Abstract
Earlier studies have shown that Tomato spotted wilt virus (TSWV) influences the biology, performance, and behavioral patterns of its vector Frankliniella occidentalis Pergande. In this study, using Capsicum annuum L. as the host plant, we aimed to determine the manipulation of F. occidentalis by TSWV through switching of the diet (+ or −TSWV) during vector’s development. Behavioral patterns, fitness, as well as vector performance were evaluated. The specific parameters investigated included longevity/survival, fecundity, development time, feeding, and preferential behavior. F. occidentalis were reared on either TSWV-infected (exposed) or healthy leaves (non-exposed) throughout their larval stages. The emerging adults were then individually transferred to either healthy or TSWV-infected leaf disks. This resulted into four treatments, consisting of exposed or non-exposed thrips reared on either infected or healthy leaf disks as adults. All F. occidentalis exposed to TSWV in their larval stages had shorter development time regardless of the adults’ diet. Whereas, the ones that were later reared on healthy leaf disks as adults recorded the highest longevity and reproduction rate. Furthermore, adults of F. occidentalis that were exposed to TSWV in their larval stages showed preference toward healthy leaf disks (−TSWV), whereas the non-exposed significantly preferred the infected leaf disks (+TSWV). These are further indications that TSWV modifies the nutritional content of its host plants, which influences vector’s biology and preferential behavior, in favor of its multiplication and dispersal. The findings offer additional explanation to the often aggressive spread of the virus in crop stands.
Keywords: Frankliniella occidentalis, Tospovirus, tomato spotted wilt virus, vector manipulation hypothesis
Tomato spotted wilt virus (TSWV) is a member of the genus Tospovirus, the only plant-infecting genus in the family Bunyaviridae (van de Wetering et al. 1999, Whitfield et al. 2005). TSWV has a wide host range and worldwide distribution (Sherwood et al. 2000, EPPO/CABI 1997c). This virus can lead to major economic losses on tomato, lettuce, pepper, papaya, eggplant, French beans, artichokes, broad beans, celery, and different ornamental plants (Roselló et al. 1996). It is transmitted exclusively by thrips, with Frankliniella occidentalis (Pergande), commonly known as western flower thrips, being the most efficient vector due to its equally large host range and worldwide distribution (Wijkamp et al. 1995, Nagata and Peters 2001, Whitfield et al. 2005).
Frankliniella occidentalis acquires the virus during feeding by sucking cell contents from infected plants. Transmission of TSWV is in a persistent and propagative manner (Wijkamp et al. 1996, Maris 2004), and virus replication within the vector is essential for high transmission efficacy. The virus is passed from the midgut lumen through the midgut cell wall and finally to the salivary glands via cell-to-cell movement (Ullman et al. 1992, Whitfield et al. 2005). Presence of nonstructural proteins (NSs) and viral inclusions in thrips body tissues and the primary salivary glands are indications of replication of the virus in the vector (Ullman et al. 1992, 1993, 1995; Wijkamp and Peter 1993). Only adults (and partly the second instars) can transmit the virus, but only if acquired in the early larval stages. The transmitting adults can vector the virus throughout their lifetime, with the highest efficacy immediately after emergence and, thereafter, at a slightly lower but steady rate (Wijkamp et al. 1996, Chatzivassiliou et al. 1999).
Viruses are reported to modify the behavior of their vectors with both direct and plant-mediated indirect effects, consequently enhancing their transmission. As the vectors are mobile and can show preferences depending on host plant infection status, virus spread within or between crop stands depends on the motivation of the vector for dispersal as well as its host selection behavior (preferences); therefore, plant-mediated indirect virus manipulation of the vector biology and behavior could change transmission and disease dynamics (Fereres and Moreno 2009, Jeger et al. 2004). Such mutual effects are reported as basically important in plant virus ecology and epidemiology (Jones 2014), and examples involving different groups of herbivorous and virus vectoring insects are already published. For instance, a study by Ingwell et al. (2012) reported that acquisition of a plant virus (Barley yellow dwarf virus) can directly alter host plant selection behavior of its vector the cereal aphid (Rhadopalosiphum padi L.). This induced preferential behavior can increase the virus spread, as non-infected aphids preferred to feed on infected plants, whereas infected aphids preferred the non-infected plants. Also, the exposure of F. occidentalis to TSWV has been reported to influence the vector’s behavior as well as fitness, for example, increased feeding rate, higher survival, shorter development time, and reduced fecundity (Sakimura 1963, Robb 1989, Wijkamp et al. 1996, Maris et al. 2004, Stafford et al. 2011, Ogada et al. 2013). Moreover, some studies have demonstrated a triggered immune response in the vector after exposure to the virus (Medeiros et al. 2004), but energy costs due to virus replication led to a kind of trade-off between vector longevity and performance in terms of reproduction (Ogada et al. 2013). Contradicting results reported on the effects of tospoviruses on survival, reproduction, development, and feeding of thrips may have arisen from the complex patterns of interaction within the triangle TSWV–plant–vector, which could be influenced by several factors such as the suitability of the host plant, virus isolate, vector gender, environmental factors, genetic constitution of individual thrips, feeding behavior, and also the interactions between these factors (Stumpf and Kennedy 2005, 2007; Ogada et al. 2013). Furthermore, infection of host plants with TSWV can be of an advantage to F. occidentalis due to lowered plant defense potential against the feeding vector, resulting in longer survival due to the readily available nutrients (Blua et al. 1994; Awmack and Leather 2002; Belliure et al. 2005, 2008, 2010; Ogada et al. 2013). As a result, TSWV not only benefits from thrips hosting and providing the resources for its propagation, but also “actively” improves vector efficacy for its own dispersal to a new host plant.
