Abstract
Giardia duodenalis is a noninvasive luminal pathogen that impairs digestive function in its host in part by reducing intestinal disaccharidase activity. This enzyme deficiency has been shown in mice to require CD8+ T cells. We recently showed that both host immune responses and parasite strain affected disaccharidase levels during murine giardiasis. However, high doses of antibiotics were used to facilitate infections in that study, and we therefore decided to systematically examine the effects of antibiotic use on pathogenesis and immune responses in the mouse model of giardiasis. We found that antibiotic treatment did not overtly increase the parasite burden but significantly limited the disaccharidase deficiency observed in infected mice. Moreover, while infected mice had more activated CD8+ αβ T cells in the small intestinal lamina propria, this increase was absent in antibiotic-treated mice. Infection also led to increased numbers of CD4+ αβ T cells in the lamina propria and activation of T cell receptor γδ-expressing intraepithelial lymphocytes (IEL), but these changes were not affected by antibiotics. Finally, we show that activated CD8+ T cells express gamma interferon (IFN-γ) and granzymes but that granzymes are not required for sucrase deficiency. We conclude that CD8+ T cells become activated in giardiasis through an antibiotic-sensitive process and contribute to reduced sucrase activity. These are the first data directly demonstrating activation of CD8+ T cells and γδ T cells during Giardia infections. These data also demonstrate that disruption of the intestinal microbiota by antibiotic treatment prevents pathological CD8+ T cell activation in giardiasis.
INTRODUCTION
The protozoan Giardia duodenalis is a major cause of parasitic diarrheal disease worldwide. Infection with G. duodenalis provides an interesting model for studying mucosal immunity, as some of the immunopathology observed in human patients and infected animals resembles that of common noninfectious intestinal disorders. The reduction of intestinal disaccharidase enzymes, for example, is a pathological hallmark of giardiasis and is also commonly observed in gastroenteritis, celiac disease, ulcerative colitis, and Crohn's disease patients (1–4). Therefore, there are likely overlapping mechanisms involved. The reduction of disaccharidase enzymes in giardiasis results from a shortening of the intestinal epithelial microvilli structures and reflects a general impairment of digestion and nutrient absorption (5–7). We have demonstrated that wild-type mice exhibit reduced disaccharidase activity following infection with G. duodenalis but that CD4−/− and β2-microglobulin−/− (β2m−/−) mice do not (8). Another study has demonstrated that the adoptive transfer of purified CD8+ T cells, but not CD4+ T cells, from Giardia muris-infected mice into athymic naive recipients is sufficient to recapitulate shortening of microvilli and intestinal disaccharidase reduction (7). Collectively, these data suggest that CD8+ T cells are involved in the pathological reduction of intestinal disaccharidases following infection with Giardia, while CD4+ T cells likely contribute to activation of the CD8+ T cells. While increased numbers of intraepithelial lymphocytes (IEL) or CD8+ T cells within the intestines of animal and human patients have been reported in giardiasis, little is known about the activation or effector phenotype(s) of these infiltrating lymphocytes (9, 10).
The intestinal microbiota is an important regulator of the underlying mucosal immune system and plays a role in giardiasis. The commensal composition of mice is a determinant of resistance or susceptibility to infection with G. duodenalis (11, 12). RAG-deficient mice obtained from one commercial vendor are resistant to infection with G. duodenalis, while mice of the same strain obtained from another vendor are susceptible (11). Further, antibiotic treatment rendered Taconic Farm mice susceptible to infection, while cohousing untreated mice renders them all resistant to Giardia infection. Commensal bacteria secrete factors that are inhibitory to G. duodenalis growth in culture (13). Probiotic treatment of mice and gerbils reduces cyst shedding and pathological markers in giardiasis (14, 15). The microbiota has profound effects on intestinal T cells as well. TH17 cell development has been linked to the presence of segmented filamentous bacteria (16), while clostridial species induce the development of regulatory Treg cells (17). Similarly, antibiotic-treated mice exhibit impaired CD4+ and CD8+ lung T cell responses to influenza virus (18). Recently, germfree and antibiotic-treated mice infected with Salmonella showed enhanced infection and increased numbers of gamma interferon (IFN-γ)-producing lymphocytes in the mesenteric lymph nodes (19). The mechanisms whereby changes in the intestinal microbiota effect Giardia infection remain unclear.
In this report we address the role of the intestinal microbiota in facilitating immune responses in giardiasis. Antibiotic-treated mice exhibited an unaltered parasite burden compared to that in untreated mice. Despite this, antibiotic-treated mice exhibited less disaccharidase deficiency. We then used flow cytometry analysis to measure intestinal T cells during infection and determined whether these cells are activated and, if so, which subsets are activated. We report that the abundance of CD4+ T cells expressing αβ T cell receptors (TCR) increases in the lamina propria (LP) at 7 days postinfection and that while the abundance of CD8+ αβ T cells does not increase, they acquire an effector (CD44hi CD69hi) phenotype. Various subsets of γδ IEL were also activated in infected mice. Disruption of the intestinal microbiota with antibiotics impaired CD8+ T cell activation but not activation of γδ IEL or the accumulation of CD4+ T cells. Thus, G. duodenalis infection leads to CD8+ T cell activation via a mechanism involving commensal bacteria. Bridging the gap between T cell activation and the immunopathology that results from it during Giardia infection may provide insights into the etiology and mechanisms of noninfectious intestinal disorders and offer novel therapeutic approaches.
MATERIALS AND METHODS
Parasites.
