Abstract
Salmonella infection profoundly affects host erythroid development, but the mechanisms responsible for this effect remain poorly understood. We monitored the impact of Salmonella infection on erythroid development and found that systemic infection induced anemia, splenomegaly, elevated erythropoietin (EPO) levels, and extramedullary erythropoiesis in a process independent of Salmonella pathogenicity island 2 (SPI2) or flagellin. The circulating EPO level was also constitutively higher in mice lacking the expression of signal-regulatory protein α (SIRPα). The expression level of EPO mRNA was elevated in the kidney and liver but not increased in the spleens of infected mice despite the presence of extramedullary erythropoiesis in this tissue. In contrast to data from a previous report, mice lacking EPO receptor (EPOR) expression on nonerythroid cells (EPOR rescued) had bacterial loads similar to those of wild-type mice following Salmonella infection. Indeed, treatment to reduce splenic erythroblasts and mature red blood cells correlated with elevated bacterial burdens, implying that extramedullary erythropoiesis benefits the host. Together, these findings emphasize the profound effect of Salmonella infection on erythroid development and suggest that the modulation of erythroid development has both positive and negative consequences for host immunity.
INTRODUCTION
In the United States, Salmonella enterica infections are an important cause of foodborne infection, where they are responsible for periodic outbreaks of gastroenteritis that can be traced to a variety of sources (1–3). Other Salmonella enterica serovars impact developing nations, where they can be transmitted from human to human and cause a systemic infection called typhoid fever (4–6). Although there are two licensed vaccines for typhoid, neither of these vaccines provides effective immunity to young children or is widely used in areas of endemicity (7, 8). An additional problem is that nontyphoidal Salmonella serovars are now a major cause of bacteremia in sub-Saharan Africa, where they are associated with HIV, poor nutrition, or malaria coinfection (9, 10). Thus, the development of new vaccines for typhoid and nontyphoidal salmonellosis (NTS) is an important public health priority. However, the generation of effective vaccines will require a greater understanding of how the innate and adaptive immune responses recognize and combat Salmonella infections (11).
Some Salmonella serovars cause disease in a single species, while other serovars infect a broad range of animals, causing local or disseminated infection depending on host susceptibility. Although Salmonella enterica serovar Typhimurium causes gastroenteritis in humans, it causes a disseminated infection in mice that displays some similarities to human typhoid and NTS (12, 13). This murine infection has been widely used as a model to understand Salmonella pathogenesis and the host immune response to infection (11, 14). Salmonella immunity in mice requires the coordinated activation of innate and adaptive immune compartments, leading to a wide variety of immune effector mechanisms that eliminate bacteria and establish protective immunity to secondary challenge (11, 15). Both CD4 and CD8 T cells contribute to the resolution of primary Salmonella infection, while CD4 T cells play a dominant role in providing secondary protective immunity (16–18). The role of B cells is more complex, since this cell population is not required for primary clearance but contributes to secondary protective immunity via antibody-dependent and antibody-independent mechanisms (19–22). In order to survive within an immunocompetent host, Salmonella has evolved multiple mechanisms to combat innate and adaptive immune responses (23–26). Some of these evasion strategies rely on the expression of Salmonella pathogenicity island (SPI) genes that encode a molecular syringe to deliver effector proteins to infected cells and disrupt cellular signaling or vesicle trafficking (27–29). Salmonella can also shut down the synthesis of flagellin soon after infecting host tissues (30), most likely because this antigen serves as a target for innate and adaptive immune responses (31, 32).
In addition to these inhibitory effects of Salmonella on host immune responses, recent studies demonstrate that Salmonella infection disrupts homeostatic erythroid development (33, 34), although the consequence of this for the host and pathogen is poorly understood. Our laboratory reported previously that Salmonella-induced splenomegaly is largely a consequence of extramedullary erythropoiesis in the spleen (33). Approximately 10 to 30% of the spleen of a healthy naive mouse is composed of erythroid cells, but this proportion rises to 80 to 85% at the peak of Salmonella-induced splenomegaly (33). This striking effect of Salmonella on erythroid development has been largely ignored despite the fact that several studies over the last 40 years have noted that bacterial endotoxin can affect erythropoiesis (35–38).
Erythrocytes develop from hematopoietic stem cells via intermediate stages, before they shed nuclei and enter the circulation (39, 40). CD71 and Ter119 define late stages in the process of erythroid development (41, 42), and Salmonella infection is known to induce a large expansion of these immature Ter119+ CD71+ reticulocytes while decreasing mature erythrocytes (33). This expansion of immature reticulocytes correlates with a large increase in the level of serum erythropoietin (EPO) that can be inhibited by EPO neutralization (33), demonstrating that dysregulated EPO production is responsible for this effect. However, the signals that drive EPO production and the location of EPO secretion during Salmonella infection are not well defined. With regard to EPO responsiveness, it has recently been appreciated that EPO receptor (EPOR) can be expressed by nonerythroid cells (43). A recent study using EPOR-rescued mice lacking EPOR on nonerythroid cells found that Salmonella bacteria were rapidly eliminated, suggesting that EPO signaling in nonerythroid cells encourages bacterial persistence (34). This effect correlated with EPO-mediated inhibition of inflammatory cytokine and nitric oxide production by activated macrophages (34), suggesting that EPO might represent a critical mediator of host immune evasion. Signal-regulatory protein α (SIRPα) is a transmembrane glycoprotein expressed by macrophages that interacts with CD47 on erythroid cells, inhibiting phagocytosis and allowing red blood cells (RBCs) to remain in the circulation (44, 45). We recently reported that SIRPα-deficient mice display basal splenomegaly and have a higher number of splenic erythroid cells, including reticulocytes (46). Furthermore, this abnormal basal erythroid development correlated with the increased susceptibility of these mice to Salmonella infection (46), thus strengthening the connection between erythroid dysfunction and Salmonella persistence.
Here, we further examine the interaction of Salmonella infection with erythroid development and show that Salmonella and lipopolysaccharide (LPS) induce extramedullary erythropoiesis and splenomegaly. While erythropoiesis was induced prominently in the spleen, increased EPO production derives from the kidney and liver of infected mice. Furthermore, we show that mice lacking EPOR expression on nonerythroid cells do not display any heightened susceptibility to infection and that treatment to reduce numbers of circulating erythrocytes actually increases EPO production and encourages bacterial persistence. Thus, the modulation of erythroid development can have both positive and negative consequences for host immunity to Salmonella infection.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD) and The Jackson Laboratory (Bar Harbor, ME). Congenic SIRPα-deficient (Sirpα−/−) mice (B6.129P2-Sirpa⟨tm1Nog⟩/Rbrc) and EPOR−/−rescued mice (EpoR−/−::HG1-EpoR) were provided by RIKEN BRC, Japan. Mice used for experiments were 6 to 12 weeks old, unless otherwise noted. All of the animal experiments were approved by the University of California, Davis, Institutional Animal Care and Use Committee.
Salmonella infection and LPS treatment.
