Abstract
Core social interaction behaviors were examined in young children 0–36 months of age who were hospitalized for accidental (n = 61) or inflicted (n = 64) traumatic brain injury (TBI) in comparison to typically developing children (n = 60). Responding to and initiating gaze and joint attention (JA) were evaluated during a semi-structured sequence of social interactions between the child and an examiner at 2 and 12 months after injury. The accidental TBI group established gaze less often and had an initial deficit initiating JA that resolved by the follow-up. Contrary to expectation, children with inflicted TBI did not have lower rates of social engagement than other groups. Responding to JA was more strongly related than initiating JA to measures of injury severity and to later cognitive and social outcomes. Compared to complicated-mild/moderate TBI, severe TBI in young children was associated with less responsiveness in social interactions and less favorable caregiver ratings of communication and social behavior. JA response, family resources, and group interacted to predict outcomes. Children with inflicted TBI who were less socially responsive and had lower levels of family resources had the least favorable outcomes. Low social responsiveness after TBI may be an early marker for later cognitive and adaptive behavior difficulties.
Keywords: Joint attention, Gaze, Developmental outcome, Social cognition, High risk, Child abuse, Communication, Adaptive behavior, Cognition, Infants
INTRODUCTION
For children who acquire brain injury early in life, identification of early markers of later neurobehavioral outcomes is critically important to guide efforts to detect and intervene with children at high risk for adverse outcomes. There is a paucity of studies examining social behavior and development in infants and young children with TBI. Using a developmental social cognitive approach, the present study examines whether accidental (aTBI) or inflicted (iTBI) traumatic brain injury disrupts the development of two core social interaction behaviors: mutual gaze and joint attention (JA). We examine whether these core social behaviors are influenced by the family’s access to resources and if they predict cognitive and social outcomes during the first year after injury.
Accidental and Inflicted Traumatic Brain Injury in Early Childhood
Infants and preschoolers are particularly vulnerable to the effects of early diffuse brain injury (St. James-Roberts, 1979). Both aTBI and iTBI sustained early in life are associated with less favorable developmental outcomes than in older children and adolescents; young children with iTBI have the poorest outcomes (Ewing-Cobbs et al., 1998; Keenan, Runyan, & Nocera, 2006). The iTBI is caused by shaking or impact injuries and occurs most commonly in children who are less than 2 years old (Duhaime, Christian, Rorke, & Zimmerman, 1998). The acceleration and deceleration forces cause wide-spread injury, including traumatic axonal injury, hemorrhages in both brain parenchyma and extracerebral spaces, as well as hypoxic-ischemic injury (Bruce & Zimmerman, 1989; Duhaime et al., 1987; Ewing-Cobbs et al., 2000; Hahn et al., 1988; Hymel, Rumack, Hay, Strain, & Jenny, 1997). The quality of developmental outcome is poorer in young children with iTBI than would be predicted based on magnetic resonance imaging findings of tissue damage and on the level of impairment in consciousness (Ewing-Cobbs et al., 1998; Keenan, Hooper, Wetherington, Nocera, & Runyan, 2007).
Social Outcomes after TBI in Young Children
As noted by Yeates and colleagues (this issue), research into the causes and correlates of social problems following pediatric TBI has lagged behind research examining cognitive and academic outcomes. Few studies have used methods validated in studies of social development; most studies rely on caregiver ratings and few studies have used direct observation of social behaviors (Yeates et al., 2007). In infants and preschoolers, studies using direct assessment of social behavior found that an array of behaviors, including joint attention (JA), gestures, and verbal communication, were affected by both iTBI and aTBI (Ewing-Cobbs et al., 2012; Landry, Swank, Steubing, Prasad, & Ewing-Cobbs, 2004).
Studies using tests and ratings of social development after preschool TBI found that social cognition was disrupted in children injured between 5 and 7 years of age; difficulties were noted on theory of mind tasks requiring intentional thinking and perspective-taking (Walz, Yeates, Wade, & Mark, 2009; Walz, Yeates, Taylor, Stancin, & Wade, 2010). In preschool-aged children, TBI has been linked to reduction in social competence and social perspective taking (Ganesalingam et al., 2011; Walz et al., 2010). Parent ratings from subacute to chronic stages of outcome indicated social problems, attention-deficit hyperactivity problems, and externalizing behaviors in preschoolers with a broad range of injury severity (Anderson et al., 2006, 1997; Beauchamp et al., 2009; Chapman et al., 2010; Goldstrohm & Arffa, 2005).
Development of Early Social Interaction: Joint Attention
Children with early brain injury may be particularly vulnerable to impairments in social interaction and communication skills. Gaze and JA are important indicators of appropriate social development (Butterworth, 1995; Carpenter, Pennington, & Rogers, 2002; Leung & Rheingold, 1981). Developmentally, early visual attention and gaze behaviors appear at around 3 to 6 months (D’Entremont & Seamans, 2007). Gaze, which involves establishing social engagement and visual attention through orienting or shifting attention to look at or follow the gaze of another person, is a foundational skill supporting later developing JA (Frischen, Bayliss, & Tipper, 2007), which in turn is a foundational skill for a variety of later-developing abilities and behaviors, including general cognitive, social communication, and psychological adjustment (Carpenter, Nagell, & Tomasello, 1998; Charman et al., 2000; Mundy & Jarrold, 2010; Scaife & Bruner, 1975).