The aim of this study, therefore, was to determine TSWV manipulation of F. occidentalis, by subjecting F. occidentalis to a diet switch (+ or − TSWV) during its development. Then evaluating performance and behavioral changes, focusing on development time, survival (longevity and mortality), reproduction, and preferential behavior, with the purpose of elucidating the importance of these virus-induced vector’s life processes in the multiplication and dispersal of the virus in a crop stand, and hence developing precise predictive models for the disease as well as control strategies of both the virus and the vector.
Materials and Methods
Host Plant
The host plant used for all the experiments with F. occidentalis and for TSWV propagation was 3–4-weeks-old Capsicum annuum L. (Solanaceae), which is a preferred and important host for F. occidentalis and susceptible to TSWV by thrips transmission as well as mechanical inoculation. Nicotiana benthamiana L. (Solanaceae) (3–4-leaf stage) served as a reservoir plant for TSWV maintenance, only because it is easy to handle for mechanical inoculation. The plants were maintained at green house conditions (28–30 °C and 70–80% RH). Phaseolus vulgaris L. plants and pods were used for the maintenance of F. occidentalis in stock culture and synchronized rearing, respectively.
Thrips Culture
Isolate of F. occidentalis (F.o 2) was obtained from Wageningen University, The Netherlands. A virus-free stock culture was established on bean plants (P. vulgaris) at 2–3-leaf stage in a thrips-proof cage maintained in climate chamber conditions. For synchronized rearing, adults from the stock culture were kept on bean pods supplemented with a commercial honey-bee pollen mixture (Naturprodukte-mv.de; Naturprodukte Lembcke, Faulenrost, Germany) in glass jars, closed on top with 64-μm thrips-proof nylon net. The bean pods were replaced at an interval of 1 d, and the harvested pods with eggs transferred to new glass jars for the emergence of larvae. Frankliniella occidentalis used in all the experiments were from the synchronized rearing. Both the stock culture and the synchronized rearing were maintained in separate climate cabins completely isolated from any other sources of thrips at 25 ± 2 °C, 60-70% (RH), and a photoperiod of 16:8 (L:D) h.
Tomato Spotted Wilt Virus Isolates and Mechanical Inoculation
The TSWV isolate (TSWV-N12) was also obtained from Wageningen University, The Netherlands, and mechanically inoculated onto 3-weeks-old C. annuum as a host plant, as well as on N. benthamiana as a reservoir plant. For mechanical inoculation of TSWV, the protocol developed by Mandal et al. (2008) was used. It involves a chilled inoculum consisting of infected leaf sap in 0.1 M phosphate buffer, 0.2% sodium sulfite and 0.01M 2-mercaptoethanol, and 1% each of Celite 545 and Carborundum 320 grit. Application to the host plant was done using a soft finger-rubbing technique. After an incubation period of 10–14 d, the first visual symptoms appeared, and samples of these plants were tested using a double-antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) to confirm successful infection by TSWV. The infected plants were maintained at 28–30 °C (greenhouse condition) and served as inoculum source for further series of mechanical inoculation.
TSWV Acquisition by F. occidentalis
For TSWV acquisition by F. occidentalis, infected leaflet of C. annuum was placed in a petri dish (Ø 15 cm) with a gypsum (CaSo4 and charcoal, 9:1 ratio) layered base. The gypsum layer was then moistened with distilled water, followed by placing a filter paper on top to absorb excess water. Afterwards, healthy or infected leaflet was placed on the filter paper and newly hatched larvae were blown softly onto the leaflet for an acquisition access period (AAP) until pupation. The lid of each petri dish had three equally spaced Ø 12-mm punched holes covered with thrips proof 64 µm nylon mesh for ventilation. The sides of the petri dishes were additionally sealed with Parafilm M (Pechiney Plastic Packaging, Inc., USA) to avoid any escape of F. occidentalis. 4–6 d after AAP (before adults’ emergence), pupae in their last stages were transferred individually onto new C. annuum leaf disks (Ø 17 mm). The leaf disks were either virus-free (healthy) or infected with TSWV-N12. This method is referred to as the “individual leaf disk assay”. Successful acquisition by F. occidentalis was checked by testing five pupae from each treatment (exposed and non-exposed) using amplified DAS-ELISA, which is an improved form of DAS-ELISA in terms of sensitivity (by amplifying the signals at least 10-fold more) using ELISA Amplification kit from Invitrogen Life Technologies GmbH (Cat. No. 19589-019).
General Setup
Two F. occidentalis cohorts were used: the term “exposed” referring to those that fed on TSWV-infected leaflet throughout their larval period only, and “non-exposed” referring to those that fed on healthy leaflet. This disregarded the adult exposure, as it was only used to distinguish the cohorts because the focus was on the diet combinations during the vector developmental. The virus status of the source plants was controlled by DAS-ELISA. The resulting adults were later reared on either virus-infected or healthy leaf disks, and the below-mentioned parameters evaluated. Leaf disk assay was used in all the experiments.
The following treatments of F. occidentalis were used (larvae/adults diet combinations):
Non-exposed larvae/adults reared on healthy leaf disks (C-C)
Non-exposed larvae/adults reared on TSWV-N12-infected leaf disks (C-N12)
Larvae exposed to TSWV-N12/adults reared on healthy leaf disks (N12-C)
Larvae exposed to TSWV-N12/adults reared on TSWV-N12-infected leaf disks (N12-N12)
Leaf disks for adult maintenance were changed at 1-d intervals. All the experiments were carried out in climate chamber conditions: 25 ± 2 °C, 60-70% (RH), and a photoperiod of 16:8 (L:D) h.
Life Parameters of F. occidentalis
Development Time
Ten 1-d-old L1 larvae were randomly selected for each treatment described above. Development time was recorded from L1 to adult emergence for each individual per treatment. This experiment was repeated two times.
Longevity and Mortality
Ten individuals for each treatment combination were controlled daily and leaf disks changed. Daily mortality was recorded throughout the adult life time, but only 15 d recording was used to calculate cumulative mortality, because beyond this time, mortality was not considered to be as a result of treatment effects only. At the same time, longevity, referring to the total number of days an individual lived, was ascertained. The experiment was repeated three times.