Salmonella enterica serovar Typhimurium strain SL1344 and a variety of mutants, including BRD509 (aroA mutant) (47), BRD509-SPI2 (aroA aroD spiB mutant) (29), and BRD509-Flagellin (aroA aroD fliC fljB mutant) (18), were grown overnight in LB broth without shaking before bacterial concentrations were estimated by using a spectrophotometer (optical density at 600 nm). All mice were infected intravenously (i.v.) in the lateral tail vein with 5 × 105 CFU of bacteria diluted in 200 μl phosphate-buffered saline (PBS). For intraperitoneal (i.p.) infection, mice were administered 500 CFU of SL1344 diluted in 500 μl PBS. The actual Salmonella bacterial dose administered was confirmed by plating serial dilutions of the bacterial culture onto MacConkey agar plates. To determine Salmonella bacterial loads in vivo, spleens and livers were removed from infected mice at various time points. Serial dilutions of organ homogenates were plated onto MacConkey agar plates, and bacterial counts per organ were calculated. For LPS treatment, C57BL/6 mice were injected i.v. with 1 to 10 μg of LPS [from Escherichia coli serotype EH100 (Ra); Enzo Life Sciences, NY] diluted in 200 μl PBS in the lateral tail vein daily for 7 consecutive days.
Measurement of packed cell volume.
C57BL/6 mice were injected intravenously with 5 × 105 CFU of BRD509. Whole blood was collected retroorbitally into heparinized capillary tubes every week after infection. Blood was transferred to BD heparinized Microtainers, briefly vortexed, and placed on ice. All samples were transported to the UC Davis Comparative Pathology Laboratory for packed cell volume (PCV) analysis.
Flow cytometry.
Spleens were harvested from naive, immunized, or infected mice, and single-cell preparations were generated in PBS supplemented with 2% fetal bovine serum (FBS). Aliquots of each single-cell suspension were stained with CD71-fluorescein isothiocyanate (FITC) (clone R17217) and Ter119-allophycocyanin (APC) (clone Ter119; eBioscience, San Diego, CA) and analyzed by using an LSRFortessa flow cytometer (BD Biosciences, San Jose, CA). Flow cytometric data were analyzed by using FlowJo software (TreeStar, Ashland, OR).
Determination of EPO levels by ELISAs.
Mice were bled retroorbitally at various time points postinfection or postimmunization, and serum was collected. Serum was analyzed for EPO protein levels by an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (R&D Systems, MN). Briefly, serial dilutions of serum samples were added to 96-well ELISA plates (Costar, Corning, NY) precoated with purified anti-EPO antibody. Bound EPO was detected by using biotinylated anti-EPO antibody, followed by the ExtrAvidin peroxidase substrate (Sigma-Aldrich, St. Louis, MO). Color change was analyzed by using a spectrophotometer (SpectraMax M5; Molecular Devices, Sunnyvale, CA), and EPO concentrations were calculated according to a standard curve.
Determination of Epo mRNA levels by real-time qPCR.
C57BL/6 mice were injected intravenously with (i) 5 × 105 CFU of BRD509 on day 0, (ii) 1 μg LPS daily for 7 days, or (iii) PBS daily for 7 days. Spleen, liver, kidney, and thymus were harvested on day 7, and total RNA was isolated by using an RNeasy kit (Qiagen, Valencia, CA). SYBR green-based real-time quantitative PCR (qPCR) for Epo mRNA was carried out by using the following primers: Epo forward primer 5′-CAGAGACCCTTCAGCTTCATATAG-3′, Epo reverse primer 5′-TCTGGAGGCGACATCAATTC-3′, Hprt forward primer 5′-CTTTCCCTGGTTAAGCAGTACA-3′, and Hprt reverse primer 5′-GCCTGTATCCAACACTTCGA-3′. Data were acquired by using a ViiA 7 real-time PCR system (Applied Biosystems) and analyzed by the comparative threshold cycle (CT) method. Expression levels of Epo mRNA were normalized to the amounts of Hprt mRNA in each sample.
CV1-hIgG4 treatment.
The high-affinity SIPRα variant (CV1-hIgG4, referred as CV1 below) was described previously (48). To deplete mature RBCs, Salmonella-infected mice were injected i.p. with 200 μg CV1 in 500 μl PBS on day 3 and day 6 postinfection. Mice were sacrificed on day 7 for analysis.
Statistical analysis.
Statistical analysis was performed by using an unpaired t test for normally distributed continuous-variable comparisons and a Mann-Whitney U test for nonparametric comparisons (Prism; GraphPad Software, Inc.).
RESULTS
Salmonella infection induces anemia and extramedullary erythropoiesis.
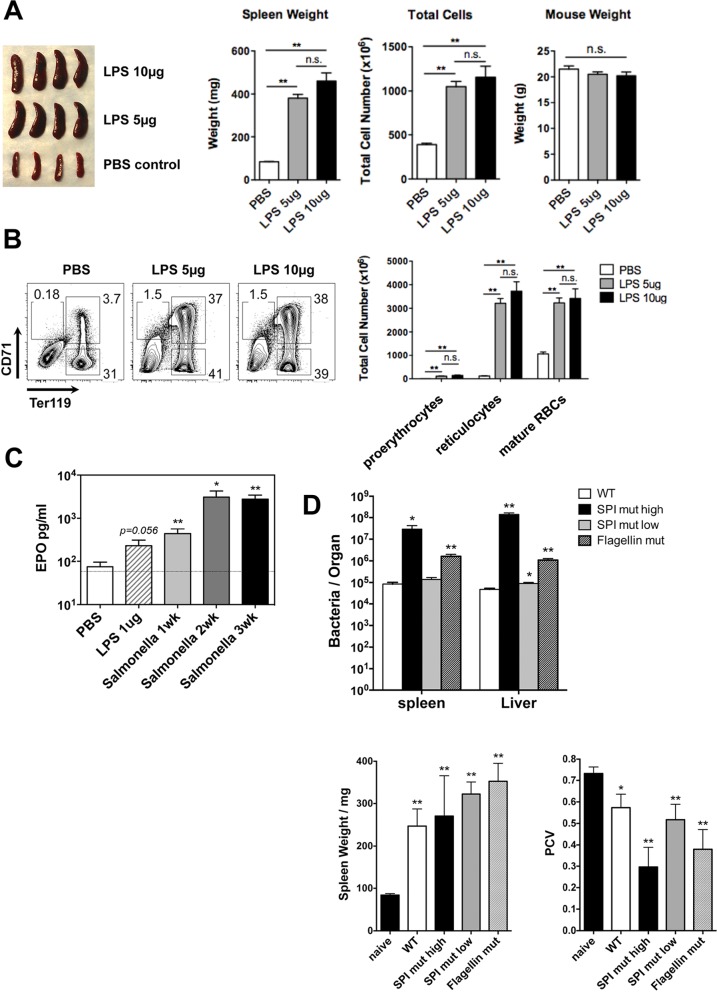
In order to examine the effect of infection on erythroid development, C57BL/6 mice were injected intravenously with 5 × 105 CFU of an attenuated strain of Salmonella (BRD509), and erythrocytes were subsequently examined in the blood and spleen. The hematocrit of whole blood was significantly reduced following Salmonella infection (Fig. 1A), while the corresponding weight of the spleen and proportion of splenic reticulocytes and their progenitors (proerythrocytes) were increased (Fig. 1B). These data are in broad agreement with results of previous reports describing Salmonella infection-induced anemia and splenic erythroid expansion and splenomegaly (33, 38, 49). Next, we examined whether LPS alone was sufficient to initiate erythroid dysfunction by regular i.v. injection of 5 or 10 μg of LPS into C57BL/6 mice. One week later, the overall size and weight of spleens of LPS-injected mice had increased substantially (Fig. 2A). This splenomegaly also correlated with markedly increased splenic cellularity and a 10-fold expansion in the percentage of CD71+ Ter119+ erythroblasts in the spleens of LPS-injected mice (Fig. 2A and B). The extramedullary splenic erythropoiesis detected in Salmonella-infected mice correlated with a significant rise in serum erythropoietin levels (Fig. 2C). In vivo survival of Salmonella is controlled by the expression of Salmonella pathogenicity island 2 (SPI2) genes and the downregulation of flagellin (50). In order to determine whether either of these virulence factors affected the development of anemia and splenomegaly, we infected mice with Salmonella strains lacking the expression of SPI2 or flagellin. Since SPI2 mutants display low levels of bacterial growth in vivo, we also infected some mice with higher doses of this mutant strain. The high doses of both the SPI2- and flagellin-deficient bacteria induced splenomegaly and anemia in C57BL/6 mice (Fig. 2D). Thus, Salmonella infection or repeated exposure to LPS can induce EPO production, splenomegaly, and extramedullary erythropoiesis in a process that does not depend on the SPI2 type III secretion system (T3SS) or flagellin.
