Abstract
Purpose:
Calcitonin receptor gene has also a polymorphism which is associated with bone mass density. This study evaluates the association between calcitonin receptor AluI (rs1801197) and Taq1 calcitonin genes polymorphism with bone density rate.
Methods:
In this descriptive-analytical study in 2013 in southwestern Iran, 200 blood samples, per the Cochran sample size formula, were taken from women aged 45 and older. DNA was extracted from the samples using the phenol– chloroform method and the genomic fragments in question were proliferated using the polymerase chain reaction (PCR) method.
Results:
The genotypic distribution of polymorphism AluI for TT, TC, and CC genotypes in control group was 31.4%, 38.6%, and 30% and in patients 25.4%, 55.4%, and 19.2%, respectively. There was no significant difference in polymorphism AluI between patients and control group and no significant association was found between this gene and bone density rate (P > 0.05). All patients and the individuals in the control group exhibited tt genotype for TaqI calcitonin gene and no significant association was found between these participants and osteoporosis.
Conclusion:
There was no association between two polymorphisms and osteoporosis, and between polymorphism of these two genes and osteoporosis development rate in the participants.
Keywords: Polymorphism, TaqI calcitonin gene, AluI calcitonin receptor gene, osteoporosis
1. INTRODUCTION
Osteoporosis is a common skeletal disorder, accompanied with decrease in bone mass, deterioration of bone microstructures, and increase in susceptibility to fracture (1). Fracture contributes to increasing morbidity and mortality and imposes high costs on individuals and community. On average, one woman per three and one man per 12 experience fracture during lifetime (2, 3). In the USA, annually 1.5 million fractures occur due to osteoporosis. More than 50% of these patients could not well recover their previous activities and 10% will need long term therapeutic practices. Deep venous thrombosis is high (20-50%) in these patients (1, 4).
Bone mass reaches to its maximum level in early adulthood and then declines gradually as age advances. In women, particularly postmenopausal, as estrogen decreases, intestinal and renal calcium absorption decreases and calcium intake by the bones reaches to its minimum level. Therefore, postmenopausal estrogen decline is one of the predisposing factors for osteoporosis in postmenopausal women (2, 5). Genetic differences could also contribute to the variety of contents in bone minerals in different ethnics (6).
Calcitonin is a polypeptide hormone, comprising 32 amino acids and contributing mainly to calcium concentration regulation. This hormone is secreted by parafollicular C cells of mammalian thymus and thyroid. A disulfide bond links cysteine 1 to cysteine 7 and a 7-amino acid circular structure is developed at the end of amine. Precursor of pre-procalcitonin has 141 amino acids. Calcitonin gene is located on short arm of chromosome 11 (11p15) (7, 8, 9).
Calcitonin declines the cells’ permeability toward calcium, stops osteoclasts’ activity, and facilitates and intensifies osteogenesis through stopping calcium’s intake from bones and intensifying osteoclastic activities. As a result, the blood’s calcium and phosphate decline, which causes decline in hydroxyproline of the blood and urine (7, 8).
Hopner et al. found that calcitonin gene (CT) holds a polymorphic site for TaqI restriction enzyme, this site is located on short arm of chromosome 11, and this polymorphism is associated with several diseases. In this gene, there is another polymorphism called dinucleotide repeat calcitonin, which is associated with bone density rate (9, 19). Miyao et al. investigated the association between the dinucleotide repeat calcitonin in calcitonin polymorphism locus in Japan (9).
Calcitonin receptor is the receptor of calcitonin hormone. Calcitonin receptor gene (CTR) is located on long arm of chromosome 7 (7q21). This receptor exists in osteoclasts, renal tract epithelium, testicles, brain, and ovary. Calcitonin activity is mediated through high-affinity receptor, i.e. calcitonin receptor (11, 12).
Calcitonin receptor gene has also polymorphism TaqI which is associated with bone density rate. Masi et al. indicated that this polymorphism was associated with bone density rate and the individuals with genotype tt had a less bone density compared with the heterozygous Tt individuals (13).
