ABSTRACT
Raman spectroscopy and phase-contrast microscopy were used to examine calcium dipicolinate (CaDPA) levels and rates of nutrient and nonnutrient germination of multiple individual Bacillus subtilis spores treated with cold atmospheric plasma (CAP). Major results for this work include the following: (i) >5 logs of spores deposited on glass surfaces were inactivated by CAP treatment for 3 min, while deposited spores placed inside an impermeable plastic bag were inactivated only ∼2 logs in 30 min; (ii) >80% of the spores treated for 1 to 3 min with CAP were nonculturable and retained CaDPA in their core, while >95% of spores treated with CAP for 5 to 10 min lost all CaDPA; (iii) Raman measurements of individual CAP-treated spores without CaDPA showed differences from spores that germinated with l-valine in terms of nucleic acids, lipids, and proteins; and (iv) 1 to 2 min of CAP treatment killed 99% of spores, but these spores still germinated with nutrients or exogenous CaDPA, albeit more slowly and to a lesser extent than untreated spores, while spores CAP treated for >3 min that retained CaDPA did not germinate via nutrients or CaDPA. However, even after 1 to 3 min of CAP treatment, spores germinated normally with dodecylamine. These results suggest that exposure to the present CAP configuration severely damages a spore's inner membrane and key germination proteins, such that the treated spores either lose CaDPA or can neither initiate nor complete germination with nutrients or CaDPA. Analysis of the various CAP components indicated that UV photons contributed minimally to spore inactivation, while charged particles and reactive oxygen species contributed significantly.
IMPORTANCE Much research has shown that cold atmospheric plasma (CAP) is a promising tool for the inactivation of spores in the medical and food industries. However, knowledge about the effects of plasma treatment on spore properties is limited, especially at the single-cell level. In this study, Raman spectroscopy and phase-contrast microscopy were used to analyze CaDPA levels and kinetics of nutrient- and non-nutrient-germinant-induced germination of multiple individual spores of Bacillus subtilis that were treated by a planar CAP device. The roles of different plasma species involved in spore inactivation were also investigated. The knowledge obtained in this study will aid in understanding the mechanism(s) of spore inactivation by CAP and potentially facilitate the development of more effective and efficient plasma sterilization techniques in various applications.
INTRODUCTION
Among low-temperature and nontoxic methods for microorganism inactivation, the application of cold atmospheric plasma (CAP) created by electrical discharge in a gas has attracted significant interest (1–5). CAP is a weakly ionized gas with a roughly zero net electrical charge and usually operates under atmospheric conditions below 40°C, with variations depending on the type of feed gas and the equipment configuration (6). Compared to conventional sterilization methods, such as wet or dry heat, irradiation, or chemical gases, CAP has many advantages. CAP produces neither high temperatures, as do dry and wet heat, nor long-lived toxic gases, as do formaldehyde and ethylene oxide. As a consequence, CAP treatments tend to only minimally alter materials' natural properties and are increasingly used for sterilization of heat-sensitive medical instruments, hard surfaces, novel polymer packaging materials in the food industry (7, 8), and biological surfaces such as animal and human tissues (4, 5).
Spores produced by some Bacillus species are a major cause of food spoilage and foodborne illness, as well as a number of other serious human diseases (9, 10). These spores can remain dormant for many years and are extremely resistant to a variety of environmental stresses, including antibiotics, heat, desiccation, radiation, and many toxic chemicals (11–13). Spores can also rapidly return to life by germination triggered through a variety of agents, including specific nutrients (amino acids and sugars), cationic surfactants (dodecylamine), and exogenously added calcium dipicolinate (a 1:1 chelate of Ca2+ and dipicolinic acid [CaDPA]) (11). Nutrients trigger germination by activating specific germinant receptors (GRs) located in the spore inner membrane (IM) (11). GR stimulation then triggers the release of the spore core's large depot (∼10% of spore dry weight) of CaDPA present in the spore core and its concomitant replacement by water. In turn, CaDPA release triggers activation of the cortex-lytic enzymes (CLEs) CwlJ and SleB, which catalyze the degradation of spores' large peptidoglycan cortex, allowing the swelling and further hydration of the spore core. Full core hydration then allows resumption of spore core enzyme activity, followed by initiation of metabolism, macromolecular synthesis, and then spore outgrowth, which leads to resumption of vegetative growth (11, 14). Notably, spores' resistance properties are lost when spores germinate and begin outgrowth.
Much research has shown that CAP can be used to inactivate many types of spores, and viabilities of spores of Bacillus spp. are typically reduced 4 to 6 logs by several minutes of treatment by different types of plasma systems (15–22). The mechanisms of spore killing by plasma are complex (18), as UV photons, charged particles, electric fields, and reactive oxygen species (ROS) (e.g., ozone, atomic oxygen, and hydroxyl radicals if water is present) are all involved in spore killing (16, 17). UV-C is the most effective UV component for microbial inactivation in some low-pressure, low-temperature gas plasma sterilization processes, with DNA as its primary target (16, 22). Changes in microbial cell walls were also observed with a low-temperature gas plasma (7), while another study found ozone and other neutral reactive species in humidified air to be the main factors for sterilization (21).
Although much work has been dedicated to investigating the details of spore inactivation mechanisms by CAP, knowledge about the changes in spore properties following plasma treatment is still rather limited at the single-cell level. In particular, important questions of whether spores retain CaDPA or are able to germinate following CAP treatments have not been thoroughly investigated. Therefore, we have chosen to use new tools to characterize these properties of individual bacterial spores after CAP treatment and to obtain new information on mechanisms of CAP inactivation of spores. Specifically, we used Raman spectroscopy and phase-contrast microscopy to analyze the CaDPA levels in individual spores and to examine the kinetics of nutrient- and non-nutrient-germinant-induced germination of multiple individual spores of Bacillus subtilis after treatment with a planar CAP device. The roles of the different plasma species from this setup in spore inactivation were also investigated. The knowledge obtained in this study will aid in understanding the mechanism(s) of spore inactivation by CAP and help facilitate the development of more effective and efficient plasma sterilization techniques in various applications.
MATERIALS AND METHODS
Cold atmospheric plasma system.
