ABSTRACT
Although dietary fibers contribute to health and physiology primarily via the fermentative actions of the gut microbiota of the hosts, few studies have focused on how these interactions influence the metabolic status of sows. Here, the effects of inclusion of konjac flour (KF) in a gestation diet on oxidative stress status, insulin sensitivity, and gut microbiota were investigated to elucidate the correlation between the microbiota and metabolic changes in sows. Sows were assigned to either control or 2.2% KF dietary treatment during gestation. The gut microbiota population in sows during gestation and lactation was assessed by 16S rRNA gene sequencing. The oxidative stress parameters, homeostasis model assessment (HOMA) values, and fatty acids in the blood of sows were also assessed. Compared to the control diet group, KF significantly reduced the serum levels of reactive oxygen species (ROS) and 8-hydroxy-deoxyguanosine (8-OHdG) but increased the serum concentrations of glutathione peroxidase (GSH-Px) in sows on day 1 in lactation. Additionally, sows in the KF group had a lower HOMA insulin resistance value but a higher HOMA insulin sensitivity (HOMA-IS) value. KF induced changes in the gut microbial composition at the phylum and genus levels. The increased relative abundances of Akkermansia and Roseburia in the KF group were positively correlated with the HOMA-IS. Overall, dietary KF alleviated oxidative stress and improved insulin sensitivity of sows, and the changes in the gut microbiota in response to KF may have been correlated with the host metabolism response.
IMPORTANCE To date, the effect of dietary fiber on metabolism responses and gut microbiota in sows has not been investigated. Here, KF supplementation of a gestation diet in sows was found to alleviate oxidative stress and to improve insulin sensitivity. Pyrosequencing analysis revealed that KF treatment induces changes in the gut microbiota composition at the phylum and genus levels. Moreover, the changes of gut microbiota in response to KF may be correlated with the host metabolism response.
INTRODUCTION
The reproductive performance (litter size, litter weight, etc.) of breeding sows and their feed intake during lactation directly affect the overall productivity of pig operations. Increased metabolic burdens on sows during late gestation and lactation cause elevated systemic oxidative stress during these important periods (1, 2). Elevated oxidative stress is reported to be associated with pregnancy complications in highly prolific sows (3, 4). Moreover, the transition from pregnancy to lactation is characterized by physiological and metabolic changes, such as a progressive decrease in insulin sensitivity during late gestation and lactation (5, 6), which may unfortunately result in decreased lactation feed intake of sows (7, 8). Our previous studies showed that sows provided with konjac flour (KF) during gestation significantly increased their voluntary feed intake during lactation and demonstrated improved litter weight at weaning (9–11), probably as a result of insulin sensitivity improvement. However, the mechanism by which dietary KF exerts the benefit is poorly understood.
KF is produced abundantly in the konjac tuber (Amorphophallus konjac), mainly containing konjac glucomannan (KGM) (12), and has been consumed in the form of rubbery jelly, noodles, and other food products by humans in Asia for centuries. KGM is a complex carbohydrate consisting of d-glucose and d-mannose units joined by β-1,4 glycosidic bond linkages (13). KGM has great viscosity and swelling capacity (10) and withstands digestion in the small bowel and has been shown to alter the colonic/fecal microbiota composition in rats (14), adult humans (12), and sows (9). Growing evidence suggests that the gut microbiota plays a vital role in driving metabolic disease development, including inflammation, and reduced insulin sensitivity in nonpregnant (15, 16) or pregnant (17) hosts. Dietary interventions are thus a potential tool to modulate gut microbiota and further impact host health (18, 19). Previous studies have shown that resistant starch (rich in nonstarch polysaccharides) improved glucose tolerance in healthy subjects with a normal body mass index (20, 21) and provided evidence of a link between colonic fermentation and glucose metabolism (20). However, to date, little information is available about the impact of KF feed consumption on the gut microbiota of pregnant females and the correlation between gut microbiota and metabolic parameters after dietary KF supplementation.
The aim of this study was to determine the effects of KF inclusion in the gestation diet on oxidative stress status and insulin sensitivity of sows. The influence of KF treatment on the taxonomic profile of the gut microbiota of sows was also investigated by high-throughput sequencing analysis. Our hypothesis was that the inclusion of KF in a gestation diet would modify the intestinal microbiota and that the modifications would be associated with changes in oxidative stress status, homeostasis model assessment (HOMA) values, and plasma concentrations of short-chain fatty acids (SCFA), free fatty acids (FFA), and inflammatory cytokine of sows.
MATERIALS AND METHODS
The experiment was approved in accordance with Huazhong Agricultural University Animal Care and Use Committee guidelines.
Animals, diets, and housing.
Fifty large white sows with an average parity of 4.95 ± 1.02 (2–6) were allocated across two dietary treatments groups based on parity and body weight (BW). During gestation, the sows were fed with two different diets consisting of a control gestation diet (n = 24) and the same basal diet supplemented with 2.2% konjac flour (KF diet; n = 26). Feeding of the sows in the two groups was restricted to their respective diets administered during gestation twice a day (07:00 and 14:30). All sows were allowed to consume the same lactation diets ad libitum (see Table S1 in the supplemental material). All diets had the same levels of net energy, crude protein (CP), acid detergent fiber (ADF), neutral detergent fiber (NDF), and insoluble fiber (ISF). The KF diet had a higher soluble fiber (SF) level than the control diet. Pregnant sows were housed individually in gestation stalls (2.2 m by 0.7 m by 1.1 m). Sows were moved from the gestation stalls to the farrowing rooms on day 107 ± 2 of gestation and then kept in individual farrowing crates with stalls (2.2 m by 0.7 m) in pens and space on both sides of the stall (2.2 m by 0.5 m) for the pigs after birth. Both sows and piglets had free access to water.
Sample collection.
Blood samples (5 ml) from ear vein were collected from the fasted sows (5 sows per dietary treatment with similar parities and BWs) before feed was given on days 10 and 109 of gestation and on days 3 and 7 of lactation for analysis of HOMA values. Blood samples were collected from the fasted sows (5 sows per dietary treatment with similar parities and BWs) before feed was given on day 109 of gestation and on day 3 of lactation for inflammatory cytokine analysis. Blood samples were collected from sows (5 sows per dietary treatment with similar parities and BWs) before feeding and at 4 h postmeal on day 109 of gestation for SCFA and FFA analyses. Blood samples (5 ml) were collected from sows (5 sows per dietary treatment with similar parities and BWs) before feeding on days 10, 60, 90, and 109 of gestation and on days 1, 3, 7, and 21 of lactation for analysis of oxidative stress parameters. Fasting sows were selected for blood sampling after an overnight fasting period of 16 h during gestation and 12 h during lactation.
Blood samples for HOMA, SCFA, FFA, and inflammatory cytokine analyses were collected in heparinized tubes (5 ml), while those for oxidative stress parameter analyses were collected in tubes without anticoagulant (5 ml). Samples for plasma assays (heparinized tubes) were kept on ice and centrifuged for 5 min at 8,500 × g at 4°C. Samples for serum assays (tubes containing no anticoagulant) were stored at room temperature for 4 h and then centrifuged for 5 min at 5,000 × g at 4°C. Serum and plasma samples were stored at −80°C until analysis.
Fresh fecal samples were individually collected using sterile 50-ml centrifuge tubes (without any treatment) from the sows (10 sows per dietary treatment with similar parities and BWs) on day 109 of gestation as well as day 21 of lactation. Samples were transported (the tubes frozen on dry ice) immediately to the laboratory and then stored at −20°C before total genomic DNA extraction (within 12 h).
Analysis of oxidative stress parameters.
