Abstract
Meningococcal outer membrane vesicles (OMVs) have been extensively investigated and successfully implemented as vaccines. They contain pathogen-associated molecular patterns, including lipopolysaccharide (LPS), capable of triggering innate immunity. However, Neisseria meningitidis contains an extremely potent hexa-acylated LPS, leading to adverse effects when its OMVs are applied as vaccines. To create safe OMV vaccines, detergent treatment is generally used to reduce the LPS content. While effective, this method also leads to loss of protective antigens such as lipoproteins. Alternatively, genetic modification of LPS can reduce its toxicity. In the present study, we have compared the effects of standard OMV isolation methods using detergent or EDTA with those of genetic modifications of LPS to yield a penta-acylated lipid A (lpxL1 and pagL) on the in vitro induction of innate immune responses. The use of detergent decreased both Toll-like receptor 4 (TLR4) and TLR2 activation by OMVs, while the LPS modifications reduced only TLR4 activation. Mutational removal of PorB or lipoprotein factor H binding protein (fHbp), two proteins known to trigger TLR2 signaling, had no effect, indicating that multiple TLR2 ligands are removed by detergent treatment. Detergent-treated OMVs and lpxL1 OMVs showed similar reductions of cytokine profiles in the human monocytic cell line MM6 and human dendritic cells (DCs). OMVs with the alternative penta-acylated LPS structure obtained after PagL-mediated deacylation showed reduced induction of proinflammatory cytokines interleukin-6 (IL-6) and IL-1β but not of IP-10, a typical TRIF-dependent chemokine. Taken together, these data show that lipid A modification can be used to obtain OMVs with reduced activation of innate immunity, similar to what is found after detergent treatment.
INTRODUCTION
Gram-negative bacteria have the ability to naturally shed vesicles from the outer membrane. These spontaneous outer membrane vesicles (sOMVs) are composed of lipids, outer membrane proteins, lipopolysaccharide (LPS), and some periplasmic proteins. Shedding of sOMVs occurs without significant disruption in the integrity of the cell, and release of vesicles is increased by bacterial stress responses (1, 2). Some of the proposed functions of shedding of outer membrane blebs include protection and transport of secreted molecules, interaction with host cells, evasion of immunity by action as a decoy for antibodies and other antibacterial molecules, and action as nucleators in biofilm formation (3).
The fact that OMVs are nonreplicating structures that contain many crucial surface components and virulence factors, in combination with pathogen-associated molecular patterns (PAMPs) that trigger innate immune responses, makes them attractive as candidate vaccines. This is evidenced by the successful control of meningococcal serogroup B epidemics using OMV vaccines (4–6) and experimental studies that have explored the vaccine potential of OMVs against other bacterial pathogens (7, 8). The isolation of OMVs from Neisseria meningitidis typically involves treatment with deoxycholate detergent to increase the yield and to reduce the amount of LPS. LPS, also known as endotoxin, is a very potent PAMP that, when bound to the pattern recognition receptor (PRR) Toll-like receptor 4 (TLR4), induces activation of innate immune responses and the release of inflammatory cytokines. TLR4 is the only TLR capable of activating both the MyD88 and TRIF downstream signaling pathways. In general, the MyD88 pathway is linked to the production of proinflammatory cytokines while the TRIF pathway induces release of type I interferons. Excessive release of inflammatory mediators can cause massive inflammation in a host, but some release of cytokines is needed for triggering an adaptive immune response. N. meningitidis contains the very potent hexa-acylated LPS structure, and sepsis is a common manifestation of meningococcal disease. Therefore, if not removed or reduced by detergent treatment, the LPS present in isolated OMVs can cause serious adverse effects. The downside of detergent treatment is the removal of potential protective antigens from the outer membrane, such as the more loosely attached surface-exposed lipoprotein factor H binding protein (fHbp) (9, 10), and a reduction of the long-term stability of the OMV vaccine (11).
Genetic modification provides many possibilities to increase the yield and properties of OMVs. Previous studies have shown that a higher yield of OMVs from N. meningitidis can be achieved by deletion of the rmpM gene (12).The RmpM protein links the outer membrane to the peptidoglycan layer, and its absence loosens the outer membrane, leading to increased OMV release. In addition, the toxicity of OMVs can be reduced by deletion of genes encoding the LpxL1 (Fig. 1) or LpxL2 enzyme of the lipid A biosynthesis pathway, resulting in a penta-acylated rather than a hexa-acylated lipid A structure (13). A penta-acylated LPS can also be formed by expression of the Bordetella bronchiseptica pagL gene, encoding a lipid A 3-O-deacylase, in N. meningitidis (14, 15) (Fig. 1). These penta-acylated lipid A forms show reduced endotoxic activity compared to that of hexa-acylated LPS (16). The combination of rmpM deletion and genetic LPS modification thus enables the isolation of high yields of so-called native OMVs (nOMVs) with reduced endotoxicity without the need for deoxycholate treatment. For application as a vaccine, it is imperative to determine the effects of the differences in composition of detergent-treated OMVs (dOMVs) versus nOMVs on the induced innate immune response, as this can determine both the reactogenicity as well as the magnitude and quality of the induced immune response. In the present study, we compared the dOMVs and nOMVs for their ability to stimulate TLR4 and TLR2 responses, to induce the release of cytokines, and to activate dendritic cells (DCs).
FIG 1.
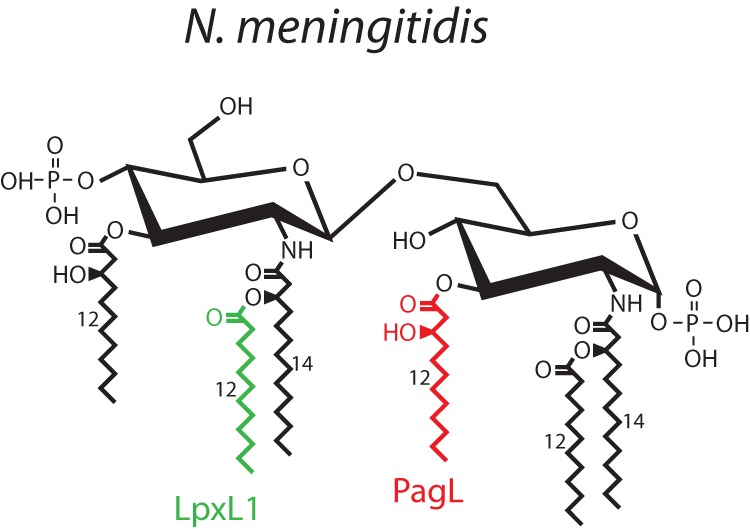
N. meningitidis lipid A architecture and structure modification by LpxL1 and PagL. N. meningitidis lipid A consists of a bisphosphorylated glucosamine disaccharide with hexa-acyl chains. Deletion of the lpxL1 gene prevents the addition of the secondary lauroyl chain at the 2′ position. Expression of PagL removes the 3-hydroxydodecanoic acid moiety at the 3 position. Both modifications result in a penta-acylated lipid A structure.