The setup of the planar CAP device is illustrated in Fig. 1a. The electrode consists of two square copper plate electrodes (60 by 60 mm) separated by a dielectric film (0.05-mm-thick polypropylene plastic) and an air gap of 1.5 mm. A 60-Hz sinusoidal-wave low-frequency power supply with ∼14-kV peak-to-peak high voltage is applied to the electrodes to generate microdischarges on the dielectric surface at atmospheric pressure (Fig. 1a). All experiments used the same parameters. The power density of the plasma discharge at the parameters noted above is estimated at ∼35 mW/cm2 (15). Exposure time was varied from 0 to 30 min, as stated below for each experiment, and the distance between the two electrodes was kept constant at 1.5 mm. The CAP electrodes were exposed to the open air at room temperature as the feedgas, and no large temperature increase in the sample coverslip was observed after the plasma treatment, as measured with an infrared thermometer. As shown in Fig. 1b, when discharged, the surface microdischarge (SMD) plasma emits purple light that is dominated by the excitation of nitrogen molecules in ambient air. The optical emission spectrum of the SMD plasma was measured with a spectrometer (TRIAX 320; Horiba Scientific, Edison, NJ) and is shown in Fig. S1a in the supplemental material; it shows the presence of primarily UV-A light (315 to 400 nm) and almost no UV-C (200 to 280 nm) or UV-B (280 to 315 nm) light (15). The optical absorbance spectra of materials used to shield spores during plasma discharge were measured with a UV-visible (UV-Vis) spectrophotometer (GENESYS 10S; Thermo Fisher Scientific, Waltham, MA). The materials included a 0.2-mm-thick microscope coverslip, a 0.04-mm-thick plastic bag, and a mixed cellulose ester (MCE) filter, as shown in Fig. S1b. The plastic bag and coverslip are nearly transparent to UV-A light from 315 to 400 nm, and the MCE filter blocks UV-A light because of its optical density (OD) of >3.5 in the UV-A region (see Fig. S1b).
FIG 1.
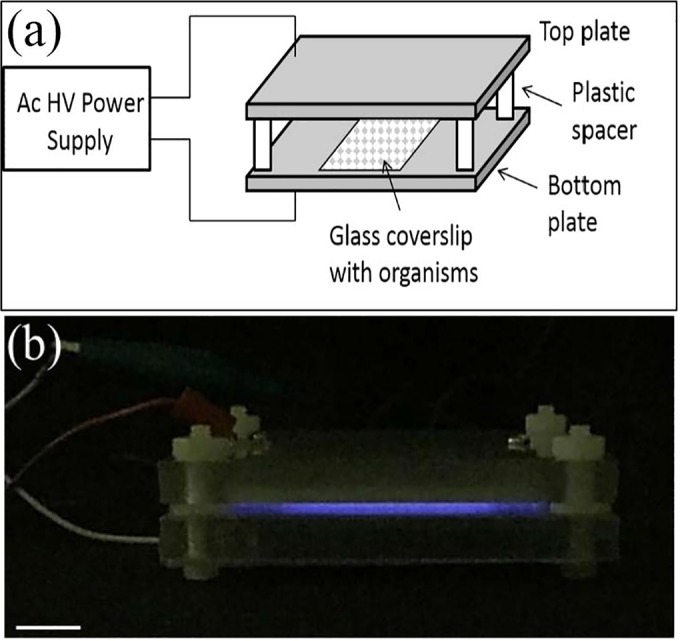
Experimental setup. (a) Schematic of the CAP apparatus with dielectric barrier discharge. Ac, alternating current; HV, high voltage. (b) Side view of the plasma discharge. When discharged, the SMD plasma emitted purple light and was photographed by a camera under dim light (scale bar = 1 cm).
Bacillus strains, growth conditions, and spore preparation.
Most work in this study was carried out with spores of B. subtilis PS533, a derivative of strain PS832 that contains plasmid pUB110, encoding resistance to kanamycin (23). To examine the effects of our CAP system on spores of concern for the food industry, we examined the effects of CAP treatment on inactivation and CaDPA release from B. cereus spores. Bacillus cereus strain T was originally obtained from H. O. Halvorson. Spores of B. subtilis were prepared at 37°C on 2× Schaeffer's glucose medium agar plates without antibiotics, and B. cereus spores were prepared at 30°C in defined liquid medium (24); spores were purified as described previously (23). All purified spores were stored at 4°C in water protected from light and were free (98%) from growing and sporulating cells, germinated spores, and cell debris as observed by phase-contrast microscopy. In order to avoid effects of high-temperature treatments on CAP-treated spore germination and viability, heat activation treatment was not applied prior to analyses of spore viability or germination.
CAP treatment and measurement of spore viability and germination.
For CAP treatment, 10-μl volumes of suspensions containing ∼107 spores/ml in deionized water were dried on one side of a sterile ∼15-mm by 5-mm glass microscope coverslip, giving ∼105 spores/slide that were spread out to reduce stacking. For determination of spore viability, the treated spores on the coverslip were put into 1 ml of sterile deionized water and vortexed at the maximum speed for 5 min to elute spores from the glass surface. Appropriate aliquots of the eluted spores were then applied to a Luria-Bertani (LB) medium agar plate, colonies were counted after 18 to 24 h of incubation at 37°C, and >95% of the spores (∼105 CFU/ml) were recovered. To assess the stress resistance of CAP-treated spores, colony formation from untreated and CAP-treated spores was also determined on LB medium plates with 1 M NaCl. This salt concentration has been shown to greatly reduce recovery of spores “damaged” by treatment with a variety of oxidizing agents (25). In order to further investigate changes in spore stress resistance after CAP treatment, untreated spores and spores eluted after only a 1-min CAP treatment were incubated at 80°C for 30 min prior to determination of spore viability as described above. When only a small number of survivors were expected following CAP, the pour plating method was performed (16). All spore viability results are shown as log of CFU of survivors on the ordinate plotted against the CAP exposure time.
To examine the influence of plasma treatment on spore germination, CAP-treated B. subtilis spores were recovered as described above. After centrifugation of eluted spores in a microcentrifuge at 4°C for 10 min, the spore pellet was suspended in 10 μl of 25 mM K-HEPES buffer (pH 7.4), and spores were prepared for germination by being allowed to adhere to a coverslip as described above and previously (26). The coverslips were incubated in 0.5 ml of either (i) 10 mM l-valine in 25 mM K-HEPES buffer (pH 7.4) at 37°C, (ii) 60 mM CaDPA in 25 mM K-HEPES buffer (pH 7.4) at 37°C, or (iii) 1.0 mM dodecylamine in 25 mM K-HEPES buffer (pH 7.4) at 50°C. Germination of multiple individual spores adhered on the microscope coverslip was monitored by phase-contrast microscopy, and data were analyzed as described previously (26, 27). Most (∼70%) of the change taking place in spore germination as observed by phase-contrast microscopy was due to the release of spores' large CaDPA depot, and the remainder was due to subsequent hydrolysis of spores' peptidoglycan cortex and spore core swelling and water uptake. At the end of germination incubations, the image intensity at the beginning of the incubation (T0) was set at 1 and the intensity at the end of measurements was set at 0 as previously described (26). Average kinetic germination parameters determined were as follows: T1, time when CaDPA leakage starts; Tlag, time after T1 when fast CaDPA release begins; Trelease,time when CaDPA release is complete; Tlysis,time when spore refractility becomes constant; ΔTleakage, Tlag − T1; ΔTrelease, Trelease − Tlag; and ΔTlysis, Tlysis − Trelease (27).