JA has been examined in terms of the child’s ability to respond to the social overture of another person and to initiate a JA interaction. Responding to JA involves following the gaze and/or gestures of another person to share a common reference point (Scaife & Bruner, 1975). Initiating JA involves the child’s use of gestures and gaze to engage another person’s attention and direct it toward objects, actions, or themselves (Landry et al., 2004; Mundy, 2003).
JA may indicate emerging understanding of social signals and understanding of the mental states of other people (Hoehl et al., 2009; Scaife & Bruner, 1975). Several core features of JA, including the reward involved in sharing an experience with another person, may predict or contribute to later social cognition and competence (Mundy & Jarrold, 2010; Vaughan Van Hecke et al., 2007). Additionally, JA involves mechanisms of executive control, including attention-shifting and inhibition, which appear to be related to social cognition and socially competent behavior (Mundy, 2003). JA has been shown to enhance several abilities, including new learning (Hirotani, Stets, Striano, & Friederici, 2009) as well as problem solving and independent thinking skills (Tomasello, Carpenter, Call, Behne, & Moll, 2005; Vygotsky, 1978).
Current Study
There are few studies examining social competencies in young children with TBI. Consequently, there is minimal information regarding early markers of later social difficulties and limited understanding of variables shaping the long-term trajectory of development of social skills. To investigate the impact of TBI on core social interaction behaviors, we completed a 1-year longitudinal study examining change in mutual gaze and JA in young children with either iTBI or aTBI relative to typically developing comparison children. We also investigated whether the frequency of JA interactions soon after the injury predicted later development of cognitive ability and everyday social and communication competence. The following hypotheses were examined:
Gaze and JA were expected to vary by group and to increase during the 1-year follow-up. We hypothesized that the highest proportion of social initiation and response behaviors would be observed in the healthy comparison children and the lowest proportion in children with iTBI.
Family resources before the injury were expected to moderate the relation of group with initiating and responding to social overtures.
JA, group membership, and family resources were expected to predict subsequent development of general cognitive ability and caregiver ratings of everyday communication and social skills.
In children with TBI, injury severity and cause of injury were expected to be related to JA and to social and communication skills.
METHODS
Participants
Gaze and JA were evaluated prospectively in 185 children 0 to 3 years of age who were enrolled in one of two cohorts recruited prospectively from 1994 to 1998 or 2001 to 2006 and were followed in longitudinal studies of neurobehavioral outcome following acquired brain injury. Sixty children were healthy community comparison participants. The remaining 125 children were hospitalized at Children’s Memorial Hermann Hospital or Texas Children’s Hospital in Houston, Texas following a TBI sustained at ages 1–36 months. For children with TBI, inclusionary criteria were: (1) mild, moderate, or severe TBI, (2) no known premorbid neurologic or metabolic disorder, (3) no history of prior TBI, and (4) gestational age ≥32 weeks. Comparison children met criteria 2–4 and were recruited from well-child clinics and community notices. Table 1 provides demographic and developmental variables for each group.
Table 1.
Comparison of demographic and developmental variables by group
| Group |
|||
|---|---|---|---|
| Comparison (n = 60) |
Inflicted TBI (n = 64) |
Accidental TBI (n = 61) |
|
| Demographic variables | |||
| Months of age at testing M (SD) | 11.7 (8.6) | 9.8 (8.0) | 12.6 (10.3) |
| Gender F/M (n) | 31/29 | 32/32 | 25/36 |
| Ethnicity (n) | |||
| African-American | 25 | 17 | 11 |
| Hispanic | 13 | 19 | 21 |
| White | 16 | 23 | 23 |
| Other/multiethnic | 6 | 5 | 6 |
| Socioeconomic status (n) | |||
| 1 (High) | 3 | 1 | 2 |
| 2 | 16 | 8 | |
| 3 | 8 | 17 | 16 |
| 4 | 20 | 25 | |
| 5 (Low) | 13 | 13 | 15 |
| Developmental variables | |||
| Family Resource Scale M (SD) | (n = 58) 120.4 (20.3) |
(n = 50) 125.7 (21.1) |
(n = 56) 119.05 (21.6) |
| Bayley Mental Development Index* M (SD) | (n = 60) 95.7 (10.8) |
(n = 55) 81.0 (17.2) |
(n = 59) 90.7 (14.6) |
| Vineland Adaptive Behavior Scales M (SD) | (n = 38) | (n = 44) | (n = 45) |
| Communication* | 99.7 (10.2) | 87.5 (11.0) | 95.0 (12.7) |
| Social | 99.6 (10.0) | 95.0 (11.7) | 97.3 (12.3) |
Note.
p<.01;
TBI = traumatic brain injury, M = mean, SD = standard deviation.
TBI was categorized as inflicted (n = 64) or accidental (n = 61) based on the determination of the Child Protection Team at each hospital and the county Department of Protective and Regulatory Services. At the baseline assessment, 32 children in the iTBI group were residing with their biological parents, 18 were placed with other family members, and 14 were in foster care.
Injury information is provided in Table 2. The severity of TBI was determined using the lowest post-resuscitation Glasgow Coma Scale (GCS) score (Teasdale & Jennett, 1974), and acute computed tomography or brain magnetic resonance imaging findings. Because the GCS was developed for adults, the motor and verbal scales were modified to accommodate the behavioral capabilities of children from birth through 35 months of age (Ewing-Cobbs et al., 1997, 1998). Severity groups were characterized as follows (Levin et al., 2008): mild—GCS = 13–15 with normal neuroimaging; complicated-mild—GCS = 13–15 with findings of parenchymal injury; moderate and severe—GCS = 9–12 and 3–8, respectively, with or without positive imaging findings. The duration of impaired consciousness was defined as the number of days that a child’s modified motor score was below 6.