MATERIALS AND METHODS
Ethics statement.
This study was conducted according to the principles of good clinical practice. Blood donors provided written informed consent for the collection of samples and subsequent analysis. Processing of the blood samples occurred anonymously.
Bacterial strains.
All N. meningitidis strains used are nonencapsulated variants of the serogroup B H44/76 strain (17) and have a truncated galE LPS, except for the lgtB pagL mutant strain, which has a truncated lgtB LPS. JB and RL strains contain one wild-type and two recombinant PorA subtypes (trivalent PorA; subtypes P1.7,16, P1.5-1,2-2, and P1.19,15) with a nonfunctional porB gene (18). JB and RL strains contain mutations in the rmpM gene, and RL strains have an additional mutation in the lpxL1 gene, as previously described (12). The lgtB pagL strain was constructed by introducing a pEN11 plasmid carrying the Bordetella pertussis pagL gene behind an IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible lac promoter (15) into an H44/76 strain with insertionally inactivated siaD and lgtB genes. Strain H44/76, lacking factor H binding protein (fHbp), was created by replacing a deleted section of the fHbp gene with a kanamycin cassette using the primers fHbp-U-Fw (5′-TCTAGACCAGCCACGGCGCATAC-3′), fHbp-U-Rv (5′-GGATCCGACGGCATTTTGTTTACAGG-3′), fHbp-D-Fw (5′-GGATCCCGCCAAGCAATAACCATTG-3′), and fHbp-D-Rv (5′-CTCGAGCAGCGTATCGAACCATGC-3′). The spontaneous PorB-deficient mutant derived from H44/76 has been described by Tommassen et al. (19) (Table 1).
TABLE 1.
Bacterial strains used in this study
| Strain | Genotype | Phenotype | Reference(s) | Properties |
|---|---|---|---|---|
| JB (H44/76 porB) | Δcps ΔrmpM | Trivalent PorA, truncated GalE LPS, capsule deficient, class 4 OMP deficient, PorB deficient | 12, 18 | Increased OMV release but toxic LPS, detergent treatment required for safe vaccine |
| RL (H44/76 porB) | Δcps ΔlpxL1 ΔrmpM | Trivalent PorA, truncated GalE-LpxL1 LPS, capsule deficient, class 4 OMP deficient, PorB deficient | 12, 18 | Increased OMV release with attenuated LPS, no detergent treatment required |
| HB-1 | Δcps | Capsule deficient, truncated GalE LPS | 36 | Toxic LPS, detergent treatment required for safe vaccine |
| HB-1 fHbp | Δcps ΔfHbp | Capsule deficient, truncated GalE LPS, fHbp deficient | This study | Toxic LPS, detergent treatment required, immunogenic lipoprotein (fHbp) deleted |
| H44/76 porB | Δcps | Capsule deficient, truncated GalE LPS, PorB deficient | 19 | Toxic LPS, detergent treatment required for safe vaccine |
| H44/76 fHbp porB | Δcps ΔfHbp | Capsule deficient, truncated GalE LPS, fHbp Deficient, PorB Deficient | This study | Toxic LPS, detergent treatment required, immunogenic lipoprotein (fHbp) deleted |
| LgtB PagL | ΔsiaD ΔlgtB with pen11 (pagL) plasmid | Capsule deficient, truncated LgtB-PagL LPS | 16 | Attenuated LPS, lgtB mutation causing increased uptake and activation by DCs, No detergent treatment required |
Isolation of OMVs.
Bacterial strains were grown in tryptic soy broth (Becton Dickinson) with shaking (140 rpm) at 37°C. Three types of OMVs were isolated during the stationary phase (>8 h). The dOMV purification process involved deoxycholate (DOC) extraction of the bacterial outer membrane in the presence of EDTA, as described previously (12, 20). The sOMVs were obtained from collected bacterial culture supernatant (20 min, 3,000 × g, 4°C) by filtration through a 0.2-μm sterile filter, followed by concentration by ultrafiltration using a 100-kDa-cutoff filter (Centricon 70-Plus Ultracell; Millipore, Billerica, MA, USA. nOMV release was stimulated by incubating the bacteria in 100 mM Tris-HCl–10 mM EDTA (pH 8.6) for 30 min with gentle shaking, followed by passage of the collected supernatant through a 0.2-μm filter, as described by Zollinger et al. (21). All types of OMV (dOMVs, sOMVs, and nOMVs) were pelleted by ultracentrifugation (60 min, 125,000 × g, 4°C). The final OMV pellets were homogenized in 10 mM Tris-HCl–3% sucrose buffer and stored at 4°C.
Cell culture.
Mono-mac-6 (MM6) cells (seeded at 1 × 105 cells per well) and HEK-blue TLR4 and TLR2 cells (InvivoGen) (seeded at 3 × 104 per well) were grown in 96-well plates in 200 μl Iscove modified Dulbecco medium (IMDM) (Gibco BRL) and Dulbecco modified Eagle medium (DMEM) (Gibco BRL), respectively, supplemented with 100 units ml−1 penicillin, 100 μg ml−1 streptomycin, 300 μg ml−1 l-glutamine (Gibco BRL), and 10% heat-inactivated fetal calf serum (FCS) (Gibco BRL). Human dendritic cells (DCs) were generated from monocytes as previously described (22, 23). Briefly, monocytes were isolated from peripheral blood mononuclear cells (PBMC) using CD14 immunomagnetic bead separation technology (Miltenyi Biotec, Surrey, United Kingdom). CD14-positive cells were cultured for 6 days in RPMI with 10% fetal calf serum, 2.4 mM l-glutamine, 100 U ml−1 penicillin, 100 mg ml−1 and streptomycin (all from Invitrogen) and 100 ng ml−1 granulocyte-macrophage colony-stimulating factor (GM-CSF) and 50 ng ml−1 interleukin-4 (IL-4) (R&D Systems). Immature DCs expressed low levels of HLA-DR, CD40, CD83, and PD-L1 as determined by flow cytometry.
OMV stimulation of cultured cell lines.
Cells were stimulated with the indicated amounts of OMVs overnight at 37°C in a humidified atmosphere containing 5% CO2. Human IL-6, IL-10, and IP-10 cytokine secretion into the supernatant of MM6 cells was quantified by enzyme-linked immunosorbent assay (ELISA) (R&D Systems). IL-1β was quantified using the Pelipair reagent kit (Sanquin). Alkaline phosphatase secreted by HEK-blue cells was quantified by measuring absorbance (630 or 649 nm) after incubation (37°C, 2 to 5 h) of 20 μl of culture supernatant from each well with 200 μl QUANTI-blue (Invivogen).