Fecundity
Five virgin females from each treatment combination were left to lay eggs on their respective leaf disks. The daily replaced leaf disks were kept for 4 d for L1 emergence. The number of emerging offspring was recorded to determine the daily realized fecundity per individual. Total fecundity was also evaluated for each individual female during its lifetime. This experiment was repeated two times.
Preferential Behavior
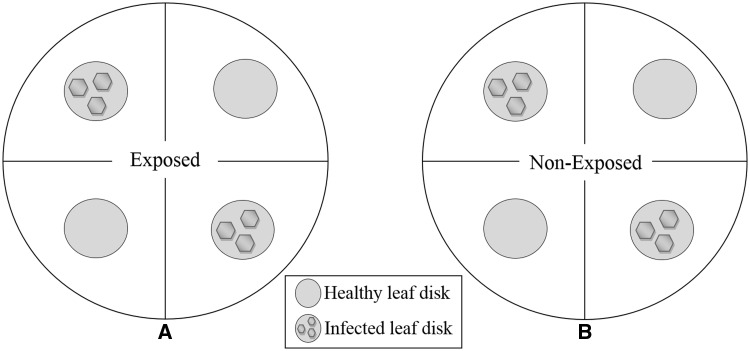
Gypsum petri dishes of Ø 18 cm were prepared by covering the moist bottom with filter paper divided virtually into four equal compartments. For each petri dish, leaf disks punched out of infected plants (TSWV-N12) and non-infected plants (C) were arranged equidistant to the center (Fig. 1). Twenty adults of F. occidentalis, either exposed or non-exposed to TSWV-N12, were released at the center of each petri dish, then the petri dishes were covered using thrips-proof (64-µm) nylon mesh lid, and sealed with Parafilm to avoid any escape. After 24 h, the thrips were anesthetized using CO2, and the number of thrips per leaf disk counted. The individuals that were not settled on any leaf disk compartment but were within the petri dish were recorded as “Out.” The experiment was repeated three times.
Fig. 1.
Example of leaf disks arrangement in petri dish arena for preference tests. (A) Exposed and (B) Non-exposed F. occidentalis adults, released at the center of each petri dish containing two virus-infected and two healthy leaf disks.
Statistical Analysis
Different measurements within each treatment repeat were tested for normal distribution (Shapiro–Wilk normality test at P > 0.05). The average values obtained from each repetition in the experiments (response variables), longevity, mortality, total lifetime fecundity, development time, and preferential behavior, were analyzed statistically using two-way analysis of variance (ANOVA), with explanatory variables (factors) being leaf disk virus status (infected vs healthy) and F. occidentalis virus status (exposed vs non-exposed). When the interaction between the two factors (second-order interaction) was significant, the Sidak correction method (α = 0.05) was used to avoid Type I error, followed by a pairwise multiple comparison procedure using Holm–Sidak method in SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA). In all the analysis, level of significance was evaluated at P = 0.05.
Results
Development Time
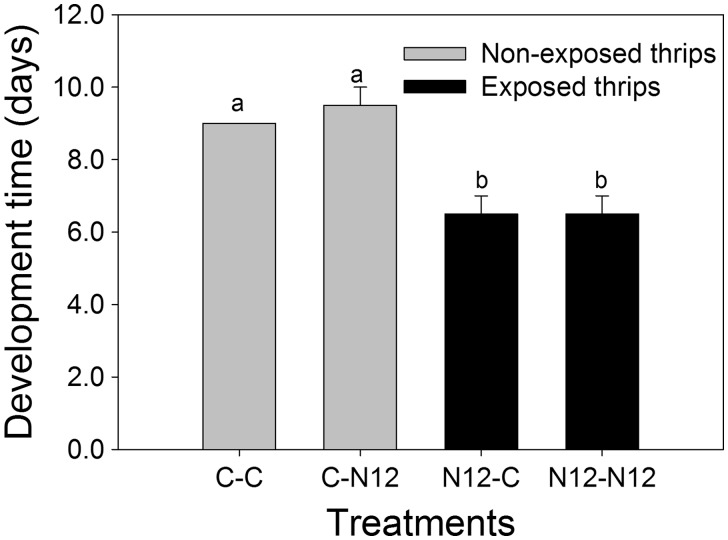
The results of this experiment revealed a significant difference in development time between TSWV-N12-exposed and non-exposed F. occidentalis. All treatments with larval development on healthy plants (non-exposed) differed significantly from the other treatments (exposed) and showed longer development time regardless of the status of the leaf disk they were transferred to as adults, healthy leaf disks (C-C), or infected leaf disks (C-N12) leaf disks (F(1,4) = 40.33, P = 0.003). In addition, there was no significant difference in development time within the treatment combinations of non-exposed (C-C and C-N12) as well as exposed F. occidentalis (N12-C and N12-N12; Holm–Sidak method, P < 0.05). We found no significant interaction between the treatments and the F. occidentalis’ exposure status (F(1,4) = 0.333, P = 0.595; Fig. 2).
Fig. 2.
Comparison of development time between F. occidentalis treatments. That is: Non-exposed reared on healthy leaf disk (C-C), non-exposed reared on infected leaf disk (C-N12), exposed reared on healthy leaf disk (N12-C), and exposed reared on infected leaf disk (N12-N12). Different letters indicate significant differences (two-way ANOVA, Holm–Sidak, P = 0.05, n = 20).