Analysis of spore CaDPA content.
Spore CaDPA content was determined by Raman spectroscopy using laser tweezers Raman spectroscopy (LTRS), as previously described (27). In brief, after plasma treatment, individual spores were suspended in water and randomly trapped at the focus of the objective by a 780-nm laser beam. Raman scattering light excited by the same laser beam was collected by the same objective and recorded by a charge-coupled device (PIXIS 400, Princeton Instruments, Trenton, NJ). A background spectrum was also acquired under the same conditions without a spore in the trap and subtracted from the spectra of spores. The spores' CaDPA content was determined from the intensity of the CaDPA-specific Raman band at 1,017 cm−1 as previously described (27). One-way analysis of variance (ANOVA) was done to compare the average CaDPA levels in spores for various durations of CAP treatment.
RESULTS
Effects of CAP treatment on spore viability and CaDPA content.
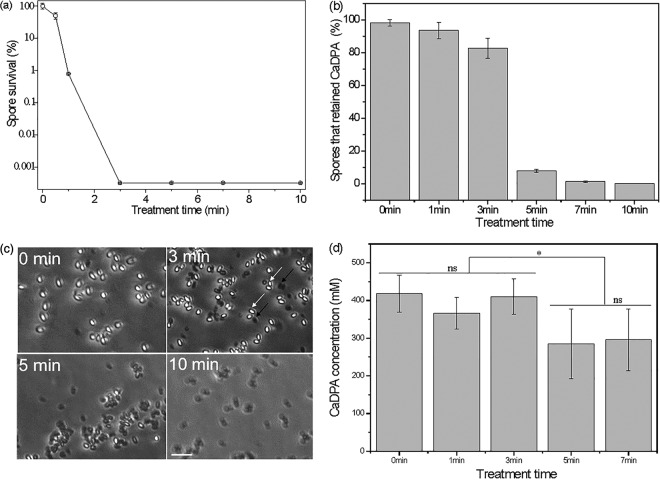
A CAP apparatus with a dielectric barrier discharge was designed (Fig. 1a) to investigate the effects of CAP treatment on spore killing, CaDPA loss, and germination. The discharge from this apparatus generated obvious purple light from UV rays (Fig. 1b). The results from CAP treatment of spores showed that increasing treatment times generally increased spore inactivation. A 3-min treatment time was sufficient to inactivate all test spores (∼5.5 logs) (Fig. 2a), although >80% of the spores given a 3-min CAP treatment retained CaDPA in their core (Fig. 2b and c). Prolonging the CAP treatment time to 5, 7, or 10 min led to corresponding decreases in the percentage of spores retaining CaDPA of ∼8%, ∼2%, and ∼0% (within the detection limit of ∼105 spores/slide), respectively (Fig. 2b and c). These results suggest that these spores incurred additional, severe damage during the prolonged treatment time. LTRS analysis of multiple individual spores estimated percentages of spores retaining CaDPA similar to the values determined by phase-contrast microscopy (data not shown).
FIG 2.
Effects of CAP treatment on B. subtilis spores. (a) Spore survival as a function of exposure time; (b) percentage of spores retaining CaDPA after CAP treatment of various durations (>300 individual spores were counted for each treatment); (c) phase-contrast images showing phase dark (black arrows) and phase bright (white arrows) for various CAP treatment times (scale bar = 5 μm); (d) average CaDPA levels estimated from ∼50 individual spores that retained CaDPA after various CAP treatment times (ns, no significant difference; *, significant difference with a P value of 0.05).
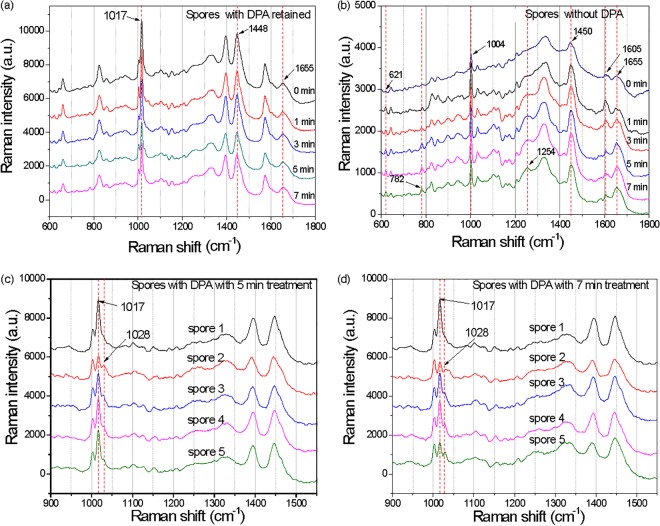
Analysis of the average intensity of the CaDPA-specific 1,017-cm−1 Raman band from ∼50 individual spores that had retained CaDPA after CAP treatment indicated that CaDPA levels were slightly lower in spores given more than 3 min of CAP treatment than in spores given 3 min of CAP treatment, and this difference was statistically significantly different (Fig. 2d). The Raman spectra of individual spores that retained CaDPA after different CAP treatment times were similar (Fig. 3a). However, compared to spectra of spores triggered to germinate with l-valine and thus having released all CaDPA, spectra of individual CAP-treated spores without CaDPA displayed some obvious differences (Fig. 3b). In particular, as CAP treatment times increased, band intensities at 621 and 782 cm−1 from nucleic acid and at 1,004 cm−1 due to lipid increased, and band intensities at 1,245, 1,605, and 1,655 cm−1 associated with proteins also changed significantly (Fig. 3b). The most interesting change is that some individual spores given 5 and 7 min of CAP treatment clearly showed partial CaDPA loss. As shown in Fig. 3c and d, spore 1 had not lost CaDPA, while spores 2 to 5 showed partial CaDPA loss, as reflected in the height of the Raman peak at 1,017 cm−1 (Fig. 3c and d). The phenomenon was not observed in previous work on spore inactivation by a number of different agents (28–30).
FIG 3.
Raman spectra of single spores of B. subtilis after various CAP treatment times. (a and b) Average Raman spectra of ∼50 individual spores that did (a) or that did not (b) retain CaDPA after CAP treatment; (c and d) Raman spectra of five individual spores that retained some CaDPA after CAP treatment for 5 (c) or 7 (d) min. Note that Raman spectra are offset for clarity. a.u., arbitrary units.