G. duodenalis strain GS/M-83-H7 was obtained from the ATCC, Manassas, VA (catalog no. 50581). Trophozoites were cultured in standard TYI-S-33 medium supplemented with bovine bile, l-cysteine, ascorbic acid, and an antibiotic-antimycotic solution (Sigma-Aldrich, St. Louis, MO) (20, 21). Prior to infection, the parasites were detached from culture flasks by icing in phosphate-buffered saline (PBS) for 15 min. The parasites were washed three times in ice-cold PBS, and 1 × 106 parasites in 0.1 ml PBS/mouse were gavaged into mice.
Mice.
C57BL/6, 129X1/SvJ, B6.129 P2-Tcrbtm1/Mom/J, B6.129 P2-Tcrdtm1/Mom/J, and 129X1/SvJ-Gzmatm1Ley Gzmbtm2.1Ley/J mice, between 6 and 8 weeks of age, were obtained from Jackson Laboratory (Bar Harbor, ME). All experiments were conducted in adherence to animal protocols approved by the Animal Care and Use Committees of Georgetown University in accordance with the National Institutes of Health guidelines. Antibiotics were used to alter the commensal microbiota without affecting the parasite. Some mice were therefore given drinking water containing 1.4 mg/ml neomycin oral solution (Durvet, Blue Spring, MO), 1 mg/ml ampicillin (Sigma-Aldrich, St. Louis, MO), and 1 mg/ml vancomycin (Hospira, Lake Forest, IL) 2 days prior to infection, and this regimen was maintained throughout the course of infection. These antibiotics are not expected to effect Giardia at these doses.
Tissue preparations.
Upon euthanasia, a 10-cm duodenal segment was obtained and pooled from all 4 mice belonging to each experimental group. The pooled duodena were then fractionated into LP and IEL using a previously described method (22). Briefly, the Peyer's patches were removed, and the remaining intestinal fragments were washed with dithioerythritol (DTE) in order to obtain IEL. EDTA washes were used to strip off the epithelium, leaving an intact LP fraction to be digested with liberase TL (Roche, Switzerland). IEL and LP suspensions were then separated on a discontinuous Percoll (Sigma-Aldrich, St. Louis, MO) gradient in order to enrich for lymphocytes and remove dead cells. The mesenteric lymph nodes (MLNs) were collected in Hanks' balanced saline solution (HBSS) supplemented with 5% fetal bovine serum (FBS) and 25 mM HEPES and passed through a 70-μm strainer.
Flow cytometry.
Fluorophore-conjugated antibodies against CD3ε, TCRβ, CD4, CD8α, CD44, CD69, TRAIL, FasL, granzyme A, and granzyme B were obtained from BioLegend (San Diego, CA). LIVE/DEAD fixable yellow stain was obtained from Invitrogen (Carlsbad, CA). Lymphocytes (5 × 106) were washed in PBS and stained with LIVE/DEAD stain for 45 min at 4°C in the dark. The cells were then washed in PBS and stained with the appropriate antibodies for 1 h at 4°C in the dark. Antibody-labeled cells were washed in PBS with 4% FBS and fixed in 4% paraformaldehyde for 20 min in the dark at room temperature (RT). The cells were then washed again in PBS with 4% FBS. The stained cells were analyzed using a Becton Dickinson FACStar Plus dual-laser system (Becton Dickinson, Franklin Lakes, NJ) and FCS Express version 4.0 software from DeNovo Software (Los Angeles, CA). Live lymphocyte populations were selected by gating on forward and side scatter along with LIVE/DEAD-negative cells (see Fig. S1A in the supplemental material).
Sucrase activity assay.
Sucrase activity was measured according to a previously described protocol (23). Briefly, 2-cm duodenal sections were extracted upon euthanasia, and the mucosae were collected by scraping. Purified mucosa was homogenized in 1 ml MilliQ water supplemented with protease inhibitor cocktail III obtained from Calbiochem (La Jolla, CA). Sucrose or lactose (56 mM) was coincubated with homogenates in maleic buffer (0.1 M, pH 6.0) for 1 h at 37°C. Chromogenic buffer containing Tris, glucose oxidase, and peroxidase was used to detect the production of glucose following the coincubation of substrate and enzyme. Samples were scanned with a BioTek Instruments (Winooski, VT) microplate reader at 450 nm. Glucose production was normalized to the protein content of each tested sample. The protein content of each sample was quantified by the Bio-Rad Bradford assay (Hercules, CA).
Statistical analysis.
Mann-Whitney tests and Student t tests were used to determine the statistical significance of parasite counts and sucrase activity assays, respectively, using PRISM GraphPad software.
RESULTS
The intestinal microbiota contributes to G. duodenalis-induced sucrase reduction.
The importance of the intestinal microbiota in gut homeostasis and immunity has been emphasized in recent years (24–26). In the adult mouse model of G. duodenalis infection, mice do not exhibit overt symptoms such as diarrhea or weight loss, but they do show reduced disaccharidase activity as well as increases in intestinal motility (8, 27, 28). In order to explore the role of the microbiota in G. duodenalis-induced immunity and immunopathology, we analyzed duodenal sucrase activity in C57BL/6 mice after a 7-day infection with and without antibiotic treatment. Antibiotic-treated mice did not exhibit altered parasite burdens (Fig. 1A). No diarrhea was observed with or without antibiotic treatment. Despite a similar parasite burden, sucrase deficiency was more severe in non-antibiotic-treated mice. Untreated mice exhibited a 50% sucrase reduction after infection, compared to only about 30% reduced sucrase activity in antibiotic-treated animals (Fig. 1B). Interestingly, antibiotic treatment had no impact on basal sucrase activity in uninfected mice. These data demonstrate that infection with G. duodenalis results in sucrase deficiency after 1 week and that disruption of the microbiota through antibiotic treatment alleviates this pathology. Given the known relationship between T cells and sucrase reduction, we hypothesized that antibiotic treatment would have a measurable impact on T cell responses following infection (8).