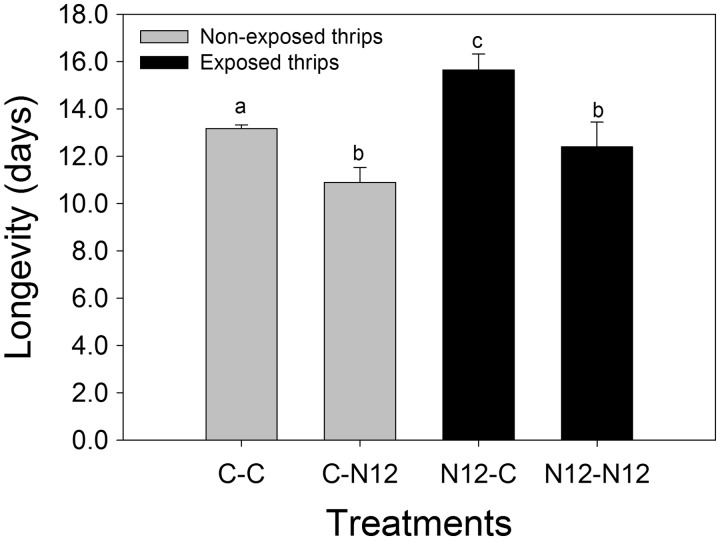
Longevity and Survival
Statistical analysis of lifetime longevity of individuals in each treatment revealed significant differences between the exposed and the non-exposed F. occidentalis (F(1,8) = 16.067, P = 0.004). Longevity of F. occidentalis exposed to TSWV-N12 in their larval stages and reared on healthy leaf disks as adults (N12-C) was significantly higher compared with those that were later reared on TSWV-N12-infected leaf disks (N12-N12), and to the non-exposed individuals that were later reared on healthy leaf disks (Fig. 3). The non-exposed F. occidentalis lived significantly longer when reared on healthy leaf disks (C-C) as adults compared with the ones that were later reared on TSWV-12-infected leaf disks (C-N12). However, there was no significant difference between the non-exposed and the exposed F. occidentalis reared on TSWV-N12-infected leaf disks, C-N12 and N12-N12. There was a significant difference between the mean values of different treatment combinations (F(1,8) = 30.761, P < 0.001), but no interaction between the treatment combinations and the F. occidentalis exposure status (F(1,8) = 0.946, P = 0.359).
Fig. 3.
Comparison of longevity between four F. occidentalis treatment combinations. Non-exposed reared on healthy leaf disk (C-C), non-exposed reared on infected leaf disk (C-N12), exposed reared on healthy leaf disk (N12-C), and exposed reared on infected leaf disk (N12-N12). Different letters indicate significant differences (two-way ANOVA, Holm–Sidak, P = 0.05, n = 30).
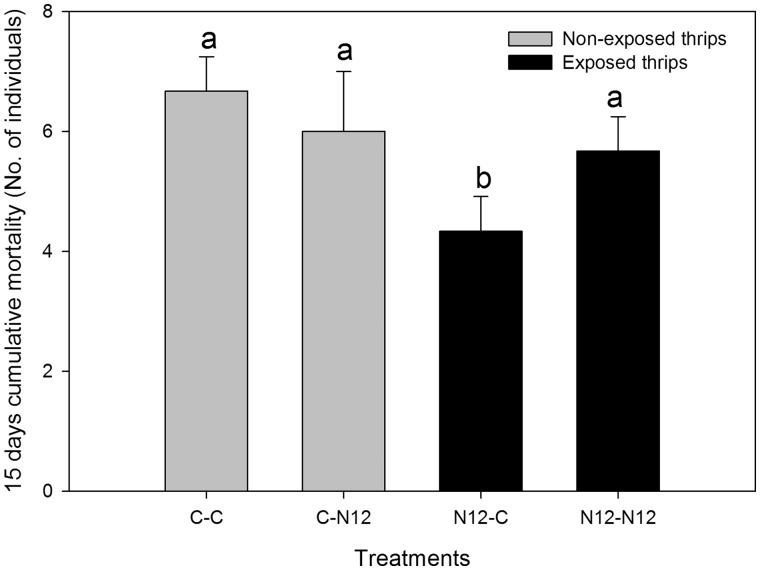
Also, the 15 d cumulative mortality analysis corroborated the longevity results, showing significantly different mortalities in the treatment combinations (F(1,4) = 16, P = 0.0285). Frankliniella occidentalis larvae exposed to TSWV-N12 and reared on healthy leaf disks as adult (N12-C) had significantly lower mortality compared with exposed F. occidentalis reared on infected leaf disks (N12-N12). There was no significant difference between the exposed F. occidentalis reared on infected leaf disks (N12-N12) and non-exposed F. occidentalis reared on infected leaf disks (C-N12). In addition, there was no significant difference between the non-exposed F. occidentalis reared on either infected (C-N12) or non-infected (C-C) leaf disks. There was no statistically significant interaction between the treatment combinations and the F. occidentalis exposure status (F(1,4) = 0.53, P = 0.488; Fig. 4).
Fig. 4.
Comparison of 15 days cumulative mortality between F. occidentalis treatment combinations. Non-exposed reared on healthy leaf disk (C-C), non-exposed reared on infected leaf disk (C-N12), exposed reared on healthy leaf disk (N12-C), and exposed reared on infected leaf disk (N12-N12). Different letters indicate significant differences (two-way ANOVA, Holm–Sidak, P = 0.05, n = 30).