Serum samples were used to measure levels of thiobarbituric acid reactive substances (TBARS), 8-hydroxy-deoxyguanosine (8-OHdG), glutathione peroxidase (GSH-Px), and reactive oxygen species (ROS). Values for TBARS, one of the most frequently used indicators of lipid peroxidation, were determined in the current study. The major marker for oxidative damage to nucleic acids, 8-OHdG, was chosen to determine the DNA damage in the current study (22). Serum samples were analyzed for the activities of antioxidant enzymes, including GSH-Px, and for TBARS using commercial kits provided by Nanjing Jiancheng Bioengineering Institute (Nanjing, China) (23). GSH-Px activity was determined by quantifying the rate of oxidation of GSH to GSSG by H2O2 catalyzed by GSH-Px. GSH reacts with 5,5′-dithiobis-p-nitrobenzoic acid (DTNB) to produce yellow 5-thio-2-nitrobenzoic acid (TNB) that can be quantified spectrophotometrically at 412 nm. TBARS was analyzed based on the reaction with 2-thiobarbituric acid. The resulting pink product was measured spectrophotometrically at 535 nm. The concentration of 8-OHdG in the serum sample was determined using an anti-8-OHdG monoclonal antibody in an enzyme-linked immunosorbent assay (ELISA) kit (Dobio Biotech Co., Ltd., Shanghai, People's Republic of China) as described by Pialoux et al. (24). Levels of ROS in serum were measured by chemiluminescence assay using luminol (5-amino-2,3-dihydro-1,4-phthalazinedione; Sigma) as a probe according to the procedure described by Du et al. (25). Briefly, 50 μl serum and 20 μl horseradish peroxidase (HRP) (Sigma) (1 U of HRP, type VI [310 U/mg]) were mixed in a tube and then supplemented with 200 μl Krebs-HEPES buffer (99 mM NaCl, 4.7 mM KCl, 1.9 mM CaCl2, 1.2 mM MgCl2, 1.00 mM KH2PO4, 25 mM NaHCO3, 20 Mm HEPES, 11.0 mM glucose; pH 7.4). All chemiluminometric counts were obtained at intervals of 0.05 s for 3 s using a LB 940 luminometer (Berthold Technologies, Bad Wildbad, Germany), and the results were expressed as areas under the curve (AUCs) of relative light units. The calculation was based on the integration of the curve using the trapezoidal rule (a linear approximation). All samples were analyzed in duplicate.
Laboratory analyses.
Diet samples were ground through a 0.45-mm-pore-size sieve by the use of a high-speed universal disintegrator before analysis. CP was determined according to AOAC guidelines (1990). ADF and NDF were analyzed as described by Van Soest et al. (26). SF and ISF levels were determined by AOAC Method 991.43 (1990). Plasma concentrations of glucose and insulin were determined using a glucose dehydrogenase activity colorimetric assay kit (BioVision Inc., CA, USA) and an insulin ELISA kit (Biosource Inc., Sunnyvale, CA, USA), respectively, according to the instructions of the manufacturers. The indirect methods were used to evaluate insulin sensitivity by HOMA as follows: HOMA-IR (insulin resistance) = [(fasting insulin, mIU/liter) × (fasting glucose, mmol/liter)]/22.5; HOMA-IS (insulin sensitivity) = 1/[(fasting insulin, mIU/liter) × (fasting glucose, mmol/liter)] (27). Plasma SCFA was analyzed using gas chromatography (Varian Inc., USA) and a capillary column (28). The ratio of the split injection used was 1:10. A flame ionization detector was used to identify the components within the sample. The column temperature was set at 110°C and increased at a rate of 18°C/min up to 200°C with T1 = 1 min and T2 = 2 min. Helium was used as a carrier gas at a flow rate of 8 ml/min with a 10-min run time for each sample. FFA was extracted from plasma samples after homogenization in a suitable excess of chloroform-methanol (2:1 [vol/vol]) (29). FFA was prepared for gas chromatography determinations using KOH-methanol. The injector and detector temperatures were held at 250 and 270°C. A total of 40 saturated, monounsaturated, and polyunsaturated fatty acid standards (NU-CHEK; Prep, United Kingdom) were used. Peaks were identified by retention time relative to individual fatty acid standard. Plasma concentrations of tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) were measured using commercially available porcine ELISA kits (Quantikine PTA00; R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. All samples were analyzed in duplicate.
DNA extraction, PCR amplification of the 16S rRNA gene, amplicon sequencing, and sequence data processing.
Microbial genomic DNA was extracted from 220 mg of each fecal sample using a QIAamp Stool DNA kit (QIAamp DNA Stool minikit 51504; Qiagen) according to the manufacturer's instructions. Successful DNA isolation was confirmed by agarose gel electrophoresis. Based on previous reports (30, 31), the bacterial 16S rRNA gene was PCR amplified using primers flanking the V3-V4 hypervariable region with barcoded fusion forward primer 338F 5′-ACTCCTACGGGAGGCAGCA-3′, and the reverse primer was 806R 5′-GGACTACHVGGGTWTCTAAT-3′. The PCR conditions were as follows: one predenaturation cycle at 94°C for 4 min, 25 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 45 s, and elongation at 72°C for 30 s, and one postelongation cycle at 72°C for 5 min. The PCR amplicon products were separated on 0.8% agarose gels and then extracted from the gels. Only the PCR products without primer dimers and contaminant bands were used for sequencing by synthesis. Barcoded V4 amplicons were sequenced using the paired-end method and Illumina MiSeq with a 7-cycle index read. Sequences with an average phred score lower than 30, ambiguous bases, homopolymer runs exceeding 6 bp, primer mismatches, and sequence lengths shorter than 100 bp were removed. Only the sequences with an overlap longer than 10 bp and without any mismatch were assembled according to their overlap sequence. Reads that could not be assembled were discarded. Barcode and sequencing primers were trimmed from the assembled sequence (30).
16S rRNA gene sequencing and bioinformatics analysis.
Taxon-dependent analysis was conducted using the Ribosomal Database Project (RDP) classifier (31). The RDP classifier is a Web-based program that assigns 16S rRNA sequences to phylogenetically consistent bacterial species with an identity cutoff of 97%. The value corresponding to the abundance of operational taxonomic units (OTUs) for each sample was generated at the genus level, log 2 transformed, and then normalized as follows: from each log-transformed measure, the arithmetic mean of all transformed values was subtracted, and the difference was divided by the standard deviation of all log-transformed values for a given sample.
Alpha diversity indices were computed using MOTHUR software. The Shannon-Wiener and Simpson's diversity index values were calculated using QIIME (32). Unweighted Unifrac principal coordinate analysis (PCoA) was performed with QIIME using the unweighted UniFrac distance matrix between the samples (33, 34).
Statistical analysis.
An individual sow was considered the experimental unit in all statistical analyses. Before analysis, the data were tested for normality and homoscedasticity using the Kolmogorov-Smirnov and Levene tests (with the significance level set at 5%). Variations of normally distributed oxidative stress parameters and HOMA values were determined by analysis of variance (ANOVA) using the procedure for repeated measurements of SAS (Institute, Inc., Cary, NC). The model included the effects of treatment, physiological stage and replicate, and the interactions. A significant interaction was specified in the text. Variations of plasma SCFA, FFA, and inflammatory cytokine data were analyzed using the MIXED procedures of SAS with treatment as a fixed effect and block as a random effect in the statistical model. BW of sows at farrowing was used as a covariate in the analysis of inflammatory cytokine during gestation. The inflammatory cytokine during lactation was subjected to covariance analysis with the litter size after cross-foster as the covariate. Data are expressed as means ± standard errors of the means (SEM) unless otherwise indicated. Significance is reported at a P value of <0.05.
The data for the relative abundance of gut microbiota were corrected by false-discovery-rate (FDR) analysis using the nonparametric Mann-Whitney tests in SAS. Data from the nonparametric tests are presented as medians and 25th to 95th percentiles. Correlations were analyzed by using Spearman's correlation in R 3.0.2 (The R Foundation) with the RStudio 0.97.310 package and gplots for the heat map. The results were considered statistically significant at a P of <0.05. Correlation results were corrected by FDR analysis according to the Benjamini-Hochberg procedure, with an α of <0.05.