Table 2.
Injury information for traumatic brain injury participants
| Group |
||
|---|---|---|
| Inflicted TBI (n = 64) |
Accidental TBI (n = 61) |
|
| Months of age at injury M (SD)* | 8.0 (7.9) | 11.3 (10.5) |
| Reported external cause of injury (n)* | ||
| Fall | 17 | 33 |
| Vehicle-related | 0 | 21 |
| Assault | 41 | 1 |
| Sports/other | 0 | 5 |
| No history | 6 | 1 |
| Glasgow Coma Scale score (n) | ||
| 3–8 | 19 | 15 |
| 9–12 | 12 | 4 |
| 13–15 | 33 | 42 |
| Severity grouping (n) | ||
| Mild | 1 | 3 |
| Complicated-mild/moderate | 44 | 43 |
| Severe | 19 | 15 |
| Days of impaired consciousness M (SD) | 2.7 (6.5) | 1.3 (3.5) |
Note.
p< .05.
Procedure
Written informed consent to participate was obtained from the children’s guardians. The protocol was approved by the Institutional Review Board at each medical school and affiliated hospital. To evaluate change in social behaviors, neuropsychological evaluations were conducted 2 and 12 months after the injury. Each child was evaluated individually by a trained examiner as part of a longitudinal study examining the development of general cognitive abilities, executive functions and social communication behaviors. Social interaction procedures were videotaped and coded by trained staff.
Family, cognitive, and social communication measures
The Family Resource Scale (Dunst & Leet, 1987) is a 31-item self-report questionnaire using a 5-point scale to rate the adequacy of tangible and intangible resources, including food, shelter, financial, transportation, health care, child care, and leisure time. The Family Resource Scale was administered at the initial visit to reflect the biological family’s resources just before the injury or enrollment. Fourteen caregivers in the iTBI group did not complete this scale; the majority were cases in which children had been removed from the home.
Long-term cognitive and adaptive behavior outcomes were assessed at the 1-year follow-up using the Bayley Scales of Infant Development-II (Bayley, 1993) Mental Development Index and the Vineland Adaptive Behavior Scales-Interview Edition (Sparrow, Balla, & Cicchetti, 1984) Social and Communication standard scores.
Social interaction outcomes were assessed using observational analyses during a semi-structured sequence of social interactions between the child and an examiner that occurred within the context of toy play. The examiner made a series of social exchanges with the child for 5min. A social exchange consisted of three steps: (1) the examiner says something to the child with or without a gesture (the social request), and then pauses to give the child an opportunity to (2) respond and then, (3) initiate. Each social request is one test item even though it may consist of multiple phases. Requests included both verbal and gestural attention-directing techniques (e.g., Examiner says “Look” then shifts gaze and gestures toward a toy). Using a time-sampling approach, the examiner pauses for 10 s following a social request. The first 5 s are the response time, and the second 5 s are coded as initiating time. Behaviors beginning in the response phase and extending into the initiate phase were coded as responding. Initiate behaviors were characterized by a distinct break in the child’s vocalization, gaze, or movement.
Gaze and JA were coded as present or absent during the respond and initiate steps of each social exchange. Gaze response was coded when the child responded to the examiner’s bid for attention by looking at the examiner’s face. Gaze initiate occurred when the child established eye contact during the last 5 s of each social exchange. JA was coded when the child and examiner shared attention to the same object/s. In the respond condition, the child looked at an object after the examiner directed attention to it; in the initiate condition, the child linked an object to the examiner’s attention and back to the object, looking either from the toy to the examiner to the toy, or from the examiner to the toy to the examiner. Coded responses ranged from 0 (no attention) to 1 (joint attention). Additional information on the validation and reliability of the scoring system may be found in Landry (Landry, Miller-Loncar, Smith, & Swank, 2002). The gaze and JA scores reflect the proportion of response and initiation exchanges in which the child engaged in the behavior divided by the number of opportunities. We also examined the number of episodes of negative affect, such as fussing or crying, the child displayed throughout the sequence of social interactions.
Statistical Approach
A general or generalized linear mixed model (GLMM) was used to examine the influence of group (comparison, iTBI, aTBI), and time of testing (baseline, 1 year) and their interaction on the gaze and JA proportion scores while controlling for age at testing. This was done using SAS software, Proc Mixed, for variables that were reasonably distributed. In some cases, the variables represented frequency counts of behaviors and were more distributed as Poisson or negative binomials and so a non-linear mixed modeling program, SAS procedure, Glimmix, was used. GLMM was also used to evaluate whether family resources before the injury moderated the relation of group with JA. To assess variables influencing later development, we examined whether initial JA scores, family resources, and group predicted cognitive and adaptive behavior 1 year after injury. Effect sizes were calculated using Cohen’s d or .
For the TBI groups, Pearson correlation coefficients were calculated to examine the relation of GCS scores and the duration of impaired consciousness with baseline and follow-up social interaction variables. To determine whether outcomes were related to external cause of TBI or to the severity of injury, we completed Group x Severity GLMs for each of the social and cognitive outcomes. Although the majority of cases had GCS scores from 13–15, 32 of 33 children in the iTBI and 39 of 42 children in the aTBI groups had complicated-mild TBI with positive neuroimaging findings. The outcome of children with complicated-mild TBI is similar to those with moderate TBI (Levin et al., 2008). Consequently, the analysis examined outcomes for children with complicated-mild/moderate and severe iTBI or aTBI.