FIG 1.
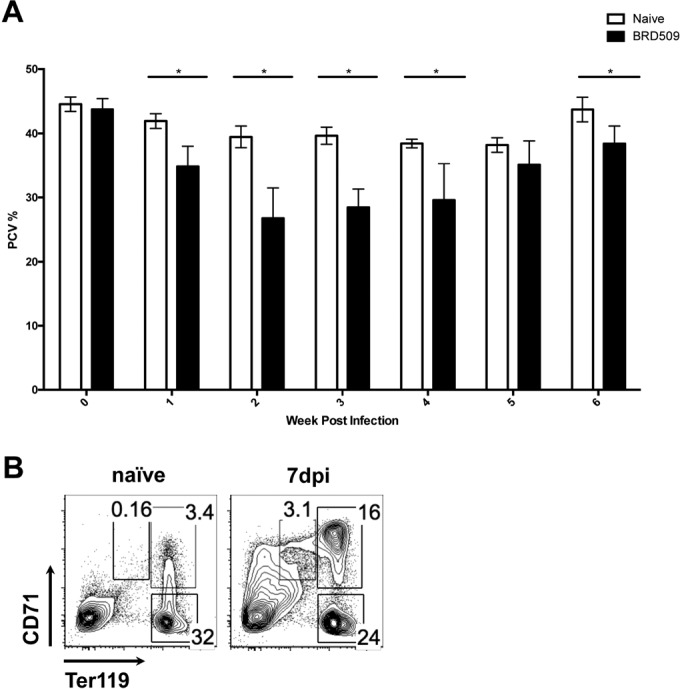
Systemic Salmonella infection induces anemia and extramedullary erythropoiesis. C57BL/6 mice were infected i.v. with 5 × 105 CFU of an attenuated Salmonella strain (BRD509). (A) The percentages peripheral blood packed cell volume (PCV%) were measured weekly after Salmonella infection. Statistical significance was established via multiple t tests. (B) Percentages of RBCs (Ter119+ CD71−), reticulocytes (Ter119+ CD71+), and proerythroblasts (Ter119med CD71+) in the peripheral blood of naive and Salmonella-infected mice were measured by flow cytometry. dpi, days postinfection; *, P < 0.05. Comparisons were made between infected and uninfected (week 0) groups.
FIG 2.
LPS induces erythropoiesis and EPO production. C57BL/6 mice were injected with 5 μg or 10 μg LPS daily for 7 consecutive days or infected i.v. with 5 × 105 CFU of attenuated Salmonella strains (BRD509 or mutant strains). (A) Sizes, weights, and total cell numbers of mouse spleens 7 days after LPS injection. (B) Total RBCs and RBC precursors recovered from the spleens of mice injected with LPS. (C) Blood EPO levels 7 days after LPS injection or Salmonella infection. (D) Bacterial counts per organ, spleen weights, and PCV in mice infected with either BRD509 or mutant strains. For the SPI2 mutant, both a low dose (5 × 105 CFU) and a high dose (5 × 107 CFU) were used for i.v. infection. Error bars represent the means ± standard errors of the means. *, P < 0.05; **, P < 0.01; n.s., not significant (by an unpaired t test). WT, wild type.
Salmonella infection and LPS injection elevate EPO production in the kidney and liver.
Previous studies demonstrated that elevated EPO production is essential for extramedullary erythropoiesis driven by Salmonella infection (33). Under homeostatic conditions, EPO is produced in the kidney (51), but the source of elevated EPO levels during Salmonella infection is currently unclear. In order to explore the source of EPO in more detail, we used ELISAs to detect EPO in organ lysates following Salmonella infection but found that this methodology gave inconsistent results. Therefore, we examined EPO mRNA expression in several different organs after C57BL/6 mice were infected with Salmonella or injected with LPS. The level of EPO mRNA was unaltered in the thymus and spleen of Salmonella-infected or LPS-injected mice (Fig. 3), indicating that splenic erythropoiesis is most likely not induced by local EPO production. In marked contrast, LPS and Salmonella infection resulted in elevated EPO mRNA levels in both the kidney and liver (Fig. 3). At baseline, the relative expression level of EPO mRNA was higher in the kidney than in the liver (Fig. 3), suggesting that this organ is the major site of EPO production.
FIG 3.