RESULTS
Plasma concentrations of TNF-α, IL-6, SCFA, and FFA and HOMA values of sows.
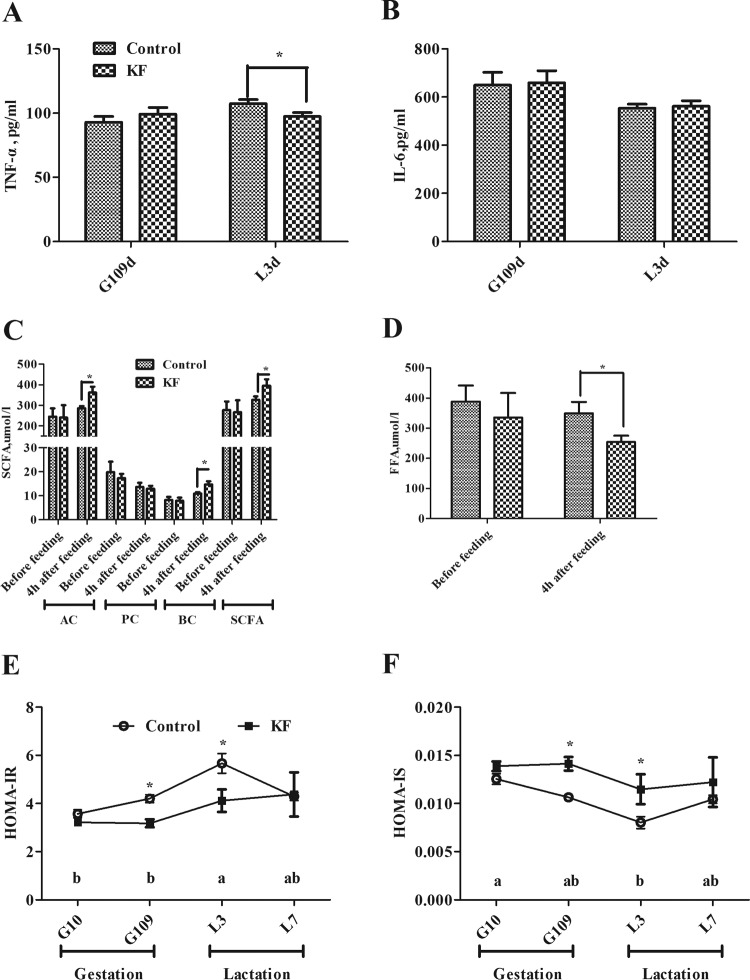
We compared 5 sows of the KF group with 5 sows of the control group for plasma TNF-α, IL-6, SCFA, FFA concentration, and HOMA values. As shown in Fig. 1A, the plasma cytokine concentration of TNF-α was significantly reduced in the KF diet group compared to the control on day 3 of lactation (P < 0.05). There were no differences between the two groups in plasma levels of SCFA before feeding on day 109 of gestation (Fig. 1C). However, inclusion of KF in the diet remarkably increased (P < 0.05) plasma concentrations of acetic acid, butyric acid, and total SCFA at 4 h after feeding on day 109 of gestation compared with the control. Despite the similar plasma concentrations of FFA in the two groups before feeding on day 109 of gestation, the inclusion of KF in gestation diet greatly reduced the plasma FFA concentration (P < 0.05) at 4 h after feeding on day 90 of gestation (Fig. 1D).
FIG 1.
Plasma concentrations of TNF-α (A), IL-6 (B), SCFA (C), and FFA (D) and HOMA-IR (E) and HOMA-IS (F) values for sows fed the control or KF diet. Data are presented as means ± SEM (n = 5). *, significant effect of dietary treatment (P < 0.05); a and b, significant effect of sampling day (P < 0.05; values with different lowercase letters are significantly different). G109d, day 109 of gestation; L3d, day 3 of lactation; SCFA, short-chain fatty acids; AC, acetate acid; PC, propionate acid; BC, butyrate acid; FFA, free fatty acid; HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-IS, homeostasis model assessment of insulin sensitivity; KF, 2.2% konjac flour diet.
In addition, the KF diet reduced the HOMA insulin resistance (HOMA-IR) of sows, while the HOMA-IS was increased on day 109 of gestation and day 3 of lactation compared with the control (P < 0.05; Fig. 1E and F).
Oxidative stress parameter of sows.
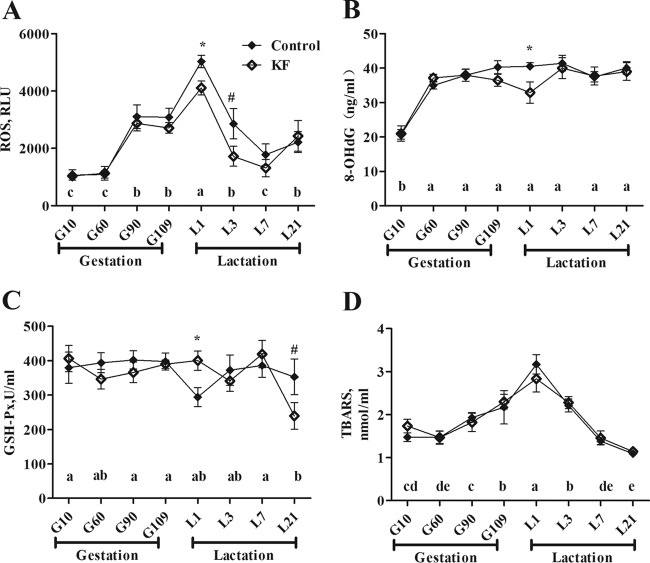
We compared 5 sows of the KF group with 5 sows of the control group for serum concentrations of oxidative stress parameter. Serum levels of ROS, TBARS, 8-OHdG, and GSH-Px on different days of gestation and lactation are shown in Fig. 2. There was a treatment-sampling day interaction for serum TBARS concentrations (P < 0.05). In both groups, the serum levels of ROS and TBARS were higher (P < 0.05) during late gestation (days 90 and 109) and lactation (days 1 and 3) than during early gestation (day 10). Additionally, the serum concentrations of 8-OHdG were higher (P < 0.05) during gestation (days 60, 90, and 109) and lactation (days 1, 3, 7, and 21) than during early gestation (day 10). Compared with the sows in the control group, sows given KF treatment had significantly lower serum levels of ROS (P < 0.05) and 8-OHdG (P < 0.05) but higher serum concentrations of GSH-Px on day 1 of lactation.
FIG 2.
Serum concentration of ROS (A), 8-OHdG (B), GSH-Px (C), and TBARS (D) of sows fed the control or KF diet. Data are presented as means ± SEM (n = 5). a to e, effect of sampling day (P < 0.05; values with different lowercase letters are significantly different), *, effect of dietary treatment (P < 0.05); #, effect of dietary treatment (P < 0.1). ROS, reactive oxygen species; RLU, relative light units; 8-OHdG, 8-hydroxy-deoxyguanosine; GSH-Px, glutathione peroxidase; TBARS, thiobarbituric acid reactive substances; KF, 2.2% konjac flour diet.
DNA sequence data and bacterial community structure.