Fecundity
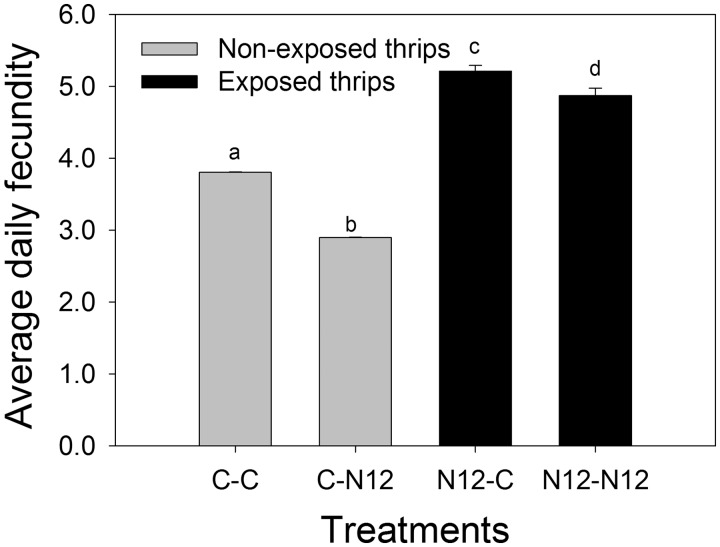
Total fecundity throughout the lifetime of individual females was significantly higher in the F. occidentalis exposed to TSWV-N12 compared with the non-exposed (F(1,4) = 673.293, P < 0.001). Also there was significant difference between the treatment combinations (F(1,4) = 90.962, P < 0.001). Non-exposed F. occidentalis larvae that were later reared on healthy leaf disks as adults (C-C) had higher fecundity compared with their counterparts that were later reared on infected leaf disks (C-N12). Also, exposed F. occidentalis reared on healthy leaf disks (N12-C) showed significantly higher fecundity than the ones reared on TSWV-N12-infected leaf disk (N12-N12). In addition, fecundity of exposed F. occidentalis was significantly higher when reared on TSWV-N12-infected leaf disks as adult (N12-N12) compared with the non-exposed reared on infected leaf disks (C-N12). Also, the fecundity of exposed F. occidentalis reared on healthy leaf disks was significantly higher compared with the non-exposed reared on healthy leaf disks (C-C; Fig. 5). The interaction between the treatment combinations and the exposure status of F. occidentalis was statistically significant (F(1,4) = 19.069, P = 0.012).
Fig. 5.
Comparison of daily fecundity between F. occidentalis treatment combinations. Non-exposed reared on healthy leaf disk (C-C), non-exposed reared on infected leaf disk (C-N12), exposed reared on healthy leaf disk (N12-C), and exposed reared on infected leaf disk (N12-N12). Different letters indicate significant differences (two-way ANOVA, Holm–Sidak, P = 0.05, n = 10).
Preferential Behavior
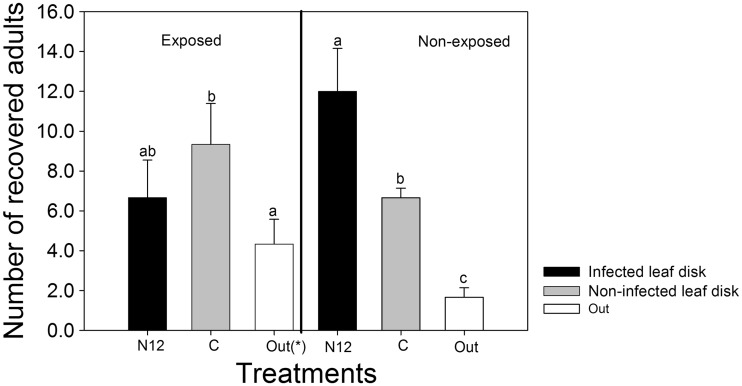
The virus status of the leaf disks significantly influenced the preferential behavior of F. occidentalis adults (F (2,12) = 18.523, P < 0.001) regardless of their exposure status, which had no significant influence (F (1,12) = 0.000, P = 1.000). The adult F. occidentalis that were not exposed to TSWV as larvae clearly preferred TSWV-infected leaf disks (N12) over the non-infected (C), with significantly high numbers being recovered from the infected leaf disks (TSWV) than from the healthy leaf disks (C) or “Out.” The numbers that were “Out” were significantly lower than the ones recovered from the healthy (C) or infected leaf disks (N12; Fig. 6). Concurrently, the amount of F. occidentalis adults exposed to TSWV in their larvae that were recovered on healthy leaf disks (C) was higher than on the TSWV-N12-infected leaf disks (N12), though the difference was not significant. However, the difference between the numbers recovered on healthy leaf disks (C) and the ones that were not on any leaf disk (“Out”) was significant. Despite higher numbers of exposed F. occidentalis being recovered on the infected leaf disks (N12) compared with the ones that were “Out,” the difference was not significant (Fig. 6). Interaction between the exposure status of F. occidentalis (exposed or non-exposed) and the infection status of the leaf disks (healthy or infected) was statistically significant (F (2,12) = 8.862, P = 0.004; Fig. 6).
Fig. 6.
Comparison of preferential behavior of exposed and non-exposed F. occidentalis towards TSWV-infected (N12) and healthy leaf disks. “Out” refers to thrips that either died or were not found in the designated compartments of the test leaf disks. Different letters indicate significant differences (two-way ANOVA, Holm–Sidak, P = 0.05, n = 60).
Discussion
In this study, several parameters of F. occidentalis biology and behavior were evaluated with respect to the influence of TSWV, by assuming that TSWV induced changes in the nutritional quality of the vector’s diet, which was varied during larvae and adult developmental stages. The results of development time study showed that all the F. occidentalis (from L1 onwards) that were initially exposed to TSWV had significantly shorter development time than the non-exposed. This may be due to the alleged physiological reactions within the vector and the host plant as a result of the replicating virus. This supports research by Ogada et al. (2013) and Maris et al. (2004), who reported shorter development time of F. occidentalis exposed to TSWV compared with the non-exposed. Furthermore, Awmack and Leather (2002) concluded that plants infected with virus have higher amino acid contents, which are especially important for development during the larval period; therefore, we can extend this conclusion to our findings. On the other hand, Wijkamp et al. (1996) did not find any influence of TSWV on the development time of F. occidentalis; however, they used different virus strains and host plants in their experiments. The importance of diet uptake or quality during the larval period in defining development time is affirmed by the observation that after transfer of the non-feeding pupa to either infected or healthy leaf disks for further development, there were no additional effects on development time. Therefore, the observed differences could only be related to the vector nutrition during the larval stages.
