type II collagen, and SOX6 were decreased, and the level of matrix metalloproteinase 13 (MMP13) was increased. These results demonstrate that IL-18 inhibited expression of aggrecan, type I collagen, type II collagen, and SOX6. Based on our experiments, we believe that IL-18 affects biosynthesis in NP and AF cells.16 Increased concentrations of IL-18 in disc degeneration patients may explain why disc degeneration patients usually feel discogenic back pain.
After combined treatment of NP and AF cells with rhBMP-2 and IL-18 for 24 hours, transcript levels of aggrecan, type II collagen, and SOX6 increased significantly compared with IL-18 treatment alone. Furthermore, protein levels of aggrecan and type II collagen also increased after combination treatment with IL-18 plus rhBMP-2 compared with treatment with IL-18 alone. In this study we have illustrated for the first time that BMP-2 may antagonise IL-18-induced IVD. Our data also revealed that BMP-2 represents a unique therapeutic option for prevention and reversal of disc degeneration, although further clinical and experimental studies are required to evaluate this hypothesis.
The results in this study suggest that IL-18 has the potential to accelerate disc degeneration. Treatment with IL-18 and BMP-2 on NP and AF cells resulted in increased aggrecan, type II collagen, and SOX6 transcript expression and increased aggrecan and type II collagen protein expression relative to treatment with IL-18 alone. BMP-2, therefore, has an anti-catabolic function in human NP and AF cells.
Footnotes
Author Contribution: S. Ye: Writing manuscript, Perfoming basic study
B. Ju: Performing clinical data collection
H. Wang: Performing basic study
K-B. Lee: Creating concept of this study, Funding and revising the manuscript
ICMJE conflict of interest: None declared
Funding Statement
A grant of the CNUH-BRI has been provided to the Biomedical Research Institute of Chonbuk National University Hospital, CNUH-BRI-2012-02-005 and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology(2012R1A1A2005729)
References
- 1. Li X, An HS, Ellman M, et al. Action of fibroblast growth factor-2 on the intervertebral disc. Arthritis Res Ther 2008;10:R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luoma K, Riihimäki H, Luukkonen R, et al. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 2000;25:487-492. [DOI] [PubMed] [Google Scholar]
- 3. Freemont TJ, LeMaitre C, Watkins A, Hoyland JA. Degeneration of intervertebral discs: current understanding of cellular and molecular events, and implications for novel therapies. Expert Rev Mol Med 2001;2001:1-10. [DOI] [PubMed] [Google Scholar]
- 4. Liuke M, Solovieva S, Lamminen A, et al. Disc degeneration of the lumbar spine in relation to overweight. Int J Obes (Lond) 2005;29:903-908. [DOI] [PubMed] [Google Scholar]
- 5. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 2011;7:33-42. [DOI] [PubMed] [Google Scholar]
- 6. Carreira AC, Lojudice FH, Halcsik E, et al. Bone morphogenetic proteins: facts, challenges, and future perspectives. J Dent Res. 2014;93:335-345. [DOI] [PubMed] [Google Scholar]
- 7. Fassbender M, Minkwitz S, Strobel C, Schmidmaier G, Wildemann B. Stimulation of bone healing by sustained bone morphogenetic protein 2 (BMP-2) delivery. Int J Mol Sci 2014;15:8539-8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Papanagiotou M, Dailiana ZH, Karachalios T, et al. RhBMP-7 for the treatment of nonunion of fractures of long bones. Bone Joint J 2015;97-B:997-1003. [DOI] [PubMed] [Google Scholar]
- 9. Papanagiotou M, Malizos KN, Vlychou M, Dailiana ZH. Autologous (non-vascularised) fibular grafting with recombinant bone morphogenetic protein-7 for the treatment of femoral head osteonecrosis: preliminary report. Bone Joint J 2014;96-B:31-35. [DOI] [PubMed] [Google Scholar]