SDS-PAGE and fHbp immunodetection.
The protein content was separated for each OMV by electrophoresis of 2.5 μg of OMV sample in a 12% Precise protein gel (Tris-HEPES-SDS; Thermo Scientific). Precision Plus protein all-blue standards (Bio-Rad) were used as molecular weight markers. After electrophoresis, the gel was stained using Coomassie brilliant blue (Thermo Scientific). Proteins separated on a different gel were transferred to a 0.2-μm nitrocellulose membrane (Thermo Scientific) for 30 min at 15 V in a semidry blotting system. Transferred proteins were incubated with primary anti-fHbp monoclonal antibody (1:1,000) (JAR4 epitope; NIBSC) for 1 h at room temperature, rinsed three times with Tris-buffered saline (TBS) plus 0.5% Tween 80, and incubated with secondary goat anti-mouse human ads-AP antibody (Southern Biotech) in TBS plus 0.5% Tween 80 plus 0.5% Protifar (1:2,000) for 1 h at room temperature. After rinsing three times, alkaline phosphatase activity was determined using the alkaline phosphatase conjugate substrate kit (Bio-Rad).
OMV stimulation of DCs.
Effects of OMVs on immature DCs were measured by incubating the isolated cells (5 × 105 ml−1) with 1 μg ml−1 OMVs for 18 to 24 h. Cells were harvested in PBS plus 1% bovine serum albumin (BSA) and 2 mM EDTA (fluorescence-activated cell sorting [FACS] buffer) and then labeled with monoclonal antibodies against CD40, CD83, HLA-DR, and PD-L1 (BD Biosciences) for 30 min at 4°C. Cells were then washed with FACS buffer and analyzed by flow cytometry using a FACS CantoII (BD). DC supernatants collected after 24 h of stimulation with OMVs were analyzed for the presence of IL-6, IL-10, and IP-10 (R&D Systems) by ELISA and for IL-23, IL-12p70, and IL-1β by MILLIplex MAP assay (Millipore) according to the manufacturer's instructions. Data are expressed as mean ± standard error of the mean (SEM) of results from 4 donors, and significance was determined using a paired t test.
RESULTS
Influence of OMV composition and LPS structure on cytokine induction in MM6 cells.
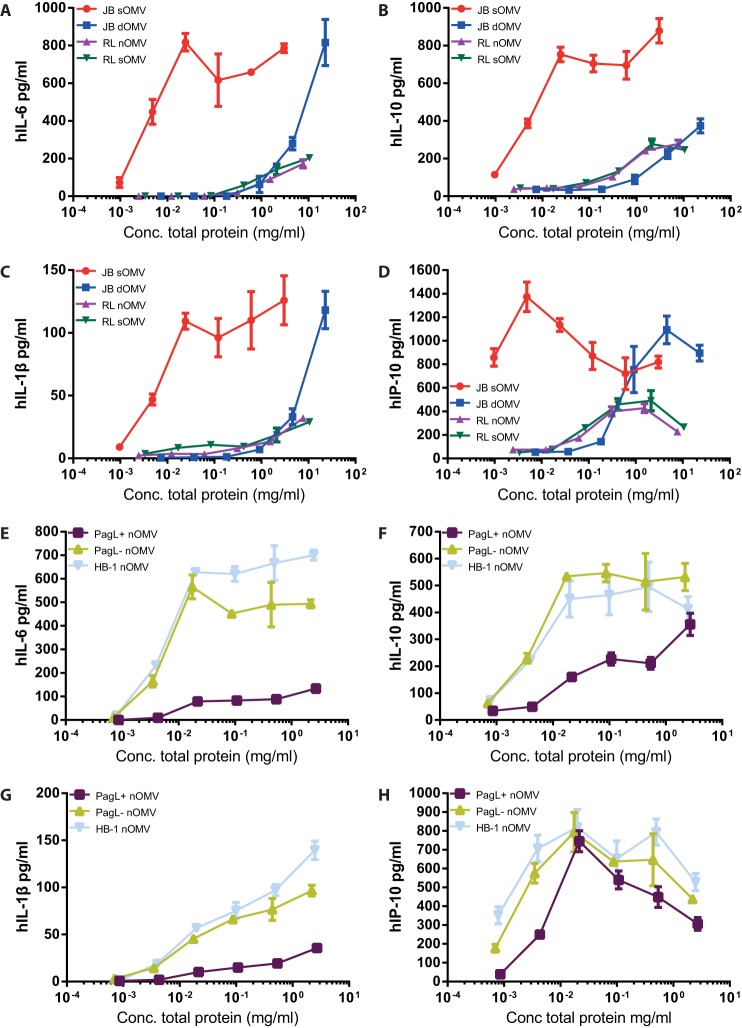
In search for the optimal OMV composition and purification procedure for vaccination purposes, we first tested different types of OMVs for the ability to produce cytokines in MM6 cells. Comparison of DOC-treated OMVs (dOMVs) with spontaneous OMVs (sOMVs) revealed a 1,000-fold reduction in IL-6, IL-1β, anti-inflammatory cytokine IL-10, and chemokine IP-10 levels for dOMVs (Fig. 2A to D). A similar reduced cytokine response was observed for sOMVs and nOMVs isolated from an lpxL1 strain, which produces less-potent penta-acylated lipid A. Stimulation of cells using different concentrations of OMVs demonstrated that sOMV levels of cytokine induction were obtained at a high concentration of dOMVs but not lpxL1 OMVs (Fig. 2A to D). For comparison, we also tested the effect of OMVs derived from the strain expressing PagL, which also has a penta-acylated LPS. As expected, nOMVs derived from an lgtB pagL strain showed reduced cytokine induction (Fig. 2E to G). However, these OMVs were still capable of inducing levels of IP-10 similar to those induced by OMVs of wild-type LPS (Fig. 2H).
FIG 2.
Production of cytokines by MM6 cells stimulated with OMVs. MM6 cells were stimulated overnight with serial dilutions of OMVs. Supernatants were collected from JB/RL (A, B, C, and D)-stimulated cells or HB-1/HB-1 pagL (E, F, G, and H)-stimulated cells, and IL-6, IP-10, IL-1β, and IL-10 production was quantified by ELISA. JB strains, capsule-deficient H44/76 wild-type strain; RL strains, LpxL1-deficient JB strain; HB-1, capsule-deficient H44/76 strain; PagL+/−, induced/noninduced pagL-carrying HB-1 strain. sOMV, nontreated spontaneous OMVs; nOMV, EDTA-treated OMVs; dOMV, deoxycholate-treated OMVs. Values are the mean values ± SEMs of triplicates. Data shown are from one representative experiment out of three independently performed experiments with similar outcomes.
Deoxycholate OMV extraction reduces TLR4 and TLR2 activation.