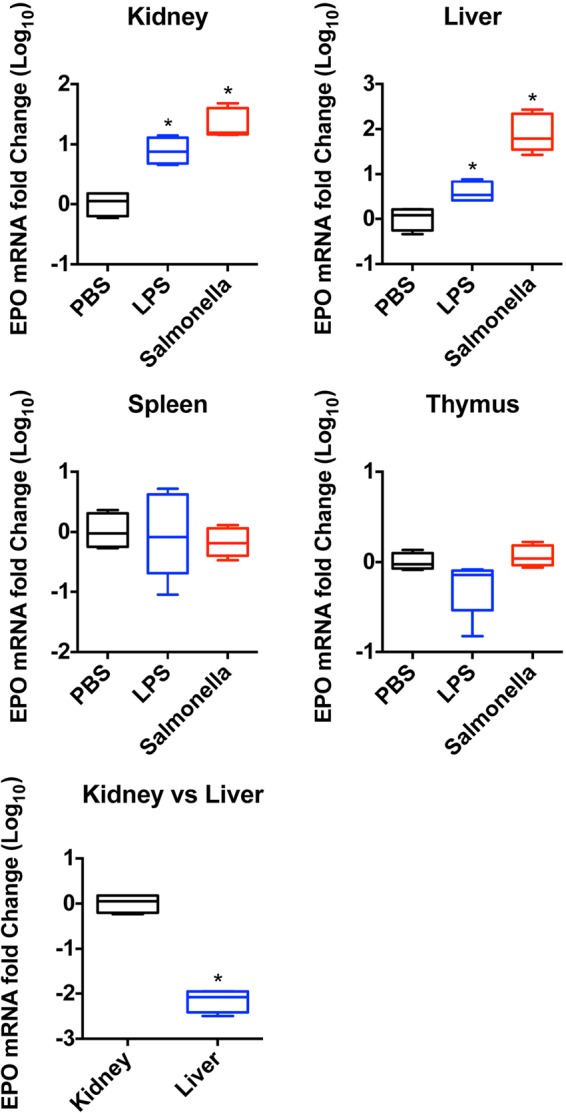
EPO mRNA levels are upregulated in kidney and liver during extramedullary erythropoiesis. C57BL/6 mice were injected with 1 μg LPS daily for 7 consecutive days or infected i.v. with 5 × 105 CFU of an attenuated Salmonella strain (BRD509). Spleen, liver, kidney, and thymus were harvested on day 7, and total mRNA was isolated as described in Materials and Methods. Graphs show the fold changes (log10) of EPO mRNA levels in mice receiving LPS or Salmonella compared with mice injected with PBS as controls. *, P < 0.05 (by a Mann-Whitney U test).
EPOR expression on nonerythroid cells does not affect bacterial persistence.
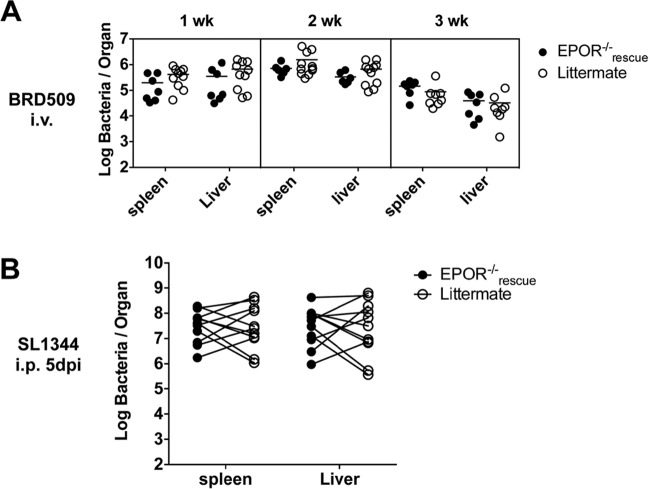
It was reported previously that mice lacking EPOR expression on nonerythroid cells (EPOR−/−rescue) display an enhanced ability to clear Salmonella infection (34). This finding suggested a novel mechanism of bacterial persistence where the induction of elevated EPO levels is able to suppress bacterial killing by macrophages and other EPO-responsive nonerythroid cells (52). We reexamined this issue by infecting EPOR−/−rescue and littermate control mice with an attenuated Salmonella strain and monitored bacterial counts at weekly intervals. Surprisingly, there was no statistically significant difference detected in bacterial loads in the spleen or liver of EPOR−/−rescue and littermate control mice at any time point postinfection (Fig. 4A). Since it was possible that any effect of EPO on persistence was masked by the slow growth of the attenuated bacteria used in our experiment, we challenged EPOR−/−rescue and littermate control mice with a virulent strain, SL1344. Again, there was no significant difference in bacterial loads in the liver or spleen between EPOR−/−rescue and littermate control mice (Fig. 4B). Thus, the expression of EPOR on nonerythroid cells does not appear to adversely affect the growth or survival of attenuated or virulent Salmonella in vivo.
FIG 4.
EPO does not appear to act on nonerythroid cell lineages to modulate host protective immunity against Salmonella. EpoR−/−rescue mice and littermate controls were infected with either 5 × 105 CFU of an attenuated Salmonella strain (BRD509) i.v. (A) or 500 CFU of a virulent Salmonella strain (SL1344) i.p. (B). Spleens and livers were recovered at 1, 2, and 3 weeks post-BRD509 infection or at 5 days post-SL1344 infection. The bacterial counts per organ were determined by serial dilution and plating. No statistical significance was determined.
Depletion of erythroid cells increases EPO production and bacterial growth.
Previous studies have shown that SIRPα-deficient (Sirpα−/−) mice exhibit enhanced basal erythropoiesis and display enhanced susceptibility to Salmonella infection (46). Given the correlation between EPO levels and susceptibility in Salmonella-infected wild-type mice, we examined EPO levels in SIRPα-deficient mice. EPO levels were markedly elevated in the sera of Salmonella-infected SIRPα-deficient mice (Fig. 5), thus providing further evidence to link circulating EPO levels with enhanced Salmonella susceptibility. As noted above, our laboratory and others previously demonstrated that neutralization of EPO reduces bacterial growth (33, 52). Since the experiments using EPOR−/−rescue mice described above suggested that any effect of EPO most likely involves erythroid responsiveness to EPO, we decided to examine whether depletion of erythroid cells could enhance bacterial clearance.
FIG 5.
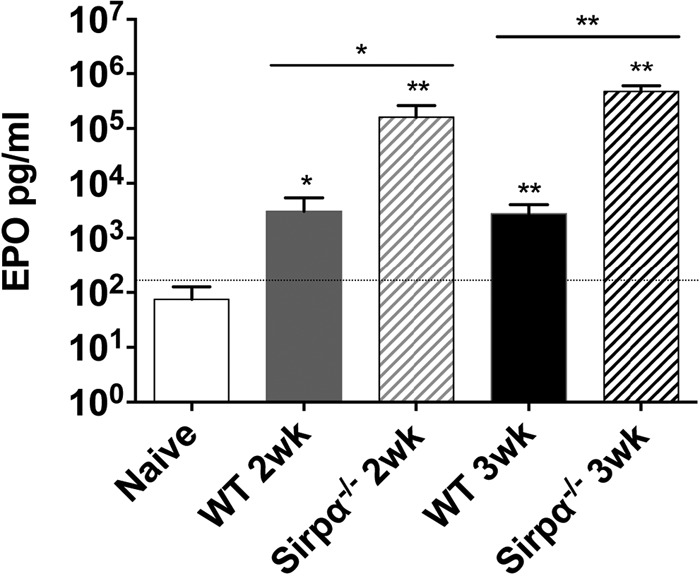
The EPO level is highly elevated in SIRPα-deficient mice after Salmonella infection. Blood EPO levels in wild-type and Sirpα−/− mice 2 and 3 weeks after infection with an attenuated Salmonella strain (BRD509) were measured by an ELISA. Error bars represent the means ± standard errors of the means. *, P < 0.05; **, P < 0.01 (by an unpaired t test).