We also found a significantly higher longevity of individuals exposed to TSWV in their larval stages and later transferred to healthy leaf disk as adults. Ogada et al. (2013) also reported similar results, where except for the TSWV isolate, the experimental conditions, host plant, and F. occidentalis strain were the same. However, this was not the case when the exposed individuals were transferred onto TSWV-infected leaf disks as adults. These results show the important influences of the host plant health status (+ or − TSWV) on F. occidentalis, especially with regards to longevity. In general, plant primary metabolism, which includes amino acids, carbohydrates, sugar, lipids, and water content, can be changed due to attack by a pathogen or insect. Nutrients available to the insects, and as a result their survival, can be relatively affected by these changes in the primary metabolism (Stout et al. 2006). It has been reported that plants infected with virus have a higher content of free amino acids, carbohydrates, and starch (Blua et al. 1994, Awmack and Leather 2002). Therefore, improvement of F. occidentalis longevity after exposure to TSWV could be due to alteration in plant primary metabolism. Furthermore, a triggered immune system in the vector as a result of exposure to the virus has also been reported, which involves transcriptional upregulation of antimicrobial peptides (Medeiros et al. 2004). This, we could also presume to be responsible for the observed increase in longevity. In addition, it can be speculated that virus infection may initiate a negative cross-talk between the metabolic signaling pathways within the plant defense systems against attack by the virus (salicylic acid pathway [SA]) and the vector (jasmonic acid pathway [JA]). Upregulation of SA, a typical defense reaction to pathogen infection, can result in downregulation of JA, which triggers defense against herbivores (Belliure et al. 2010). Therefore, increase in longevity and thus survival of the vector could be linked to the compromised plant defense against thrips attack, and thus easy access to nutrients by the vector (Belliure et al. 2005, 2008; Abe et al. 2012, Nachappa et al. 2013) during larval development. However, for the exposed F. occidentalis individuals that spent their entire lives on TSWV-infected leaf disks, the longevity was significantly lower compared with those that were later reared on healthy leaf disks as adults. This result could be explained as a negative effect of TSWV on the longevity of F. occidentalis. We assume that the replicating virus, and the continued acquisition of the virus, increased the virus load within the vector, overwhelming the initially triggered defense system, leading to pathological effects. In addition, Stumpf and Kennedy (2005) hypothesized a compromised gut function of the vector, as a result of the virus infection, leading to the observed adverse effects on fitness and performance. These assumptions are comparable with the results reported by De Angelis et al. (1993) and Wijkamp et al. (1996), where the thrips spent their entire lives or a significant part of it on infected plants. The positive effect was only found when F. occidentalis were exposed to TSWV for AAP throughout their larval development until pupation, but later spent the rest of their adult lives on healthy leaf disks.
Reproduction rate was also significantly higher in treatments where F. occidentalis were exposed to TSWV, regardless of the leaf disk status they were reared on as adults. This could be due to the already-mentioned improved nutritional condition in the infected plants. A research done by Nachappa et al. (2013) showed that infection of tomato plants with TSWV increased the fecundity of two-spotted spider mites by 30%. Differences in fecundity could also be as a result of differences in host plant acceptance or discrimination behavior of F. occidentalis, especially in the case of the non-exposed individuals that were transferred on infected leaf disks. Earlier studies have reported varying results on fecundity of F. occidentalis whether thrips were exposed to TSWV or not. Wijkamp et al. (1996) reported no significant difference between TSWV-exposed and non-exposed F. occidentalis; however, the host plant for their experiments was not a convenient for F. occidentalis, and the thrips spent their entire lifetime on infected or non-infected leaf disks. The study by Ogada et al. (2013) reported a negative effect of TSWV on F. occidentalis fecundity, which they attributed to the replicating virus in the vector system leading to competition for resources, which are highly needed during reproduction period. The larvae were reared on infected leaflets for AAP and later pupae were transferred onto healthy leaf disks for egg laying. In addition, Awmack and Leather (2002) suggested that during adult stage, there is an influence of nutritional status of the host plant on the rate of fecundity. These authors concluded that poor quality of host plant could alter the oviposition behavior of herbivorous insects by reducing the number of eggs deposited per plant to avoid food shortage for the offspring. Therefore, the difference in experimental setups and the host plant quality (Stumpf and Kennedy 2005) could have led to the reported differences in fecundity.
Exposure or non-exposure to TSWV greatly influenced the preferential behavior of F. occidentalis toward either infected or non-infected host plant. This result can be explained by an evolutionary mechanism that leads to enhancement of acquisition and transmission of TSWV, as shown also for other host–virus–vector systems. Differences in color between the virus-infected and healthy plants could be a reason for herbivorous insect to be attracted to the infected plants. In our experiments, we observed yellowing of the infected leaves compared with the non-infected ones. Döring et al. (2009) demonstrated that, for instance, aphids are more attracted to the infected plants with virus due to color changes as a result of virus infection. Ogada et al. (2013) also showed that non-exposed F. occidentalis preferred infected leaf disks to non-infected ones. Similarly, Maris et al. (2004) demonstrated that significantly higher numbers of F. occidentalis were recovered on plants infected with TSWV in choice experiments compared with the non-infected ones. However, in our study, only the non-exposed F. occidentalis preferred the infected leaf disks, and thus the color changed influence. The exposed F. occidentalis significantly preferred the non-infected, hence “normal colored” leaf disks; we are therefore cautious to deduce any color-triggered preference mechanism for F. occidentalis. Thus, the observed switch in preferential behavior of the TSWV-exposed F. occidentalis toward the non-infected host plant could be linked to what Ingwell et al. (2012) referred to as “Vector Manipulation Hypothesis,” whereby the virus manipulates its vector in such a way to promote its dispersal between host plants. Preference of the non-exposed F. occidentalis toward the infected plants increases the probability of acquisition that would lead to transmission, as they will lay eggs on the infected plants, whereas preference of the exposed F. occidentalis for non-infected plants enhances the transmission probability (Ingwell et al. 2012). The restlessness of the virus-carrying F. occidentalis could also be seen during the preferential behavior study, as high numbers were recovered outside the choice arenas after the 24 h period, in case of the setup where the exposed individuals were released, compared with the setup with the non-exposed. Several studies have shown that the exposure to plant viruses can change the preferential behavior of the vector. For instance, acquisition of Barley yellow dwarf virus and Potato leaf-roll virus can alter the preferential behavior of their vectors, Rhopalosiphum padi and Myzus persicae, respectively (Eigenbrode et al. 2002, Jimenez-Martinez 2004, Medina Ortega et al. 2009, Werner et al. 2009, Bosque-Pérez and Eigenbrode 2011). In addition, it has been shown that apart from the virus load of the host plant, other factors such as host plant resistance or susceptibility can influence thrips preferential behavior (Maris et al. 2004).