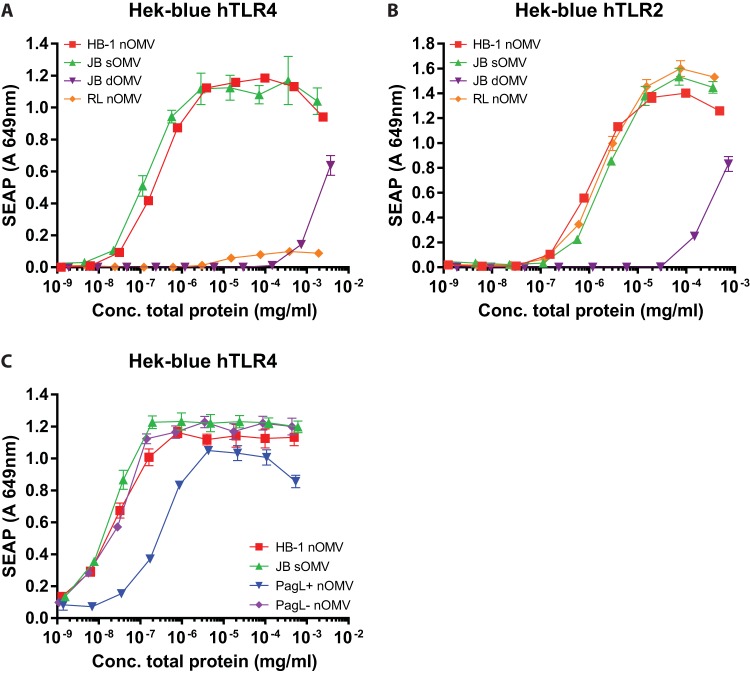
To investigate whether the reduced cytokine response observed for dOMVs was caused by the presence of reduced amounts of LPS or other changes in innate immune stimuli, we compared the capacities of sOMVs, dOMVs, and lpxL1-derived nOMVs to activate both TLR4- and TLR2-expressing cells. First, we compared the TLR4-stimulatory properties of lpxL1 nOMVs with those of dOMVs and sOMVs containing wild-type LPS to test whether lpxL1 mutation reduces TLR4 activation to a similar degree as DOC treatment. Compared to the wild-type sOMVs, the lpxL1 nOMVs and wild-type dOMVs showed strongly reduced activation of TLR4 in HEK293-TLR4 cells (Fig. 3A). pagL nOMVs also showed a reduced induction of TLR4 activation, although this reduction was not as strong as that observed for lpxL1 nOMVs and wild-type dOMVs (Fig. 3A and C). The difference between dOMVs and lpxL1 nOMVs in TLR4 activation was minor, with the former showing higher activation in the higher concentration range (Fig. 3A). This suggests that at least for potential toxicity caused by TLR4 activation, lpxL1 nOMVs are as safe as, if not safer than, wild-type dOMVs.
FIG 3.
Comparison of TLR4 and TLR2 activation by OMVs. HEK-blue cell lines expressing either human TLR4-MD-2-CD14 (A and C) or human TLR2 (B) were stimulated overnight with serial dilutions of OMVs. Supernatant was collected, and TLR activation was determined by secreted embryonic alkaline phosphatase (SEAP) in the supernatant after stimulation with OMVs. JB strains, capsule-deficient H44/76 wild-type strain; RL strains, LpxL1-deficient JB strain; HB-1, capsule-deficient H44/76 strain. Data are expressed as mean optical density at 649 nm ± SEM of triplicates. Data shown are from one representative experiment out of three independently performed experiments with similar outcomes.
While DOC treatment of OMVs is thought primarily to reduce the amount of LPS, it also removes loosely attached lipoproteins from the outer membrane, such as the factor H binding protein (fHbp), which is a well-established inducer of protective bactericidal antibodies (10, 24, 25). Lipoproteins are known to be ligands for TLR2. Activation of TLR2 also triggers innate immune responses, and thus lipoproteins can also be potential adjuvants of the OMV vaccine. To investigate whether the removal of these lipoproteins by DOC treatment results in a reduced TLR2 activation, we measured the abilities of the different types of OMVs to activate HEK293-TLR2 cells. Interestingly, sOMVs and nOMVs showed equal TLR2-activating potential, whereas dOMVs showed a clear reduction of the TLR2 response (Fig. 3B).
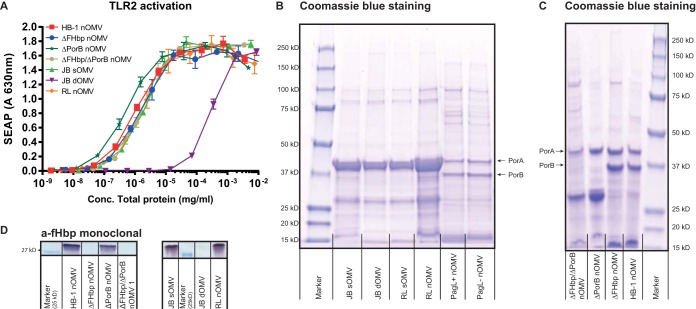
PorB and fHbp are not the major activators of TLR2 in OMVs.
Previous studies have shown that PorB, a major protein present in the outer membrane of N. meningitidis, is a TLR2 ligand (26, 27). To test the contribution of PorB and of the lipoprotein fHbp to TLR2 activation, we created an fHbp knockout strain, used a porB-deficient strain, and created a double mutant fHbp porB strain. PorB deficiency and the composition of all OMVs were verified by protein Coomassie blue staining after separation by gel electrophoresis (Fig. 4B and C), and the lack of fHbp was determined using a monoclonal antibody against fHbp (Fig. 4D). HEK293-TLR2 cells were then stimulated with serial dilutions of nOMVs collected from H44/76 fHbp- and H44/76 porB-deficient strains, lpxL1 nOMVs, and, for comparison, wild-type sOMVs and dOMVs. No significant reduction of TLR2 activation was found for fHbp, porB, and fHbp porB double mutant strains compared to wild-type strains (Fig. 4A). The only significant reduction of TLR2 activation was measured for dOMVs. These results demonstrate that removal of PorB and fHbp is not sufficient to cause a measurable reduction in TLR2 activation, presumably because of redundancy of TLR2 ligands that are present in nOMV. The reduction seen for dOMVs can be explained by the loss of multiple lipoproteins in addition to fHbp.
FIG 4.
Induction of TLR2 activation by PorB-deficient and/or fHbp-deficient OMVs. (A) HEK-blue cells expressing human TLR2 were stimulated overnight with serial dilutions of OMVs. Supernatant was collected, and TLR2 activation was determined by secreted embryonic alkaline phosphatase (SEAP) in the supernatant after stimulation with OMVs. JB strains, capsule-deficient H44/76 wild-type strain; RL strains, LpxL1-deficient JB strain; HB-1, capsule-deficient H44/76 strain. Data are expressed as mean optical density at 649 nm. Results are from one representative experiment of two independent experiments. (B and C) Protein composition of all OMVs after SDS-PAGE and Coomassie blue staining. (D) A monoclonal antibody against fHbp was used to confirm the lack of fHbp or removal of fHbp in the case of dOMVs.