RESULTS
As indicated in Table 1, the three groups did not differ significantly in terms of age at assessment, gender, ethnicity, or socioeconomic status (SES) (Hollingshead, 1975). Although the inflicted group tended to be somewhat younger than the other groups, the differences were not statistically significant, either by analysis of variance or the Kruskal-Wallis test, p = .23. The groups did not differ on SES (p = .32), nor by gender (p = .45) or ethnicity (p = .16).
Age at injury differed for the two TBI groups, F(1,123) = 4.13; p = .04; the inflicted group was younger than the accidental group. However, because the standard deviations were similar to the means, a Kruskal-Wallis test was conducted which was not significant (p = .18). The external cause of injury varied across TBI groups, χ2 (7, N = 125) = 74.52, p<.0001. Falls and vehicle-related incidents predominated in the aTBI group. For participants with iTBI, assault was the most common cause of injury, followed by children presenting with changes in level of consciousness with no reported history of injury who were subsequently diagnosed with TBI. The distribution of both GCS scores and the duration of impaired consciousness was similar in the TBI groups.
Social Interaction by Group, Time, and Family Resources
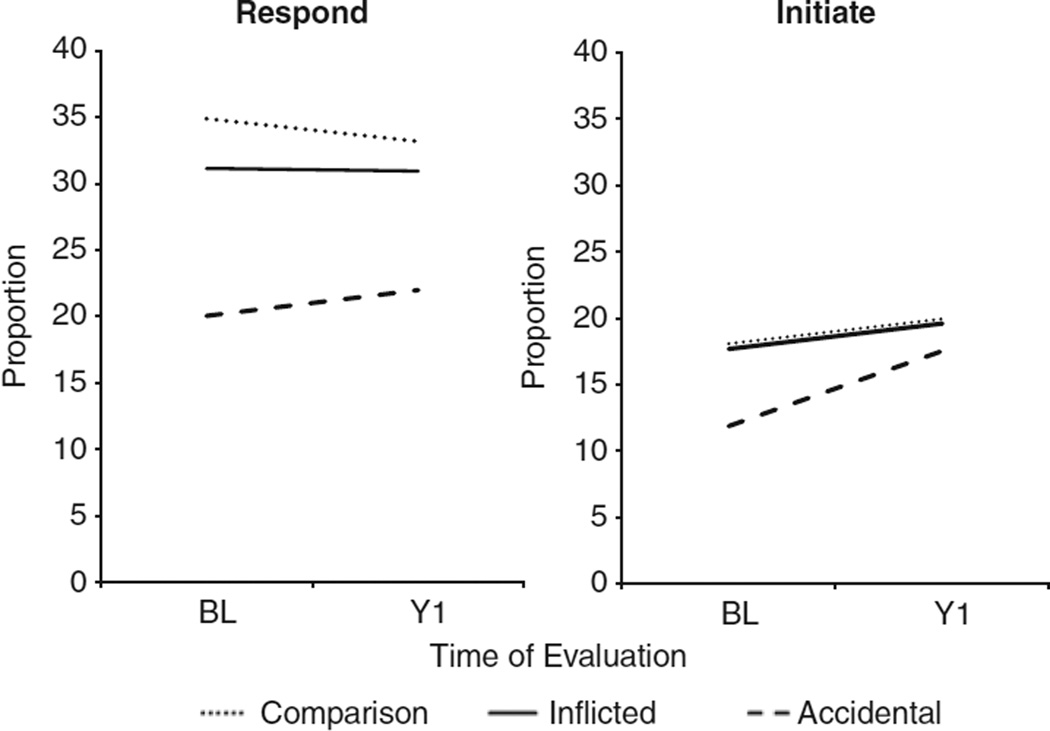
The model for the social interaction variables was a repeated measures design across two time points with group, time, and the group by time interaction. In addition, age at baseline was included as a time invariant covariate. Age was significantly related only to the JA initiation score, F(1,181) = 7.70, p = .006; older children initiated more JA interactions than younger children but did not differ on gaze or responding to JA. Figure 1 depicts the gaze variables across time for each group. For the gaze variables, responding to the examiner’s eye contact differed significantly by group, F(2,181) = 6.35, p = .002. Neither time nor the group by time interaction accounted for significant variability in responding to the examiner’s gaze. On average, the aTBI group responded to social overtures with a significantly lower proportion of eye contact than either the iTBI (t(181) = −2.84; p = .005; d = .36) or comparison groups, (t(181) = −3.49; p = .0006; d = .50), which did not differ from each other, (t(181) = 0.80; p = .423). For initiating eye contact, the group and group by time interaction terms were not significant. The time effect approached significance, F(1,181) = 3.75; p = .054; d = .17.
Fig. 1.
Responding to and initiating mutual gaze varied by group. The accidental traumatic brain injury (TBI) group responded to social overtures with a lower proportion of eye contact than the other groups. Eye contact tended to increase in all groups over time.