Effects of CAP treatment on spore stress resistance.
After a 1-min CAP treatment, ∼1% of spores were still viable (Fig. 2a). Previous work has shown that spores surviving a number of potentially lethal treatments can be damaged and less able to resist additional stress, such as high salt content in the spore recovery medium or a normally sublethal heat treatment (25, 31). To examine the resistance of spores surviving 1-min CAP treatments, the viability of CAP-treated spores was determined on LB medium plates with or without 1 M NaCl, or on LB medium plates with and without a heat treatment of spores at 80°C prior to plating. The results showed that the CAP-treated spores had not become sensitive to the 1 M NaCl-containing medium (100% recovery of spores) but had become sensitive to the treatment with 80°C for 30 min (10% recovery), which had no effects on untreated control spores (see Fig. S2 in the supplemental material).
Germination kinetics of CAP-treated spores.
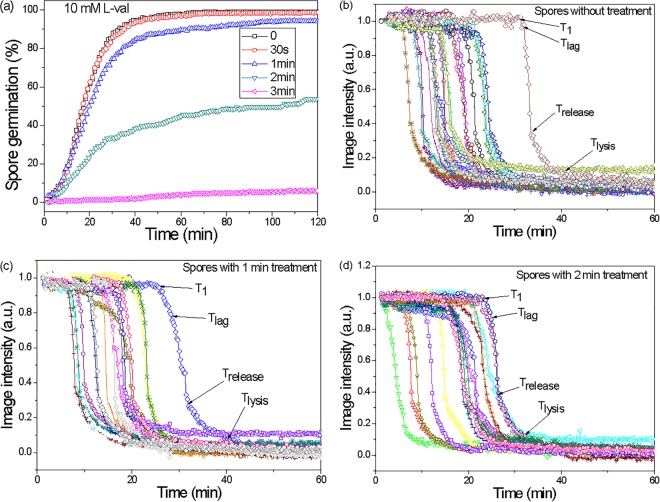
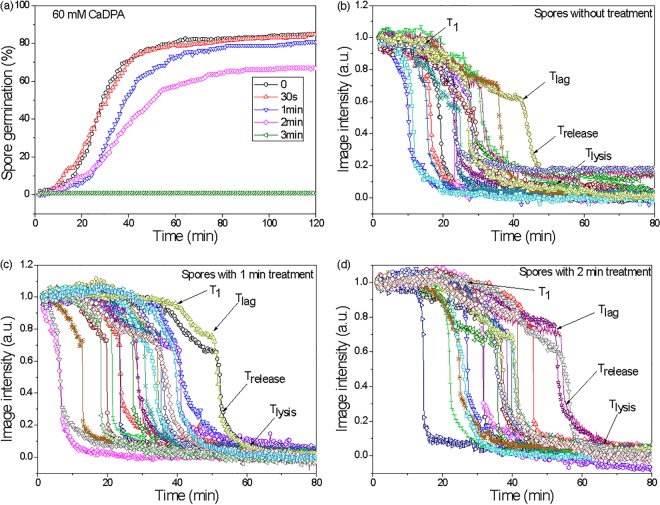
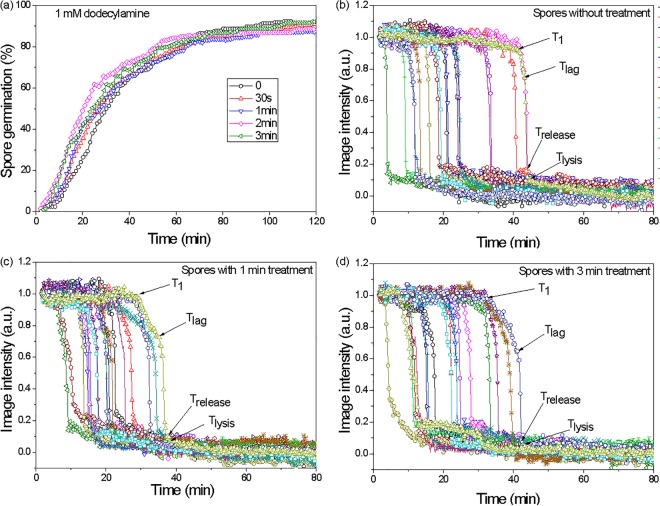
As noted above, a 3-min CAP treatment killed essentially all (105) of the test spores, but the majority (>80%) retained CaDPA. An obvious question is whether the treated spores that retained CaDPA are able to germinate like untreated spores. Therefore, the responses of multiple individual spores (untreated spores and CAP-treated spores that had retained CaDPA) were examined with exposure to germinants that initiate germination through different pathways. These germinants were (i) l-valine (acts via GRs), (ii) CaDPA (bypasses the GRs and activates the CLE CwlJ [11, 14]), and (iii) dodecylamine (directly triggers CaDPA release [11, 14]). Notably, with l-valine and CaDPA as germinants (Fig. 4 and 5, respectively), a prior 1-min CAP treatment had almost no effect on the observed spore germination kinetics (100% germination after 2 min). A 2-min CAP treatment, however, reduced the germination of spores to 54% and 67% after exposure to l-valine or the nonnutrient germinant CaDPA, respectively. A 3-min CAP treatment almost completely abolished subsequent spore germination with either l-valine or CaDPA (Fig. 4 and 5). Average values of T1, Tlag, and Trelease from individual spore germination curves with l-valine also increased with increasing CAP treatment times, but ΔTleakage, ΔTrelease, and ΔTlysis did not change (Table 1). Similar results were obtained for spore germination with CaDPA, except for T1 and ΔTleakage, which also increased for CaDPA-triggered germination with increasing CAP treatment times (Table 2). Interestingly, while CAP treatment for 3 min abolished l-valine and CaDPA spore germination, this CAP treatment had no effect on spore germination triggered by dodecylamine, a germinant that directly triggers CaDPA release (11, 14). Kinetic parameters of dodecylamine germination were similar in untreated spores and spores treated for 3 min with CAP (Fig. 6; Table 3).
FIG 4.
l-Valine germination of spores after CAP treatment. (a) l-Valine germination kinetics for various CAP treatment times (0, 0.5, 1, 2, and 3 min; >268 spores examined); (b to d) estimated kinetic parameters (T1, Tlag, Trelease, and Tlysis) of single spores (average of ∼20 individual spores) after various CAP treatment times (0, 1, and 2 min are shown).
FIG 5.
CaDPA germination of spores after CAP treatment. (a) CaDPA germination kinetics for various CAP treatment times (0, 0.5, 1, 2, and 3 min; >219 spores examined); (b to d) estimated kinetic parameters (T1, Tlag, Trelease, and Tlysis) of single spores (average of ∼20 individual spores) after various CAP treatment times (0, 1, and 2 min are shown).