A high-affinity SIRPα variant (CV1) has been developed and used as a therapeutic to enhance tumor-specific monoclonal antibodies (48). CV1 antagonizes the “do not eat me” signal delivered by CD47 and thus has the effect of enhancing macrophage phagocytosis of CD47-expressing tumor cells. Since this same CD47 signal regulates erythroid homeostasis in the spleen, we injected Salmonella-infected C57BL/6 mice with CV1 and examined the effect on erythroid expansion. While Salmonella-infected mice displayed a marked expansion of erythroblasts (CD71+ Ter119+) and mature red blood cells (CD71− Ter119+) in the spleen, this expansion was curtailed in Salmonella-infected mice administered CV1 (Fig. 6A). Thus, CV1 effectively prevented infection-induced splenic erythroid expansion by antagonizing CD47. Since EPO production and erythroid expansion correlate with enhanced bacterial persistence (33, 52), our expectation was that this CV1-mediated reduction in erythroid expansion would limit bacterial growth. However, mice injected with CV1 actually displayed significantly higher bacterial burdens in the spleen despite the fact that extramedullary erythropoiesis was effectively curtailed (Fig. 6B). A possible explanation for this increased bacterial growth was that CV1-injected mice displayed elevated levels of EPO in the circulation (Fig. 6C). Thus, directly blocking erythroid expansion or the availability of EPO itself has differential effects on bacterial growth in vivo.
FIG 6.
Targeted depletion of erythrocytes from the spleen increases host susceptibility to Salmonella. C57BL/6 mice were infected i.v. with 5 × 105 CFU of an attenuated Salmonella strain (BRD509). Cohorts of mice were treated with high-affinity SIRPα variants (CV1) on day 3 and day 6 after infection. (A) Percentages and total cell numbers of RBCs (Ter119+ CD71−), reticulocytes (Ter119+ CD71+), and proerythroblasts (Ter119med CD71+) in the spleens of naive, Salmonella-infected, and Salmonella-infected and CV1-treated mice as measured by flow cytometry. (B) Bacterial counts in the spleens and livers of Salmonella-infected mice at 7 days postinfection with or without CV1 treatment. (C) Blood EPO levels in naive, Salmonella-infected, and Salmonella-infected and CV1-treated mice as measured by an ELISA. An unpaired t test was used to calculate statistical significance for panels A to C. Error bars represent the means ± standard errors of the means. *, P < 0.05; **, P < 0.01; n.d., not detected.
DISCUSSION
Salmonella infection of inbred mice causes profound enlargement and increased cellularity of the spleen, which is sometimes ascribed to the proliferation of innate and adaptive immune cells responding to infection. Although the numbers of splenic macrophages, NK cells, dendritic cells, T cells, and B cells increase during Salmonella infection, the largest increase is actually found among erythroid cells, which expand to account for 80 to 85% of the spleen at the peak of infection (33). This extramedullary erythropoiesis causes a large expansion of the red pulp, which disrupts the splenic architecture and has the potential to influence innate and adaptive immunity to infection (53). The relationship between Salmonella infection, extramedullary erythropoiesis, and host immunity therefore represents an important but poorly understood component of the host-pathogen dynamic.
Previous studies have shown that Toll-like receptor (TLR) recognition of Salmonella increases EPO production and red blood cell expansion and that EPO neutralization alone can decrease bacterial growth (33, 52). Our current data confirm that extramedullary erythropoiesis is a consequence of Salmonella infection and further show that this expansion is accompanied by a reduction in circulating red cells. Furthermore, these erythroid alterations in the spleen are not a direct consequence of the type III secretion system encoded by SPI2 or bacterial expression of flagellin. Thus, erythroid dysregulation is induced in the spleen by virulent or highly attenuated Salmonella and is likely a direct consequence of host recognition of bacterial pathogen-associated molecular patterns (PAMPs) (35, 36). In support of this idea, injection of mice with LPS alone also induced splenomegaly and extramedullary erythropoiesis. This finding conflicts somewhat with previously reported data showing that LPS or the cytokines gamma interferon (IFN-γ) and interleukin-1 (IL-1) can inhibit circulating EPO and EPO mRNA production (54–56). Conversely, our data show that the levels of EPO mRNA in the kidney and liver were markedly elevated during Salmonella infection, suggesting that these two organs are the primary sources of elevated EPO levels. Indeed, both of these organs were previously shown to account for elevated EPO levels in response to reduced oxygen tension (52, 57). As expected, the EPO mRNA level was not increased in primary or secondary lymphoid tissues during infection. It is not yet clear whether kidney and liver cells are responding to hypoxia during Salmonella infection or whether EPO is enhanced by the detection of PAMPs or inflammatory cytokines. The fact that EPO production is highly dependent on TLR signaling (33) suggests that the latter pathway may be more important than hypoxia. Whatever the stimulus, the erythroid dysfunction detected in the spleen during Salmonella infection is likely to be driven by elevated EPO production from the kidney and liver.
It is clear from previous studies that TLR-dependent EPO production drives splenic erythropoiesis and that this correlates with enhanced bacterial growth (33, 52). However, the actual mechanism accounting for enhanced bacterial growth is substantially less clear. One possibility comes from the understanding that EPO has pleiotropic effects on a broad range of cell types in vivo (52). Indeed, various effects of EPO and/or EPOR expression on T cells, B cells, neutrophils, dendritic cells, and macrophages have been reported (52, 58). Most importantly, EPO was found to have direct inhibitory effects on macrophage killing of Salmonella in vitro (34). Furthermore, mice that lacked EPOR expression on nonerythroid cells were able to control bacterial growth more efficiently (34). Indeed, some of our data add some support to this mechanism since CV1 treatment effectively blocked splenic erythrocyte expansion while maintaining high levels of circulating EPO, and the consequence was enhanced bacterial growth. Similarly, our data demonstrate that circulating EPO levels are elevated in SIRPα-deficient mice, which also display enhanced susceptibility to Salmonella (46). Together, these findings reinforce the idea that EPO, rather than extramedullary erythropoiesis, is the critical variable for inhibiting bacterial clearance. Importantly, however, we were unable to detect any difference in the bacterial burdens in wild-type and EPOR−/−rescue mice, which lack the expression of EPO on nonerythroid cells. This negative result was confirmed by using both attenuated and virulent bacteria, neither of which displayed enhanced clearance in EPOR−/−rescue mice. Thus, this data set conflicts with a model where EPOR expression by nonerythroid cells would be responsible for encouraging Salmonella growth in vivo. Although our mice were obtained from the same source as the mice used in a previous study, it remains possible that minor differences in bacterial strains, infection doses, or host microbiota could account for the divergent results. Indeed, it should also be noted that EPO was previously reported to have proinflammatory and anti-inflammatory effects on cell signaling and cytokine production, and thus, small alterations in the experimental setup between these two studies could have accentuated these effects.
Overall, our data are consistent with a model where (i) increased EPO production is responsible for encouraging Salmonella persistence and (ii) the primary effect of EPO is on cells of an erythroid lineage but that (iii) erythroid cell expansion in the spleen is not absolutely required to encourage bacterial growth. A potential model that focuses more on erythroid cell responsiveness to EPO can be generated from the understanding that extramedullary erythropoiesis encourages splenic hemophagocytosis, a pathological process whereby local macrophages engulf red blood cells and lymphocytes (59, 60). The process of hemophagocytosis can be induced by injection of mice with heat-killed Brucella or a variety of TLR ligands (61–63). Indeed, a recent report showed that stimulation of macrophages with heat-killed Salmonella or LPS induces hemophagocytosis (64).