OMV-induced activation of monocyte-derived DCs.
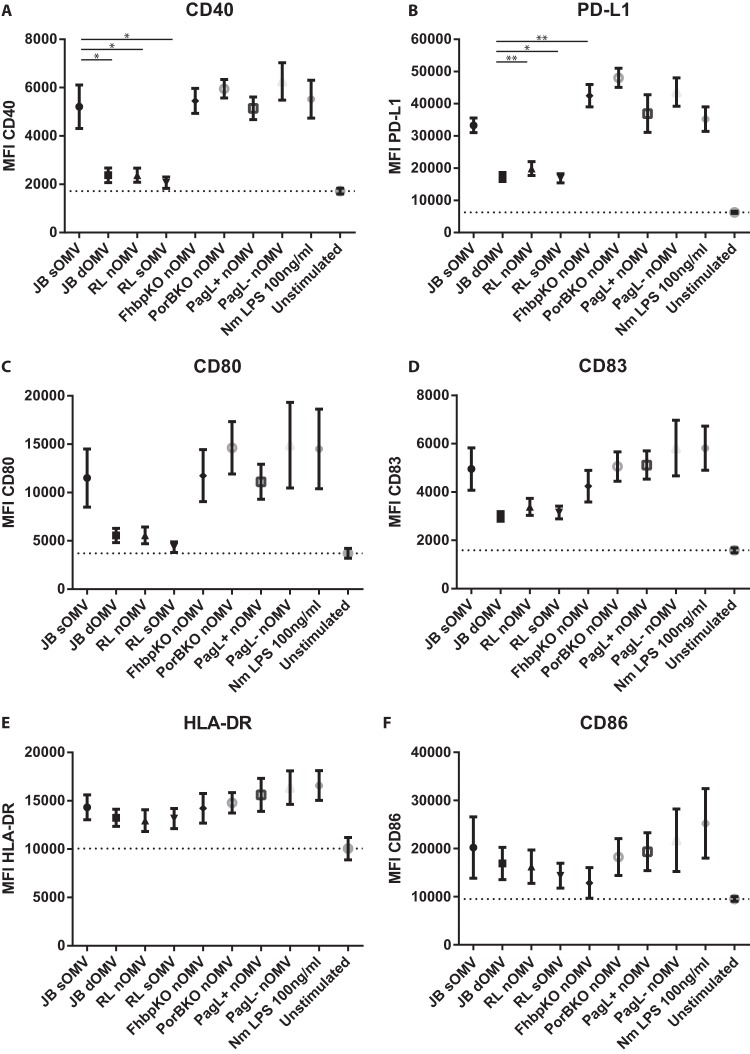
Dendritic cells (DCs) are key antigen-presenting cells and important drivers of the adaptive immune response. To determine the effect of OMV composition and OMVs carrying a penta-acylated LPS on DCs, we stimulated 6-day-old monocyte-derived dendritic cells with OMVs (1 μg ml−1 total protein) for 24 h and then determined the expression of activation markers by flow cytometry. OMVs lacking fHbp or PorB protein were also included. Wild-type sOMVs induced high expression of the CD40, PD-L1, CD83, CD80, CD86, and HLA-DR activation markers (Fig. 5). dOMVs and lpxL1 deletion nOMVs and sOMVs induced very similar expression levels of these activation markers, which in the case of CD40 and PD-L1 were significantly less than after stimulation with wild-type sOMVs (Fig. 5A and B). The absence of fHbp or PorB in OMVs did not result in any significant difference in DC activating potential, which corroborates our observation with HEK cells that these proteins are not major contributors to the overall TLR2 activity. All OMVs did induce activation of DCs compared to unstimulated DCs.
FIG 5.
Dendritic cell expression of surface markers following stimulation with OMVs. Human monocyte-derived DCs were stimulated with 1 μg/ml of the indicated OMV for 20 to 24 h or with 100 ng ml−1 LPS (positive control). Cells were analyzed by flow cytometry for the expression of surface proteins CD40 (A), PD-L1 (B), CD80 (C), CD83 (D), HLA-DR (E), and CD86 (F). Data are expressed as the mean ± SEM for four individual donors. Statistical significance was determined using a paired t test between two groups. *, P < 0.05; **, P < 0.01.
Cytokine induction by OMV-stimulated DCs.
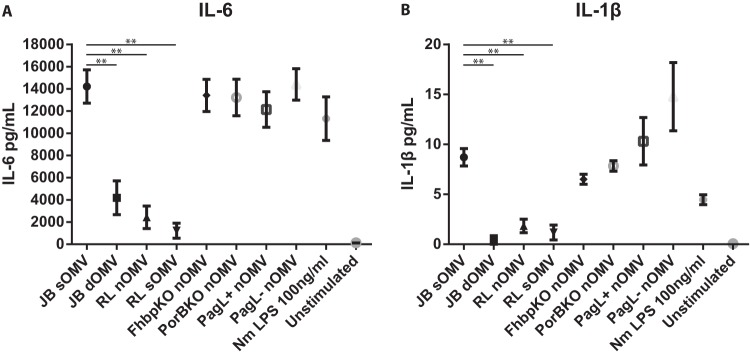
To assess whether the OMVs influenced functional cytokine and chemokine production in DCs in addition to DC surface activation markers, we determined inflammatory cytokine responses in human DCs. dOMVs and lpxL1 nOMVs and sOMVs showed a significantly reduced IL-6 and IL-1β response compared to wild-type sOMV (Fig. 6). However, penta-acylated LPS in OMVs of the lgtB pagL+ strain did not show significant differences in inflammatory cytokine production compared to wild-type sOMVs, which is different from what was observed in MM6 cells. PorB or fHbp deficiency did not result in significant differences in IL-6 or IL-1β production.
FIG 6.
Production of proinflammatory cytokines by DCs following stimulation with OMVs. Human monocyte-derived DCs were stimulated with 1 μg/ml of the indicated OMV for 20 to 24 h. Supernatants were collected from OMV-stimulated DCs, and IL-6 (A) and IL-1b (B) production was quantified by ELISA. Data are expressed as the mean ± SEM for four individual donors. Statistical significance was determined using a paired t test between two groups. **, P < 0.01.