In calcitonin and calcitonin receptor genes, the ability to develop cutting by the enzymes TaqI and AluI causes development of the following genotypes: AluI genotypes CC, TC and TT (14, 15) and TaqI genotypes TT, Tt and tt (16).
The main purpose in treating osteoporosis is to prevent fractures. Recent advances in detecting genetic basis of diseases have opened new horizons. Disease diagnosis through genotype determination is increasing. The pharmacologic and interventional methods available for preventing osteoporosis in the individuals at risk add to the significance of the issue. Also, no study has been yet conducted on the association between molecular basis of bone density decline and the polymorphisms AluI calcitonin receptor in Iran. Therefore, this study was conducted to investigate the association between AluI (rs1801197) calcitonin receptor gene and bone density rate in 45- and above 45-year-old women in southwestern Iran.
2. MATERIALS AND METHODS
In this descriptive, analytical study conducted between December, 2012 and September, 2013. This research was approved at research at council and ethics committee of Shahrekord University of medical Sciences-Iran. Ethics code: 92-12-17. sample size was calculated by Cochrane equation. 45- and over 45-year-old women referring the centers for bone density measurement in southwestern Iran were enrolled based on convenience sampling after the consent to participate in the study was obtained. The questionnaires of clinical and demographic data were filled out. Exclusion criteria were corticosteroids consumption history, ovariectomy or premature ovarian failure, thyroid disease, calcium intake disorders, gastrointestinal and renal diseases, etc. Bone density rate in lumbar vertebrae and femoral neck was measured using dual-energy X-ray absorptiometry (DEXA) and the values were determined by t-score. A 5-ml peripheral blood was taken from each patient in the tubes containing EDTA (0.5 M). DNA was extracted using phenol-chloroform for molecular experimentations (17). Quality and quantity of the extracted DNA were examined by spectrophotometry using Nanodrop.
Sequence of calcitonin and calcitonin receptor genes was extracted from NCBI database. The primers for the polymorphism AluI and TaqI were designed using online Primer3 software. The used primers are shown in Table 1.
Table 1.
The sequence of used primers in the study
Amplification of fragments containing TaqI calcitonin gene and AluI calcitonin receptor gene polymorphisms was done by PCR. Investigation of PCR products and determination of the genotypes were done using electrophoresis technique on polyacrylamide gel 8% by means of Tris-Borate EDTA (TBE) and loading 6X (Fermentas) buffers (18).
In each PCR, a vial was considered as negative control to show no contamination with DNA sample. The final volume of the reaction mixture was determined 25 µl per each micro-tube were considered. Thermocycler (PC818, ASTEC, Japan) was used.
The fragments were treated using restriction enzymes of AluI and TaqI (Fermentas). The marker of DNA size used was GeneRuler™ DNA Ladder, ready-to-use. For observing the obtained bands, silver nitrate staining was used.
Restriction fragment length polymorphism (RFLP): RFLP is a method based on longitudinal study of differences in a specific fragment of DNA that is highly capable of detecting some loci. Three cutting enzymes of AluI and Taq1 (Fermentase, Germany) were used for RFLP. For this purpose, a mixture containing 10 ml of PCR product, 1 µl of AluI and Taq1, and 2 µl of each enzyme buffer was prepared in a final volume of 30 µl and incubated for 16 h at 37°C. Restriction digestion products obtained in the acrylamide gel were electrophoresed and stained using silver nitrate.
In this study, t-score > − 1 was determined as normal, t-score ≤ − 1 to ≥ − 2 as mild osteopenia, t-score < − 2 to ≥ − 2.5 as severe osteopenia, and t-score < − 2.5 as osteoporosis (19).
The data were analyzed by SPSS software using chi-square and ANOVA. The association between different genotypes of the polymorphisms and bone density rate was investigated by ANOVA. The CI in all experiments was considered 95% and P<0.05 was considered significant.
3. RESULTS
Since all women over 45 years old have some bone density decline, we assigned, based on radiographs’ t-scores, the individuals with normal bone density and/or mild osteopenia as the control group (70 individuals) and those with severe osteopenia and osteoporosis as the patient group (130 individuals) (Table 2).
