Abstract
Background:
Alopecia areata (AA) is a common, recurrent, autoimmune hair disorder. It has been found that vitamin D deficiency is associated with many autoimmune diseases.
Aims:
The current study aimed to estimate serum levels of 25-hydroxy vitamin D in patients with AA.
Materials and Methods:
This case–control study included 60 patients with AA and 60 age, gender, skin phototype, and body mass index-matched healthy subjects as a control group. Levels of serum 25-hydroxy vitamin D were estimated using ELISA technique.
Results:
Serum 25-hydroxy vitamin D levels were significantly lower in AA cases when compared with healthy controls (P < 0.001). The least values were significantly associated with alopecia totalis/universalis compared with patchy AA (P < 0.001) and ophiasis (P = 0.04). Severe AA showed significantly the lowest vitamin D levels compared with cases with mild (P = 0.002) and moderate disease (P = 0.03). A significant inverse correlation was found between 25-hydroxy vitamin D levels and age of the patients (r = −0.38; P = 0.03). There was no significant association between serum 25-hydroxy vitamin D levels and gender, disease duration, disease recurrence, nail affection, duration of sun exposure/day, or positive family history of AA.
Conclusion:
AA patients have lower levels of 25-hydroxy vitamin D than healthy subjects. More studies are required to assess the value of vitamin D supplementation in the treatment of that disease.
Keywords: Alopecia areata, autoimmunity, pathogenesis, Vitamin D
INTRODUCTION
Alopecia areata (AA) is one of the most common skin diseases, leading to chronic and relapsing hair loss. The onset may be at any age and there is no known race or gender preponderance. It usually presents as patches of hair loss on the scalp but any hair-bearing skin may also be involved.[1]
The etiology of AA is not exactly known; however, genetic predisposition, autoimmunity, and environmental factors have been suggested to play a role.[2] Autoimmune etiology has been proposed on the basis of its association with various autoimmune diseases, the presence of autoantibodies, the presence of inflammatory lymphocytes around and within the affected hair follicles, and the ability to promote hair regrowth with the use of immunosuppressive agents.[3]
Vitamin D is a secosteroid hormone that plays an important role in calcium homeostasis and bone health. It has three sources: endogenous synthesis in the skin, which is induced by UVB radiation, dietary intake, and vitamin D supplementation.[4]
Vitamin D is a modulator of both the innate and adaptive immune systems through its varied effects on T and B lymphocytes, dendritic cells (DCs), and macrophages. The connection between vitamin D deficiency and some autoimmune diseases, including type I diabetes mellitus, rheumatoid arthritis, systemic lupus erythematosis, vitiligo, psoriasis, multiple sclerosis, and inflammatory bowel disease has been reported.[5] This suggests that vitamin D deficiency might be an environmental trigger for the induction of autoimmunity.[6]
On the other hand, it has been demonstrated that vitamin D receptors (VDRs) are strongly expressed in the key structures of hair follicles. Expression of VDRs on keratinocytes is necessary for maintenance of the normal hair cycle.[7] It has also been shown that a lack of VDRs reduces epidermal differentiation and hair follicle growth.
[8] Several studies were done to evaluate the role of vitamin D in different hair disorders with contrasting results.[4,9,10,11,12,13,14]
The aim of this work is to assess serum 25-hydroxy vitamin D [25(OH)D] levels in patients with AA in an attempt to evaluate its possible role in the pathogenesis of the disease.
MATERIALS AND METHODS
Studied population
This case–control study was conducted on 60 AA patients and 60 age, gender, and body mass index (BMI)-matched healthy control subjects. Cases were selected during the period from October 2013 to March 2014. All the selected cases and control subjects had skin phototype III or IV.
Diagnosis was made clinically and by dermoscopy. Disease duration was calculated from onset to the time of sampling. For cases with recurrent disease, the duration was calculated from the onset of relapse to the time of sampling.
Clinical data describing patients’ demographics (age and gender) as well as the clinical variables (site, disease recurrence, disease duration, positive family history, and duration of sun exposure/day) were all documented.