FIG 1.

Parasite counts and sucrase activity following infection in C57BL/6J mice. (A) Quantification of luminal trophozoites from 3-cm duodenal segments. (B) Sucrase activity in uninfected and day 7 G. duodenalis-infected mice with (black bars) and without (white bars) antibiotic pretreatment. Two centimeters of the duodenal mucosa (immediately distal to segments used for parasite counts) from uninfected and infected mice was tested for sucrase activity as described in Materials and Methods. Mean parasite counts and enzyme activity are presented for 4 mice per group, along with standard error of the mean (SEM). A Mann-Whitney test was used to determine significance for parasite counts. An unpaired Student t test was used to determine significance for sucrase activity.
Infection with G. duodenalis increases intestinal CD4+ T cell numbers independently of the microbiota.
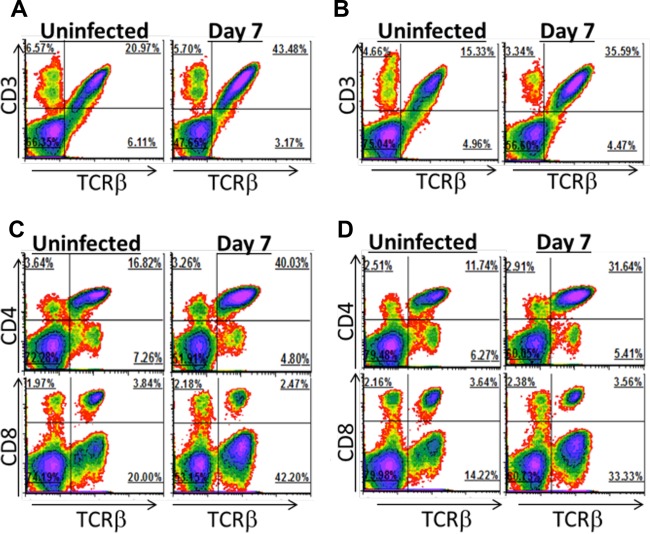
Disruption of the intestinal microbiota with antibiotics lessened the severity of sucrase deficiency in infected mice, and this may be due to an impairment of T cell responses. In order to address this, we asked if infection with G. duodenalis leads to changes in the relative abundance of duodenal T cell subsets and if the intestinal microbiota plays a role in this process. We assessed the abundance of duodenal T cells in uninfected and day 7 infected C57BL/6 mice with and without antibiotic treatment. We used flow cytometric analysis on intestinal cell suspensions to quantify T cells relative to all living intestinal cells for each group of mice. The LP of uninfected mice consists of 21% αβ T cells (Fig. 2A). Infection with G. duodenalis increased the relative abundance of αβ T cells to 43% of the LP. This increase was observed in antibiotic-treated and untreated animals and is thus likely independent of the intestinal microbiota (Fig. 2A and B). These increasing T cells were almost exclusively CD4+ (Fig. 2C). CD4+ and CD8+ αβ T cells accounted for 17% and 4% of the LP in uninfected mice, respectively. Infection with G. duodenalis increased CD4+ T cells to 40% of the LP, while the proportion of CD8+ T cells was unaltered. Antibiotic treatment did not overtly impact this increase in LP CD4+ T cells (Fig. 2D). In repeat experiments, increases in the proportion of CD4+ T cells in the LP were consistently observed, with or without antibiotic treatment of mice (see Fig. S1B, S2A, and S3A in the supplemental material). These data are consistent with previous data that giardiasis is met with a robust and protective CD4+ T cell response (29) and that this response occurs independently of the intestinal microbiota.
FIG 2.
Intestinal lamina propria T cell abundance. Lamina propria lymphocytes were isolated from uninfected and day 7 infected C57BL/6J mice given autoclaved drinking water (A and C) or treated with antibiotics from 2 days prior to infection and throughout the infection (B and D). The proportions of CD3ε+ and TCRβ+ cells (A and B) and of CD4+ and CD8+ T cells (C and D) after gating on viable cells are shown. Four mice were pooled for each density plot. Increased proportions of LP CD4+ T cells were observed in infected mice in at least 3 independent experiments.
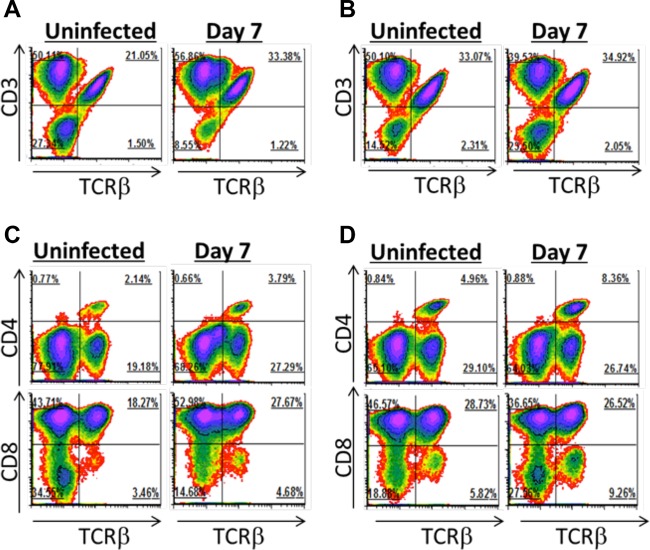
The intestinal epithelium is rich in IEL, which consist of both αβ and γδ T cells. αβ IEL monitor the epithelium for pathogens as well as stressed or transformed cells. Although the biology of γδ T cells is poorly understood, they are known to protect against some enteric pathogens and also maintain epithelial integrity (for a review, see reference 30). We quantified the proportions of αβ and γδ IEL in uninfected and G. duodenalis-infected mice (Fig. 3). The proportion of αβ T cells increased from 21% to 33% of the IEL following infection, and most of these αβ T cells were CD8+ (Fig. 3A and C). Since IEL are constantly exposed to commensal antigens, we asked if antibiotic treatment altered IEL numbers. Interestingly, antibiotic treatment alone raised αβ IEL numbers to those in of infected non-antibiotic-treated mice (Fig. 3B). No further increase in αβ IEL was observed during infection of antibiotic-treated mice (Fig. 3B). IEL expressing the γδ T cell receptor (CD3+ Tcrβ− cells) outnumbered their αβ counterparts 2:1 within the epithelium (Fig. 3). Infection with G. duodenalis had little impact on total γδ IEL numbers, as they were maintained at between 50 and 57% of total IEL (Fig. 3A). The biological relevance of γδ T cells in our model is unclear, as γδ T cell-deficient mice clear infection with normal kinetics (31). There is also no known role for γδ T cells in the pathology of giardiasis, as β2m−/− mice contain normal numbers of γδ T cells and fail to develop sucrase deficiency (8).