Table 2.
Characteristics of control and patient groups under study., * Bone mineral densityThe number of patient group is 130 and control group is 70.
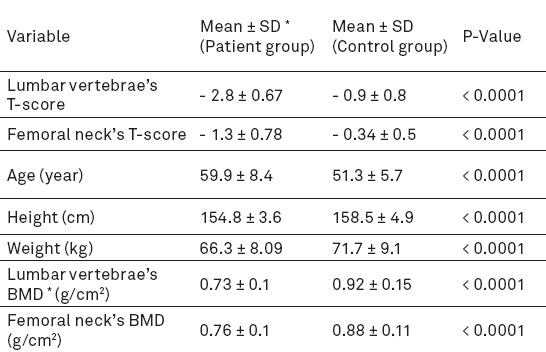
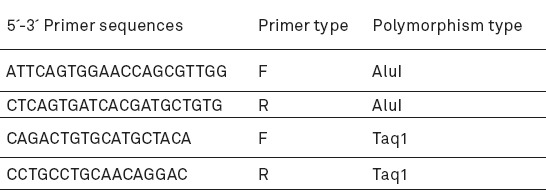
The quality of the extracted DNA samples was mostly desirable and A260/A280 between 1.75 and 2. The size of fragments obtained from PCR in the polymorphism TaqI calcitonin gene was 280 bp which produced two 111 and 169-bp fragments after digestion by enzyme TaqI, if the detection site of the enzyme was present. In addition, the length of amplified fragment in the polymorphism AluI of receptor calcitonin gene was 228 bp which produced two 108 and 120-bp fragments after digestion by enzyme AluI, if the restriction site of the enzyme was present (Figures 1, 2).
Figure 1.
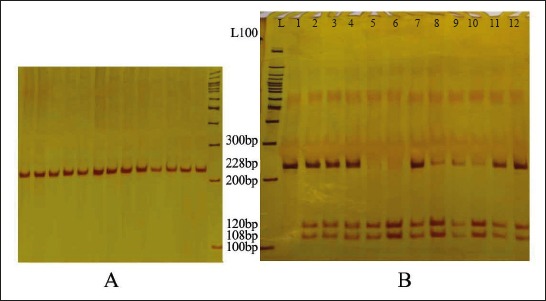
A: PCR product of polymorphism AluI (The size of amplified fragment was 228 bp); B: Enzymatic dissolution of polymorphism AluI (L: DNA ladder. The samples 2, 3, 4, 7-12 had three 228, 108, and 120-bp bands [genotype TC], Sample1 has two 228-bp bands [the individuals with homozygous genotype CC], and samples 5 and 6 had two 108- and 120-bp fragments [genotype TT]).
Figure 2.
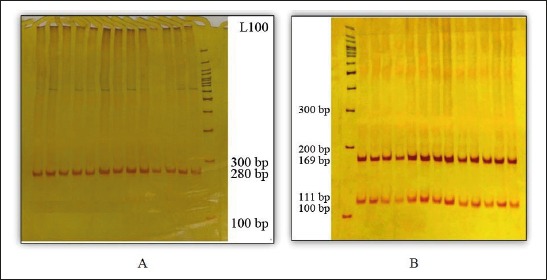
A: The product of polymorphism TaqI PCR (the size of amplified fragments was 280 bp). B: Enzymatic dissolution of polymorphism TaqI (All individuals in case and control groups had genotype tt).
Genotypic frequency was calculated for the polymorphisms in the two groups (Tables 3-4). For polymorphism AluI in both groups, degree of freedom (df) was considered 2 and p value was obtained > 0.05 (P = 0.16 in the control group and P = 0.443 in the patients); that is, there was no statistically significant deviation for the polymorphism AluI and Hardy-Weinberg equilibrium was noted.
Table 3.