Subgrouping of studied AA cases
-
Alopecia areata was graded according to the disease severity into:[15]
- Mild: The presence of three or less patches of alopecia with a maximum diameter of 3 cm or less, or when the disease is limited to the eyelashes and eyebrows
- Moderate: The existence of more than three patches of alopecia, or a patch greater than 3 cm at the widest diameter without alopecia totalis or alopecia universalis
- Severe: Alopecia totalis or alopecia universalis.
Cases were categorized according to the pattern of hair loss into patchy AA, ophiasis and alopecia totalis/universalis.
Exclusion criteria
Patients with a history of topical or systemic treatment (corticosteroids, intralesional steroid injection, immunosuppressive therapy, vitamin D or calcium supplementation, bisphosphonates, hepatic enzyme inducers, anticonvulsants and anticancer medications, and cholesterol-lowering drugs) within 4 weeks of the study, and patients receiving phototherapy within 6 months of the study were excluded. Patients with a history of diabetes mellitus, anemia, thyroid disorders, chronic liver or renal diseases, atopy, parathyroid disorders, patients with known autoimmune diseases or cancer, patients with congenital or acquired errors of calcium or phosphorus metabolism, subjects with inadequate sun exposure and obese subjects were excluded. Pregnant or lactating women, subjects with skin phototypes V, and VI, and smokers were also excluded from the study.
Ethics
A written consent form approved by the Local Ethical Research Committee was obtained from every patient and control subject after the procedure had been fully explained. This was also in accordance with the Helsinki Declaration of 1975 (revised in 2000).
Sample taking
Three milliliters of venous blood were withdrawn from antecubital vein of each participant. Samples were then transferred into a plain tube, left to stand for 30 min, and then centrifuged. All reagents and samples were used at room temperature (18–26°C) and mixed gently to avoid foam formation. The separated serum was used for determination of 25(OH)D.
Serum 25(OH)D concentration, the major circulating form of vitamin D, was measured using commercial Enzyme-Linked Immunosorbent Assay (ELISA) kits (Immunodiagnostic Systems Limited, Bolden, UK). This test is a competitive protein-binding assay for the measurement of 25(OH)D. It is based on competition between 25(OH)D present in the sample and 25(OH)D tracers for the binding pocket of vitamin D-binding protein (VDBP) (Gc-globulin).
Since all circulating 25(OH)D is bound to VDBP in vivo, samples have to be precipitated with precipitation reagent to extract the analyst. The supernatant can be used without further treatment within the test.
The results were calculated from the typical calibration curve given in the ELISA kit's pamphlet.
Interpretation of 25(OH)D level
<50 nmol/L: Deficient, 50–75 nmol/L: Insufficient, >75 nmol/L: Sufficient.[16]
Statistical analysis
Data were collected, tabulated, and statistically analyzed using a personal computer with “(SPSS) version 22” program (SPSS Inc., Chicago, Illinois, USA). Chi-square test and Fisher's exact test were used for comparison of qualitative variables. Mann–Whitney U test and Kruskal–Wallis test were used for comparison between quantitative variables. Spearman's correlation was used to measure the correlation between two quantitative variables. Differences were considered as statistically significant with P values < 0.05.
RESULTS
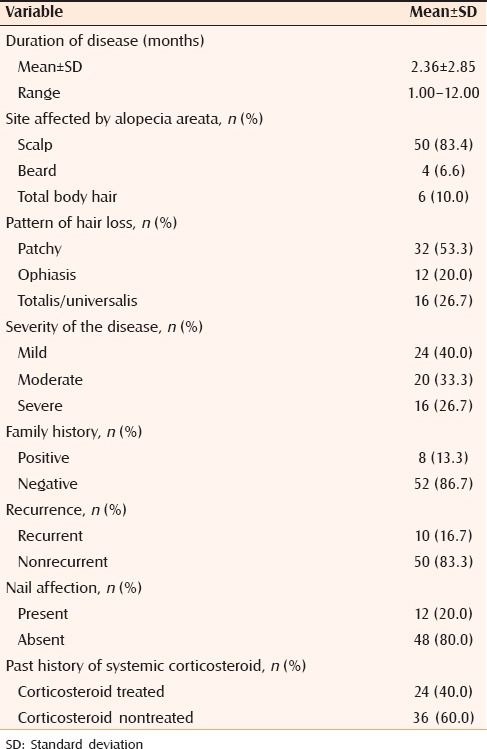
Clinical data of selected cases and control subjects are summarized in Table 1. Disease criteria in the studied cases are summarized in Table 2.