Hemophagocytic macrophages display an anti-inflammatory phenotype and appear to be permissive for Salmonella growth in a mouse model of chronic Salmonella infection (65). Thus, any increase in the number of hemophagocytes would be expected to enhance bacterial persistence in vivo. While conceptually appealing, some of our data indicate that this model may not fully explain the effect of EPO and erythropoiesis on bacterial growth. In particular, our use of the high-affinity SIRPα variant (CV1) demonstrates that splenomegaly and erythroblast formation can be blocked in the spleen during Salmonella infection and that this correlates with enhanced bacterial growth. However, it seems possible that CV1 would enhance erythrocyte uptake by macrophages and therefore increase the number of hemophagocytes in the spleen. Since hemophagocytes have an anti-inflammatory phenotype (65), any expansion in the number of these cells should provide a niche for enhanced bacterial growth. Mice infected with Salmonella also show a loss of iron from the spleen and high levels of expression of ferroportin-1 on macrophages (66), which should limit the availability of iron for intracellular bacterial growth. The enhancement of bacterial growth after CV1 treatment could therefore reflect an increased access of Salmonella to iron as a consequence of hemophagocytosis (67).
In addition to the contribution of hemophagocytes to bacterial growth, EPO is also known to have effects on erythroid cells other than driving erythroid expansion. In particular, EPO can affect iron homeostasis as a consequence of modulating transferrin receptor expression in erythroid cells. In human and murine erythroid cell lines, EPO was shown to increase transferrin receptor expression on the cell surface and consequently to increase iron uptake into cells (68). Such iron sequestration by erythroid cells could deliver more iron to hemophagocytes and thus compensate for reduced erythroid expansion during CV1 treatment. Overall, we suggest that the critical variables for understanding the effect of EPO on Salmonella growth might relate to the ability of EPO to increase hemophagocytosis and the availability of iron for subsequent bacterial growth.
Together, our data show that both Salmonella and LPS induce extramedullary erythropoiesis and splenomegaly. Furthermore, we show that mice lacking EPOR expression on nonerythroid cells do not display heightened susceptibility to infection but that directly reducing circulating erythrocytes increased EPO production and encouraged bacterial persistence. Thus, interventions that modulate erythroid development can have both positive and negative consequences for host immunity to Salmonella infection.
ACKNOWLEDGMENTS
We thank the UC Davis Comparative Pathology Laboratory for PCV analysis and the members of the McSorley laboratory for helpful discussions.
Funding Statement
K.W. and K.C.G. declare U.S. patent applications pertaining to CD47-blocking therapies for human disease, including “High affinity SIRP alpha reagents.” K.W. declares consulting and/or equity ownership in Forty Seven Inc. and Alexo Therapeutics, Inc. K.C.G. declares consulting and/or equity ownership in Alexo Therapeutics, Inc.
REFERENCES
- 1.Anonymous. 2007. Multistate outbreaks of Salmonella infections associated with raw tomatoes eaten in restaurants—United States, 2005-2006. MMWR Morb Mortal Wkly Rep 56:909–911. [PubMed] [Google Scholar]
- 2.Anonymous. 2008. Outbreak of Salmonella serotype Saintpaul infections associated with multiple raw produce items—United States, 2008. MMWR Morb Mortal Wkly Rep 57:929–934. [PubMed] [Google Scholar]
- 3.Anonymous. 2009. Multistate outbreak of Salmonella infections associated with peanut butter and peanut butter-containing products—United States, 2008-2009. MMWR Morb Mortal Wkly Rep 58:85–90. [PubMed] [Google Scholar]
- 4.Jones BD, Falkow S. 1996. Salmonellosis: host immune responses and bacterial virulence determinants. Annu Rev Immunol 14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 5.Crump JA, Mintz ED. 2010. Global trends in typhoid and paratyphoid fever. Clin Infect Dis 50:241–246. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keestra-Gounder AM, Tsolis RM, Baumler AJ. 2015. Now you see me, now you don't: the interaction of Salmonella with innate immune receptors. Nat Rev Microbiol 13:206–216. doi: 10.1038/nrmicro3428. [DOI] [PubMed] [Google Scholar]
- 7.Fraser A, Paul M, Goldberg E, Acosta CJ, Leibovici L. 2007. Typhoid fever vaccines: systematic review and meta-analysis of randomised controlled trials. Vaccine 25:7848–7857. doi: 10.1016/j.vaccine.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 8.McGregor AC, Waddington CS, Pollard AJ. 2013. Prospects for prevention of Salmonella infection in children through vaccination. Curr Opin Infect Dis 26:254–262. doi: 10.1097/QCO.0b013e32835fb829. [DOI] [PubMed] [Google Scholar]
- 9.Gordon MA, Kankwatira AM, Mwafulirwa G, Walsh AL, Hopkins MJ, Parry CM, Faragher EB, Zijlstra EE, Heyderman RS, Molyneux ME. 2010. Invasive non-typhoid salmonellae establish systemic intracellular infection in HIV-infected adults: an emerging disease pathogenesis. Clin Infect Dis 50:953–962. doi: 10.1086/651080. [DOI] [PubMed] [Google Scholar]
- 10.Morpeth SC, Ramadhani HO, Crump JA. 2009. Invasive non-Typhi Salmonella disease in Africa. Clin Infect Dis 49:606–611. doi: 10.1086/603553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McSorley SJ. 2014. Immunity to intestinal pathogens: lessons learned from Salmonella. Immunol Rev 260:168–182. doi: 10.1111/imr.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos RL, Zhang S, Tsolis RM, Kingsley RA, Adams LG, Baumler AJ. 2001. Animal models of salmonella infections: enteritis versus typhoid fever. Microbes Infect 3:1335–1344. doi: 10.1016/S1286-4579(01)01495-2. [DOI] [PubMed] [Google Scholar]
- 13.Griffin AJ, McSorley SJ. 2011. Development of protective immunity to Salmonella, a mucosal pathogen with a systemic agenda. Mucosal Immunol 4:371–382. doi: 10.1038/mi.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsolis RM, Xavier MN, Santos RL, Baumler AJ. 2011. How to become a top model: impact of animal experimentation on human Salmonella disease research. Infect Immun 79:1806–1814. doi: 10.1128/IAI.01369-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tam MA, Rydstrom A, Sundquist M, Wick MJ. 2008. Early cellular responses to Salmonella infection: dendritic cells, monocytes, and more. Immunol Rev 225:140–162. doi: 10.1111/j.1600-065X.2008.00679.x. [DOI] [PubMed] [Google Scholar]
- 16.Hess J, Ladel C, Miko D, Kaufmann SH. 1996. Salmonella typhimurium aroA− infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J Immunol 156:3321–3326. [PubMed] [Google Scholar]