In conclusion, we found strong indications that the proposed hypothesis of “Vector Manipulation” by Ingwell et al. (2012) is relevant for the studied system C. annuum–TSWV–F. occidentalis, as the discussed influences, both in the vector developmental parameters and preferential behavior, could lead to a predictable enhancement of acquisition and transmission of TSWV.
Acknowledgments
This research was funded by German Research Foundation (DFG - Deutsche Forschungsgemeinschaft; Project number: 207/37-1). We thank the Wageningen University, The Netherlands, for the supply of F. occidentalis and the TSWV isolates, and also the entire technical support team, Department of Phytomedicine, Leibniz Universität, Hannover. We would also like to thank the anonymous reviewers whose corrections and suggestions greatly helped to improve this manuscript.
We declare no conflict of interest among the authors of this paper.
References Cited
- Abe H., Tomitaka Y., Shimoda T., Seo S., Sakurai T., Kugimiya S., Tsuda S., Kobayashi M. 2012. Antagonistic plant defense system regulated by phytohormones assists interactions among vector insect, thrips and a tospovirus. Plant Cell Physiol. 53: 204–212. [DOI] [PubMed] [Google Scholar]
- Awmack C. S., Leather S. R. 2002. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 47: 817–844. [DOI] [PubMed] [Google Scholar]
- Belliure B., Janssen A., Maris P. C., Peters D., Sabelis M. W. 2005. Herbivore arthropods benefit from vectoring plant viruses. Ecol. Lett. 8: 70–79. [Google Scholar]
- Belliure B., Janssen A., Sabelis M. W. 2008. Herbivore benefits from vectoring plant virus through reduction of period of vulnerability to predation. Oecologia 156: 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliure B., Janssen A., Sabelis M. W. 2010. Vector and virus induce plant responses that benefit a non-vector herbivore. Basic Appl. Ecol. 11: 162–169. [Google Scholar]
- Blua M. J., Perring T. M., Madore M. A. 1994. Plant virus-induced changes in aphid population development and temporal fluctuations in plant nutrients. J. Chem. Ecol. 20: 691–707. [DOI] [PubMed] [Google Scholar]
- Bosque-Pérez N. A., Eigenbrode S. D. 2011. The influence of virus-induced changes in plants on aphid vectors: Insights from luteovirus patho systems. Virus Res. 159: 201–205. [DOI] [PubMed] [Google Scholar]
- Chatzivassiliou E. K., Nagata T., Katis N. I., Peters D. 1999. Transmission of Tomato spotted wilt virus by Thrips tabaci populations originating from leek. Plant Pathol. 48: 700–706. [Google Scholar]
- De Angelis J. D., Sether D. M., Rossignol P. A. 1993. Survival, development, and reproduction in Western flower thrips (Thysanoptera: Thripidae) exposed to impatiens necrotic spot virus. Environ. Entomol. 22: 1308–1312. [Google Scholar]
- Döring T. F., Archetti M., Hardie J. 2009. Autumn leaves seen through herbivore eyes. Proc. Biol. Sci. 276: 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenbrode S. D., Ding H., Shiel P., Berger P. H. 2002. Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae). Proc. R. Soc. Lond. Ser. B. Biol. Sci. 269: 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO/CABI 1997c. Tomato spotted wilt virus in: Quarantine pests for Europe (2nd ed), CAB International, Wallingford (GB: ), pp. 1379–1387. [Google Scholar]
- Fereres A., Moreno A. 2009. Behavioural aspects influencing plant virus transmission by homopteran insects. Virus Res. 141: 158–168. [DOI] [PubMed] [Google Scholar]
- Ingwell L. L., Eigenbrode S. D., Bosque-PéRez N. A., 2012. Plant viruses alter insect behaviour to enhance their spread. Sci. Rep. 2: 578.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeger M. J., Holt J., van den Bosch F., Madden L. V. 2004. Epidemiology of insect-transmitted plant viruses: modelling disease dynamics and control interventions. Physiol Entomol. 29: 291–304. [Google Scholar]
- Jimenez-Martinez E. S. 2004. Volatile cues influence the response of Rhopalosiphum padi (Homoptera: Aphididae) to Barley yellow dwarf virus-infected transgenic and untransformed wheat. Environ. Entomol. 33: 1207–1216. [Google Scholar]
- Jones R.A.C. 2014. Plant virus ecology and epidemiology: Historical perspectives, recent progress and future prospects. Annu. Appl. Biol. 164: 320–347. [Google Scholar]
- Mandal B., Csinos A. S., Martinez-Ochoa N., Pappu H. R. 2008. A rapid and efficient inoculation method for Tomato spotted wilt virus. J. Virol. Met. 149: 195–198. [DOI] [PubMed] [Google Scholar]
- Maris P. C. 2004. Evaluation of thrips resistance in pepper to control Tomato spotted wilt virus infection. PhD thesis, Wageningen University, Wageningen. [Google Scholar]
- Maris P. C., Joosten N. N., Goldbach R. W., Peters D. 2004. Tomato spotted wilt virus infection improves host suitability for its vector Frankliniella occidentalis. Phytopathology 94: 706–711. [DOI] [PubMed] [Google Scholar]