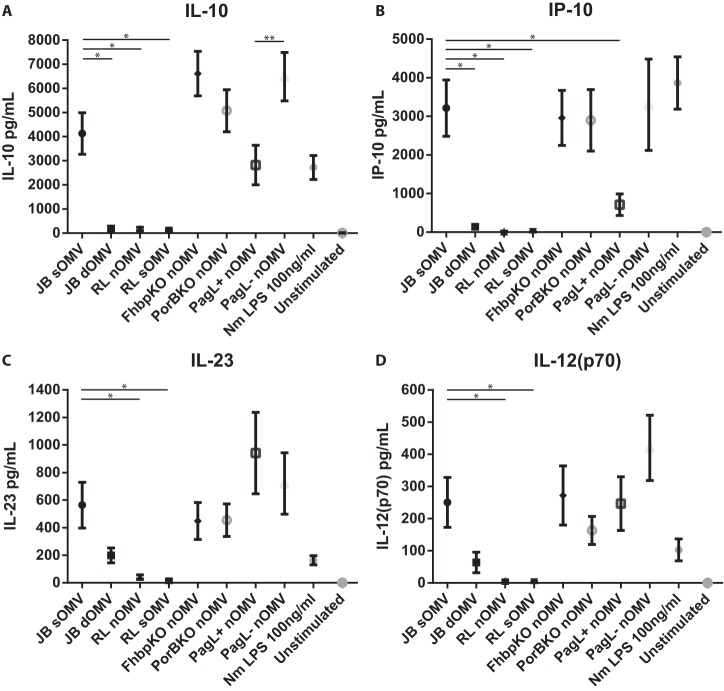
We also investigated whether different OMV compositions influenced the production of T-cell-stimulatory cytokines IL-23, IP-10, IL-10, and IL-12(p70) by DCs (Fig. 7). In MM6 cells (Fig. 2), lgtB pagL+ nOMVs showed a reduced IL-6 production but retained the ability to induce IP-10. In DCs, the lgtB pagL+ nOMVs showed hardly any reduction in proinflammatory cytokines compared to wild-type sOMVs, while the amount of IP-10 produced by DCs was reduced. However, the IP-10 induction was still higher than was detected with dOMVs or lpxL1 nOMVs. Although IL-23 and IL-12(p70) were both slightly induced by dOMVs containing wild-type LPS, they were barely detectable with lpxL1 mutant OMVs. IL-10 was significantly reduced in dOMV- and lpxL1 OMV-stimulated DCs. PagL expression caused reduction of IP-10 and IL-10 production in DCs but did not reduce the IL-23 and IL-12(p70) production. Overall, the results show that both DOC treatment and LPS attenuation have a great impact on the magnitude of the human DC response.
FIG 7.
Production of T-cell-polarizing cytokines by DCs following stimulation with OMVs. Human monocyte-derived DCs were stimulated with 1 μg/ml OMVs for 20 to 24 h. Supernatants were collected from OMV-stimulated DCs, and IL-10 (A), IP-10 (B), IL-23 (C), and IL-12(p70) (D) production was quantified by ELISA (IL-10 and IP-10) or MILLIplex assay [IL-23 and IL-12(p70)]. Data are expressed as the mean ± SEM for four individual donors. Statistical significance was determined using a paired t test between two groups. *, P < 0.05; **, P < 0.01.
DISCUSSION
Traditionally, OMV vaccines against N. meningitidis utilize DOC treatment during preparation to reduce toxic LPS content as well as to increase the efficiency of OMV extraction. Although this method has been used successfully for OMV vaccines to control meningococcal outbreaks and is the basis for the fourth component of the recently approved Bexsero 4CMenB vaccine (28), it has the downside that DOC treatment complicates processing and removes potentially protective lipoproteins such as fHbp, an antigen of N. meningitidis known to induce serum bactericidal antibodies (9–11). To circumvent the need for DOC treatment, genetic modification of the LPS structure, such as by deletion of the lpxL1 gene, has been shown to result in a safe vaccine based on spontaneously released OMVs (29, 30). These different methods to prepare OMV vaccines raise the question of the impact of OMV composition on the induction of an immune response. Since all OMV compositions used in this study are capable of inducing immunity through the induction of bactericidal antibodies and since both LPS and lipoproteins, major activators of innate immunity, are removed or retained depending on the OMV preparation method used, we compared the nature of their induced innate immune responses in order to tailor OMVs for use as safe vaccines. Our results clearly show that both the use of DOC during the OMV preparation and modification of hexa-acylated into penta-acylated LPS reduce the ability of the resulting OMVs to stimulate TLR4 and TLR2 responses and induce cytokine release from monocyte-derived dendritic cells. Considering that DOC removes potentially protective lipoproteins such as fHbp, LPS attenuation may yield OMV vaccines that are as safe as dOMVs, while inducing a broader protection.
Our finding that both dOMVs and lpxL1 nOMVs yield low TLR4 responses is consistent with the fact that the amount of hexa-acylated LPS is reduced in dOMVs and with the reduced potency of the penta-acylated LPS in lpxL1 and pagL nOMVs to activate TLR4. Interestingly, the two different penta-acylated types of LPS (lpxL1 and pagL) induced different levels of TLR4 activation and a qualitatively different cytokine release in MM6 cells. MM6 cells do not express DC-SIGN (data not shown), and thus the oligosaccharide would not influence binding, internalization, and activity. The basis for the limited effect of lgtB pagL+ nOMVs on the IP-10 response compared to the reduced release of proinflammatory cytokines IL-6 and IL-1β is unclear, but this has been previously observed for purified pagL LPS (16). Activation of TLR4 and MD-2 very much depends on how the lipid A structure is situated inside the MD-2 pocket and whether the two dimerization interfaces can be formed (31). The dimerization interfaces consist of a slightly exposed fatty acid chain interacting with TLR4 residues and the interaction of the phosphate groups with TLR4 (31). Thus, it is conceivable that removal of a certain fatty acid chain could change the position of the lipid A in the MD-2/TLR4 complex in such a way that the dimerization interfaces are not ideal, causing a reduced TLR4 activation. Crystal structure analysis of the TLR4/MD-2 complex with the antagonist lipid IVa revealed that indeed the lipid IVa structure is oriented differently deep inside the MD-2 hydrophobic pocket, making it impossible to provide the dimerization interfaces necessary for TLR4 activation and downstream signaling (32). Unlike the MyD88 pathway, the TRIF pathway is induced through endocytosis, and it is possible that upon endocytosis, different lipid A structures influence TRIF signaling. In human DCs, the bias of lgtB pagL+ nOMVs toward a more TRIF-dependent cytokine release was not seen, as these cells showed a clear reduction in IP-10 production. Also, the reduction in proinflammatory cytokine induction was less pronounced in DCs than in MM6 cells. A possible explanation may be the presence of additional receptors such as DC-SIGN in DCs but not in MM6 cells, which influences the response to OMVs. The lgtB pagL+ nOMVs used can bind and activate DC-SIGN (33), leading to increased uptake and internalization of OMVs and increased IL-10 and IL-23 cytokine production (34). However, these studies did not investigate whether OMV binding to DC-SIGN influenced the differential activation of MyD88-dependent and -independent pathways. The lpxL1 OMVs provide a clear method of detoxifying OMVs for use in humans; however, the adjuvant activity of the modified LPS might be too low, as it barely activates TLR4. lgtB pagL+ OMVs obviously do activate TLR4 and induce increased release of cytokines. The presence of a more active intrinsic LPS adjuvant translates to an increased immune response. Thus, if both OMVs are equally safe in humans, inclusion of the PagL+ and lgtB− LPS would be preferred.