FIG 3.
Intestinal IEL abundance. Intraepithelial lymphocytes were isolated from uninfected and day 7 infected C57BL/6J mice given autoclaved drinking water (A and C) or treated with antibiotics from 2 days prior to infection and throughout the infection (B and D). The proportions of CD3ε+ and TCRβ+ cells (A and B) and of CD4+ and CD8+ T cells (C and D) after gating on viable cells are shown. Four mice were pooled for each density plot.
The intestinal microbiota contributes to CD8+ T cell activation following infection with G. duodenalis.
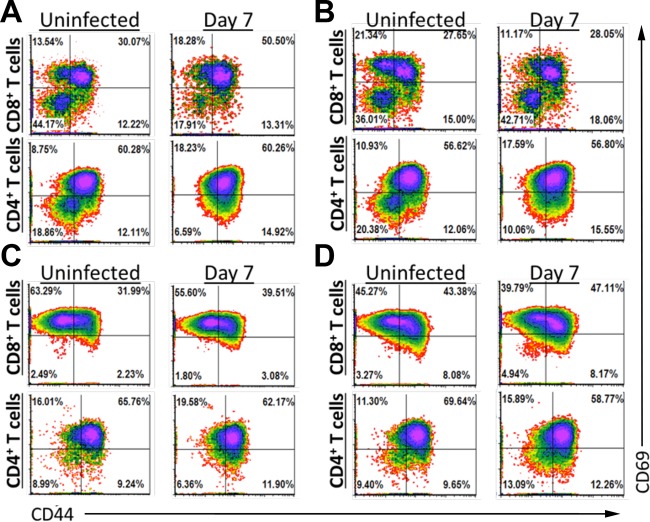
Infection with G. duodenalis led to an increase of duodenal αβ CD4+ T cells, and this was unaffected by antibiotic treatment. We hypothesized that this expansion would coincide with increased T cell activation in infected mice. Activated T cells express greater amounts of cell surface proteins, such as CD44 and CD69, distinguishing them from their naive counterparts. We therefore conducted flow cytometric analysis on LP and intraepithelial T cells from uninfected mice at day 7 of infection with Giardia to quantify the relative abundances of activated and naive T cells. In the LP and IEL, we observed similar percentages of αβ CD4+ T cells with an activated phenotype, defined as CD44hi CD69hi, in both infected and uninfected mice. This was largely unaffected by antibiotic treatment (Fig. 4; see Fig. S1C, S2D, and S3C in the supplemental material). In contrast, after 7 days of infection in the absence of antibiotics, the percentage of CD44hi CD69hi CD8+ αβ T cells increased from 30% to 50% of the entire CD8+ αβ T cell population within the LP (Fig. 5A; see Fig. S2D and S3C in the supplemental material). Antibiotic treatment completely ablated the G. duodenalis-mediated increase of CD44hi CD69hi CD8+ αβ T cells within the LP (Fig. 4B; see Fig. S1C in the supplemental material). Antibiotic treatment also increased the abundance of a CD44lo CD69hi CD8+ αβ T cell population within the LP of uninfected mice from 14% to 21% (Fig. 4A and B; see Fig. S1C, S2D, and S3C in the supplemental material). αβ IEL are largely CD69hi regardless of infection and consist mainly of CD8+ T cells (Fig. 4C). Infection increased the intraepithelial CD44hi αβ CD8+ T cell population from 34% to 42%. Antibiotic treatment alone increased the proportion of intraepithelial CD44hi αβ CD8+ T cells from 34% to 51% in uninfected mice (Fig. 5D), and infection with G. duodenalis further raised the percentage of intraepithelial CD44hi CD8+ αβ T cells to 55% in antibiotic-treated mice. Increased abundance of activated CD8+ αβ T cells in the IEL of infected mice was also observed in repeated experiments in the absence of antibiotics (see Fig. S2D and S3C in the supplemental material). Together, these data clearly reveal an increased abundance of activated CD8+ αβ T cells in the LP and IEL of mice infected with G. duodenalis.
FIG 4.
αβ T cells are activated following infection with G. duodenalis. Lamina propria lymphocytes (A and B) and intraepithelial lymphocytes (C and D) were isolated from uninfected or day 7 infected C57BL/6J mice. Mice were given autoclaved water (A and C) or treated with antibiotics (B and D). Expression of CD44 and CD69 on T cells following gating for CD3ε+ TCRβ+ cells and either CD8+ (CD4− CD8α+) or CD4+ (CD4+ CD8α−) cells is shown. Four mice were pooled for each density plot. CD8+ T cell activation was observed in at least 3 independent experiments without antibiotics.