- 17.Lee SJ, Dunmire S, McSorley SJ. 2012. MHC class-I-restricted CD8 T cells play a protective role during primary Salmonella infection. Immunol Lett 148:138–143. doi: 10.1016/j.imlet.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Donnell H, Pham OH, Li LX, Atif SM, Lee SJ, Ravesloot MM, Stolfi JL, Nuccio SP, Broz P, Monack DM, Baumler AJ, McSorley SJ. 2014. Toll-like receptor and inflammasome signals converge to amplify the innate bactericidal capacity of T helper 1 cells. Immunity 40:213–224. doi: 10.1016/j.immuni.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McSorley SJ, Jenkins MK. 2000. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar Typhimurium. Infect Immun 68:3344–3348. doi: 10.1128/IAI.68.6.3344-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mastroeni P, Simmons C, Fowler R, Hormaeche CE, Dougan G. 2000. Igh-6−/− (B-cell-deficient) mice fail to mount solid acquired resistance to oral challenge with virulent Salmonella enterica serovar Typhimurium and show impaired Th1 T-cell responses to Salmonella antigens. Infect Immun 68:46–53. doi: 10.1128/IAI.68.1.46-53.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barr TA, Brown S, Mastroeni P, Gray D. 2009. B cell intrinsic MyD88 signals drive IFN-gamma production from T cells and control switching to IgG2c. J Immunol 183:1005–1012. doi: 10.4049/jimmunol.0803706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nanton MR, Way SS, Shlomchik MJ, McSorley SJ. 2012. Cutting edge: B cells are essential for protective immunity against Salmonella independent of antibody secretion. J Immunol 189:5503–5507. doi: 10.4049/jimmunol.1201413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hornef MW, Wick MJ, Rhen M, Normark S. 2002. Bacterial strategies for overcoming host innate and adaptive immune responses. Nat Immunol 3:1033–1040. doi: 10.1038/ni1102-1033. [DOI] [PubMed] [Google Scholar]
- 24.Bueno SM, Gonzalez PA, Schwebach JR, Kalergis AM. 2007. T cell immunity evasion by virulent Salmonella enterica. Immunol Lett 111:14–20. doi: 10.1016/j.imlet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Bedoui S, Kupz A, Wijburg OL, Walduck AK, Rescigno M, Strugnell RA. 2010. Different bacterial pathogens, different strategies, yet the aim is the same: evasion of intestinal dendritic cell recognition. J Immunol 184:2237–2242. doi: 10.4049/jimmunol.0902871. [DOI] [PubMed] [Google Scholar]
- 26.Kullas AL, McClelland M, Yang HJ, Tam JW, Torres A, Porwollik S, Mena P, McPhee JB, Bogomolnaya L, Andrews-Polymenis H, van der Velden AW. 2012. l-Asparaginase II produced by Salmonella Typhimurium inhibits T cell responses and mediates virulence. Cell Host Microbe 12:791–798. doi: 10.1016/j.chom.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abrahams GL, Hensel M. 2006. Manipulating cellular transport and immune responses: dynamic interactions between intracellular Salmonella enterica and its host cells. Cell Microbiol 8:728–737. doi: 10.1111/j.1462-5822.2006.00706.x. [DOI] [PubMed] [Google Scholar]
- 28.Srinivasan A, Nanton M, Griffin A, McSorley SJ. 2009. Culling of activated CD4 T cells during typhoid is driven by Salmonella virulence genes. J Immunol 182:7838–7845. doi: 10.4049/jimmunol.0900382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nanton MR, Lee SJ, Atif SM, Nuccio SP, Taylor JJ, Baumler AJ, Way SS, McSorley SJ. 2015. Direct visualization of endogenous Salmonella-specific B cells reveals a marked delay in clonal expansion and germinal center development. Eur J Immunol 45:428–441. doi: 10.1002/eji.201444540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cummings LA, Wilkerson WD, Bergsbaken T, Cookson BT. 2006. In vivo, fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol Microbiol 61:795–809. doi: 10.1111/j.1365-2958.2006.05271.x. [DOI] [PubMed] [Google Scholar]
- 31.McSorley SJ, Cookson BT, Jenkins MK. 2000. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J Immunol 164:986–993. doi: 10.4049/jimmunol.164.2.986. [DOI] [PubMed] [Google Scholar]
- 32.Letran SE, Lee SJ, Atif SM, Uematsu S, Akira S, McSorley SJ. 2011. TLR5 functions as an endocytic receptor to enhance flagellin-specific adaptive immunity. Eur J Immunol 41:29–38. doi: 10.1002/eji.201040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson A, Nanton MR, O'Donnell H, Akue AD, McSorley SJ. 2010. Innate immune activation during Salmonella infection initiates extramedullary erythropoiesis and splenomegaly. J Immunol 185:6198–6204. doi: 10.4049/jimmunol.1001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nairz M, Schroll A, Moschen AR, Sonnweber T, Theurl M, Theurl I, Taub N, Jamnig C, Neurauter D, Huber LA, Tilg H, Moser PL, Weiss G. 2011. Erythropoietin contrastingly affects bacterial infection and experimental colitis by inhibiting nuclear factor-kappaB-inducible immune pathways. Immunity 34:61–74. doi: 10.1016/j.immuni.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fruhman GJ. 1966. Bacterial endotoxin: effects on erythropoiesis. Blood 27:363–370. [PubMed] [Google Scholar]
- 36.Reissmann KR, Udupa KB, Labedzki L. 1976. Induction of erythroid colony forming cells (CFU-E) in murine spleen by endotoxin. Proc Soc Exp Biol Med 153:98–101. doi: 10.3181/00379727-153-39488. [DOI] [PubMed] [Google Scholar]
- 37.Staber FG, Metcalf D. 1980. Cellular and molecular basis of the increased splenic hemopoiesis in mice treated with bacterial cell wall components. Proc Natl Acad Sci U S A 77:4322–4325. doi: 10.1073/pnas.77.7.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyland L, Villarreal-Ramos B, Clarke B, Baaten B, Hou S. 2005. Bone marrow immunosuppression in Salmonella-infected mice is prolonged following influenza virus infection. Exp Hematol 33:1477–1485. doi: 10.1016/j.exphem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Koury MJ, Sawyer ST, Brandt SJ. 2002. New insights into erythropoiesis. Curr Opin Hematol 9:93–100. doi: 10.1097/00062752-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Elliott S, Pham E, Macdougall IC. 2008. Erythropoietins: a common mechanism of action. Exp Hematol 36:1573–1584. doi: 10.1016/j.exphem.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Socolovsky M, Gross AW, Lodish HF. 2003. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood 102:3938–3946. doi: 10.1182/blood-2003-05-1479. [DOI] [PubMed] [Google Scholar]
- 42.Gordon AR, Outram SV, Keramatipour M, Goddard CA, Colledge WH, Metcalfe JC, Hager-Theodorides AL, Crompton T, Kemp PR. 2008. Splenomegaly and modified erythropoiesis in KLF13−/− mice. J Biol Chem 283:11897–11904. doi: 10.1074/jbc.M709569200. [DOI] [PubMed] [Google Scholar]