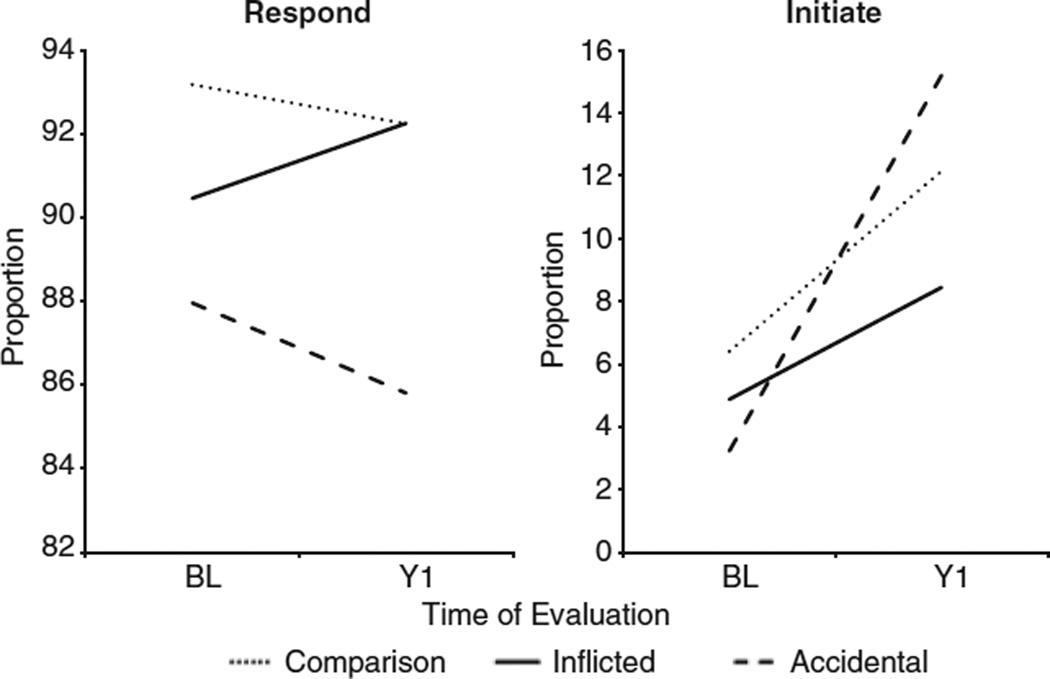
A different pattern of findings emerged for the JA variables. Responding to the examiner’s JA overtures did not differ significantly by either group, F(2,181) = 2.37, p = .097, or time F(1,181) = 0.02, p = .894. In contrast, the group by time interaction for children’s initiation of JA was significant, F(2,181)= 3.60, p = .029. Although the main effect for group was not significant, the proportion of JA initiation increased from the baseline to the 1 year evaluation. At baseline, the comparison group initiated a higher proportion of JA interactions than the aTBI group, (t(181) = 2.21; p = .028; d = .33), and had higher JA scores than the iTBI group, (t(181) = 2.13; p = .035; d = .30). Comparison of change between baseline and the 1-year follow-up by group revealed that significant gains were noted for the aTBI group relative to the both the comparison group, (t(181) = −2.30; p = .023; d = .68) and the iTBI group, (t(181) = 2.45; p = .015; d = .82), which did not differ from each other. Figure 2 shows the proportion of JA initiation for each group over time. Because the groups had comparable levels of negative affect during the social interactions, F(2,104.5) = 1.29, p = .28, it is unlikely that group differences were related to situational distress.
Fig. 2.
Responding to joint attention did not vary by group or time. Initiating joint attention by the accidental group was lower at baseline and higher at the 1-year follow-up than in either the inflicted traumatic brain injury (TBI) or comparison groups.
We then examined whether the impact of SES or family resources before the injury exerted a main effect or moderated the relation of group with the social interaction behaviors. Family resource data were available in 164 children. Neither SES nor resources interacted with group or time; nonsignificant interactions were trimmed from each model. The main effect of SES was nonsignificant for each model, each F < 1.0, p >.60. The family resources variable was positively related to gaze behaviors. More resources predicted children’s responding to the examiner’s gaze over and above the effect of group, F(1,210) = 8.96, p = .003, . When added to the model for gaze initiation, resources were positively related, F(1,228) = 4.35, p = .038; , whereas the group effect was no longer significant. Regarding JA, resources positively predicted both responding, F(1,194) = 7.00, p = .009, , and initiation F(1,204) = 4.61, p = .033, , over and above the effect of group.