FIG 5.
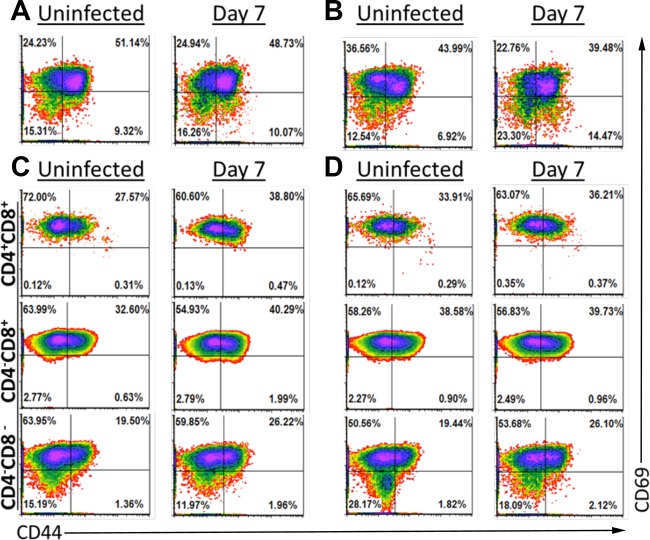
γδ T cells are activated in the epithelial layer following infection with G. duodenalis. Lamina propria lymphocytes (A and B) and intraepithelial lymphocytes (C and D) were isolated from uninfected or day 7 infected C57BL/6J mice. Mice were given autoclaved water (A and C) or treated with antibiotics (B and D). Expression of CD44 and CD69 on T cells following gating for viable CD3ε+ TCRβ− cells is shown. For IEL (C and D), cells were further gated based on expression of CD4 and CD8. Four mice were pooled for each density plot. γδ T cell activation has been observed in at least 3 independent experiments without antibiotics.
We further hypothesized that G. duodenalis may trigger stress responses within the intestinal epithelium by activating γδ T cells. These cells were largely CD69hi in the LP and intraepithelial compartment, irrespective of infection (Fig. 5; see Fig. S1D, S2E, and S3D in the supplemental material). Infection did not reproducibly lead to increased CD44hi γδ cells within the LP (Fig. 5A and B; see Fig. S1D, S2E, and S3D in the supplemental material). There was, however, a slight increase in CD44hi γδ T cell subsets within the intraepithelial compartment following infection (Fig. 5C; see Fig. S2E and S3D in the supplemental material). Antibiotic treatment increased the abundance of CD44hi γδ T cells within the CD4− CD8+ and CD4+ CD8+ subpopulations, while the CD4− CD8− cells were unaffected with respect to CD44 expression (Fig. 5D). The biological relevance of this is not clear. However, it is likely that the effect of antibiotics on the activation status of intraepithelial γδ T cells impacts intestinal function.
Phenotype and function of activated CD8+ T cells.
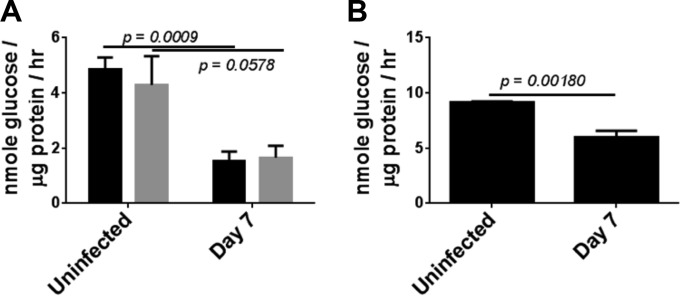
To determine the functional status of these activated CD8+ T cells and to gain insights into how they contribute to immunopathology following infection, we next used flow cytometry to examine expression of molecules associated with cytotoxic T cell functions. CD8+ αβ T cells from the LP of infected mice exhibited increased expression of granzymes A and B, as well as the cytokine gamma interferon (Fig. 6). Interestingly, we could not detect increased expression of perforin, FasL, or TRAIL, three additional molecules involved in cytotoxic responses following infection (data not shown; see Fig. S4 in the supplemental material). To determine if granzymes were involved in disaccharidase activity reduction in this model, we infected mice lacking both granzymes A and B. Sucrase activity decreased by more than 50% in both wild-type mice and doubly granzyme-deficient mice at day 7 postinfection (Fig. 7A), indicating that these proteases are not involved in immunopathology following G. duodenalis infection. Similarly, since γδ T cells also become activated following infection, we tested mice lacking the TCR δ locus. Again, the reduction in sucrase activity was unaffected in these mice following infection (Fig. 7B).
FIG 6.