TABLE 1.
Mean values and standard deviations for kinetic parameters of l-valine germination of untreated and CAP-treated B. subtilis sporesa
| Treatment time | T1 (min) | Tlag (min) | ΔTleakage (min) | Trelease (min) | ΔTrelease (min) | ΔTlysis (min) | % germination (no. of germinated spores) |
|---|---|---|---|---|---|---|---|
| 0 min | 16.9 ± 10.9 | 18.5 ± 11.2 | 1.5 ± 1.3 | 22.0 ± 11.6 | 3.5 ± 1.1 | 8.9 ± 4.1 | 99.9 (438) |
| 30 s | 17.7 ± 12.6 | 19.1 ± 12.1 | 1.4 ± 1.0 | 22.5 ± 12.2 | 3.4 ± 1.3 | 8.3 ± 4.1 | 98.7 (608) |
| 1 min | 20.1 ± 16.2 | 22.0 ± 16.4 | 1.3 ± 1.3 | 25.9 ± 16.7 | 3.9 ± 1.4 | 9.1 ± 4.0 | 94.5 (379) |
| 2 min | 33.7 ± 32.9 | 35.2 ± 33.2 | 1.6 ± 1.5 | 38.9 ± 33.3 | 3.6 ± 1.3 | 10.5 ± 3.4 | 53.7 (144) |
The kinetic germination parameters T1, Tlag, Trelease, ΔTleakage (Tlag − T1), ΔTrelease (Trelease − Tlag), and ΔTlysis (Tlysis − Trelease) were determined for l-valine germination of B. subtilis spores with and without CAP treatment as described in Materials and Methods.
TABLE 2.
Mean values and standard deviations for kinetic parameters of CaDPA germination of untreated and CAP-treated B. subtilis sporesa
| Treatment time | T1 (min) | Tlag (min) | ΔTleakage (min) | Trelease (min) | ΔTrelease (min) | ΔTlysis (min) | % germination (no. of germinated spores) |
|---|---|---|---|---|---|---|---|
| 0 min | 16.7 ± 6.9 | 26.1 ± 10.3 | 9.4 ± 7.2 | 28.0 ± 10.4 | 1.9 ± 0.9 | 9.8 ± 5.1 | 84.8 (476) |
| 30 s | 21.4 ± 8.9 | 31.3 ± 17.7 | 10.1 ± 7.6 | 32.9 ± 17.5 | 1.6 ± 1.0 | 9.1 ± 4.6 | 83.4 (337) |
| 1 min | 23.7 ± 8.6 | 37.0 ± 17.9 | 13.3 ± 11.3 | 38.7 ± 17.7 | 1.8 ± 1.1 | 9.6 ± 5.4 | 80.8 (177) |
| 2 min | 26.2 ± 9.7 | 39.9 ± 16.5 | 13.7 ± 10.3 | 41.6 ± 16.4 | 1.7 ± 1.0 | 9.4 ± 5.1 | 66.8 (266) |
The kinetic germination parameters T1, Tlag, Trelease, ΔTleakage (Tlag − T1), ΔTrelease (Trelease − Tlag), and ΔTlysis (Tlysis − Trelease) were determined for CaDPA germination of B. subtilis spores with and without CAP treatment as described in Materials and Methods.
FIG 6.
Dodecylamine germination of spores after CAP treatment. (a) Dodecylamine kinetics after various CAP treatment times (0, 0.5, 1, 2, and 3 min; >213 spores examined); (b to d) estimated kinetic parameters (T1, Tlag, Trelease, and Tlysis) of single spores (average of ∼20 individual spores) after various CAP treatment times (0, 1, and 3 min are shown).
TABLE 3.
Mean values and standard deviations of kinetic parameters of dodecylamine germination of untreated and CAP-treated B. subtilis sporesa
| Treatment time | T1 (min) | Tlag (min) | ΔTleakage (min) | Trelease (min) | ΔTrelease (min) | ΔTlysis (min) | % germination (no. of germinated spores) |
|---|---|---|---|---|---|---|---|
| 0 min | 28.9 ± 18.7 | 30.5 ± 19.1 | 1.6 ± 1.9 | 32.9 ± 19.1 | 2.3 ± 0.7 | 1.8 ± 2.1 | 92.3 (199) |
| 30 s | 29.1 ± 22.3 | 31.3 ± 17.7 | 2.2 ± 3.2 | 33.0 ± 23.4 | 2.6 ± 1.0 | 2.1 ± 4.6 | 90.9 (411) |
| 1 min | 29.6 ± 19.2 | 32.0 ± 23.6 | 2.4 ± 1.0 | 34.4 ± 23.3 | 2.4 ± 1.0 | 1.6 ± 1.6 | 87.6 (247) |
| 2 min | 29.2 ± 16.9 | 32.4 ± 19.0 | 2.2 ± 1.5 | 35.5 ± 18.9 | 2.1 ± 1.5 | 1.8 ± 5.1 | 88.5 (243) |
| 3 min | 32.7 ± 25.3 | 35.5 ± 28.6 | 2.8 ± 4.1 | 41.6 ± 16.4 | 2.6 ± 1.4 | 1.0 ± 1.6 | 92.2 (196) |
The kinetic germination parameters T1, Tlag, Trelease, ΔTleakage (Tlag − T1), ΔTrelease (Trelease − Tlag), and ΔTlysis (Tlysis − Trelease) were determined for dodecylamine germination of B. subtilis spores with and without CAP treatment as described in Materials and Methods.
Spore inactivation and CaDPA loss due to various CAP components.