Relation of Joint Attention with Mental Development and Adaptive Behavior Scores
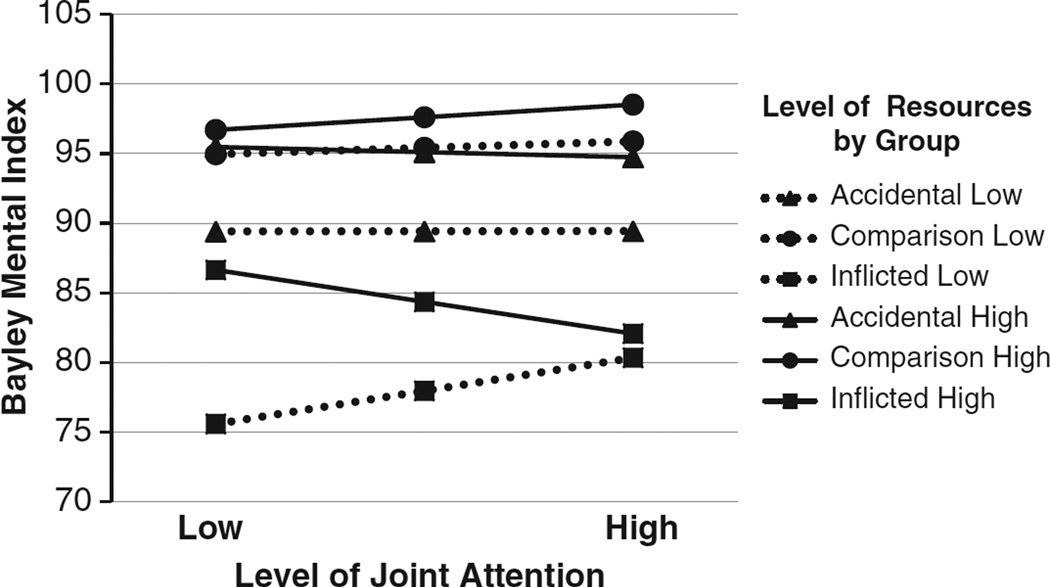
We examined whether the baseline proportion of JA initiation or response, level of family resources, and group predicted cognitive and adaptive behavior scores at the 1-year follow-up. Bayley Mental Development scores were not related to JA initiation; however, they were predicted by group, F(2,153) = 15.35, p < .0001, , and family resources, F(1,153) = 9.13, p = .003, . In contrast, a three-way interaction of JA response behaviors, resources, and group predicted Bayley scores, F(2,146) = 3.24, p = .042, . Figure 3 shows that for the comparison group, neither JA nor resources were associated with Bayley scores. However, for children with aTBI, lower family resources were associated with lower Bayley scores independent of JA. For the iTBI group, developmental quotients were related to both JA and resources. High resources were associated with higher scores in children with low JA but conferred no benefit in children with high JA. Low resources and low JA were associated with the least favorable scores.
Fig. 3.
Joint attention and family resources influenced the level of Bayley Mental Development scores at the 1 year follow-up for both traumatic brain injury (TBI) groups. In the inflicted group, high resources buffered the impact of injury on cognitive scores in children with low levels of responding to joint attention but conferred no benefit in children with higher responsiveness in joint attention interactions.
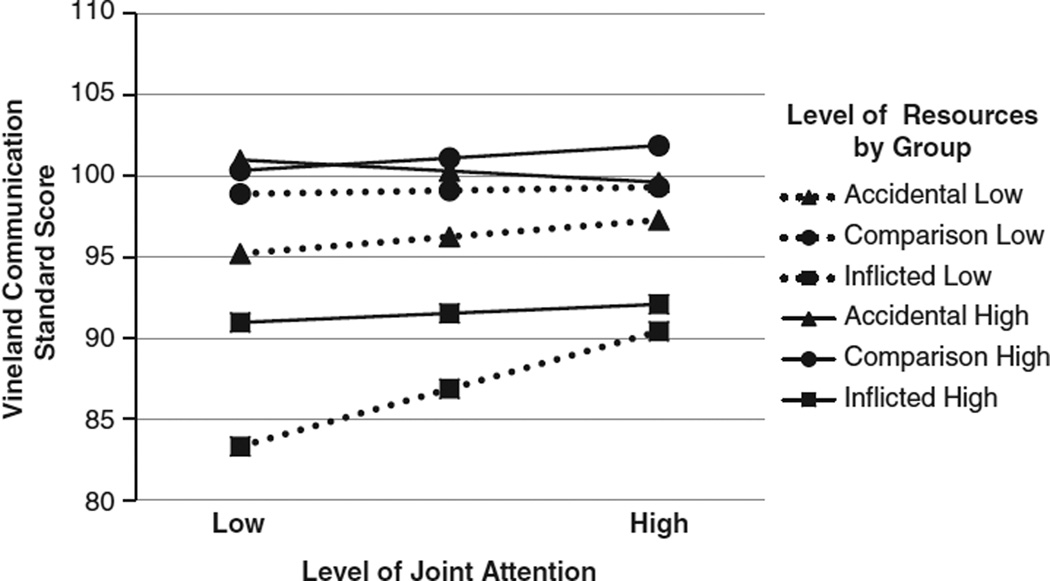
Family resources and baseline JA scores were also examined in relation to parent ratings of everyday communication and social skills obtained 1 year after TBI in 127 children. JA initiation did not predict Vineland Communication scores, F(1,112) = 0.00, p = .99; although main effects were significant for group, F(2,112) = 11.13, p < .0001, , and family resources, F(1,112) = 5.14, p = .025, . As depicted in Figure 4, Communication standard scores were predicted by the interaction of JA responding, group, and resources, F(2,116) = 4.31, p = .016, . In children with low JA, higher family resources buffered the impact of TBI on communication ratings. Family resources had less impact in children with higher JA.
Fig. 4.
Family resources tended to interact with the level of joint attention to influence Vineland Communication scores. For children with inflicted traumatic brain injury (TBI), higher family resources had a protective effect and lower resources had a detrimental effect on everyday communication in children with lower responsiveness in social interactions.
Family resources did not significantly moderate the effect of JA and group on Vineland Socialization scores. For JA initiate scores, there was a trend for main effects of JA, F(1,112) = 3.00, p = .086, , and resources, F(1,112) = 2.99, p = .086, , on the Socialization scores. For JA respond scores, a trend was obtained for a three-way interaction of, JA, resources, and group on the Socialization scores, F(2,105) = 2.70, p = .072, . The pattern of scores by group was similar to that depicted in Figure 4 for the Communication score: higher family resources had a protective effect and lower resources had a detrimental effect in children with iTBI who responded to fewer JA overtures.