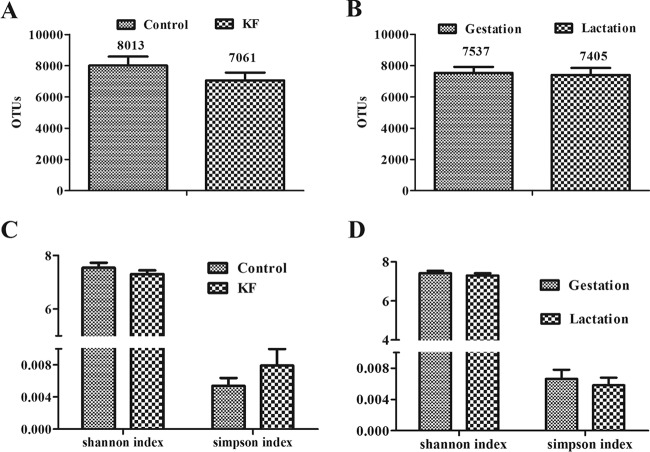
After quality filtering was performed as described above, a total of 745,743 reads were analyzed for assignment of OTUs (≥97% identity level) and taxonomic nomenclature from 40 bacterial communities (see Table S2 in the supplemental material). A mean of 8,013 OTUs of 19,862 valid sequence reads were assigned to the control diet group versus a mean of 7,061 OTUs of 16,092 valid reads assigned to the KF diet group (Fig. 3A and B). Means of 7,537 and 7,405 OTUs of 17,977 and 19,310 valid reads were assigned to sows during the gestation and lactation periods, respectively. The α-diversity of microbial communities was measured using Shannon index and Simpson index analysis. The index values corresponding to the diet (control versus KF) treatments or the reproductive periods (gestation versus lactation) were similar (Fig. 3).
FIG 3.
Number of observed OTUs (≥97% identity level) and diversity index (Shannon and Simpson) values in the fecal samples of sows. (A and B) Numbers of observed OTUs of the fecal communities of sows in dietary treatment period (A [n = 10]) or reproductive period (B [n = 20]). (C and D) Diversity index of the fecal communities of sows in dietary treatment period (C [n = 10]) or reproductive period (D [n = 20]). Data are presented as means ± SEM. OTUs, operational taxonomic units; KF, 2.2% konjac flour diet.
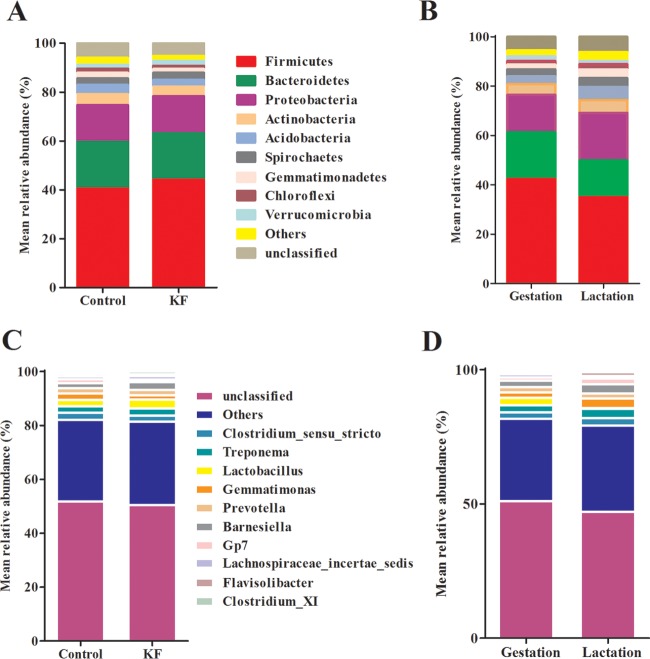
The results of the phylum distributions are shown in Fig. 4. Taxonomic assignment of the OTU identified 28 phyla in the fecal samples of sows tested in this study. Two phyla, Firmicutes and Bacteroidetes, were dominant in the fecal samples regardless of the diet treatment groups (KF and control) or time periods (gestation and lactation) of sows. In the present study, after correction via a FDR calculation according to the Mann-Whitney test, we found that KF treatment profoundly affected the abundance of different phyla. For instance, KF treatment resulted in significant increases in the abundance of Bacteroidetes, Firmicutes, Spirochaetes, and Verrucomicrobia and significant decreases in the abundance of Actinobacteria and Proteobacteria compared to the control (Table 1). Compared with the levels seen in the gestation period, the abundance of Firmicutes, Bacteroidetes, and Verrucomicrobia was significantly increased and the abundance of Acidobacteria, Actinobacteria, Gemmatimonas, Proteobacteria, and Spirochaetes was significantly decreased in sows during lactation (Table 2).
FIG 4.
The distribution of bacterial phyla (A and B) and genera (C and D) in fecal samples under conditions of dietary treatment during gestation and lactation (relative abundances of bacterial taxa accounting for more than 1%). Values represent the mean proportions at the phylum or genus level for each group. KF, 2.2% konjac flour diet.
TABLE 1.
Abundant taxa in the gut microbiome of sows fed the konjac flour or control dieta
| Taxon | Median % (% in first quartile, % in third quartile) |
P value (Mann-Whitney test) | FDR | |
|---|---|---|---|---|
| Control | KF | |||
| Acidobacteria | 4.06 (0.09, 5.97) | 3.70 (0.04, 4.56) | 0.00011562 | 0.16 |
| Actinobacteria | 5.53 (1.30, 5.96) | 3.96 (2.35, 5.75) | 0.0000319 | 0.04 |
| Bacteroidetes | 17.42 (15.21, 24.19) | 19.83 (12.17, 23.87) | 0.0000319 | 0.04 |
| Barnesiella | 1.67 (1.01, 2.64) | 2.66 (1.79, 3.58) | 0.00037339 | 0.5 |
| Prevotella | 1.55 (1.06, 2.52) | 2.04 (0.95, 2.97) | 0.0000319 | 0.04 |
| Bacteroides | 0.39 (0.23, 1.02) | 0.57 (0.15, 0.89) | 0.0000319 | 0.04 |
| Chloroflexi | 1.72 (0.02, 2.40) | 1.45 (0.01, 2.03) | 0.00037559 | 0.51 |
| Firmicutes | 40.78 (32.35, 50.52) | 44.85 (34.21, 51.92) | 0.0000319 | 0.04 |
| Clostridium sensu stricto | 2.57 (0.82, 3.35) | 2.60 (0.68, 3.50) | 0.00011487 | 0.15 |
| Eubacterium | 0.19 (0.13, 0.23) | 0.29 (0.17, 0.46) | 0.0000319 | 0.04 |
| Blautia | 0.34 (0.27, 0.47) | 0.30 (0.17, 0.78) | 0.000031 | 0.04 |
| Roseburia | 0.10 (0.08, 0.16) | 0.19 (0.10, 0.29) | 0.000031 | 0.04 |
| Clostridium cluster XI | 0.70 (0.38, 1.35) | 1.02 (0.58, 1.22) | 0.0000315 | 0.04 |
| Clostridium cluster IV | 0.83 (0.70, 0.94) | 0.92 (0.80, 1.10) | 0.0000319 | 0.04 |
| Ruminococcus | 0.27 (0.22, 0.31) | 0.43 (0.17, 0.67) | 0.0000286 | 0.04 |
| Lactobacillus | 1.09 (0.05, 4.41) | 2.16 (0.02, 4.31) | 0.0000317 | 0.04 |
| Lachnospiraceae incertae sedis | 1.16 (0.91, 1.76) | 1.26 (0.97, 1.70) | 0.0000319 | 0.04 |
| Gemmatimonadetes | 2.66 (0.05, 3.71) | 2.11 (0.06, 2.67) | 0.00037559 | 0.51 |
| Proteobacteria | 17.08 (7.41, 19.62) | 13.28 (10.27, 17.93) | 0.0000319 | 0.04 |
| Pseudomonas | 0.55 (0.20, 1.10) | 0.66 (0.10, 2.57) | 0.00011562 | 0.16 |
| Spirochaetes | 1.93 (1.17, 3.66) | 3.16 (1.33, 4.72) | 0.0000319 | 0.04 |
| Treponema | 1.66 (1.09, 3.45) | 2.78 (1.21, 4.54) | 0.0000317 | 0.04 |
| Verrucomicrobia | 1.39 (1.19, 2.10) | 1.70 (1.32, 2.67) | 0.0000319 | 0.04 |
| Akkermansia | 0.40 (0.18, 0.73) | 0.74 (0.16, 1.45) | 0.0000319 | 0.04 |
Data are presented as treatment medians (with 25th and 75th percentiles). The control column shows abundances in the gut microflora of sows fed the control diet during gestation (n = 10). The KF column shows abundances in the gut microflora of sows fed the konjac flour diet during gestation (n = 10). P values are from the Mann-Whitney test. KF, 2.2% konjac flour diet; FDR, false-discovery rate.
