Abstract
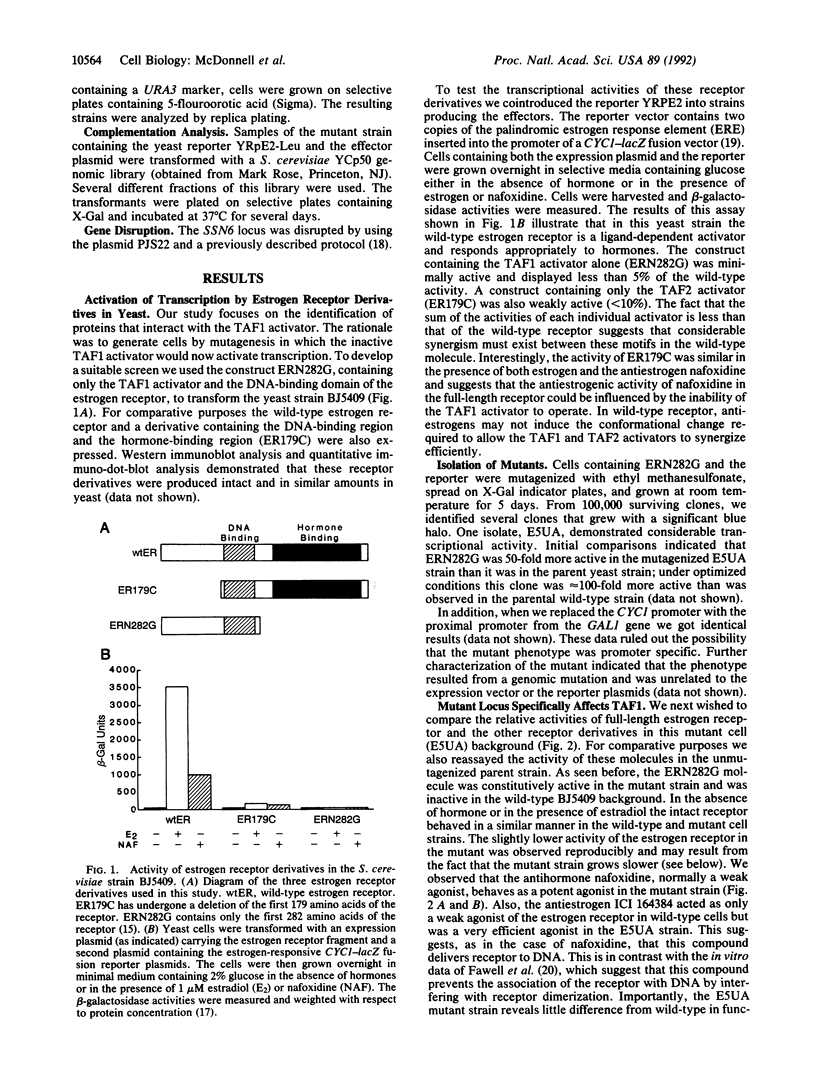
This report describes the identification of a negative regulator of estrogen and progesterone receptor function. Using a reconstituted estrogen-responsive transcription system in Saccharomyces cerevisiae, we have identified a "repressor function," which when mutated, increases the transcriptional activity of the estrogen and progesterone receptors. In the case of the estrogen receptor this mutation increases the sensitivity of estrogen-mediated activation by at least four orders of magnitude. Analysis of derivatives of the estrogen receptor indicated that this repressor specifically affects the transcription activity of the TAF1 activation domain of the estrogen receptor. The repressor was cloned by complementation and identified as SSN6, a previously described mediator of glucose repression in yeast. Our results indicate that SSN6 is likely to be involved also in the repression of other cellular activators. Interestingly, deletion of the SSN6 protein allows the antiestrogens ICI 164384 and nafoxidine to behave as more potent agonists of estrogen receptor function, while RU486 also becomes a more potent activator of progesterone receptor function. These data suggest that in wild-type cells the role of hormone is twofold: it promotes DNA binding of the receptor and it also induces a conformational change in the receptor which overcomes the effects of this repressor function.