In addition to the reduced TLR4 response, dOMVs but not lpxL1 nOMVs displayed a reduced TLR2 response compared to that of sOMVs with wild-type LPS. This is in agreement with DOC treatment removing not only the toxic LPS but also lipoproteins that are typically involved in activating TLR2. It remains to be identified which TLR2 ligands are present in the OMVs. A mutant lacking the fHbp lipoprotein still activated TLR2, indicating that this antigen is not a major contributor to the TLR2 response. Similarly, PorB-deficient nOMVs did not cause a reduced TLR2 activation, despite the fact that meningococcal PorB has been reported to be a TLR2 activator (26, 27). This inconsistency might be attributed to differences in conformational structure and/or availability of free versus membrane-bound PorB. Our results are in line with those of Biagini et al., who reported decreased activation of TLR2 by vesicle-bound lipoproteins (35). On the other hand, one should take into account that OMVs will contain a variety of (lipo)proteins in addition to fHbp and PorB, and the contribution of each of these individually to the overall TLR2 activation may not be sufficiently high to make a noticeable difference. Even a double knockout strain deficient in fHbp and PorB did not result in a significant reduction of TLR2 activity. Apparently, DOC treatment removes many different lipoproteins to such an extent that overall TLR2 activation becomes strongly reduced.
The present data show that the lpxL1 nOMVs closely resemble wild-type dOMVs in their capacity to activate DCs and induce the production of proinflammatory cytokines and T-cell-polarizing cytokines. This indicates that it is possible to have two different tactics for producing OMVs to induce similar immune responses. The lpxL1 nOMVs have the added benefit over wild-type dOMVs of being easier to produce and to better preserve potential antigens (11). The use of EDTA is known to stimulate OMV release but does not seem to influence the immune-stimulating properties, as in all the experiments where we used both types of OMVs, we did not observe any significant differences between lpxL1 sOMVs and nOMVs. Therefore, LPS modification is a promising alternative for detergent extraction, as it reduces adverse effects to the same degree while at the same time providing additional opportunities to direct the magnitude and type of immune response.
ACKNOWLEDGMENT
We declare no conflicts of interest.
REFERENCES
- 1.McBroom AJ, Johnson AP, Vemulapalli S, Kuehn MJ. 2006. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J Bacteriol 188:5385–5392. doi: 10.1128/JB.00498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McBroom AJ, Kuehn MJ. 2007. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol Microbiol 63:545–558. doi: 10.1111/j.1365-2958.2006.05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kulp A, Kuehn MJ. 2010. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol 64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjune G, Hoiby EA, Gronnesby JK, Arnesen O, Fredriksen JH, Halstensen A, Holten E, Lindbak AK, Nokleby H, Rosenqvist E, Solberg LK, Closs O, Froholm LO, Lystrad A, Bakketeig LS, Hareide B, Halstensen A, Holten E. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093–1096. doi: 10.1016/0140-6736(91)91961-S. [DOI] [PubMed] [Google Scholar]
- 5.Sierra GV, Campa HC, Varcacel NM, Garcia IL, Izquierdo PL, Sotolongo PF, Casanueva GV, Rico CO, Rodriguez CR, Terry MH. 1991. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann 14:195–207. (Discussion, 14:208–110.) [PubMed] [Google Scholar]
- 6.Thornton V, Lennon D, Rasanathan K, O'Hallahan J, Oster P, Stewart J, Tilman S, Aaberge I, Feiring B, Nokleby H, Rosenqvist E, White K, Reid S, Mulholland K, Wakefield MJ, Martin D. 2006. Safety and immunogenicity of New Zealand strain meningococcal serogroup B OMV vaccine in healthy adults: beginning of epidemic control. Vaccine 24:1395–1400. doi: 10.1016/j.vaccine.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 7.Leitner DR, Feichter S, Schild-Prufert K, Rechberger GN, Reidl J, Schild S. 2013. Lipopolysaccharide modifications of a cholera vaccine candidate based on outer membrane vesicles reduce endotoxicity and reveal the major protective antigen. Infect Immun 81:2379–2393. doi: 10.1128/IAI.01382-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossi O, Pesce I, Giannelli C, Aprea S, Caboni M, Citiulo F, Valentini S, Ferlenghi I, MacLennan CA, D'Oro U, Saul A, Gerke C. 2014. Modulation of endotoxicity of Shigella generalized modules for membrane antigens (GMMA) by genetic lipid A modifications: relative activation of TLR4 and TLR2 pathways in different mutants. J Biol Chem 289:24922–24935. doi: 10.1074/jbc.M114.566570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Waterbeemd B, Mommen GP, Pennings JL, Eppink MH, Wijffels RH, van der Pol LA, de Jong AP. 2013. Quantitative proteomics reveals distinct differences in the protein content of outer membrane vesicle vaccines. J Proteome Res 12:1898–1908. doi: 10.1021/pr301208g. [DOI] [PubMed] [Google Scholar]
- 10.Koeberling O, Seubert A, Granoff DM. 2008. Bactericidal antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed factor H-binding protein and genetically attenuated endotoxin. J Infect Dis 198:262–270. doi: 10.1086/589308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Waterbeemd B, Zomer G, Kaaijk P, Ruiterkamp N, Wijffels RH, van den Dobbelsteen GP, van der Pol LA. 2013. Improved production process for native outer membrane vesicle vaccine against Neisseria meningitidis. PLoS One 8:e65157. doi: 10.1371/journal.pone.0065157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van de Waterbeemd B, Streefland M, van der Ley P, Zomer B, van Dijken H, Martens D, Wijffels R, van der Pol L. 2010. Improved OMV vaccine against Neisseria meningitidis using genetically engineered strains and a detergent-free purification process. Vaccine 28:4810–4816. doi: 10.1016/j.vaccine.2010.04.082. [DOI] [PubMed] [Google Scholar]