Table 1.
Clinical data of studied cases and control subjects
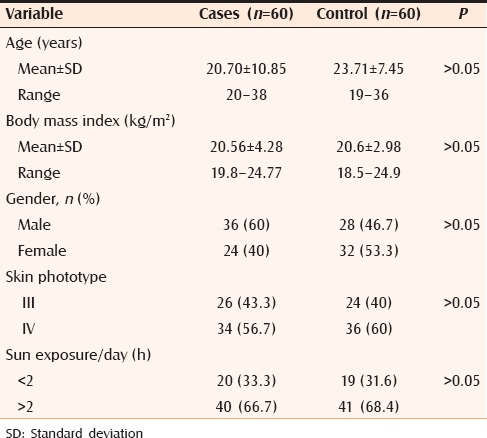
Table 2.
Disease criteria of the studied cases
Serum 25(OH)D in the studied groups
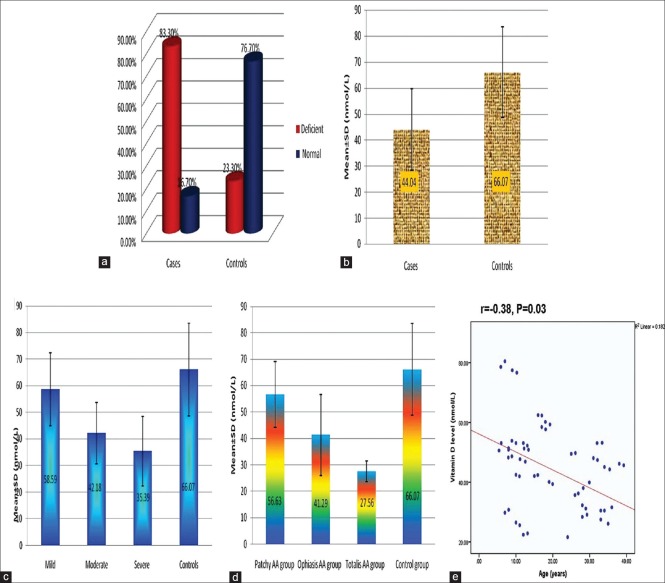
Fifty cases (83.3%) had deficient 25(OH)D level and 10 cases (16.7%) had normal level. 25(OH)D, among cases, ranged from 21.60 to 80.50 nmol/mL with mean ± SD value of 44.04 ± 15.61 nmol/mL. Regarding 25(OH)D level among controls, 14 subjects (23.3%) had deficient and 46 subjects (76.7%) had normal level. 25(OH)D level, among controls, ranged from 29.80 to 95.50 nmol/mL with mean ± SD value of 66.07 ± 17.40 nmol/mL [Figure 1a and b].
Figure 1.
(a, b) Comparison between cases and controls with regard to vitamin D level. (c) Relationship between vitamin D level and severity of hair loss. (d) Relationship between vitamin D level and pattern of hair loss. (e) Correlation between vitamin D level and age of studied cases
There was a significant difference between cases and controls regarding quantitative (P < 0.001) and qualitative (P < 0.001) 25(OH)D level as lower values were significantly associated with cases [Figure 1a and b].
Relationship between serum 25(OH)D level and clinical parameters of studied cases
Regarding the relationship between 25(OH)D level and disease severity, cases with severe AA showed significantly the lowest vitamin level compared with cases with mild (P = 0.002) and moderate disease (P = 0.03) [Figure 1c].
Serum 25(OH)D level showed gradual decline from patchy AA to alopecia totalis/universalis. The least values were significantly associated with alopecia totalis/universalis compared with patchy AA (P < 0.001) and ophiasis (P = 0.04) [Figure 1d].
A significant negative correlation between serum 25(OH)D levels and age of patients was detected (r = −0.38, P = 0.03) [Figure 1e].
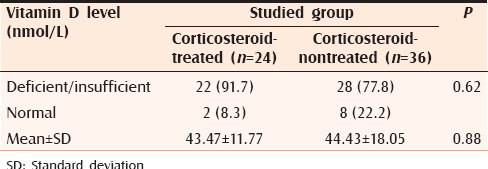
There was no significant difference in serum 25(OH)D levels between patients with previous history of systemic corticosteroid therapy and patients who did not receive systemic corticosteroid treatment [Table 3].