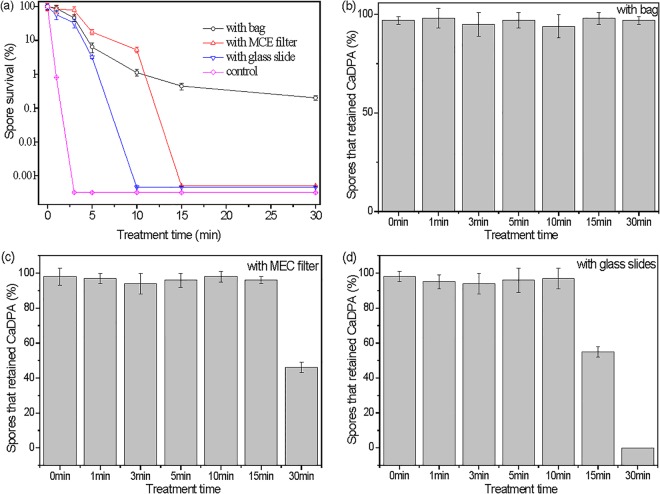
As noted above, UV photons, charged particles, and ROS are three important plasma components involved in microbial inactivation. To distinguish how these three CAP components contribute to spore inactivation and CaDPA loss, different methods were used to eliminate or reduce exposure of spores to UV-A photons, charged particles, and ROS during CAP treatments. If spores are put inside a 40-μm-thick, sealed plastic bag, this plastic bag will prevent charged particle and ROS penetration and will also limit the amount of air inside the bag, and thus, there will likely be less ROS produced inside the bag, although passage of UV-A light produced by the CAP apparatus should be unaffected (see Fig. S1b in the supplemental material). Indeed, CAP treatment of spores in the plastic bag gave only an ∼2.7-log reduction in spore viability in 30 min, and the treated spores retained CaDPA (Fig. 7a and b; note that without the bag, a 10-min CAP treatment inactivated all spores and none retained CaDPA). In a second test, the spores were covered by an MCE filter with 0.2-μm pores that prevents UV-A penetration (see Fig. S1b) but likely allows charged particles and ROS to pass through under the influence of the intense electric field between two electrodes. The result was that CAP spore inactivation was faster than in the plastic bag, with complete spore inactivation (∼ 5.3 logs) occurring with 15 min of CAP treatment (Fig. 7a). However, even 30 min of CAP treatment with this filter caused only ∼50% of spores to lose CaDPA (Fig. 7c). In a third experimental setup, the spores were covered with a 100-μm-thick glass slide in a sandwich configuration, which allowed UV-A light to pass through but not charged particles. However, ROS generated during CAP treatment can likely enter the gap between the glass slide and the coverslip with spores and interact with the spores. The CAP treatment with the glass slide shield gave the unexpected result of more spore killing (100% in 10 min) and more CaDPA loss (100% in 30 min) than treatment with the MEC filter (Fig. 7a and d).
FIG 7.
B. subtilis spore inactivation and CaDPA loss by CAP treatment with various materials shielding the spores from the different plasma components. (a) Variations in spore inactivation kinetics with different materials; (b to d) percentages of spores retaining CaDPA after various treatment times with different materials for shielding (>300 individual spores were analyzed for each time point).
Effects of CAP treatment on B. cereus spore inactivation and loss of CaDPA.
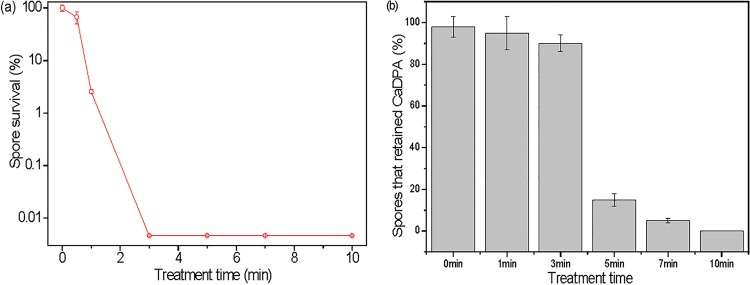
With increasing CAP treatment times, B. cereus spore inactivation increased and a 3-min treatment inactivated all test spores (∼4.4 logs) (Fig. 8a). In addition, ∼90% of the B. cereus spores given a 3-min CAP treatment retained CaDPA, 5-min and 7-min treatments caused CaDPA loss from the majority of spores, and a 10-min treatment resulted in loss of CaDPA from all spores examined (Fig. 8b). All these results with CAP treatment of B. cereus spores were very similar to those with CAP treatment of B. subtilis spores.
FIG 8.
Effects of CAP on B. cereus spores. (a) Spore inactivation kinetics with CAP; (b) CaDPA loss after various CAP treatment times of 0 to 10 min (>300 individual spores were analyzed for each time point).
DISCUSSION
The work in this communication has shown that almost all B. cereus and B. subtilis spores treated for ≥5 min in a CAP apparatus were killed at a rate of ≥4.4 logs and lost all or almost all CaDPA. The relative agreement between the effects of the CAP on B. cereus and B. subtilis spores strongly suggests that the mechanism(s) of effects of CAP on spores of these two species are likely to be similar. In addition, that spores subjected to CAP treatment for ≥5 min had lost almost all CaDPA indicates that these dead spores had accumulated some severe defect(s), as CaDPA loss from dormant spores requires breakdown of spores' IM permeability barrier, which prevents CaDPA release from the spore core (13, 25). The inability of almost all B. subtilis spores subjected to 3 min of CAP treatment and that retained CaDPA to germinate with either CaDPA or l-valine further indicates that one or more of the spores' crucial germination proteins were severely damaged in these treated spores. Spores' crucial germination proteins include the SpoVA proteins that likely comprise the channel for CaDPA efflux during germination, as well as GRs and CLEs (11, 14). However, the SpoVA protein channel does not seem to be severely damaged, as germination by dodecylamine, which triggers germination most likely by activating the mechanosensitive SpoVAC protein (32, 33), was normal in CAP-treated spores that retained CaDPA. The ∼2-fold increase in the T1, Tlag, and Trelease for l-valine germination with increasing CAP treatment is certainly consistent with CAP treatment partially inactivating the GerA GR, which triggers l-valine germination. The minimal effects of ≤2 min of CAP treatment on ΔTrelease and ΔTlysis in l-valine germination also suggest that this CAP treatment has minimal effects on the CLEs CwlJ and SleB. Indeed, complete loss of CwlJ increases ΔTrelease in GR-dependent germination almost 10-fold (11, 14). Notably, CaDPA germination, which absolutely requires the CLE CwlJ (11, 14), was inhibited less than l-valine germination, again consistent with at least the GerA GR being the primary CAP target that abolishes l-valine germination. It will be interesting to determine in the future if CAP inactivates GRs for other nutrient germinants similarly.
The findings noted above indicate that CAP treatments of ≥3 min do severe damage to some B. subtilis spore structure and germination proteins, and severe visible damage has been observed in CAP-treated spores (7, 20, 34, 35). However, it is important to note that B. subtilis spores given these CAP treatment times in the current work were killed at a rate of >5 logs. Thus, the loss of CaDPA and the ability to germinate in these CAP-treated spores tell us little about how the particular CAP treatments used in this work actually kill spores. Indeed, >90% of spores given a 2-min CAP treatment retained CaDPA, and >50% of these spores germinated with l-valine or CaDPA, and yet these spores had been killed at a rate of ∼4 logs. This finding indicates that these CAP treatments do not kill B. subtilis spores by destroying either spores' ability to germinate or spores' IM permeability barrier. However, while CAP undoubtedly does these types of damage, they appear to occur only after spores are already dead due to other causes.