- 10. Kim H, Lee JU, Moon SH, et al. Zonal responsiveness of the human intervertebral disc to bone morphogenetic protein-2. Spine (Phila Pa 1976) 2009;34:1834-1838. [DOI] [PubMed] [Google Scholar]
- 11. Li J, Yoon ST, Hutton WC. Effect of bone morphogenetic protein-2 (BMP-2) on matrix production, other BMPs, and BMP receptors in rat intervertebral disc cells. J Spinal Disord Tech 2004;17:423-428. [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y, Phillips FM, Thonar EJ, et al. Cell therapy using articular chondrocytes overexpressing BMP-7 or BMP-10 in a rabbit disc organ culture model. Spine (Phila Pa 1976) 2008;33:831-838. [DOI] [PubMed] [Google Scholar]
- 13. Masuda K, Takegami K, An H, et al. Recombinant osteogenic protein-1 upregulates extracellular matrix metabolism by rabbit annulus fibrosus and nucleus pulposus cells cultured in alginate beads. J Orthop Res 2003;21:922-930. [DOI] [PubMed] [Google Scholar]
- 14. Takegami K, Thonar EJ, An HS, Kamada H, Masuda K. Osteogenic protein-1 enhances matrix replenishment by intervertebral disc cells previously exposed to interleukin-1. Spine (Phila Pa 1976) 2002;27:1318-1325. [DOI] [PubMed] [Google Scholar]
- 15. Lee JM, Song JY, Baek M, et al. Interleukin-1β induces angiogenesis and innervation in human intervertebral disc degeneration. J Orthop Res 2011;29:265-269. [DOI] [PubMed] [Google Scholar]
- 16. Lee S, Moon CS, Sul D, et al. Comparison of growth factor and cytokine expression in patients with degenerated disc disease and herniated nucleus pulposus. Clin Biochem 2009;42:1504-1511. [DOI] [PubMed] [Google Scholar]
- 17. Siemionow K, An H, Masuda K, Andersson G, Cs-Szabo G. The effects of age, sex, ethnicity, and spinal level on the rate of intervertebral disc degeneration: a review of 1712 intervertebral discs. Spine (Phila Pa 1976). 2011;36:1333-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murakami H, Yoon ST, Attallah-Wasif ES, et al. The expression of anabolic cytokines in intervertebral discs in age-related degeneration. Spine (Phila Pa 1976) 2006;31:1770-1774. [DOI] [PubMed] [Google Scholar]
- 19. Scuderi GJ, Brusovanik GV, Anderson DG, et al. Cytokine assay of the epidural space lavage in patients with lumbar intervertebral disk herniation and radiculopathy. J Spinal Disord Tech 2006;19:266-269. [DOI] [PubMed] [Google Scholar]
- 20. Masuda K, An HS. Growth factors and the intervertebral disc. Spine J 2004;4(suppl):330S-340S. [DOI] [PubMed] [Google Scholar]
- 21. Igarashi A, Kikuchi S, Konno S, Olmarker K. Inflammatory cytokines released from the facet joint tissue in degenerative lumbar spinal disorders. Spine (Phila Pa 1976). 2004;29(19):2091-2095. [DOI] [PubMed] [Google Scholar]
- 22. Brisby H, Olmarker K, Larsson K, Nutu M, Rydevik B. Proinflammatory cytokines in cerebrospinal fluid and serum in patients with disc herniation and sciatica. Eur Spine J 2002;11:62-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tolonen J, Grönblad M, Vanharanta H, et al. Growth factor expression in degenerated intervertebral disc tissue. An immunohistochemical analysis of transforming growth factor beta, fibroblast growth factor and platelet-derived growth factor. Eur Spine J 2006;15:588-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alini M, Li W, Markovic P, et al. The potential and limitations of a cell-seeded collagen/hyaluronan scaffold to engineer an intervertebral disc-like matrix. Spine (Phila Pa 1976) 2003;28:446-454. [DOI] [PubMed] [Google Scholar]
- 25. Pattison ST, Melrose J, Ghosh P, Taylor TK. Regulation of gelatinase-A (MMP-2) production by ovine intervertebral disc nucleus pulposus cells grown in alginate bead culture by Transforming Growth Factor-beta(1)and insulin like growth factor-I. Cell Biol Int 2001;25:679-689. [DOI] [PubMed] [Google Scholar]