Table 3.
Relationship between Vitamin D levels among the studied cases with regard to previous corticosteroid treatment
There was no significant relationship between the 25(OH)D level and gender of patients, disease duration, nail affection, disease recurrence, duration of sun exposure/day, or family history of AA (data not shown in tables).
DISCUSSION
In the present study, 25(OH)D deficiency was detected in 23.3% of normal subjects. Vitamin D deficiency among normal population was variably detected in different studies. Tangpricha et al. reported vitamin D deficiency in 30% of their studied normal cohort.[17] Mansour et al., demonstrated 90% prevelance of vitamin D deficiency in apparently healthy hospital staff and health care professionals.[18] Zargar et al., found that 82% of healthy subjects had vitamin D defficiency.[19] Osullivan et al., and Al-Kinidireported a high prevalence of vitamin D deficiency in healthy Irish adults and Omani women, respectively.[20,21]
Several conditions may contribute to low serum levels of vitamin D in the general population, including poor dietary intake of vitamin D, sun avoidance and / or negligible sun exposure, possibly related also to impaired quality of life, and malabsorption due to inflammatory bowel disease, gluten enteropathy, gastric surgery, biliary disease, or intestinal bacteria overgrowth.[22]
In the current study, serum vitamin D level was significantly lower in AA cases when compared with healthy controls. This was in agreement with previous similar studies.[4,9,13] This may provide evidence about the role of vitamin D deficiency in AA pathogenesis.
This role was suggested to be through its effects on the hair cycle and the immune system. Vitamin D is a modulator of both the innate and adaptive immunity and inhibits the proinflammatory process in autoimmune reactions. This is brought through its varied effects on T and B lymphocytes, DCs, and macrophages, all of which express VDRs and the vitamin D-activating enzyme, 1α-hydroxylase.[4]
Vitamin D inhibits the differentiation and maturation of DCs, reduces the expression of major histocompatibility complex class II, CD40, CD80, and CD86 and inhibits the secretion of proinflammatory cytokines, including interleukin (IL)-1, IL-2, IL-6, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α.[5,22] It inhibits IL-12, which is an immunostimulatory cytokine crucial for polarizing the immune system toward the Th1 phenotype. Therefore, it shifts the immune response toward the Th2 phenotype. Thus, Th2 cytokine (IL-4, IL-5, and IL-10) secretion increases. It was suggested that inhibition of the Th1 phenotype and potentiation of the Th2 phenotype comprise one of the mechanisms by which vitamin D suppresses Th1-mediated autoimmune diseases.[22,23]
In addition, vitamin D may suppress autoimmune diseases by enhancing the production and function of regulatory T cells. Vitamin D inhibits IL-6 and IL-17 secretion, thus impending Th17 function and differentiation. Th17 cells were shown to play a significant role in autoimmune reactions.[5,24]
VDR is expressed in epidermal keratinocytes, the outer root sheath keratinocytes, and dermal papilla cells of hair follicles.[25] Furthermore, the enzyme for synthesizing 1,25(OH)2D3, 25-hydroxyvitamin D-1α hydroxylase, is expressed in both the basal layer of the epidermis and the matrix cells of hair follicles in the dermis, suggesting that keratinocytes in hair follicles both make and respond to their own 1,25(OH)2D3.[26]
Expression of VDRs in keratinocytes is necessary for maintenance of the normal hair cycle.[7] In both the late anagen and catagen stages, there is an increase in VDR expression, which is associated with decreased proliferation and increased differentiation of keratinocytes. These changes are thought to promote the progression of the hair cycle.[27]
Contrary to the mentioned results, Nassiri et al. reported no significant difference between AA patients and controls with regard to serum 25(OH)D when the data was adjusted for gender.[14] d’Ovidio et al. confirmed the presence of 25(OH)D insufficiency/deficiency in AA patients but the results were not significantly different when compared with controls.[12] This may be due to the high prevalence of vitamin D deficiency in the normal population.