Relation of Social Interaction Scores with Injury Variables
Partial correlation of the social interaction scores at the initial and 1-year follow-up with the GCS score and impaired consciousness revealed that only the JA respond score at baseline was significantly correlated with the injury variables. Higher GCS scores (r = 0.47; p =.0001) and shorter impaired consciousness (r = −0.52; p = .0001) were associated with greater social responsiveness.
For children with complicated–mild/moderate and severe TBI, we also examined whether outcomes were related to external cause of TBI or to severity of TBI. Descriptive and inferential statistics for the social interaction, cognitive and adaptive behavior outcomes are provided in Table 3. There were no significant interactions of group (inflicted, accidental) and TBI severity (complicated–mild/moderate, severe) for any of the outcomes. At baseline, children with iTBI responded to (d = 0.57) and initiated (d = 0.45) more eye contact than children with aTBI. At follow-up, gaze variables were not significantly related to either group or severity. In both TBI groups, children with severe TBI were less responsive to JA overtures than children with complicated–mild/moderate TBI at both baseline (d = 0.82) and 1 year (d = 0.58) follow-ups. Children’s initiation of JA interactions was not related to either group or severity of injury at either time point.
Table 3.
Social interaction, cognitive, and adaptive behavior scores for children with complicated-mild/moderate and severe TBI
| Group |
||||||
|---|---|---|---|---|---|---|
| Inflicted TBI |
Accidental TBI |
|||||
| Statistics |
||||||
| Outcomes M (SD) | Complicated- mild/moderate (n = 44) |
Severe (n = 19) |
Complicated- mild/moderate (n = 29) |
Severe (n = 15) |
Group F-value |
Severity F-value |
| Social interaction | df(1,103) | |||||
| Baseline | ||||||
| Gaze respond | 28.9 (26.3) | 37.8 (28.0) | 18.5 (19.3) | 18.2(19.1) | 8.88** | 0.73 |
| Gaze initiate | 16.2 (16.6) | 19.8(17.1) | 9.9 (11.2) | 12.6 (13.3) | 4.63* | 1.02 |
| JA respond | 94.8 (8.6) | 80.0 (29.3) | 91.3 (13.4) | 74.0(31.5) | 1.42 | 15.99*** |
| JA initiate | 5.7 (9.6) | 3.3 (6.1) | 3.4 (7.2) | 4.2 (7.8) | 0.17 | 0.21 |
| Follow-up | ||||||
| Gaze respond | 30.3 (18.0) | 35.8 (27.6) | 25.5 (19.4) | 23.1 (25.2) | 2.04 | 0.07 |
| Gaze initiate | 17.4(12.1) | 22.4 (19.3) | 19.4(17.1) | 14.2 (19.8) | 0.46 | 0.00 |
| JA respond | 94.4 (9.0) | 86.4 (26.1) | 96.0 (5.3) | 82.9 (27.5) | 0.04 | 4.65* |
| JA initiate | 8.4 (9.9) | 6.9 (9.6) | 16.8 (18.0) | 8.7 (10.5) | 2.38 | 2.16 |
| Cognitive and adaptive behavior follow-up | ||||||
| Bayley Mental Development Index | df(1,96) | |||||
| 82.1 (15.8) | 76.9 (21.6) | 91.4(15.3) | 86.3 (12.5) | 6.49* | 1.99 | |
| Vineland Adaptive Behavior Scales | df(1,71) | |||||
| Communication | 89.9 (10.7) | 81.7(10.1) | 98.3 (10.7) | 88.2(15.1) | 7.04** | 10.62** |
| Social | 98.0(11.0) | 88.0(11.1) | 99.8 (11.9) | 89.7 (14.1) | 0.37 | 12.14*** |
Note.
p < .05,
p <.01,
p < .001.
JA = joint attention.
Cognitive and adaptive behavior scores at the 1-year follow-up were differentially related to group and severity. Children with complicated–mild/moderate or severe iTBI had lower Bayley MDI scores than the accidental group (d = −0.56). Vineland Communication scores were significantly lower in the iTBI than the aTBI group (d = −0.55) and were lower in children with severe than complicated–mild/moderate TBI (d = 0.99). Vineland Social scores were significantly lower in children with severe than complicated–mild/moderate TBI (d = 1.00), but did not vary by external cause of injury.
DISCUSSION
Joint Attention during the First Year after TBI
During the first year after injury, aspects of social interaction differed in young children with aTBI or iTBI relative to healthy comparison children. Regarding gaze, initiating eye contact was similar across the three groups and tended to increase over time. Responding to the examiner by establishing mutual gaze was reduced in the aTBI group relative to the iTBI and comparison groups. The pattern of findings differed for JA. Initiation of JA occurred significantly less frequently in the aTBI group than other groups at the baseline evaluation. All groups increased JA initiations over time; however, children with aTBI showed significantly more growth and initiated the highest proportion of JA interactions at the 1-year follow-up. Given the nature of the coding system, it is not possible to determine whether the high rate of social initiation behaviors was appropriate to the interaction or whether it reflected disinhibited or attention-demanding behavior.
Contrary to expectation, children with inflicted injuries did not have lower rates of social engagement than the other groups. Our initial study found that infants with iTBI initiated fewer JA interactions and were less responsive using gaze and JA than a community comparison group (Landry et al., 2004). However, the sample of children in the present study did not show the same pattern of reduced social interaction. This discrepant finding may be due in part to the larger sample size of the current study.