The genotypic number and percent of polymorphisms in the two groups
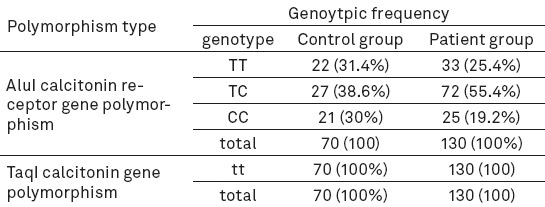
Table 4.
The number of expected and observed individuals for polymorphism AluI in the two groups
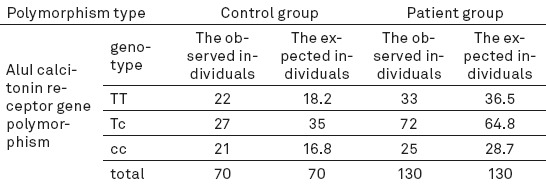
Mean t-score for lumbar vertebrae was–2.933 in the participants with genotype TT,–2.819 in those with genotype CC, and–2.712 in those with genotype TC. df for both lumbar vertebrae and femoral neck was 2 and P value for lumbar vertebrae 0.466 and that of femoral neck 0.959, indicating that the polymorphism AluI was not significantly associated with t-score, i.e. bone density.
It is noteworthy that for TaqI all individuals in both groups had the geno6type tt, such analyses were not done for this restriction site and its possible association with bone density.
4. DISCUSSION
Osteoporosis is one of the most prevalent metabolic disorders of bone minerals. This disease is multifactorial and is developed by interaction among environmental factors, different genes, and aging. In this study, the association between polymorphism TaqI of calcitonin gene and bone density was investigated in 45- and above 45-year-old women. In other studies, this polymorphism was associated with other diseases, but the association between this polymorphism and osteoporosis has not yet been examined. If this polymorphism is noted in the individuals with osteoporosis, three genotypes are developed. However, the polymorphism of this site, throughout the study, was not noted in the osteoporotic individuals, and all participants had homozygous genotype tt and cutting site was not polymorphic.
Also, in the present study, existence of two allelic forms of calcitonin receptor gene was confirmed due to polyphormistic site of AluI. We found that genotypic frequency of heterozygous TC individuals in the patients was higher compared with other genotypes. We also found that heterozygous TC individuals had a higher bone density compared with other genotypes. Therefore, heterozygot genotype of calcitonin receptor gene is more frequent than other hemozygot genotypes. Homozygous TT individuals had a lower bone density compared with homozygous CC. However, there was generally no significant association between bone density and no statistically considerable difference in genotypic distribution and allelic frequency between the patients and the control group.
Several studies have been conducted on association between several genes polymorphisms and osteoporosis, including vitamin D receptor gene, collagen Type 1, calcitonin receptor, growth factor, insulin-like, interleukin 6, etc (20).
Nakamura et al. in a study in Japan found an RFLP in calcitonin receptor gene nucleotide 1377 which caused substitution of a leucine amino acid in 463 site with proline. The genotype of heterozygous individuals is manifested as TC, leucin hemozygpus individuals as TT, and homozygous proline as CC (21). Lack of the remaining proline could change the second structure of calcitonin receptor. This change could decrease calcitonin hormone bond to calcitonin receptor gene and adenylate cyclase response decreases. Therefore, C domain of calcitonin receptor terminal is especially important. Heterozygous genotype or proline/leucine existence changes calcitonin receptor biological activity. Since individuals with heterozygous genotypes produce both alleles, they are somehow superior over other homozygous genotypes.
Some researchers found similar results. For example, Massi et al. in Italy found that frequency of heterozygous TC and homozygous TT genotypes was high. Also, homozygot TT genotype decreased lumbar bone mineral density (BMD) considerably compared with the genotype CC (Masi, 1998). In Taboulet et al. study, femoral neck’s BMD was higher considerably in heterozygous TC individuals compared with homozygous leucine TT and homozygous proline CC. Fracture risk also was lower in heterozygous individuals (22).