The current study showed gradual decline of 25(OH)D level with increased AA severity with significant difference in the vitamin level between different AA subgroups. This is in line with the study by Cerman et al. who reported an inverse correlation between vitamin D level and AA severity.[4]
Contrary to our results, Yilmaz et al. reported lack of association between 25(OH)D level and disease severity.[9] This controversy can be explained by different sample size, different clinical data of selected cases and geographic differences in sun exposure as serum 25(OH)D exhibits great seasonal variation. Yilmaz et al.'s study was performed during summer, when it is possible that these patients were less exposed outdoors than the healthy population for reasons of psychological distress.[12] In addition, diseases that could compromise serum vitamin D levels were not excluded in their study.
The present study shows that 25(OH)D gradually declined from patchy AA to alopecia totalis/universalis. The least values were present in alopecia totalis/universalis. This conflicted with d’Ovidio et al. and Mahamid et al. who reported lack of association between vitamin D level and AA pattern.[12,13] The cause of variation may be due to unequal number of examined cases in every study, geographic differences in sun exposure and methodological variations.
In current study, there was significant negative correlation between 25(OH)D levels and the age of the patients. On the contrary, Cerman et al. and Yilmaz et al. reported that there was no significant correlation between age and 25(OH)D levels in the studied groups.[4,9] Further large-scaled studies incorporating cases of different ages are needed for a definitive conclusion.
The lack of association between 25(OH)D levels and disease duration reported in the present study was in agreement with others.[4,9]
The present work showed no significant association between 25(OH)D levels and gender of the studied cases. The relationship between serum vitamin D level and patients’ gender is a matter of debate. Cerman et al. and Nassiri et al. reported that vitamin D deficiency was more prevalent among female cases than male cases.[4,14] This may be explained by women's limited exposure to UVR due to religious and social factors and the reduction in outdoor exposure time. On the contrary, d’Ovidio et al. reported that vitamin D deficiency was more prevalent among males.[12]
As different localities, cultural, and dietary habits may affect serum vitamin D levels in both genders, different research outcomes are expected and further large-scaled work is needed for more clarification.
In current study, there was no significant association between 25(OH)D levels and nail affliction. This was in agreement with the results of the study by Yilmaz et al.[9] However, Nassiri et al. reported that the serum level of 25(OH)D was significantly lower in patients with nail involvement when compared with patients without nail involvement.[14]
The current study demonstrated lack of association between serum 25(OH)D level and disease recurrence or family history of AA. There was no comment on these findings in similar studies done previously and more investigation is warranted.
The nonsignificant association between the duration of sun exposure/day and 25(OH)D levels, reported in the current work, was not commented upon earlier in previous similar studies. However, Jacobs et al. proved that sun exposure is not the only determinant of vitamin D status as individuals living at lower latitudes in relatively sunny environments are also at risk of vitamin D insufficiency.[28] Need et al. proved that intentional unprotected sun exposure is unnecessary and inefficient for populations at a risk of vitamin D deficiency.[29] This may be due to the fact that there is a threshold dose of UVB required to induce vitamin D production. The exact amount of UVB required for optimal vitamin D levels is not known as it will be affected by several factors.[30,31,32,33] Conversely, Giovannucci et al. postulated that the duration of sun exposure positively correlated with vitamin D concentrations.[34] More investigation is needed to prove or deny the current observation.
From the aforementioned results, a question arises; what is the place of vitamin D in AA treatment? Kim et al. reported successful treatment of a case of AA with topical calcipotriol, a strong vitamin D analog.[35] This was explained by the upregulation of VDR expression in the lesion. However, further studies are needed to assess the effectiveness of this therapeutic modality in a greater number of patients. In addition, systemic vitamin D supplementation had been shown to be therapeutically effective in different autoimmune diseases, such as inflammatory bowel disease, Behcet's disease, rheumatoid arthritis, and systemic lupus erythematosus.[6,36,37] Therefore, as we do not know at present whether vitamin D supplementation would be effective in the therapy of AA or not; further clinical trials are needed to evaluate its efficacy in treating this common dermatosis.