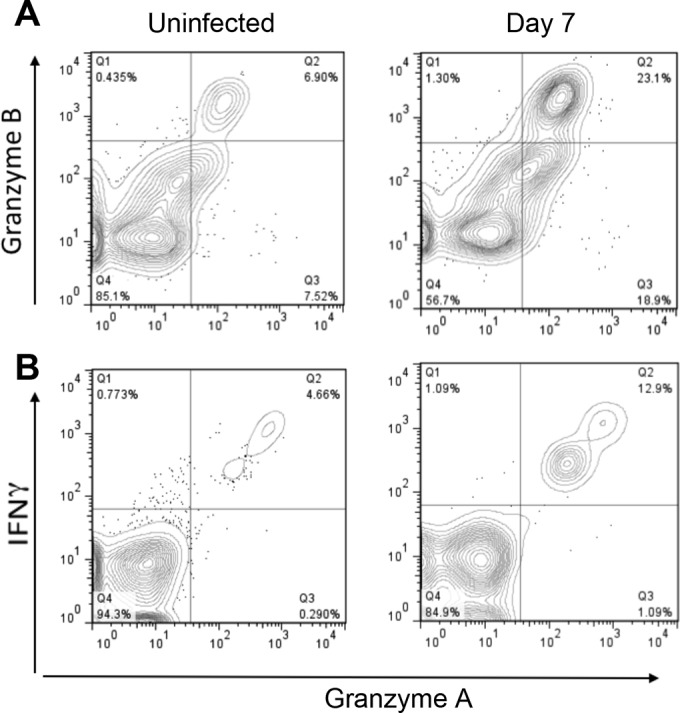
Phenotype of activated lamina propria CD8+ T cells. (A) Freshly isolated lamina propria cells from uninfected and infected C57BL/6J mice were stained and analyzed by flow cytometry. Data were gated on live TCRβ+ CD8+ cells, and staining for granzyme A and granzyme B was plotted as a topographic map. (B) Lamina propria cells were incubated on anti-CD3-coated dishes for 8 h in the presence of brefeldin A before staining and analysis. Data were gated on live CD8+ cells. Four mice were pooled for each group.
FIG 7.
Sucrase activity in TCRδ- and granzyme-deficient mice. (A) 129X1/SvJ mice (black bars) and transgenic mice on this background lacking both granzymes A and B (gray bars) were infected with G. duodenalis, and sucrase activity was determined at day 7 of infection and in uninfected controls. (B) C57BL/6J mice with a deletion in the Tcrd locus were infected and sucrase activity determined at day 7 and in uninfected controls. n = 4 mice/group. P values for Student t tests are shown.
DISCUSSION
We have known for many years that the microbiota could impact the ability of G. duodenalis to colonize mice (11). In this study, we now show that the microbiota also plays a role in the development of CD8+ T cell-mediated disaccharidase deficiency, one of the hallmark symptoms of this infection in humans. This study is also the first to directly describe the activation of T cell responses in the intestinal lamina propria and intraepithelial lymphocyte compartments during infection with this intestinal protozoan. Specifically, we report that infection with Giardia leads to an increased proportion of αβ CD4+ T cells in the LP of infected mice and that this is not affected by antibiotic treatment. We also found an increased abundance of CD8+ T cells with an activated CD44hi CD69hi phenotype in the LP of infected mice, and this shift is extremely sensitive to antibiotics. Activated CD8+ T cells in the LP express IFN-γ and granzymes A and B but not perforin, FasL, or TRAIL. Finally, while the ablation of CD8+ T cell activation through antibiotic treatment leads to a less severe sucrase deficiency in infected mice, deletion of granzymes has no effect on sucrase deficiency.
This study relied on treating mice with a cocktail of antibiotics in order to perturb the microbiota. This approach has been used by us and others to investigate the role of the microbiota during Giardia and other infections (8, 11, 18, 19). The cocktail of ampicillin, vancomycin, and neomycin is similar to that used by Rakoff-Nahoum et al. to demonstrate a role for the microbiota in maintaining intestinal homeostasis (32). We have removed metronidazole from the cocktail used in their studies due to its antiparasitic effects on Giardia. In related work, we have performed 16S rRNA sequencing-based analysis of the microbiome composition throughout the gastrointestinal tract before and during treatment with these antibiotics as well as during infection with Giardia. Detailed results will be published elsewhere, but major changes include an increase in the relative abundance of gammaproteobacteria, while firmicutes were much less abundant, following 2 weeks of treatment with antibiotics (N. Barash, J. Maloney, and S. Dawson, unpublished data). Several studies have examined inhibitory effects of lactobacilli on Giardia (13–15). Further studies are necessary to identify the exact role of the microbiota in modulating immune responses against the parasite.
The activation of CD8+ T cells is highly sensitive to antibiotic treatment in our system. The intestinal microbiota is of great importance in establishing intestinal T cells, as germfree mice exhibit reduced CD4+ and CD8+ T cell numbers compared to those in normal mice (33). Germfree or antibiotic-treated mice also exhibit impaired TH1 and TH17 responses upon infection with various pathogens, and the introduction of single commensal strains can restore proper immune function (16, 18, 34). In contrast, both antibiotic-treated mice and germfree mice infected with Salmonella were recently shown to have greater numbers of IFN-γ-producing lymphocytes in the mesenteric lymph nodes than conventional mice (19). Our data clearly show a role for antibiotic-dependent activation of CD8+ T cells during Giardia infections.
Infection with G. duodenalis results in increased CD4+ T cells within the duodenal LP after 7 days in both antibiotic-treated and untreated mice. To date, increases in CD4+ T cells have been reported only in the Peyer's patches of G. muris-infected mice, and increased IEL numbers have been found in both G. duodenalis and G. muris infections (6, 10, 35, 36). The relative abundances of T cell populations within the IEL, however, are unaltered throughout infection in our model. The proliferation of Giardia-specific CD4+ T cells has been reported in mice and humans (37–39). Several studies have shown that CD4+ T cells are necessary for clearance of Giardia infections, and recent data have suggested that interleukin-17 (IL-17) is a key cytokine in mediating this protective effect (reviewed in reference 40). Our lab has previously shown that antibiotic-treated mice resolve infection within 3 weeks and that CD4+ T cells from these mice produce IFN-γ, IL-13, and IL-17 after in vitro restimulation (8). We also showed in the same study that CD4−/− mice failed to develop sucrase deficiency following infection (8). Given the ability of isolated CD8+ T cells to induce this effect following adoptive transfer, we suggest that CD4+ T cells may be needed for activation of the CD8+ T cell response (7) Thus, the impact of antibiotic treatment in this model appears to be mainly restricted to the activation of CD8+ T cells.