In a study in Turkey, no statistically significant difference was found in genotype and allelic frequency between the patients and the control group (23), which is consistent with the present study. Braga et al. found that polymorphism AluI calcitonin receptor gene in lower ages was associated with vertebral column BMD. That is, this polymorphism was more correlated with bone mass compared with bone loss due to aging (24), which is in contrast to our study. In addition, Tsai, in a study in Taiwan, found that this polymorphism was associated with osteoporosis prevalence and the individuals with the genotype TT were at high risk of osteoporosis in lumbar vertebrae and femoral neck (25). In Drews et al. study, BMD was very high in heterozygous individuals and this polymorphism was associated with osteopenia and osteoporosis progress (Drews, 2005). In two studies in Korea and Spain, the polymorphism AluI and BMD were associated (26, 27). In addition, in Charopoulos study, this polymorphism was associated with brachial BMD (28).
However, study of the association between several polymorphic sites of vitamin D receptor and bone density has revealed different findings worldwide. Liu et al. argued that polymorphism TaqI vitamin D receptor was far unlikely to be associated with body mass index variations, but could be only an indicator in a strong, unbalanced link with a close functional mutation (29). Mitra et al. study of the polymorphisms of vitamin D receptor (Apa1, TaqI, Fok1, Bsm1) and bone density in Indian postmenopausal women indicated that individuals with the genotypes aa, FF, and TT had a higher bone density compared with the genotypes AA, BB, ff, and tt (30). Zhang et al. did not observe any significant association between different polymorphisms of vitamin D genes and bone density in postmenopausal period (31). However, in some studies, polymorphism TaqI was associated with BMD in femoral neck and lumbar neck (7, 27). This inconsistency of the findings could be due to the difference in sample size or inadequate number of the samples, as well as racial differences among different communities. However, the important finding in the present study and other studies is higher bone density in the individuals with heterozygous TC genotype of calcitonin receptor gene compared with homozygous genotypes.
5. CONCLUSION
This study indicated no statistically significant deviation for polymorphism TaqI, AluI gene, and calcitonin receptor in the patients and control group. In this study, we used RFLP method to make the findings consistent with those of other studies conducted on this field. The association between other polymorphisms involved in osteogenesis regulation and bone density should be studied. Also, further research is recommended to study calcitonin gene TaqI polymorphism.
Acknowledgments
Hereby, we thank Research Deputy of University of Shahrekord, Research and Technology Deputy of Shahrekord University of Medical Sciences, and Cellular and Molecular Research Center of Shahrekord University of Medical Sciences for cooperating in this study.
Footnotes
• Conflict of Interest: The authors declare no conflict of interests.
REFERENCES
- 1.Lindsay R, Cosman F. Osteoporosis. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Larry Jameson J, Joseph Loscalzo J, editors. Harrison's principles of internal medicine. 17th ed. New Yourk: MC Graw Hill; 2008. pp. 2397–2408. [Google Scholar]
- 2.Kung AW, Huang QY. Genetic and environmental determinants of osteoporosis. J Musculoskelet Neuronal Interact. 2007;7(1):26–32. [PubMed] [Google Scholar]