TABLE 2.
Abundant taxa in the gut microbiome of the sows during gestation and lactationa
| Taxon | Median % (% in first quartile, % in third quartile) |
P value (Mann-Whitney test) | FDR | |
|---|---|---|---|---|
| Gestation | Lactation | |||
| Acidobacteria | 5.56 (0.01, 6.94) | 3.70 (0.06, 5.48) | 1.65E−06 | 0.002223 |
| Actinobacteria | 5.31 (1.52, 6.86) | 4.62 (2.07, 5.85) | 3.99E−09 | 5.37E−06 |
| Bacteroidetes | 13.09 (10.63, 20.72) | 18.09 (13.70, 22.33) | 4.00E−09 | 5.39E−06 |
| Bacteroides | 0.07 (0.04, 0.16) | 0.44 (0.19, 0.91) | 1.72E−07 | 0.000232 |
| Barnesiella | 2.27 (1.41, 5.19) | 2.17 (1.13, 3.10) | 1.75E−07 | 0.000236 |
| Prevotella | 1.30 (0.82, 2.55) | 1.89 (1.02, 2.76) | 1.74E−07 | 0.000234 |
| Chloroflexi | 1.90 (0.00, 2.81) | 1.57 (0.02, 2.25) | 4.71E−06 | 0.006344 |
| Firmicutes | 36.26 (30.70, 53.82) | 43.02 (34.08, 51.08) | 4.00E−09 | 5.39E−06 |
| Bacillus | 0.15 (0.01, 0.22) | 0.05 (0.00, 0.17) | 1.64E−06 | 0.002209 |
| Blautia | 0.15 (0.08, 0.29) | 0.33 (0.24, 0.47) | 1.74E−07 | 0.000234 |
| Clostridium cluster IV | 0.73 (0.47, 0.93) | 0.89 (0.77, 0.99) | 3.96E−09 | 5.33E−06 |
| Clostridium sensu stricto | 2.68 (1.12, 3.90) | 2.57 (0.78, 3.18) | 4.00E−09 | 5.39E−06 |
| Eubacterium | 0.12 (0.07, 0.24) | 0.21 (0.16, 0.33) | 5.21E−08 | 7.02E−05 |
| Faecalibacterium | 0.01 (0.00, 0.02) | 0.02 (0.01, 0.05) | 0.000195 | 0.263325 |
| Lachnospiraceae incertae sedis | 0.89 (0.58, 1.14) | 1.16 (0.97, 1.71) | 1.75E−07 | 0.000236 |
| Lactobacillus | 0.23 (0.09, 0.35) | 0.40 (0.03, 4.22) | 1.75E−07 | 0.000236 |
| Roseburia | 0.06 (0.01, 0.18) | 0.10 (0.07, 0.19) | 1.63E−06 | 0.002196 |
| Ruminococcus | 0.27 (0.10, 0.46) | 0.30 (0.19, 0.49) | 3.97E−09 | 5.35E−06 |
| Streptococcus | 0.03 (0.00, 0.07) | 0.18 (0.11, 0.71) | 4.65E−06 | 0.006264 |
| Gemmatimonas | 3.32 (0.02, 4.39) | 2.28 (0.07, 3.17) | 1.65E−06 | 0.002223 |
| Proteobacteria | 19.96 (8.19, 23.11) | 15.84 (9.71, 18.39) | 4.00E−09 | 5.39E−06 |
| Escherichia-Shigella | 0.04 (0.02, 0.11) | 0.33 (0.10, 1.37) | 1.70E−07 | 0.000229 |
| Pseudomonas | 0.37 (0.23, 0.84) | 0.55 (0.15, 1.40) | 4.00E−09 | 5.39E−06 |
| Spirochaetes | 2.59 (1.11, 6.87) | 2.30 (1.26, 4.05) | 4.00E−09 | 5.39E−06 |
| Treponema | 2.51 (1.01, 6.77) | 2.04 (1.16, 3.84) | 5.26E−08 | 7.09E−05 |
| Verrucomicrobia | 1.34 (0.84, 1.65) | 1.43 (1.30, 2.14) | 3.99E−09 | 5.37E−06 |
| Akkermansia | 0.05 (0.00, 0.33) | 0.57 (0.18, 0.88) | 1.64E−06 | 0.002209 |
Data are presented as treatment medians (with 25th to 75th percentiles). The gestation column shows abundances in the gut microflora of sows during gestation (n = 20). The lactation column shows abundances in the gut microflora of sows during lactation (n = 20). P values are from the Mann-Whitney test. FDR, false-discovery rate.
At the genus level, a total of 658 genera were identified from all the samples, regardless of diet treatment and reproductive period of sows. The following 10 genera were defined as the most abundant, with more than 1% of the total DNA sequences: Clostridium sensu stricto, Treponema, Lactobacillus, Gemmatimonas, Prevotella, Barnesiella, Gp7, Lachnospiraceae incertae sedis, Flavisolibacter, and Clostridium cluster XI (Fig. 4). A total of 12 differentially abundant genera were identified between the dietary treatments at the genus level, which included four abundant (>1% of the total sequences) and eight less abundant genera (Table 1). In addition, a total of 19 differentially abundant genera were identified between the reproductive periods at the genus level which included five abundant (>1% of the total sequences) and 14 less abundant genera (Table 2).
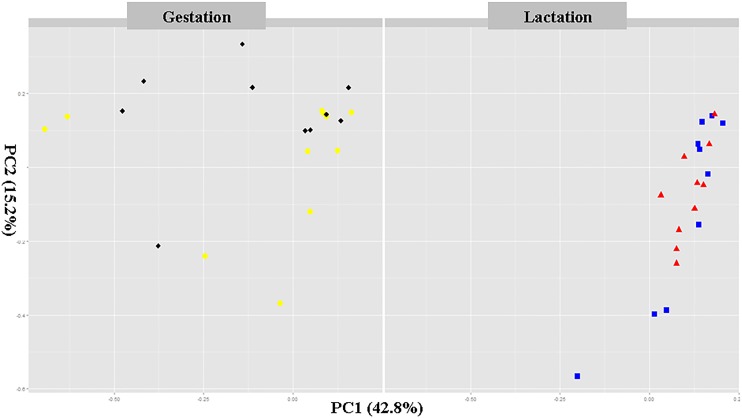
PCoA revealed the structure administration of gut microbiota. KF administration differences were mainly observed in the first principal coordinate (PC1), which accounted for the largest proportion (42.8%) of total variation (Fig. 5). After the KF administration during gestation, the PCoA indicated that different diets promoted the development of different gut microbial communities. However, during lactation, the control and KF groups were clustered along the PCA1 due to the use of the same lactation diet. This indicates that the KF administration can change the diversity of the fecal microbiota in sows.
FIG 5.
Principal coordinate analysis (PCoA) of the dissimilarities between the microbial samples. Individual sow fecal samples for each period (gestation and lactation) are labeled for the cows in the control group (black) and konjac flour group (yellow) during gestation and for the cows in the control group (red) and konjac flour group (blue) during lactation in the intervention period.
Correlations between gut microbiota and blood metabolic parameters.