- 43.Broxmeyer HE. 2011. Erythropoietin surprises: an immune saga. Immunity 34:6–7. doi: 10.1016/j.immuni.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Barclay AN. 2009. Signal regulatory protein alpha (SIRPalpha)/CD47 interaction and function. Curr Opin Immunol 21:47–52. doi: 10.1016/j.coi.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishikawa-Sekigami T, Kaneko Y, Okazawa H, Tomizawa T, Okajo J, Saito Y, Okuzawa C, Sugawara-Yokoo M, Nishiyama U, Ohnishi H, Matozaki T, Nojima Y. 2006. SHPS-1 promotes the survival of circulating erythrocytes through inhibition of phagocytosis by splenic macrophages. Blood 107:341–348. doi: 10.1182/blood-2005-05-1896. [DOI] [PubMed] [Google Scholar]
- 46.Li LX, Atif SM, Schmiel SE, Lee SJ, McSorley SJ. 2012. Increased susceptibility to Salmonella infection in signal regulatory protein alpha-deficient mice. J Immunol 189:2537–2544. doi: 10.4049/jimmunol.1200429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strugnell R, Dougan G, Chatfield S, Charles I, Fairweather N, Tite J, Li JL, Beesley J, Roberts M. 1992. Characterization of a Salmonella typhimurium aro vaccine strain expressing the P.69 antigen of Bordetella pertussis. Infect Immun 60:3994–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiskopf K, Ring AM, Ho CC, Volkmer JP, Levin AM, Volkmer AK, Ozkan E, Fernhoff NB, van de Rijn M, Weissman IL, Garcia KC. 2013. Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science 341:88–91. doi: 10.1126/science.1238856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown DE, McCoy MW, Pilonieta MC, Nix RN, Detweiler CS. 2010. Chronic murine typhoid fever is a natural model of secondary hemophagocytic lymphohistiocytosis. PLoS One 5:e9441. doi: 10.1371/journal.pone.0009441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salazar-Gonzalez RM, Srinivasan A, Griffin A, Muralimohan G, Ertelt JM, Ravindran R, Vella AT, McSorley SJ. 2007. Salmonella flagellin induces bystander activation of splenic dendritic cells and hinders bacterial replication in vivo. J Immunol 179:6169–6175. doi: 10.4049/jimmunol.179.9.6169. [DOI] [PubMed] [Google Scholar]
- 51.Hattangadi SM, Wong P, Zhang L, Flygare J, Lodish HF. 2011. From stem cell to red cell: regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications. Blood 118:6258–6268. doi: 10.1182/blood-2011-07-356006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nairz M, Sonnweber T, Schroll A, Theurl I, Weiss G. 2012. The pleiotropic effects of erythropoietin in infection and inflammation. Microbes Infect 14:238–246. doi: 10.1016/j.micinf.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McSorley SJ, Asch S, Costalonga M, Rieinhardt RL, Jenkins MK. 2002. Tracking Salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity 16:365–377. doi: 10.1016/S1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 54.Jelkmann W. 1998. Proinflammatory cytokines lowering erythropoietin production. J Interferon Cytokine Res 18:555–559. doi: 10.1089/jir.1998.18.555. [DOI] [PubMed] [Google Scholar]
- 55.Vannucchi AM, Grossi A, Rafanelli D, Statello M, Cinotti S, Rossi-Ferrini P. 1994. Inhibition of erythropoietin production in vitro by human interferon gamma. Br J Haematol 87:18–23. doi: 10.1111/j.1365-2141.1994.tb04864.x. [DOI] [PubMed] [Google Scholar]
- 56.Frede S, Fandrey J, Pagel H, Hellwig T, Jelkmann W. 1997. Erythropoietin gene expression is suppressed after lipopolysaccharide or interleukin-1 beta injections in rats. Am J Physiol 273:R1067–R1071. [DOI] [PubMed] [Google Scholar]
- 57.Boutin AT, Weidemann A, Fu Z, Mesropian L, Gradin K, Jamora C, Wiesener M, Eckardt KU, Koch CJ, Ellies LG, Haddad G, Haase VH, Simon MC, Poellinger L, Powell FL, Johnson RS. 2008. Epidermal sensing of oxygen is essential for systemic hypoxic response. Cell 133:223–234. doi: 10.1016/j.cell.2008.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lifshitz L, Prutchi-Sagiv S, Avneon M, Gassmann M, Mittelman M, Neumann D. 2009. Non-erythroid activities of erythropoietin: functional effects on murine dendritic cells. Mol Immunol 46:713–721. doi: 10.1016/j.molimm.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 59.Nix RN, Altschuler SE, Henson PM, Detweiler CS. 2007. Hemophagocytic macrophages harbor Salmonella enterica during persistent infection. PLoS Pathog 3:e193. doi: 10.1371/journal.ppat.0030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silva-Herzog E, Detweiler CS. 2008. Intracellular microbes and haemophagocytosis. Cell Microbiol 10:2151–2158. doi: 10.1111/j.1462-5822.2008.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gardenghi S, Renaud TM, Meloni A, Casu C, Crielaard BJ, Bystrom LM, Greenberg-Kushnir N, Sasu BJ, Cooke KS, Rivella S. 2014. Distinct roles for hepcidin and interleukin-6 in the recovery from anemia in mice injected with heat-killed Brucella abortus. Blood 123:1137–1145. doi: 10.1182/blood-2013-08-521625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Behrens EM, Canna SW, Slade K, Rao S, Kreiger PA, Paessler M, Kambayashi T, Koretzky GA. 2011. Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J Clin Invest 121:2264–2277. doi: 10.1172/JCI43157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohyagi H, Onai N, Sato T, Yotsumoto S, Liu J, Akiba H, Yagita H, Atarashi K, Honda K, Roers A, Muller W, Kurabayashi K, Hosoi-Amaike M, Takahashi N, Hirokawa M, Matsushima K, Sawada K, Ohteki T. 2013. Monocyte-derived dendritic cells perform hemophagocytosis to fine-tune excessive immune responses. Immunity 39:584–598. doi: 10.1016/j.immuni.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 64.McDonald EM, Pilonieta MC, Nick HJ, Detweiler CS. 2016. Bacterial stimulation of Toll-like receptor 4 drives macrophages to hemophagocytose. Infect Immun 84:47–55. doi: 10.1128/IAI.01149-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCoy MW, Moreland SM, Detweiler CS. 2012. Hemophagocytic macrophages in murine typhoid fever have an anti-inflammatory phenotype. Infect Immun 80:3642–3649. doi: 10.1128/IAI.00656-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown DE, Nick HJ, McCoy MW, Moreland SM, Stepanek AM, Benik R, O'Connell KE, Pilonieta MC, Nagy TA, Detweiler CS. 2015. Increased ferroportin-1 expression and rapid splenic iron loss occur with anemia caused by Salmonella enterica serovar Typhimurium infection in mice. Infect Immun 83:2290–2299. doi: 10.1128/IAI.02863-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagy TA, Moreland SM, Detweiler CS. 2014. Salmonella acquires ferrous iron from haemophagocytic macrophages. Mol Microbiol 93:1314–1326. doi: 10.1111/mmi.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weiss G, Houston T, Kastner S, Johrer K, Grunewald K, Brock JH. 1997. Regulation of cellular iron metabolism by erythropoietin: activation of iron-regulatory protein and upregulation of transferrin receptor expression in erythroid cells. Blood 89:680–687. [PubMed] [Google Scholar]