- 3.Duncan EL, Brown MA. Genetic studies in osteoporosis - the end of the beginning. Arthritis Res Ther. 2008;10(5):214. doi: 10.1186/ar2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pocock NA, Eisman JA, Hopper JL, Yeates MG, Sambrook PN, Eberl S. Genetic determinants of bone mass in adults. A twin study. J Clin Inves. 1987;80(3):706–10. doi: 10.1172/JCI113125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prince RL, Dick I. Oestrogen effects on calcium membrane transport: a new view of the inter-relationship between oestrogen deficiency and age-related osteoporosis. Osteoporos Int. 1997;7(3):S150–S154. doi: 10.1007/BF03194362. [DOI] [PubMed] [Google Scholar]
- 6.Gong G, Haynatzki G, Haynatzka V, Howell R, Kosoko-Lasaki S, Fu YX, Yu F, Gallagher JC, Wilson MR. Bone mineral density-affecting genes in Africans. J Natl Med Assoc. 2006;98(7):1102–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Masi L, Brandi ML. Calcitonin and calcitonin receptors. Clin Cases Miner Bone Metab. 2007;4(2):117–22. [PMC free article] [PubMed] [Google Scholar]
- 8.Becker KL, Nylen ES, Cohen R, Snider RH Calcitonin: structure, molecular biology, and actions. Principle of bone biology. San Diego: Academic Press; 1996. pp. 471–4. [Google Scholar]
- 9.Miyao M, Hosoi T, Emi M, Nakajima T, Inoue S, Hoshino S, Shiraki M, Orimo H, Ouchi Y. Association of bone mineral density with a dinucleotide repeat polymorphism at the calcitonin (CT) locus. J Hum Genet. 2000;45(6):346–50. doi: 10.1007/s100380070006. [DOI] [PubMed] [Google Scholar]
- 10.Hoppener JWM, Steenbergh PH, Zandberg J, Bakker E, Pearson PL, Geurts van Kessel AHM, Jansz HS, Lips CJM. Localization of the polymorphic human calcitonin gene on chromosome 11. Hum Genet. 1984;66(4):309–12. doi: 10.1007/BF00287635. [DOI] [PubMed] [Google Scholar]
- 11.Drews K, Seremak-Mrozikiewicz A, Bartkowiak-Wieczorek J, Pienkowski W, Dubiel M, Mrozikiewicz PM. [Genetic polymorphism of the calcitonin receptor gene and bone mineral density in Polish population of postmenopausal women] Ginekol Pol. 2005;76(8):612–8. [PubMed] [Google Scholar]
- 12.Braga V, Sangalli A, Malerba G, Mottes M, Mirandola S, Gatti D, Rossini M, Zamboni M, Adami S. Relationship among VDR (BsmI and FokI), COLIA1, and CTR polymorphisms with bone mass, bone turnover markers, and sex hormones in men. Calcif Tissue Int. 2002;70(6):457–62. doi: 10.1007/s00223-001-1088-9. [DOI] [PubMed] [Google Scholar]
- 13.Masi L, Becherini L, Colli E, Gennari L, Mansani R, Falchetti A, Becorpi AM, Cepollaro C, Gonnelli S, Tanini A, et al. Polymorphisms of the calcitonin receptor gene are associated with bone mineral density in postmenopausal Italian women. Biochem Biophys Res Commun. 1998;248(1):190–5. doi: 10.1006/bbrc.1998.8880. [DOI] [PubMed] [Google Scholar]
- 14.Mittal RD, Bid HK, Kumar R, Kumar A, Bhandari M. Is calcitonin receptor gene polymorphism an appropriate marker for calcium oxalate urolithiasis? Int J Hum Genet. 2004;4(1):57–60. [Google Scholar]
- 15.Lkhagvasuren U, Jav S, Batjargal O, Batsukh M. Association between osteoporosis and polymorphisms of the bone estrogen receptor 1, calcitonin receptor genes in Mongolian postmenopausal women. Peer J Pre Prints. 2014;2:e191v1. [Google Scholar]
- 16.Atoum MF, Tchoporyan MN. Association between circulating vitamin D, the Taq1 vitamin D receptor gene polymorphism and colorectal cancer risk among Jordanians. Asian Pac J Cancer Prev. 2014;15(17):7337–41. doi: 10.7314/apjcp.2014.15.17.7337. [DOI] [PubMed] [Google Scholar]
- 17.Dale J, Schantz M. Purification and separation of nucleic acid. In: Dale JW, Schantz MV, editors. From Genes to genomes. 2nd ed. West Sussex: John Wiley press; 2002. pp. 31–3. [Google Scholar]
- 18.Walker JM, Rapley R. Molecular biology and biotechnology. Royal Society of Chemistry. 2000 [Google Scholar]