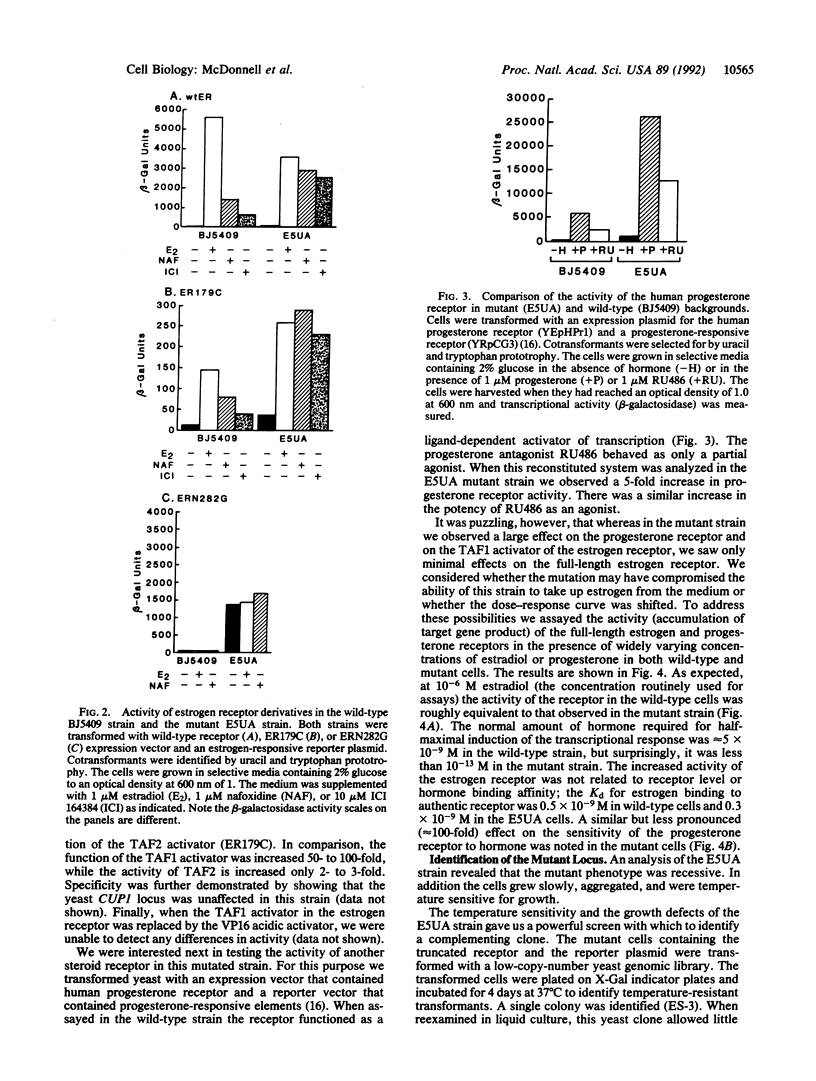
Full text
PDF
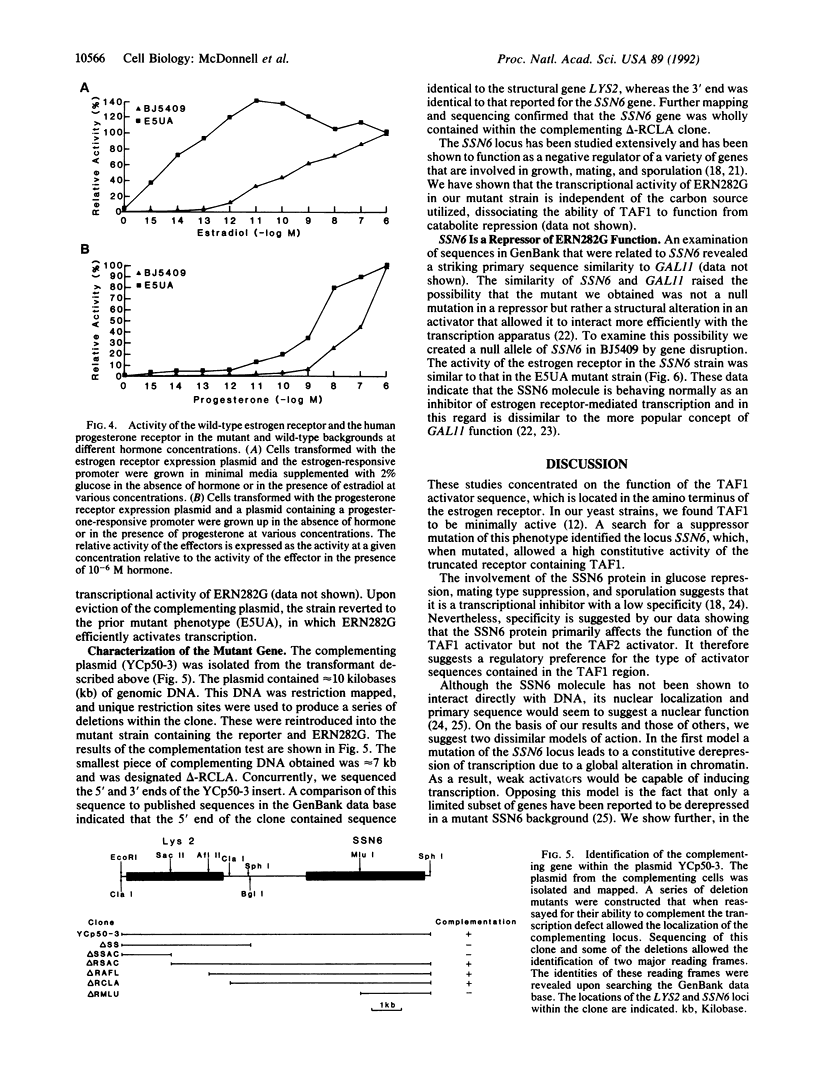
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
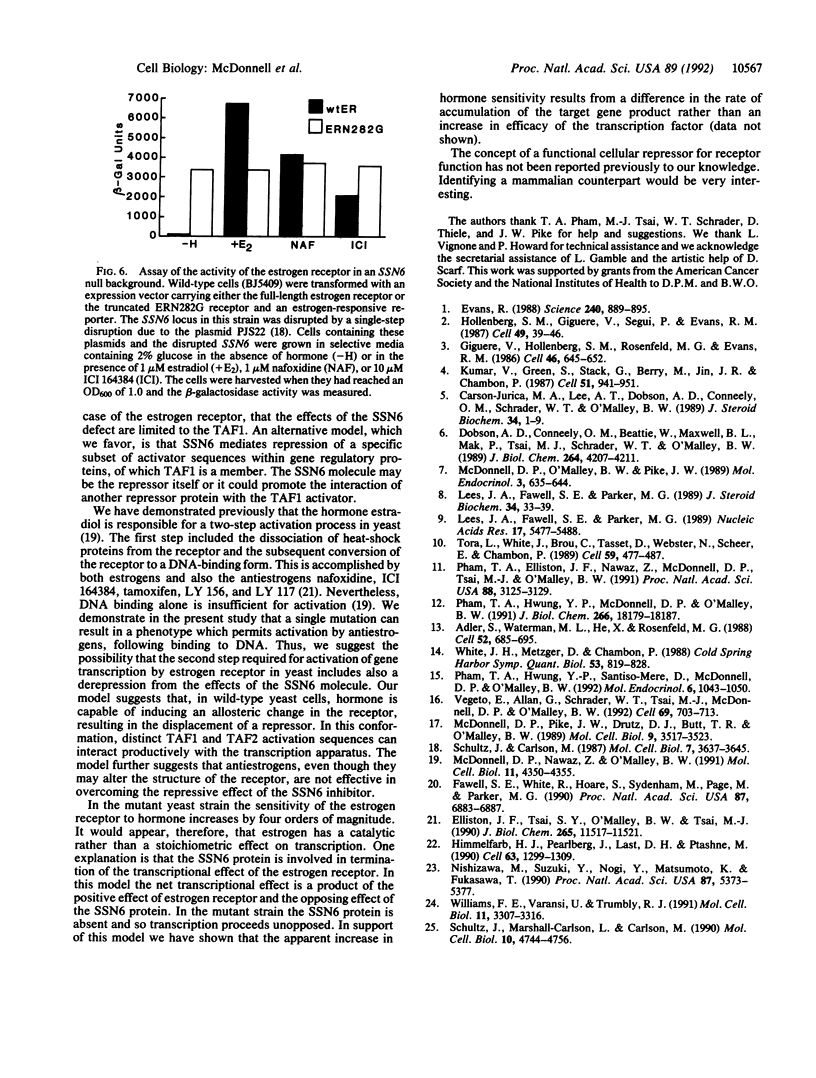
- Adler S., Waterman M. L., He X., Rosenfeld M. G. Steroid receptor-mediated inhibition of rat prolactin gene expression does not require the receptor DNA-binding domain. Cell. 1988 Mar 11;52(5):685–695. doi: 10.1016/0092-8674(88)90406-0. [DOI] [PubMed] [Google Scholar]
- Carson-Jurica M. A., Lee A. T., Dobson A. W., Conneely O. M., Schrader W. T., O'Malley B. W. Interaction of the chicken progesterone receptor with heat shock protein (HSP) 90. J Steroid Biochem. 1989;34(1-6):1–9. doi: 10.1016/0022-4731(89)90060-5. [DOI] [PubMed] [Google Scholar]
- Dobson A. D., Conneely O. M., Beattie W., Maxwell B. L., Mak P., Tsai M. J., Schrader W. T., O'Malley B. W. Mutational analysis of the chicken progesterone receptor. J Biol Chem. 1989 Mar 5;264(7):4207–4211. [PubMed] [Google Scholar]
- Elliston J. F., Tsai S. Y., O'Malley B. W., Tsai M. J. Superactive estrogen receptors. Potent activators of gene expression. J Biol Chem. 1990 Jul 15;265(20):11517–11521. [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell S. E., White R., Hoare S., Sydenham M., Page M., Parker M. G. Inhibition of estrogen receptor-DNA binding by the "pure" antiestrogen ICI 164,384 appears to be mediated by impaired receptor dimerization. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6883–6887. doi: 10.1073/pnas.87.17.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère V., Hollenberg S. M., Rosenfeld M. G., Evans R. M. Functional domains of the human glucocorticoid receptor. Cell. 1986 Aug 29;46(5):645–652. doi: 10.1016/0092-8674(86)90339-9. [DOI] [PubMed] [Google Scholar]