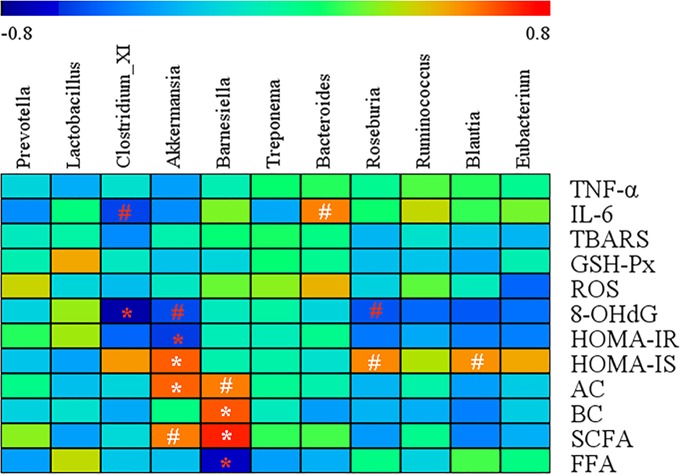
A Spearman correlation analysis was performed to evaluate the potential link between significant changes in gut microbiota composition induced by KF and host metabolism (Fig. 6). Akkermansia was negatively correlated with HOMA-IR (r = −0.61, P < 0.05) but positively correlated with HOMA-IS (r = 0.63, P < 0.05) and acetate acid (r = 0.62, P < 0.05). Barnesiella was negatively correlated with FFA (r = −0.64, P < 0.05) but positively correlated with butyrate acid (r = 0.64, P < 0.05) and SCFA (r = 0.73, P < 0.05). Additionally, Clostridium cluster XI was negatively correlated with the 8-OHdG (r = −0.74, P < 0.05).
FIG 6.
Heat map of the Spearman r correlations between the gut microbiota significantly modified by the konjac flour treatment and metabolic parameters of sows. Correlation analyses were performed on differential values for each sow in both groups (control diet group and konjac flour diet group during gestation). *, P < 0.05; #, P < 0.1 (following the Spearman correlation analysis). TBARS, thiobarbituric acid reactive substances; GSH-Px, glutathione peroxidase; ROS, reactive oxygen species; 8-OHdG, 8-hydroxy-deoxyguanosine; HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-IS, homeostasis model assessment of insulin sensitivity; AC, acetate acid; BC, butyrate acid; SCFA, short-chain fatty acids; FFA, free fatty acid.
DISCUSSION
In the current study, the sows were found to suffer from increased oxidative stress during late gestation and early lactation, as indicated by their elevated ROS, 8-OHdG, and TBARS levels, which were similar to those reported by Berchieri-Ronchi et al. (2). The peripartum period, particularly the delivery, is a critical time for maintaining a balance between the production of free radicals and the incompletely developed antioxidative protection of the fetus and the newborn (35). Additionally, the KF diet group significantly reduced the level of both ROS and 8-OHdG but significantly increased the concentrations of GSH-Px of sows on day 1 of lactation, which is probably attributable to the greatest oxidative stress that the sows suffered then.
Excessive production of ROS has been shown to affect the insulin signaling cascade by inducing insulin receptor substrate phosphorylation and altering mitochondrial activity (36, 37). The results of the present study suggested that the insulin sensitivity of sows was decreased during late gestation and early lactation relative to early gestation, which was consistent with the increased systemic oxidative stress present during the respective periods of time. Interestingly, sows fed the KF diet improved their insulin sensitivity (HOMA values; Fig. 1) during the two periods of time, implying that KF supplementation may improve insulin sensitivity by alleviating the oxidative stress of sows.
The mechanism by which dietary supplementation of fiber during gestation improved insulin sensitivity of sows is poorly understood. Colonic fermentation of dietary fiber may exert an insulin-sensitizing effect through the production of the SCFA, especially acetic acid. SCFA may modulate insulin sensitivity by reducing fatty acid flux (38–40). Feeding fermentable fiber reduces serum FFA concentrations (41–43). In the present study, sows fed the KF diet had an increase in plasma concentrations of acetic acid, butyric acid, and total SCFA and a decrease in the plasma concentration of FFA. This result could be explained by the results of a previous study reporting that, 4 h after the consumption of 24 g inulin, the subjects showed an increase of the acetic acid concentration in the systemic circulation, which may reduce lipolysis and plasma FFA concentrations and thus improve insulin sensitivity in human (39).
A previous study also indicated that the gut microbial community composition and structure are profoundly altered over the course of pregnancy, which is associated with metabolic disease, including insulin resistance, and lower levels of inflammation (17). To our knowledge, this is probably the first report presenting the results of high-throughput analysis of the effects of KF on the gut microbiota and its association with the metabolism of sows. Here, we used a 16S rRNA gene-based high-throughput sequencing approach to demonstrate a role of KF inclusion in gestation diet in modulating the composition of the gut microbiota of sows. The PCoA showed that the sow microbiota was highly influenced by the administration of KF during gestation.
KF supplementation significantly changed the gut microbiota composition, with an increased proportion of Firmicutes and Bacteroidetes at the phylum level. These phyla have been previously reported to be associated with type 2 diabetes and insulin resistance in human beings and swine. The abundance of Firmicutes was lower in diabetic persons than in their nondiabetic counterparts (44). Lean Ossabaw minipigs with lower fasting insulin levels (15 pmol/liter versus 34.7 pmol/liter) had a higher abundance of Bacteroidetes in colon than obese pigs (45). In the present study, a lower relative abundance of Proteobacteria was observed in the KF group, which can be considered beneficial, as increased Proteobacteria levels are linked with intestinal inflammation (46), and this phylum encompasses bacteria known to cause intestinal pathology in humans and animals (47, 48). Interestingly, this reduction in Proteobacteria levels in the KF diet group was associated with a decrease in the plasma TNF-α concentration during lactation in the same sows. Additionally, the relative abundance of Spirochaetes was increased in the KF group. Anaerobic intestinal Spirochaetes of the genus Brachyspira commonly colonizes the large intestines of pigs. Of the five main species found in swine, two are considered important pathogens: Brachyspira hyodysenteriae is the causative agent of swine dysentery (49), while B. pilosicoli causes porcine intestinal spirochetosis (50). However, both species showed no differences in levels in the results from the two dietary treatment groups.
Moreover, KF addition affected several genera that have been previously reported to be associated with oxidative stress (Lactobacillus [51]) and insulin resistance (Akkermansia [52] and Roseburia [53]). It is worth noting that Akkermansia is a mucin-degrading, Gram-negative anaerobe residing in intestinal mucus layers and associated with obesity and insulin resistance in humans and mice (54). A recent study has demonstrated that oral administration of Akkermansia in a high-fat diet significantly enhanced glucose tolerance (52). In the present study, a Spearman correlation analysis showed that Akkermansia was negatively correlated with HOMA-IR but positively correlated with HOMA-IS. These results suggested that the KF supplementation to the diet of sows during gestation improved their insulin sensitivity, which may be attributable to the elevated Akkermansia abundance.
The relative abundances of butyrate-producing Roseburia and Ruminococcus, belonging to the phylum Firmicutes, were higher in sows fed a KF diet in this study. The expansion of the populations of these two genera may explain the elevated plasma concentration of butyric acid observed in KF-fed sows. Butyric acid serves as the main energy source for colonocytes and protects against inflammation (55, 56). Thus, it can be speculated that, in response to KF, the gut microbiota may contribute to the host metabolism. However, whether these genera directly contribute to the phenotype needs further investigation. For instance, we found an increase in levels of Clostridium cluster XI in KF-supplemented sows and a negative correlation between Clostridium cluster XI and 8-OHdG levels.
As the sow experiences dietary and physiological changes during the transition period, an investigation of the impact of these changes on the gut microbiota can provide specific insights into the biological and metabolic functions of the sows. The current study showed that, with the transition from a gestation diet to a lactation diet, the most noticeable shifts for the sows were an increase in the proportions of Firmicutes and Bacteroidetes and a decrease in the proportion of Proteobacteria. A similar finding was reported by Pitta et al. (57), i.e., an increase in the proportion of Bacteroidetes and a decrease in the proportion of Proteobacteria in ruminal microbiome with the transition of the cows from gestation to lactation. These specific changes in the gut microbiota may have a beneficial influence on host metabolism. For instance, Proteobacteria organisms are often associated with inflammatory conditions (46).