The precise mechanisms whereby the B. subtilis spores are killed by the CAP generated in this work are not completely clear. However, five possible ways that spores are killed by a variety of treatments have been suggested (13, 36), including (i) destruction of spores' IM permeability barrier, (ii) inactivation of one or more essential spore germination proteins, (iii) damage to spore DNA, (iv) damage to spores' IM such that this defect becomes acute when spores germinate, and (v) damage to one or more essential spore proteins. Clearly, the first two killing mechanisms cannot be significant with the CAP used in this work, as noted above. It also seems unlikely that the CAP used in this work causes major DNA damage, for several reasons. First, the CAP used has minimal, if any, UV-C light, the UV wavelengths most effective in damaging DNA. There is some UV-A, and UV-A does generate lesions in spore DNA that can lead to mutations and spore death (37). However, when CAP components other than UV-A were removed or minimized by CAP exposure in a plastic bag, CAP spore killing was greatly reduced. Thus, it seems likely that the UV-A light generated by the CAP in this work contributes only minimally to spore killing. In the experiment with the spores in a plastic bag, some ROS were generated inside the bag, which was not permeable to ROS. Since the amount of oxygen gas inside the bag was limited because the bag was sealed, ROS generation in the bag decreased with time. This is consistent with the shape of spore killing kinetics in the plastic bag (Fig. 7a), compared to the killing curves with other shielding or no shielding. Overall, these results suggested that ROS were the CAP component causing most spore killing, with UV-A photons and charged particles being of lesser importance.
It is of course possible that CAP components such as ROS and free radicals could also damage spore DNA, as these molecular species can cause damage to DNA in growing cells that can lead to mutations and death (38). However, DNA in dormant spores is very well protected against these types of damaging agents by its saturation with spore-specific DNA binding proteins (13). Indeed, agents such as hydrogen peroxide, organic peroxides, peroxynitrite, and many other oxidizing agents that are effective in killing spores do not kill spores by DNA damage, because of DNA's layer of protective proteins. However, when this layer of protective protein is removed, at least hydrogen peroxide kills these mutant spores by DNA damage (13). Notably, several studies have shown that these DNA binding proteins are quite important in protecting spores from several types of plasma, in particular plasma types that have high levels of UV-C (16, 35).
It is more difficult to decide whether it is through damage to spores' IM or a specific crucial spore protein that the CAP kills spores. Previous work has indicated that high-temperature treatment of spores in water likely kills spores by damaging one or more essential proteins, probably in the spore core (36). This also may be how hydrogen peroxide kills spores (13). However, for almost all other oxidizing agents that have been used for spore killing, the mechanisms by which these agents kill spores appear to be by damaging the spores' IM such that when these spore germinate, the damaged IM ruptures, leading to spore death.
Currently we know much about B. subtilis spore killing by agents other than plasma because of (i) the many mutant strains that lack specific elements important in spore resistance, including spore-specific DNA binding proteins and the large proteinaceous spore coat, and (ii) ways to modify the lipid content and composition of the spore IM. Consequently, it seems likely that a detailed study of the effects of the CAP used in this work on spores of various mutant strains as well as properties of spores killed only 90 to 95%, such as their germination, outgrowth, and mutagenesis and the strength of the treated spores' IM barrier, could well determine the precise mechanisms of spore killing by various CAP components.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the U.S. Army Research Office under contract number W911NF-12-2-0024 and by a Department of Defense Multi-disciplinary University Research Initiative through the U.S. Army Research Laboratory and the U.S. Army Research Office under contract number W911F-09-1-0286 (P.S. and Y.-Q.L.).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01669-16.
REFERENCES
- 1.Mitra A, Li Y-F, Klämpfl TG, Shimizu T, Jeon J, Morfill GE, Zimmermann JL. 2013. Inactivation of surface-borne microorganisms and increased germination of seed specimen by cold atmospheric plasma. Food Bioproc Tech 7:645–653. [Google Scholar]
- 2.Mertens N, Mahmoodzada M, Helmke A, Grünig P, Laspe P, Emmert S, Viöl W. 2014. Inactivation of microorganisms using cold atmospheric pressure plasma with different temporal discharge characteristics. Plasma Process Polym 11:910–920. doi: 10.1002/ppap.201300184. [DOI] [Google Scholar]
- 3.Lacombe A, Niemira BA, Gurtler JB, Fan X, Sites J, Boyd G, Chen H. 2015. Atmospheric cold plasma inactivation of aerobic microorganisms on blueberries and effects on quality attributes. Food Microbiol 46:479–484. doi: 10.1016/j.fm.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 4.De Geyter N, Morent R. 2012. Nonthermal plasma sterilization of living and nonliving surfaces. Annu Rev Biomed Eng 14:255–274. doi: 10.1146/annurev-bioeng-071811-150110. [DOI] [PubMed] [Google Scholar]
- 5.Kong MG, Kroesen G, Morfill G, Nosenko T, Shimizu T, van Dijk J, Zimmermann JL. 2009. Plasma medicine: an introductory review. N J Phys 11:115012. doi: 10.1088/1367-2630/11/11/115012. [DOI] [Google Scholar]
- 6.Denes FS, Manolache S. 2004. Macromolecular plasma-chemistry: an emerging field of polymer science. Prog Polym Sci 29:815–885. doi: 10.1016/j.progpolymsci.2004.05.001. [DOI] [Google Scholar]
- 7.Muranyi P, Wunderlich J, Langowski HC. 2010. Modification of bacterial structures by a low-temperature gas plasma and influence on packaging material. J Appl Microbiol 109:1875–1885. doi: 10.1111/j.1365-2672.2010.04815.x. [DOI] [PubMed] [Google Scholar]
- 8.Shintani H. 2016. Inactivation of bacterial spore, endotoxin, lipid A, normal prion and abnormal prion by exposures to several sorts of gases plasma. Biocontrol Sci 21:1–12. doi: 10.4265/bio.21.1. [DOI] [PubMed] [Google Scholar]
- 9.Logan NA. 2012. Bacillus and relatives in foodborne illness. J Appl Microbiol 112:417–429. doi: 10.1111/j.1365-2672.2011.05204.x. [DOI] [PubMed] [Google Scholar]