- Himmelfarb H. J., Pearlberg J., Last D. H., Ptashne M. GAL11P: a yeast mutation that potentiates the effect of weak GAL4-derived activators. Cell. 1990 Dec 21;63(6):1299–1309. doi: 10.1016/0092-8674(90)90425-e. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Giguere V., Segui P., Evans R. M. Colocalization of DNA-binding and transcriptional activation functions in the human glucocorticoid receptor. Cell. 1987 Apr 10;49(1):39–46. doi: 10.1016/0092-8674(87)90753-7. [DOI] [PubMed] [Google Scholar]
- Kumar V., Green S., Stack G., Berry M., Jin J. R., Chambon P. Functional domains of the human estrogen receptor. Cell. 1987 Dec 24;51(6):941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- Lees J. A., Fawell S. E., Parker M. G. Identification of constitutive and steroid-dependent transactivation domains in the mouse oestrogen receptor. J Steroid Biochem. 1989;34(1-6):33–39. doi: 10.1016/0022-4731(89)90063-0. [DOI] [PubMed] [Google Scholar]
- Lees J. A., Fawell S. E., Parker M. G. Identification of two transactivation domains in the mouse oestrogen receptor. Nucleic Acids Res. 1989 Jul 25;17(14):5477–5488. doi: 10.1093/nar/17.14.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell D. P., Nawaz Z., O'Malley B. W. In situ distinction between steroid receptor binding and transactivation at a target gene. Mol Cell Biol. 1991 Sep;11(9):4350–4355. doi: 10.1128/mcb.11.