- 13.van der Ley P, Steeghs L, Hamstra HJ, ten Hove J, Zomer B, van Alphen L. 2001. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect Immun 69:5981–5990. doi: 10.1128/IAI.69.10.5981-5990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geurtsen J, Steeghs L, Hamstra HJ, Ten Hove J, de Haan A, Kuipers B, Tommassen J, van der Ley P. 2006. Expression of the lipopolysaccharide-modifying enzymes PagP and PagL modulates the endotoxic activity of Bordetella pertussis. Infect Immun 74:5574–5585. doi: 10.1128/IAI.00834-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geurtsen J, Steeghs L, Hove JT, van der Ley P, Tommassen J. 2005. Dissemination of lipid A deacylases (pagL) among gram-negative bacteria: identification of active-site histidine and serine residues. J Biol Chem 280:8248–8259. doi: 10.1074/jbc.M414235200. [DOI] [PubMed] [Google Scholar]
- 16.Pupo E, Hamstra HJ, Meiring H, van der Ley P. 2014. Lipopolysaccharide engineering in Neisseria meningitidis: structural analysis of different pentaacyl lipid A mutants and comparison of their modified agonist properties. J Biol Chem 289:8668–8680. doi: 10.1074/jbc.M114.554345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holten E. 1979. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J Clin Microbiol 9:186–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Ley P, van der Biezen J, Poolman JT. 1995. Construction of Neisseria meningitidis strains carrying multiple chromosomal copies of the porA gene for use in the production of a multivalent outer membrane vesicle vaccine. Vaccine 13:401–407. doi: 10.1016/0264-410X(95)98264-B. [DOI] [PubMed] [Google Scholar]
- 19.Tommassen J, Vermeij P, Struyve M, Benz R, Poolman JT. 1990. Isolation of Neisseria meningitidis mutants deficient in class 1 (porA) and class 3 (porB) outer membrane proteins. Infect Immun 58:1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claassen I, Meylis J, van der Ley P, Peeters C, Brons H, Robert J, Borsboom D, van der Ark A, van Straaten I, Roholl P, Kuipers B, Poolman J. 1996. Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine 14:1001–1008. doi: 10.1016/0264-410X(96)00020-5. [DOI] [PubMed] [Google Scholar]
- 21.Zollinger WD, Mandrell RE, Griffiss JM, Altieri P, Berman S. 1979. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest 63:836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dixon GL, Newton PJ, Chain BM, Katz D, Andersen SR, Wong S, van der Ley P, Klein N, Callard RE. 2001. Dendritic cell activation and cytokine production induced by group B Neisseria meningitidis: interleukin-12 production depends on lipopolysaccharide expression in intact bacteria. Infect Immun 69:4351–4357. doi: 10.1128/IAI.69.7.4351-4357.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sallusto F, Lanzavecchia A. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Arico B, Brunelli B, Pieri A, Santini L, Savino S, Serruto D, Litt D, Kroll S, Welsch JA, Granoff DM, Rappuoli R, Pizza M. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J Exp Med 197:789–799. doi: 10.1084/jem.20021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welsch JA, Rossi R, Comanducci M, Granoff DM. 2004. Protective activity of monoclonal antibodies to genome-derived neisserial antigen 1870, a Neisseria meningitidis candidate vaccine. J Immunol 172:5606–5615. doi: 10.4049/jimmunol.172.9.5606. [DOI] [PubMed] [Google Scholar]
- 26.Massari P, Visintin A, Gunawardana J, Halmen KA, King CA, Golenbock DT, Wetzler LM. 2006. Meningococcal porin PorB binds to TLR2 and requires TLR1 for signaling. J Immunol 176:2373–2380. doi: 10.4049/jimmunol.176.4.2373. [DOI] [PubMed] [Google Scholar]
- 27.Singleton TE, Massari P, Wetzler LM. 2005. Neisserial porin-induced dendritic cell activation is MyD88 and TLR2 dependent. J Immunol 174:3545–3550. doi: 10.4049/jimmunol.174.6.3545. [DOI] [PubMed] [Google Scholar]
- 28.Perrett KP, McVernon J, Richmond PC, Marshall H, Nissen M, August A, Percell S, Toneatto D, Nolan T. 2015. Immune responses to a recombinant, four-component, meningococcal serogroup B vaccine (4CMenB) in adolescents: a phase III, randomized, multicentre, lot-to-lot consistency study. Vaccine doi: 10.1016/j.vaccine.2015.06.103. [DOI] [PubMed] [Google Scholar]
- 29.Keiser PB, Biggs-Cicatelli S, Moran EE, Schmiel DH, Pinto VB, Burden RE, Miller LB, Moon JE, Bowden RA, Cummings JF, Zollinger WD. 2011. A phase 1 study of a meningococcal native outer membrane vesicle vaccine made from a group B strain with deleted lpxL1 and synX, over-expressed factor H binding protein, two PorAs and stabilized OpcA expression. Vaccine 29:1413–1420. doi: 10.1016/j.vaccine.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 30.van der Ley P, van den Dobbelsteen G. 2011. Next-generation outer membrane vesicle vaccines against Neisseria meningitidis based on nontoxic LPS mutants. Hum Vaccin 7:886–890. doi: 10.4161/hv.7.8.16086. [DOI] [PubMed] [Google Scholar]
- 31.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. 2009. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 32.Ohto U, Fukase K, Miyake K, Shimizu T. 2012. Structural basis of species-specific endotoxin sensing by innate immune receptor TLR4/MD-2. Proc Natl Acad Sci U S A 109:7421–7426. doi: 10.1073/pnas.1201193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steeghs L, van Vliet SJ, Uronen-Hansson H, van Mourik A, Engering A, Sanchez-Hernandez M, Klein N, Callard R, van Putten JP, van der Ley P, van Kooyk Y, van de Winkel JG. 2006. Neisseria meningitidis expressing lgtB lipopolysaccharide targets DC-SIGN and modulates dendritic cell function. Cell Microbiol 8:316–325. doi: 10.1111/j.1462-5822.2005.00623.x. [DOI] [PubMed] [Google Scholar]
- 34.Jones HE, Copland A, Hamstra HJ, Cohen J, Brown J, Klein N, van der Ley P, Dixon G. 2014. LOS oligosaccharide modification enhances dendritic cell responses to meningococcal native outer membrane vesicles expressing a non-toxic lipid A. Cell Microbiol 16:519–534. doi: 10.1111/cmi.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biagini M, Garibaldi M, Aprea S, Pezzicoli A, Doro F, Becherelli M, Taddei AR, Tani C, Tavarini S, Mora M, Teti G, D'Oro U, Nuti S, Soriani M, Margarit I, Rappuoli R, Grandi G, Norais N. 2015. The human pathogen Streptococcus pyogenes releases lipoproteins as lipoprotein-rich membrane vesicles. Mol Cell Proteomics 14:2138–2149. doi: 10.1074/mcp.M114.045880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frosch M, Schultz E, Glenn-Calvo E, Meyer TF. 1990. Generation of capsule-deficient Neisseria meningitidis strains by homologous recombination. Mol Microbiol 4:1215–1218. doi: 10.1111/j.1365-2958.1990.tb00697.x. [DOI] [PubMed] [Google Scholar]