CONCLUSION
Serum 25(OH)D levels is lower in patients with AA than healthy controls. These levels were negatively correlated with disease severity and pattern of hair loss. These data may provide evidence about the role of vitamin D deficiency in the pathogenesis of the disease. Future studies are needed to clarify the association between 25(OH)D and nail affection, disease recurrence, duration of sun exposure/day, and a positive family history. Clinical trials to evaluate the use of vitamin D in disease treatment are also needed.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare no conflict of interest.
REFERENCES
- 1.Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med. 2012;366:1515–25. doi: 10.1056/NEJMra1103442. [DOI] [PubMed] [Google Scholar]
- 2.Islam N, Leung PS, Huntley AC, Gershwin ME. The autoimmune basis of alopecia areata: A comprehensive review. Autoimmun Rev. 2015;14:81–9. doi: 10.1016/j.autrev.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Bakry OA, El Shazly RM, Basha MA, Mostafa H. Total serum immunoglobulin E in patients with alopecia areata. Indian Dermatol Online J. 2014;5:122–7. doi: 10.4103/2229-5178.131076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aksu Cerman A, Sarikaya Solak S, Kivanc Altunay I. Vitamin D deficiency in alopecia areata. Br J Dermatol. 2014;170:1299–304. doi: 10.1111/bjd.12980. [DOI] [PubMed] [Google Scholar]
- 5.Hewison M. An update on Vitamin D and human immunity. Clin Endocrinol (Oxf) 2012;76:315–25. doi: 10.1111/j.1365-2265.2011.04261.x. [DOI] [PubMed] [Google Scholar]
- 6.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: New aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66:1137–42. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikle DD. Vitamin D metabolism and function in the skin. Mol Cell Endocrinol. 2011;347:80–9. doi: 10.1016/j.mce.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malloy PJ, Feldman D. The role of Vitamin D receptor mutations in the development of alopecia. Mol Cell Endocrinol. 2011;347:90–6. doi: 10.1016/j.mce.2011.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yilmaz N, Serarslan G, Gokce C. Vitamin D concentrations are decreased in patients with alopecia areata. Vitam Trace Elem. 2012;1:1–4. [Google Scholar]
- 10.Iyanda AA. Serum Vitamin levels in different categories of androgenetic alopecia subjects. Open Access Sci Rep. 2010;1:137–41. [Google Scholar]
- 11.Rasheed H, Mahgoub D, Hegazy R, El-Komy M, Abdel Hay R, Hamid MA, et al. Serum ferritin and Vitamin D in female hair loss: Do they play a role? Skin Pharmacol Physiol. 2013;26:101–7. doi: 10.1159/000346698. [DOI] [PubMed] [Google Scholar]
- 12.d’Ovidio R, Vessio M, d’Ovidio FD. Reduced level of 25-hydroxyvitamin D in chronic/relapsing alopecia areata. Dermatoendocrinol. 2013;5:271–3. doi: 10.4161/derm.24411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahamid M, Abu-Elhija O, Samamra M, Mahamid A, Nseir W. Association between Vitamin D levels and alopecia areata. Isr Med Assoc J. 2014;16:367–70. [PubMed] [Google Scholar]
- 14.Nassiri S, Saffarian Z, Younespour S. Association of Vitamin D level with alopecia areata. Iran J Dermatol. 2013;16:1–5. [Google Scholar]
- 15.Kavak A, Baykal C, Ozarmagan G, Akar U. HLA in alopecia areata. Int J Dermatol. 2000;39:589–92. doi: 10.1046/j.1365-4362.2000.00921.x. [DOI] [PubMed] [Google Scholar]
- 16.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of Vitamin D deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 17.Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112:659–62. doi: 10.1016/s0002-9343(02)01091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansoor S, Habib A, Ghani F, Fatmi Z, Badruddin S, Mansoor S, et al. Prevalence and significance of Vitamin D deficiency and insufficiency among apparently healthy adults. Clin Biochem. 2010;43:1431–5. doi: 10.1016/j.clinbiochem.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 19.Zargar AH, Ahmad S, Masoodi SR, Wani AI, Bashir MI, Laway BA, et al. Vitamin D status in apparently healthy adults in Kashmir valley of Indian subcontinent. Postgrad Med J. 2007;83:713–6. doi: 10.1136/pgmj.2007.059113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Sullivan M, Nic Suibhne T, Cox G, Healy M, O’Morain C. High prevalence of Vitamin D insufficiency in healthy Irish adults. Ir J Med Sci. 2008;177:131–4. doi: 10.1007/s11845-008-0129-z. [DOI] [PubMed] [Google Scholar]