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell D. P., Pike J. W., Drutz D. J., Butt T. R., O'Malley B. W. Reconstitution of the vitamin D-responsive osteocalcin transcription unit in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Aug;9(8):3517–3523. doi: 10.1128/mcb.9.8.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell D. P., Scott R. A., Kerner S. A., O'Malley B. W., Pike J. W. Functional domains of the human vitamin D3 receptor regulate osteocalcin gene expression. Mol Endocrinol. 1989 Apr;3(4):635–644. doi: 10.1210/mend-3-4-635. [DOI] [PubMed] [Google Scholar]
- Nishizawa M., Suzuki Y., Nogi Y., Matsumoto K., Fukasawa T. Yeast Gal11 protein mediates the transcriptional activation signal of two different transacting factors, Gal4 and general regulatory factor I/repressor/activator site binding protein 1/translation upstream factor. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5373–5377. doi: 10.1073/pnas.87.14.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham T. A., Elliston J. F., Nawaz Z., McDonnell D. P., Tsai M. J., O'Malley B. W. Antiestrogen can establish nonproductive receptor complexes and alter chromatin structure at target enhancers. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3125–3129. doi: 10.1073/pnas.88.8.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham T. A., Hwung Y. P., McDonnell D. P., O'Malley B. W. Transactivation functions facilitate the disruption of chromatin structure by estrogen receptor derivatives in vivo. J Biol Chem. 1991 Sep 25;266(27):18179–18187. [PubMed] [Google Scholar]
- Pham T. A., Hwung Y. P., Santiso-Mere D., McDonnell D. P., O'Malley B. W. Ligand-dependent and -independent function of the transactivation regions of the human estrogen receptor in yeast. Mol Endocrinol. 1992 Jul;6(7):1043–1050. doi: 10.1210/mend.6.7.1508220. [DOI] [PubMed] [Google Scholar]