- Medeiros R. B., Resende R. O., Avila A. C. 2004. The plant virus Tomato spotted wilt tospovirus activates the immune system of its main insect vector, Frankliniella occidentalis. J. Virol. 78: 4976–4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina Ortega K. J., Bosque-Perez N. A., Ngumbi E., Jimenez-Martinez E. S., Eigenbrode S. D. 2009. Rhopalosiphum padi (Hemiptera: Aphididae) responses to volatile cues from Barley yellow dwarf virus-infected wheat. Environ. Entomol. 38: 836–845. [DOI] [PubMed] [Google Scholar]
- Nachappa P., Margolies D. C., Nechols J. R., Whitefield A. E., Rotenberg D. 2013. Tomato spotted wilt virus benefits a non-vector arthropod, Tetranychus urticae, by modulating different plant responses in tomato. PLoS ONE 8: e75909.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T., Peters D. 2001. An anatomical perspective of Tospovirus transmission, pp. 51–67. In Harris K. F., Smith O. P., Duffus J. E. (eds.), Virus-insect-plant interactions, Academic Press, New York. [Google Scholar]
- Ogada P. A., Maiss E., Poehling H. M. 2013. Influence of Tomato spotted wilt virus on performance and behaviour of western flower thrips (Frankliniella occidentalis). J. Appl. Entomol. 137: 488–498. [Google Scholar]
- Robb K. L. 1989. Analysis of Franklineilla occidentalis (Pergande) as a pest of floricultural crops in California greenhouses. PhD Thesis. University of California, Riverside, p. 135. [Google Scholar]
- Roselló S., Diaz M. J., Nuez F. 1996. Viral diseases causing economic losses to the tomato crop. I. The Tomato spotted wilt virus - a review. Sci. Hortic. 67: 117–150. [Google Scholar]
- Sakimura K. 1963. Frankliniella fusca, an additional vector for the Tomato spotted wilt virus, with notes on Thrips tabaci, another vector. Phytopathology 53: 412–415. [Google Scholar]
- Sherwood J., German T. L., Moyer J. W., Ullman D. E., Whitfield A. E. 2000. Tomato spotted wilt, pp. 1030–1031. In Maloy O. C., Murray T. D. (eds.), Encyclopedia of plant pathology. John Wiley & Sons, New York. [Google Scholar]
- Stafford C. A., Walker G. P., Ullman D. 2011. Infection with a plant virus modifies vector feeding behaviour. Proc. Natl. Acad. Sci. 108: 9350–9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout M. J., Thaler J. S., Thomma B.P.H.J. 2006. Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annu. Rev. Entomol. 51: 663–689. [DOI] [PubMed] [Google Scholar]
- Stumpf C. F., Kennedy G. G., 2005. Effects of tomato spotted wilt virus (TSWV) isolates, host plants, and temperature on survival, size, and development time of Frankliniella fusca. Entomol. Exp. Appl. 144: 215–225. [Google Scholar]
- Stumpf C. F., Kennedy G. G., 2007. Effects of tomato spotted wilt virus isolates, host plants, and temperature on survival, size, and development time of Frankliniella occidentalis. Entomol. Exp. Appl. 123: 139–147. [Google Scholar]
- Ullman D. E., Cho J. J., Mau R.F.L., Westcot D. M., Custer D. M. 1992. Midgut epithelial cells act as a barrier to tomato spotted wilt virus acquisition by adult western flower thrips. Phytopathology 82: 1333–1342. [Google Scholar]
- Ullman D. E., German T. L., Sherwood J. L., Westcot D. B., Cantone F. A. 1993. Tospovirus replication in insect vector cells: Immunocytochemical evidence that the nonstructural protein encoded by the S RNA of tomato spotted wilt tospovirus is present in thrips vector cells. Phytopathology 86: 900–905. [Google Scholar]
- Ullman D. E., German T. L., Sherwood J. L., Westcot D. M. 1995. Thrips transmission of tospoviruses: Future possibilities for management, pp. 135–151. In: Thrips biology and management, the series NATO ASI series, Vol. 276, Springer, Burlington, Vermont. [Google Scholar]
- van de Wetering F., Hulshof J., Posthuman K., Harrewijn P., Goldbach R., Peters D. 1999. Distinct feeding behaviour between sexes of Frankliniella occidentalis results in higher scar production and lower Tospovirus transmission by females. Entomol. Exp. Appl. 88: 9–15. [Google Scholar]
- Werner B. J., Mowry T. M., Bosque-Perez N. A., Ding H., Eigenbrode S. 2009. Changes in green peach aphid responses to potato leafroll virus-induced volatiles emitted during disease progression. Environ. Entomol. 38: 1429–1438. [DOI] [PubMed] [Google Scholar]
- Whitfield A. E., Ullman D. E., German T. L. 2005. Tospovirus-thrips interactions. Annu. Rev. Phytopathol. 43: 459–489. [DOI] [PubMed] [Google Scholar]
- Wijkamp I., Peters D. 1993. Determination of the median latent period of two tospoviruses in Frankliniella occidentalis, using a novel leaf disk assay. Phytopathology 83: 986–991. [Google Scholar]
- Wijkamp I., Almarza N., Goldbach R., Peters D. 1995. Distinct levels of specificity in thrips transmission of tospoviruses. Phytopathology 85: 1069–1074. [Google Scholar]
- Wijkamp I., Goldbach R., Peters D. 1996. Propagation of Tomato spotted wilt virus in Frankliniella occidentalis does neither result in pathological effects nor in transovarial passage of virus. Entomol. Exp. Appl. 81: 285–292. [Google Scholar]