Our previous study showed that sows fed the KF diet showed increased lactation feed intake (5.81 kg versus 5.39 kg), improved in the number of piglets weaned per litter (9.68 versus 9.50), and weaned piglets of significantly higher litter weight (67.37 kg versus 61.25 kg) than those fed the control diet (9). The addition of KF during gestation could have a substantial financial impact on sow operations. For example, assuming the average price for a weaned pig is 500 Chinese Yuan (¥ 500), an additional 0.18 weaned pigs per sow (9.68 weaned − 9.50 weaned = 0.18 weaned per litter) could result in ¥ 90 of additional income, and, assuming that a weaned pig sells for ¥ 70 per kg, an additional 6.12 kg per litter (67.37 kg − 61.25 kg= 6.12 kg per litter) could result in ¥ 428.4 of additional income. Since the cost of supplementing KF is ¥ 255.3 (¥ 45 [per kg KF] × 2.2% [dosage] × 2.41 kg per day [feed intake during gestation] × 107 days [duration of gestation] = ¥ 255.3) and the cost of additional feed intake during lactation is ¥ 30.9 (¥ 3.5 [per kg lactation diet] × 0.42 kg [additional feed intake] × 21 days [duration of lactation] = ¥ 30.9), the income per sow could be ¥ 232.2 (¥ 90 + ¥ 428.4 − ¥ 255.3 − ¥ 30.9 = ¥ 232.2).
In conclusion, our results have demonstrated that there is increased systemic oxidative stress during late gestation and early lactation of sows. The KF supplementation to their gestation diet may change the gut microbiota, alleviate oxidative stress and oxidative damage, and improve their insulin sensitivity. Additionally, putative correlations were observed between genera and several metabolic markers, implying that the changes of gut microbiota in response to KF may also be correlated with the host metabolism response.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Fundamental Research Funds for Innovation Position in Agricultural Science and Technology Innovation Center of Hubei Province (2007-062) and International Cooperation Projects of Ministry of Science and Technology of the People's Republic of China (2013DFG32510).
We thank Jiajian Tan, Zhaowei Xu, and Haibo Gao at GuoXiang Pig Farm for their support during the feeding trial. We also thank YangXiang Joint Stock Company for providing sow feeding facilities.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01374-16.
REFERENCES
- 1.Tan C, Wei H, Sun H, Ao J, Long G, Jiang S, Peng J. 11 October 2015. Effects of dietary supplementation of oregano essential oil to sows on oxidative stress status, lactation feed intake of sows, and piglet performance. Biomed Res Int doi: 10.1155/2015/525218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berchieri-Ronchi C, Kim S, Zhao Y, Correa C, Yeum K-J, Ferreira A. 2011. Oxidative stress status of highly prolific sows during gestation and lactation. Animal 5:1774–1779. doi: 10.1017/S1751731111000772. [DOI] [PubMed] [Google Scholar]
- 3.Pereira AC, Martel F. 2014. Oxidative stress in pregnancy and fertility pathologies. Cell Biol Toxicol 30:301–312. doi: 10.1007/s10565-014-9285-2. [DOI] [PubMed] [Google Scholar]
- 4.Kim SW, Weaver AC, Shen YB, Zhao Y. 2013. Improving efficiency of sow productivity: nutrition and health. J Anim Sci Biotechnol 4:26. doi: 10.1186/2049-1891-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Père MC, Etienne M. 2007. Insulin sensitivity during pregnancy, lactation, and postweaning in primiparous gilts. J Anim Sci 85:101–110. doi: 10.2527/jas.2006-130. [DOI] [PubMed] [Google Scholar]
- 6.Père M, Etienne M, Dourmad J. 2000. Adaptations of glucose metabolism in multiparous sows: effects of pregnancy and feeding level. J Anim Sci 78:2933–2941. [DOI] [PubMed] [Google Scholar]
- 7.Mosnier E, Le Floc'h N, Etienne M, Ramaekers P, Sève B, Père MC. 2010. Reduced feed intake of lactating primiparous sows is associated with increased insulin resistance during the peripartum period and is not modified through supplementation with dietary tryptophan. J Anim Sci 88:612–625. doi: 10.2527/jas.2008-1768. [DOI] [PubMed] [Google Scholar]
- 8.van der Peet-Schwering CM, Kemp B, Plagge JG, Vereijken PF, den Hartog LA, Spoolder HA, Verstegen MW. 2004. Performance and individual feed intake characteristics of group-housed sows fed a nonstarch polysaccharides diet ad libitum during gestation over three parities. J Anim Sci 82:1246–1257. [DOI] [PubMed] [Google Scholar]
- 9.Tan CQ, Wei HK, Sun HQ, Long G, Ao JT, Jiang SW, Peng J. 2015. Effects of supplementing sow diets during two gestations with konjac flour and Saccharomyces boulardii on constipation in peripartal period, lactation feed intake and piglet performance. Anim Feed Sci Technol 210:254–262. doi: 10.1016/j.anifeedsci.2015.10.013. [DOI] [Google Scholar]
- 10.Sun H, Tan C, Wei H, Zou Y, Long G, Ao J, Xue H, Jiang S, Peng J. 2015. Effects of different amounts of konjac flour inclusion in gestation diets on physio-chemical properties of diets, postprandial satiety in pregnant sows, lactation feed intake of sows and piglet performance. Anim Reprod Sci 152:55–64. doi: 10.1016/j.anireprosci.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Sun HQ, Zhou YF, Tan CQ, Zheng LF, Peng J, Jiang SW. 2014. Effects of konjac flour inclusion in gestation diets on the nutrient digestibility, lactation feed intake and reproductive performance of sows. Animal 8:1089–1094. doi: 10.1017/S175173111400113X. [DOI] [PubMed] [Google Scholar]
- 12.Chen H-L, Cheng H-C, Liu Y-J, Liu S-Y, Wu W-T. 2006. Konjac acts as a natural laxative by increasing stool bulk and improving colonic ecology in healthy adults. Nutrition 22:1112–1119. doi: 10.1016/j.nut.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Li B, Xia J, Wang Y, Xie B. 2005. Grain-size effect on the structure and antiobesity activity of konjac flour. J Agric Food Chem 53:7404–7407. doi: 10.1021/jf050751q. [DOI] [PubMed] [Google Scholar]
- 14.Young W, Roy NC, Lee J, Lawley B, Otter D, Henderson G, Tannock GW. 2013. Bowel microbiota moderate host physiological responses to dietary konjac in weanling rats. J Nutr 143:1052–1060. doi: 10.3945/jn.113.174854. [DOI] [PubMed] [Google Scholar]
- 15.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. 2010. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. 2007. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 17.Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Bäckhed HK, Gonzalez A, Werner JJ, Angenent LT, Knight R, Bäckhed F, Isolauri E, Salminen S, Ley RE. 2012. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150:470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovatcheva-Datchary P, Arora T. 2013. Nutrition, the gut microbiome and the metabolic syndrome. Best Pract Res Clin Gastroenterol 27:59–72. doi: 10.1016/j.bpg.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Dewulf EM, Cani PD, Claus SP, Fuentes S, Puylaert PG, Neyrinck AM, Bindels LB, de Vos WM, Gibson GR, Thissen JP, Delzenne NM. 2013. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 62:1112–1121. doi: 10.1136/gutjnl-2012-303304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson EV, Nilsson AC, Östman EM, Björck IM. 2013. Effects of indigestible carbohydrates in barley on glucose metabolism, appetite and voluntary food intake over 16 h in healthy adults. Nutr J 12:46. doi: 10.1186/1475-2891-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilsson A, Granfeldt Y, Ostman E, Preston T, Björck I. 2006. Effects of GI and content of indigestible carbohydrates of cereal-based evening meals on glucose tolerance at a subsequent standardised breakfast. Eur J Clin Nutr 60:1092–1099. doi: 10.1038/sj.ejcn.1602423. [DOI] [PubMed] [Google Scholar]