- Schultz J., Carlson M. Molecular analysis of SSN6, a gene functionally related to the SNF1 protein kinase of Saccharomyces cerevisiae. Mol Cell Biol. 1987 Oct;7(10):3637–3645. doi: 10.1128/mcb.7.10.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J., Marshall-Carlson L., Carlson M. The N-terminal TPR region is the functional domain of SSN6, a nuclear phosphoprotein of Saccharomyces cerevisiae. Mol Cell Biol. 1990 Sep;10(9):4744–4756. doi: 10.1128/mcb.10.9.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora L., White J., Brou C., Tasset D., Webster N., Scheer E., Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989 Nov 3;59(3):477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Vegeto E., Allan G. F., Schrader W. T., Tsai M. J., McDonnell D. P., O'Malley B. W. The mechanism of RU486 antagonism is dependent on the conformation of the carboxy-terminal tail of the human progesterone receptor. Cell. 1992 May 15;69(4):703–713. doi: 10.1016/0092-8674(92)90234-4. [DOI] [PubMed] [Google Scholar]
- White J. H., Metzger D., Chambon P. Expression and function of the human estrogen receptor in yeast. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):819–828. doi: 10.1101/sqb.1988.053.01.093. [DOI] [PubMed] [Google Scholar]
- Williams F. E., Varanasi U., Trumbly R. J. The CYC8 and TUP1 proteins involved in glucose repression in Saccharomyces cerevisiae are associated in a protein complex. Mol Cell Biol. 1991 Jun;11(6):3307–3316. doi: 10.1128/mcb.11.6.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]