- 21.Al-Kindi MK. Vitamin D status in healthy Omani women of childbearing age: Study of female staff at the Royal hospital, Muscat, Oman. Sultan Qaboos Univ Med J. 2011;11:56–61. [PMC free article] [PubMed] [Google Scholar]
- 22.DeLuca HF. Overview of general physiologic features and functions of Vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S–96S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 23.Ersoy-Evans S. Commentary: Vitamin D and autoimmunity: Is there an association? J Am Acad Dermatol. 2010;62:942–4. doi: 10.1016/j.jaad.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: A helpful immuno-modulator. Immunology. 2011;134:123–39. doi: 10.1111/j.1365-2567.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reichrath J, Schilli M, Kerber A, Bahmer FA, Czarnetzki BM, Paus R. Hair follicle expression of 1,25-dihydroxyvitamin D3 receptors during the murine hair cycle. Br J Dermatol. 1994;131:477–82. doi: 10.1111/j.1365-2133.1994.tb08547.x. [DOI] [PubMed] [Google Scholar]
- 26.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–94. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 27.Amor KT, Rashid RM, Mirmirani P. Does D matter? The role of Vitamin D in hair disorders and hair follicle cycling. Dermatol Online J. 2010;16:3. [PubMed] [Google Scholar]
- 28.Jacobs ET, Alberts DS, Foote JA, Green SB, Hollis BW, Yu Z, et al. Vitamin D insufficiency in Southern Arizona. Am J Clin Nutr. 2008;87:608–13. doi: 10.1093/ajcn/87.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Need AG, Morris HA, Horowitz M, Nordin C. Effects of skin thickness, age, body fat, and sunlight on serum 25-hydroxyvitamin D. Am J Clin Nutr. 1993;58:882–5. doi: 10.1093/ajcn/58.6.882. [DOI] [PubMed] [Google Scholar]
- 30.Highwood EJ, Kinnersley RP. When smoke gets in our eyes: The multiple impacts of atmospheric black carbon on climate, air quality and health. Environ Int. 2006;32:560–6. doi: 10.1016/j.envint.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Webb AR, Engelsen O. Ultraviolet exposure scenarios: Risks of erythema from recommendations on cutaneous Vitamin D synthesis. Adv Exp Med Biol. 2014;810:406–22. [PubMed] [Google Scholar]
- 32.Engelsen O, Brustad M, Aksnes L, Lund E. Daily duration of Vitamin D synthesis in human skin with relation to latitude, total ozone, altitude, ground cover, aerosols and cloud thickness. Photochem Photobiol. 2005;81:1287–90. doi: 10.1562/2004-11-19-RN-375. [DOI] [PubMed] [Google Scholar]
- 33.Holick MF. The cutaneous photosynthesis of previtamin D3: A unique photoendocrine system. J Invest Dermatol. 1981;77:51–8. doi: 10.1111/1523-1747.ep12479237. [DOI] [PubMed] [Google Scholar]
- 34.Giovannucci E. The epidemiology of Vitamin D and colorectal cancer: Recent findings. Curr Opin Gastroenterol. 2006;22:24–9. doi: 10.1097/01.mog.0000196150.36701.c2. [DOI] [PubMed] [Google Scholar]
- 35.Kim DH, Lee JW, Kim IS, Choi SY, Lim YY, Kim HM, et al. Successful treatment of alopecia areata with topical calcipotriol. Ann Dermatol. 2012;24:341–4. doi: 10.5021/ad.2012.24.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antico A, Tampoia M, Tozzoli R, Bizzaro N. Can supplementation with Vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun Rev. 2012;12:127–36. doi: 10.1016/j.autrev.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Abou-Raya A, Abou-Raya S, Helmii M. The effect of Vitamin D supplementation on inflammatory and hemostatic markers and disease activity in patients with systemic lupus erythematosus: A randomized placebo-controlled trial. J Rheumatol. 2013;40:265–72. doi: 10.3899/jrheum.111594. [DOI] [PubMed] [Google Scholar]