- 19.Bundred NJ, Campbell ID, Davidson N, DeBoer RH, Eidtmann H, Monnier A, Neven P, von Minckwitz G, Miller JC, Schenk NL, Coleman RE. Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: ZO-FAST Study results. Cancer. 2008;112(5):1001–10. doi: 10.1002/cncr.23259. [DOI] [PubMed] [Google Scholar]
- 20.Ralston SH, de Crombrugghe B. Genetic regulation of bone mass and susceptibility to osteoporosis. Genes Dev. 2006;20(18):2492–2506. doi: 10.1101/gad.1449506. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura M, Zhang ZQ, Shan L, Hisa T, Sasaki M, Tsukino R, Yokoi T, Kaname A, Kakudo K. Allelic variants of human calcitonin receptor in the Japanese population. Hum Genet. 1997;99(1):38–41. doi: 10.1007/s004390050307. [DOI] [PubMed] [Google Scholar]
- 22.Taboulet J, Frenkian M, Frendo JL, Feingold N, Jullienne A, de Vernejoul MC. Calcitonin receptor polymorphism is associated with a decreased fracture risk in post-menopausal women. Hum Mol Genet. 1998;7(13):2129–33. doi: 10.1093/hmg/7.13.2129. [DOI] [PubMed] [Google Scholar]
- 23.Tural S, Kara N, Alayli G, Tomak L. Association between osteoporosis and polymorphisms of the bone Gla protein, estrogen receptor 1, collagen 1-A1 and calcitonin receptor genes in Turkish postmenopausal women. Gene. 2013;515(1):167–72. doi: 10.1016/j.gene.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 24.Braga V, Mottes M, Mirandola S, Lisi V, Malerba G, Sartori L, Bianchi G, Gatti D, Rossini M, Bianchini D, et al. Association of CTR and COLIA1 alleles with BMD values in peri- and postmenopausal women. Calcif Tissue Int. 2000;67(5):361–6. doi: 10.1007/s002230001160. [DOI] [PubMed] [Google Scholar]
- 25.Tsai FJ, Chen WC, Chen HY, Tsai CH. The ALUI calcitonin receptor gene polymorphism (TT) is associated with low bone mineral density and susceptibility to osteoporosis in postmenopausal women. Gynecol Obstet Invest. 2003;55(2):82–7. doi: 10.1159/000070179. [DOI] [PubMed] [Google Scholar]
- 26.Kim JG KS, Jee BC, Suh CS, Kim SH, Choi YM, Moon SY. The relationship between calcitonin receptor gene AluI polymorphism, bone mineral density and bone responsiveness to hormone replacement therapy in postmenopausal Korean women. Korean J Obstet Gynecol. 2005;48(6):1476–83. [Google Scholar]
- 27.Bandres E, Pombo I, Gonzalez-Huarriz M, Rebollo A, Lopez G, Garcia-Foncillas J. Association between bone mineral density and polymorphisms of the VDR, ERalpha, COL1A1 and CTR genes in Spanish postmenopausal women. J Endocrinol Invest. 2005;28(4):312–21. doi: 10.1007/BF03347196. [DOI] [PubMed] [Google Scholar]
- 28.Charopoulos I, Trovas G, Stathopoulou M, Kyriazopoulos P, Galanos A, Dedoussis G, Antonogiannakis E, Lyritis GP. Lack of association between vitamin D and calcitonin receptor gene polymorphisms and forearm bone values of young Greek males. J Musculoskelet Neuronal Interact. 2008;8(2):196–203. [PubMed] [Google Scholar]
- 29.Liu YZ, Liu YJ, Recker RR, Deng HW. Molecular studies of identification of genes for osteoporosis: the 2002 update. J Endocrinol. 2003;177(2):147–96. doi: 10.1677/joe.0.1770147. [DOI] [PubMed] [Google Scholar]
- 30.Mitra S, Desai M, Ikram Khatkhatay M. Vitamin D receptor gene polymorphisms and bone mineral density in postmenopausal Indian women. Maturitas. 2006;20(55(1)):27–35. doi: 10.1016/j.maturitas.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Zhang ZL, Zhao JX, Meng XW, Zhou XY, Xing XP, Xia WB. [Association of polymorphisms of vitamin D receptor gene start codon and 3'-end region with bone mineral density in postmenopausal women] Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2003;20(1):5–8. [PubMed] [Google Scholar]