- 22.Van PY, Hamilton GJ, Kremenevskiy IV, Sambasivan C, Spoerke NJ, Differding JA, Watters JM, Schreiber MA. 2011. Lyophilized plasma reconstituted with ascorbic acid suppresses inflammation and oxidative DNA damage. J Trauma 71:20–25. doi: 10.1097/TA.0b013e3182214f44. [DOI] [PubMed] [Google Scholar]
- 23.Hou FL, Zhang RF, Zhang MW, Su DX, Wei ZC, Deng YY, Zhang Y, Chi JW, Tang XJ. 2013. Hepatoprotective and antioxidant activity of anthocyanins in black rice bran on carbon tetrachloride-induced liver injury in mice. J Funct Foods 5:1705–1713. doi: 10.1016/j.jff.2013.07.015. [DOI] [Google Scholar]
- 24.Pialoux V, Hanly PJ, Foster GE, Brugniaux JV, Beaudin AE, Hartmann SE, Pun M, Duggan CT, Poulin MJ. 2009. Effects of exposure to intermittent hypoxia on oxidative stress and acute hypoxic ventilatory response in humans. Am J Respir Crit Care Med 180:1002–1009. doi: 10.1164/rccm.200905-0671OC. [DOI] [PubMed] [Google Scholar]
- 25.Du D, Shi YH, Le GW. 2010. The effect of diet with different glycemic index on the redox status of duodenums in mice and its underlying mechanism. Eur Food Res Technol 230:935–941. doi: 10.1007/s00217-010-1240-8. [DOI] [Google Scholar]
- 26.Van Soest PJ, Robertson J, Lewis B. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- 27.Wallace TM, Levy JC, Matthews DR. 2004. Use and abuse of HOMA modeling. Diabetes Care 27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 28.Bosch G, Pellikaan W, Rutten P, van der Poel A, Verstegen M, Hendriks W. 2008. Comparative in vitro fermentation activity in the canine distal gastrointestinal tract and fermentation kinetics of fiber sources. J Anim Sci 86:2979–2989. doi: 10.2527/jas.2007-0819. [DOI] [PubMed] [Google Scholar]
- 29.Folch J, Lees M, Sloane Stanley GH. 1957. A simple method for the isolation and purification of total lipids from animal tissue. J Biol Chem 226:497–509. [PubMed] [Google Scholar]
- 30.Meng H, Zhang Y, Zhao L, Zhao W, He C, Honaker CF, Zhai Z, Sun Z, Siegel PB. 2014. Body weight selection affects quantitative genetic correlated responses in gut microbiota. PLoS One 9:e89862. doi: 10.1371/journal.pone.0089862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lan Y, Wang Q, Cole JR, Rosen GL. 2012. Using the RDP classifier to predict taxonomic novelty and reduce the search space for finding novel organisms. PLoS One 7:e32491. doi: 10.1371/journal.pone.0032491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. 2012. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6:610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saker M, Mokhtari NS, Merzouk SA, Merzouk H, Belarbi B, Narce M. 2008. Oxidant and antioxidant status in mothers and their newborns according to birthweight. Eur J Obstet Gynecol Reprod Biol 141:95–99. doi: 10.1016/j.ejogrb.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Morino K, Petersen KF, Shulman GI. 2006. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55(suppl 2):S9–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bloch-Damti A, Bashan N. 2005. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid Redox Signal 7:1553–1567. doi: 10.1089/ars.2005.7.1553. [DOI] [PubMed] [Google Scholar]
- 38.Fernandes J, Vogt J, Wolever TM. 2012. Intravenous acetate elicits a greater free fatty acid rebound in normal than hyperinsulinaemic humans. Eur J Clin Nutr 66:1029–1034. doi: 10.1038/ejcn.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandes J, Vogt J, Wolever TMS. 2011. Inulin increases short-term markers for colonic fermentation similarly in healthy and hyperinsulinaemic humans. Eur J Clin Nutr 65:1279–1286. doi: 10.1038/ejcn.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. 2005. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr 82:559–567. [DOI] [PubMed] [Google Scholar]
- 41.Tarini J, Wolever TM. 2010. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab 35:9–16. doi: 10.1139/H09-119. [DOI] [PubMed] [Google Scholar]
- 42.Brighenti F, Benini LRD, Casiraghi C, Pellegrini N, Scazzina F, Jenkins DJ, Vantini I. 2006. Colonic fermentation of indigestible carbohydrates contributes to the second-meal effect. Am J Clin Nutr 83:817–822. [DOI] [PubMed] [Google Scholar]
- 43.Ferchaud-Roucher V, Pouteau E, Piloquet H, Zaïr Y, Krempf M. 2005. Colonic fermentation from lactulose inhibits lipolysis in overweight subjects. Am J Physiol Endocrinol Metab 289:E716–E720. doi: 10.1152/ajpendo.00430.2004. [DOI] [PubMed] [Google Scholar]
- 44.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. 2010. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pedersen R, Ingerslev HC, Sturek M, Alloosh M, Cirera S, Christoffersen BØ, Moesgaard SG, Larsen N, Boye M. 2013. Characterisation of gut microbiota in Ossabaw and Gottingen minipigs as models of obesity and metabolic syndrome. PLoS One 8:e56612. doi: 10.1371/journal.pone.0056612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mukhopadhya I, Hansen R, El-Omar EM, Hold GL. 2012. IBD—what role do Proteobacteria play? Nat Rev Gastroenterol Hepatol 9:219–230. doi: 10.1038/nrgastro.2012.14. [DOI] [PubMed] [Google Scholar]
- 47.De Cock HE, Marks SL, Stacy BA, Zabka TS, Burkitt J, Lu G, Steffen DJ, Duhamel GE. 2004. Ileocolitis associated with Anaerobiospirillum in cats. J Clin Microbiol 42:2752–2758. doi: 10.1128/JCM.42.6.2752-2758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salyers AA, Whitt DD. 2002. Bacterial pathogenesis: a molecular approach, 2nd ed. ASM Press, Washington, DC. [Google Scholar]
- 49.Taylor DJ, Alexander TJ. 1971. The production of dysentery in swine by feeding cultures containing a spirochaete. Brit Vet J 127:58–61. [DOI] [PubMed] [Google Scholar]
- 50.Taylor DJ, Simmons JR, Laird HM. 1980. Production of diarrhoea and dysentery in pigs by feeding pure cultures of a spirochaete differing from Treponema hyodysenteriae. Vet Rec 106:326–332. doi: 10.1136/vr.106.15.326. [DOI] [PubMed] [Google Scholar]
- 51.Sun J, Hu XL, Le GW, Shi YH. 2010. Lactobacilli prevent hydroxy radical production and inhibit Escherichia coli and Enterococcus growth in system mimicking colon fermentation. Lett Appl Microbiol 50:264–269. doi: 10.1111/j.1472-765X.2009.02786.x. [DOI] [PubMed] [Google Scholar]
- 52.Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae JW. 2014. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 63:727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 53.Neyrinck AM, Possemiers S. 2012. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin-glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J Nutr Biochem 23:51–59. doi: 10.1016/j.jnutbio.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 54.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. 2013. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. 2011. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol 17:1519–1528. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogawa H, Iimura M, Eckmann L, Kagnoff MF. 2004. Regulated production of the chemokine CCL28 in human colon epithelium. Am J Physiol Gastrointest Liver Physiol 287:G1062–G1069. doi: 10.1152/ajpgi.00162.2004. [DOI] [PubMed] [Google Scholar]
- 57.Pitta DW, Kumar S, Vecchiarelli B, Shirley DJ, Bittinger K, Baker LD, Ferguson JD, Thomsen N. 2014. Temporal dynamics in the ruminal microbiome of dairy cows during the transition period. J Anim Sci 92:4014–4022. doi: 10.2527/jas.2014-7621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.