We have, for the first time, directly demonstrated that αβ CD8+ T cells are activated following infection with G. duodenalis. Previous studies have implicated these cells in Giardia-induced sucrase reduction by looking at infection outcomes in knockout mice and by adoptive transfer of these cells from infected animals into naive athymic recipients (7). Our lab found that sucrase deficiency occurs in wild-type, but not SCID, CD4−/− or β2m−/− mice (8). Given that Giardia is an extracellular pathogen, it is unclear how CD8+ T cells become activated during this infection. It is possible that parasite antigens are taken up by dendritic cells and cross-presented to naive CD8+ T cells. It is also possible that the parasite promotes breakdown of the intestinal barrier and that translocation of luminal bacteria into the mucosa leads to activation of CD8+ T cells (41). The reduction of CD8+ T cell activation following antibiotic treatment suggests that bacterial translocation may be playing a role in this process, but additional studies are needed to support this conclusion.
The clinical presentation and outcomes of Giardia infection vary widely in humans. Many studies have attempted to correlate symptoms of disease with differences in parasite genetics (29). Our prior work supports roles for differences among parasite strains but also demonstrates important roles for the immune response in contributing to disease symptoms (8, 27, 28). These new data suggest that differences in the intestinal microbiota can contribute to differences in immune responses and may therefore also contribute to differences in clinical outcomes. Understanding the variation in clinical presentation of humans with Giardia is a major question in the field, and the importance of the microbiota in this variation remains to be explored.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by funding from the National Institutes on Health (AI 094492) and an American Association of Immunologists Career Fellowship to S.M.S. The Georgetown Lombardi Shared Resources are partially supported by grant CA-051008 from the National Cancer Institute and the Georgetown University Barrier Animal Facility by grant RR-025828 from the National Center for Research Resources.
We extend special thanks to Karen Creswell (Georgetown University Flow Cytometry Core facility) and Dragana Jankovic for helpful suggestions.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00348-16.
REFERENCES
- 1.Barnes GL, Townley RR. 1973. Duodenal mucosal damage in 31 infants with gastroenteritis. Arch Dis Child 48:343–349. doi: 10.1136/adc.48.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lubos MC, Gerrard JW, Buchan DJ. 1967. Disaccharidase activities in milk-sensitive and celiac patients. J Pediatr 70:325–331. doi: 10.1016/S0022-3476(67)80128-8. [DOI] [PubMed] [Google Scholar]
- 3.Arvanitakis C. 1979. Abnormalities of jejunal mucosal enzymes in ulcerative colitis and Crohn's disease. Digestion 19:259–266. [DOI] [PubMed] [Google Scholar]
- 4.Dunne WT, Cooke WT, Allan RN. 1977. Enzymatic and morphometric evidence for Crohn's disease as a diffuse lesion of the gastrointestinal tract. Gut 18:290–294. doi: 10.1136/gut.18.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buret A, Gall DG, Olson ME. 1990. Effects of murine giardiasis on growth, intestinal morphology, and disaccharidase activity. J Parasitol 76:403–409. doi: 10.2307/3282675. [DOI] [PubMed] [Google Scholar]
- 6.Scott KG, Logan MR, Klammer GM, Teoh DA, Buret AG. 2000. Jejunal brush border microvillous alterations in giardia muris-infected mice: role of T lymphocytes and interleukin-6. Infect Immun 68:3412–3418. doi: 10.1128/IAI.68.6.3412-3418.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott KG, Yu LC, Buret AG. 2004. Role of CD8+ and CD4+ T lymphocytes in jejunal mucosal injury during murine giardiasis. Infect Immun 72:3536–3542. doi: 10.1128/IAI.72.6.3536-3542.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solaymani-Mohammadi S, Singer SM. 2011. Host immunity and pathogen strain contribute to intestinal disaccharidase impairment following gut infection. J Immunol 187:3769–3775. doi: 10.4049/jimmunol.1100606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruest N, Couture Y, Faubert GM, Girard C. 1997. Morphological changes in the jejunum of calves naturally infected with Giardia spp. and Cryptosporidium spp. Vet Parasitol 69:177–186. doi: 10.1016/S0304-4017(96)01121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oberhuber G, Vogelsang H, Stolte M, Muthenthaler S, Kummer AJ, Radaszkiewicz T. 1996. Evidence that intestinal intraepithelial lymphocytes are activated cytotoxic T cells in celiac disease but not in giardiasis. Am J Pathol 148:1351–1357. [PMC free article] [PubMed] [Google Scholar]
- 11.Singer SM, Nash TE. 2000. The role of normal flora in Giardia lamblia infections in mice. J Infect Dis 181:1510–1512. doi: 10.1086/315409. [DOI] [PubMed] [Google Scholar]
- 12.Benyacoub J, Perez PF, Rochat F, Saudan KY, Reuteler G, Antille N, Humen M, De Antoni GL, Cavadini C, Blum S, Schiffrin EJ. 2005. Enterococcus faecium SF68 enhances the immune response to Giardia intestinalis in mice. J Nutr 135:1171–1176. [DOI] [PubMed] [Google Scholar]
- 13.Perez PF, Minnaard J, Rouvet M, Knabenhans C, Brassart D, De Antoni GL, Schiffrin EJ. 2001. Inhibition of Giardia intestinalis by extracellular factors from lactobacilli: an in vitro study. Appl Environ Microbiol 67:5037–5042. doi: 10.1128/AEM.67.11.5037-5042.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shukla G, Sidhu RK. 2011. Lactobacillus casei as a probiotic in malnourished Giardia lamblia-infected mice: a biochemical and histopathological study. Can J Microbiol 57:127–135. doi: 10.1139/W10-110. [DOI] [PubMed] [Google Scholar]