- 10.Mallozzi M, Viswanathan VK, Vedantam G. 2010. Spore-forming Bacilli and Clostridia in human disease. Future Microbiol 5:1109–1123. doi: 10.2217/fmb.10.60. [DOI] [PubMed] [Google Scholar]
- 11.Setlow P. 2013. When the sleepers wake: the germination of spores of Bacillus species. J Appl Microbiol 115:1251–1268. doi: 10.1111/jam.12343. [DOI] [PubMed] [Google Scholar]
- 12.Paredes-Sabja D, Setlow P, Sarker MR. 2011. Germination of spores of Bacillales and Clostridiales species: mechanisms and proteins involved. Trends Microbiol 19:85–94. doi: 10.1016/j.tim.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Setlow P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol 101:514–525. doi: 10.1111/j.1365-2672.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- 14.Setlow P. 2014. Germination of spores of Bacillus species: what we know and do not know. J Bacteriol 196:1297–1305. doi: 10.1128/JB.01455-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klämpfl TG, Isbary G, Shimizu T, Li Y-F, Zimmermann JL, Stolz W, Schlegel J, Morfill GE, Schmidt H-U. 2012. Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Appl Environ Microbiol 78:5077–5082. doi: 10.1128/AEM.00583-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth S, Feichtinger J, Hertel C. 2010. Characterization of Bacillus subtilis spore inactivation in low-pressure, low-temperature gas plasma sterilization processes. J Appl Microbiol 108:521–531. doi: 10.1111/j.1365-2672.2009.04453.x. [DOI] [PubMed] [Google Scholar]
- 17.Deng X, Shi J, Kong MG. 2006. Physical mechanisms of inactivation of Bacillus subtilis spores using cold atmospheric plasmas. IEEE Trans Plasma Sci 34:1310–1316. doi: 10.1109/TPS.2006.877739. [DOI] [Google Scholar]
- 18.Laroussi M. 2005. Low temperature plasma-based sterilization: overview and state-of-the-art. Plasma Process Polym 2:391–400. doi: 10.1002/ppap.200400078. [DOI] [Google Scholar]
- 19.Hertwig C, Steins V, Reineke K, Rademacher A, Klocke M, Rauh C, Schlüter O. 2015. Impact of surface structure and feed gas composition on Bacillus subtilis endospore inactivation during direct plasma treatment. Front Microbiol 6:774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Bokhorst-van de Veen H, Xie H, Esveld E, Abee T, Mastwijk H, Nierop Groot M. 2015. Inactivation of chemical and heat-resistant spores of Bacillus and Geobacillus by nitrogen cold atmospheric plasma evokes distinct changes in morphology and integrity of spores. Food Microbiol 45:26–33. doi: 10.1016/j.fm.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Eto H, Ono Y, Ogino A, Nagatsu M. 2008. Low-temperature sterilization of wrapped materials using flexible sheet-type dielectric barrier discharge. Appl Phys Lett 93:221502. doi: 10.1063/1.3039808. [DOI] [Google Scholar]
- 22.Reineke K, Langer K, Hertwig C, Ehlbeck J, Schlüter O. 2015. The impact of different process gas compositions on the inactivation effect of an atmospheric pressure plasma jet on Bacillus spores. Innov Food Sci Emerg Technol 30:112–118. doi: 10.1016/j.ifset.2015.03.019. [DOI] [Google Scholar]
- 23.Setlow B, Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J Bacteriol 178:3486–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clements MO, Moir A. 1998. Role of the gerI operon of Bacillus cereus 569 in the response of spores to germinants. J Bacteriol 180:6729–6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortezzo DE, Koziol-Dube K, Setlow B, Setlow P. 2004. Treatment with oxidizing agents damages the inner membrane of spores of Bacillus subtilis and sensitizes spores to subsequent stress. J Appl Microbiol 97:838–852. doi: 10.1111/j.1365-2672.2004.02370.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang S, Faeder JR, Setlow P, Li Y-Q. 2015. Memory of germinant stimuli in bacterial spores. mBio 6:e01859-15. doi: 10.1128/mBio.01859-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S, Setlow P, Li Y-Q. 2015. Slow leakage of Ca-dipicolinic acid from individual Bacillus spores during initiation of spore germination. J Bacteriol 197:1095–1103. doi: 10.1128/JB.02490-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang SS, Chen D, Pelczar PL, Vepachedu VR, Setlow P, Li YQ. 2007. Levels of Ca2+-dipicolinic acid in individual Bacillus spores determined using microfluidic Raman tweezers. J Bacteriol 189:4681–4687. doi: 10.1128/JB.00282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang PF, Kong LB, Setlow P, Li YQ. 2010. Characterization of wet-heat inactivation of single spores of Bacillus species by dual-trap Raman spectroscopy and elastic light scattering. Appl Environ Microbiol 76:1796–1805. doi: 10.1128/AEM.02851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong LB, Doona CJ, Setlow P, Li YQ. 2014. Monitoring rates and heterogeneity of high pressure germination of Bacillus spores using phase contrast microscopy of individual spores. Appl Environ Microbiol 80:345–353. doi: 10.1128/AEM.03043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurst A. 1977. Bacterial injury: a review. Can J Microbiol 23:935–944. [DOI] [PubMed] [Google Scholar]
- 32.Velásquez J, Schuurman-Wolters Birkner JP, Abee T, Poolman B. 2014. Bacillus subtilis spore protein SpoVAC functions as a mechanosensitive channel. Mol Microbiol 92:813–823. doi: 10.1111/mmi.12591. [DOI] [PubMed] [Google Scholar]
- 33.Vepachedu VR, Setlow P. 2007. Role of SpoVA proteins in release of dipicolinic acid during germination of Bacillus subtilis spores triggered by dodecylamine or lysozyme. J Bacteriol 189:1565–1672. doi: 10.1128/JB.01613-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moisan M, Barbeau J, Moreau S, Pelletier J, Tabrizian M, Yahia LH. 2001. Low-temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. Int J Pharm 226:1–21. doi: 10.1016/S0378-5173(01)00752-9. [DOI] [PubMed] [Google Scholar]
- 35.Philip N, Saoudi B, Crevier MC, Moisan M, Barbeau J, Pelletier J. 2002. The respective roles of UV photons and oxygen atoms in plasma sterilization at reduced gas pressure: the case of N2/O2 mixtures. IEEE Trans Plasma Sci 30:1429–1436. doi: 10.1109/TPS.2002.804203. [DOI] [Google Scholar]
- 36.Coleman WH, Chen D, Li Y-Q, Cowan AE, Setlow P. 2007. How moist heat kills spores of Bacillus subtilis. J Bacteriol 189:8458–8466. doi: 10.1128/JB.01242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slieman TA, Nicholson WL. 2000. Artificial and solar UV radiation induces strand breaks and cyclobutane pyrimidine dimers in Bacillus subtilis spore DNA. Appl Environ Microbiol 66:199–205. doi: 10.1128/AEM.66.1.199-205.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang FC. 2004. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol 2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.