- 15.Humen MA, De Antoni GL, Benyacoub J, Costas ME, Cardozo MI, Kozubsky L, Saudan KY, Boenzli-Bruand A, Blum S, Schiffrin EJ, Perez PF. 2005. Lactobacillus johnsonii La1 antagonizes Giardia intestinalis in vivo. Infect Immun 73:1265–1269. doi: 10.1128/IAI.73.2.1265-1269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. 2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. 2011. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. 2011. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A 108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Santoscoy M, Wenzel UA, Yrlid U, Cardell S, Backhed F, Wick MJ. 2015. The gut microbiota reduces colonization of the mesenteric lymph nodes and IL-12-independent IFN-gamma production during Salmonella infection. Front Cell Infect Microbiol 5:93. doi: 10.3389/fcimb.2015.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nash TE, Aggarwal A. 1986. Cytotoxicity of monoclonal antibodies to a subset of Giardia isolates. J Immunol 136:2628–2632. [PubMed] [Google Scholar]
- 21.Keister DB. 1983. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg 77:487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- 22.Sheridan BS, Lefrancois L. 2012. Isolation of mouse lymphocytes from small intestine tissues. Curr Protoc Immunol Chapter 3:Unit3.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahlqvist A, Hammond JB, Crane RK, Dunphy JV, Littman A. 1968. Intestinal lactase deficiency and lactose intolerance in adults. Preliminary report. Gastroenterology 54(Suppl):807–810. [PubMed] [Google Scholar]
- 24.Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M. 2014. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fonseca DM, Hand TW, Han SJ, Gerner MY, Glatman Zaretsky A, Byrd AL, Harrison OJ, Ortiz AM, Quinones M, Trinchieri G, Brenchley JM, Brodsky IE, Germain RN, Randolph GJ, Belkaid Y. 2015. Microbiota-dependent sequelae of acute infection compromise tissue-specific immunity. Cell 163:354–366. doi: 10.1016/j.cell.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng MY, Cisalpino D, Varadarajan S, Hellman J, Warren HS, Cascalho M, Inohara N, Nunez G. 2016. Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity 44:647–658. doi: 10.1016/j.immuni.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li E, Zhou P, Singer SM. 2006. Neuronal nitric oxide synthase is necessary for elimination of Giardia lamblia infections in mice. J Immunol 176:516–521. doi: 10.4049/jimmunol.176.1.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li E, Zhao A, Shea-Donohue T, Singer SM. 2007. Mast cell mediated changes in smooth muscle contractility during mouse giardiasis. Infect Immun 75:4514–4518. doi: 10.1128/IAI.00596-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solaymani-Mohammadi S, Singer SM. 2010. Giardia duodenalis: the double-edged sword of immune responses in giardiasis. Exp Parasitol 126:292–297. doi: 10.1016/j.exppara.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komori HK, Meehan TF, Havran WL. 2006. Epithelial and mucosal gamma delta T cells. Curr Opin Immunol 18:534–538. doi: 10.1016/j.coi.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Singer SM, Nash TE. 2000. T-cell-dependent control of acute Giardia lamblia infections in mice. Infect Immun 68:170–175. doi: 10.1128/IAI.68.1.170-175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. 2004. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, Reading NC, Villablanca EJ, Wang S, Mora JR, Umesaki Y, Mathis D, Benoist C, Relman DA, Kasper DL. 2012. Gut immune maturation depends on colonization with a host-specific microbiota. Cell 149:1578–1593. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. 2005. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Abdul-Wahid A, Faubert G. 2008. Characterization of the local immune response to cyst antigens during the acute and elimination phases of primary murine giardiasis. Int J Parasitol 38:691–703. doi: 10.1016/j.ijpara.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Koot BG, ten Kate FJ, Juffrie M, Rosalina I, Taminiau JJ, Benninga MA. 2009. Does Giardia lamblia cause villous atrophy in children? A retrospective cohort study of the histological abnormalities in giardiasis. J Pediatr Gastroenterol Nutr 49:304–308. doi: 10.1097/MPG.0b013e31818de3c4. [DOI] [PubMed] [Google Scholar]
- 37.Ebert EC. 1999. Giardia induces proliferation and interferon gamma production by intestinal lymphocytes. Gut 44:342–346. doi: 10.1136/gut.44.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Astiazaran-Garcia H, Quintero J, Vega R, Briceno P, Oviedo C, Rascon L, Garibay-Escobar A, Castillo-Yanez FJ, Robles-Zepeda R, Hernandez J, Velazquez C. 2009. Identification of T-cell stimulating antigens from Giardia lamblia by using Giardia-specific T-cell hybridomas. Parasite Immunol 31:132–139. doi: 10.1111/j.1365-3024.2008.01083.x. [DOI] [PubMed] [Google Scholar]
- 39.Hanevik K, Kristoffersen E, Svard S, Bruserud O, Ringqvist E, Sornes S, Langeland N. 2011. Human cellular immune response against Giardia lamblia 5 years after acute giardiasis. J Infect Dis 204:1779–1786. doi: 10.1093/infdis/jir639. [DOI] [PubMed] [Google Scholar]
- 40.Singer SM. 2016. Control of giardiasis by interleukin-17 in humans and mice—are the questions all answered? Clin Vaccine Immunol 23:2–5. doi: 10.1128/CVI.00648-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halliez MC, Motta JP, Feener TD, Guerin G, LeGoff L, Francois A, Colasse E, Favennec L, Gargala G, Lapointe TK, Altier C, Buret AG. 2016. Giardia duodenalis induces para-cellular bacterial translocation and causes post-infectious visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol doi: 10.1152/ajpgi.00144.2015:ajpgi0014402015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.