We also hypothesized that the proportion of social behaviors would increase from the initial to the 1-year follow-up in all groups. Partial support was obtained for this hypothesis; initiating JA was the only measure that increased over time and with age. Responding to JA in response to the examiner’s overtures was similar in all groups at baseline and 1-year time points.
Joint Attention Initiation and Responding as Predictors of Outcomes
Do levels of initiating and responding to JA predict later developing cognitive abilities and social skills after early brain injury? Interestingly, JA initiation scores did not predict either cognitive ability or caregiver ratings of everyday social and communication behaviors. These outcomes were predicted by both group membership and family resources. In contrast, the child’s response to JA overtures interacted with both group and resources to predict cognitive and adaptive behavior outcomes. These findings suggest that JA response, but not JA initiation, is a core social cognitive ability that accounted for variability in longer-term outcomes following TBI. Responding to JA has been related more strongly than initiating JA to disengagement of attention and self-regulation (Vaughan Van Hecke et al., 2012, 2007). Interestingly, in our sample, responding to JA was more strongly related than initiating JA to initial measures of injury severity and to later cognitive and social outcomes.
Several longitudinal studies have found that initiating and responding to JA are differentially related to outcomes in both typically developing and clinical samples. Vaughan Van Hecke et al. (2007) noted that infants who either initiated and/or responded to JA overtures more frequently at 12 months of age were rated as showing fewer externalizing behaviors and more favorable social and behavioral competence at 30 months of age. Moreover, initiating JA was not correlated with measures of temperament, cognition, or language; responding to JA was positively correlated with language comprehension and negatively correlated with ratings of inhibitory control (Vaughan Van Hecke et al., 2007). In a sample of children with prenatal exposure to cocaine, Sheinkopf, Mundy, Claussen, and Willoughby (2004) reported that initiating JA at 12 to 18 months of age was negatively related to general cognitive and language skills, as well as to caregiver ratings indicating lower positive social behaviors at 36 months. In contrast, responding to JA was positively related to ratings of positive social behavior (Sheinkopf et al., 2004). Overall, these studies suggest that high rates of initiating JA may be associated with lower IQ and language scores and with increased disruptive behavior. High rates of responding to JA were associated with more favorable language, cognition, and behavior regulation. In the present study, the higher levels of initiating JA in the aTBI group than the comparison group may be an indicator of increased risk for long-term social difficulties.
Family Resources
During the first year after TBI, social interaction behaviors were influenced by the family’s access to material and social support, but not by socioeconomic status. Not surprisingly, the more proximal indicator of the family’s resources was more strongly related to outcomes than the more distal measure of parental education and occupation. Across groups, children from families with more resources engaged in a higher proportion of gaze and JA interactions than those from families with lower resources. Contrary to expectation, the level of resources did not differentially affect the growth of social behaviors in children with TBI and comparison children across the follow-up. Greater resources enhanced social communication in all children in an additive manner but did not interact with group or time. Similar to findings of other studies (Kurowski et al., 2011; Yeates, Taylor, Walz, Stancin, & Wade, 2010), family variables influenced, but did not moderate, the relation of group and social competence during the first year after injury in young children.
For typically developing children, the level of family resources did not exert a major impact on the Bayley Mental Development Index. Resources had an additive effect on the developmental scores of children with aTBI; greater access to resources was associated with higher scores at all levels of JA. In contrast, outcomes in children with iTBI varied depending on the level of JA and resources. In children with low social responsiveness, high resources buffered the adverse impact of TBI on Bayley scores. Children with iTBI with low resources and low JA were disproportionately disadvantaged and had the lowest developmental scores of any group. A similar pattern of findings was obtained for caregiver ratings of adaptive behavior. Children with iTBI who were less responsive and had lower levels of family resources had the lowest communication and socialization scores. Clearly, young children with iTBI and low social responsiveness are extremely sensitive to environmental variables, which can either enhance positive outcomes or potentiate poor long-term outcomes.
Severity of Traumatic Brain Injury
Severity of TBI and external cause of injury had medium to large effects on several outcomes, which is particularly salient because the analyses compared children with complicated-mild/moderate to severe TBI to each other rather than to a healthy comparison group. During the first year after complicated-mild/moderate to severe TBI, JA interactions were similar in children with iTBI and aTBI; in both groups, children with complicated-mild/moderate TBI were significantly more responsive than those with severe TBI. Children with iTBI had significantly lower general cognitive and communication scores than children with aTBI. In both groups, children with severe TBI were rated as less competent than children with complicated-mild/moderate TBI in communication and social domains.
Infants and preschoolers who sustain complicated-mild/moderate to severe TBI are clearly at high risk for deficits in core social interaction and communication abilities that are likely to influence their future social development and health-related quality of life. Because JA is a foundational skill, disruption in JA may contribute to a cascade of difficulties in later-developing cognitive, social, and communication abilities. Reduced responsivity to social cues may contribute to the chronic social problems (Rosema et al., 2012) and poor vocational outcomes (Koskinicmi, Kyyka, Nybo, & Jarho, 1995; Nybo, Sainio, & Muller, 2004) reported following TBI in young children.
Limitations and Future Directions
Findings of the present study are limited by several factors. First, the sample is based on two cohorts of children enrolled into longitudinal studies from 1994 to 1999 and 2001 to 2006. Although every effort was made to recruit children admitted consecutively, there is no information available regarding whether children who enrolled in the study were representative of all children admitted to the hospital. Inclusion of a comparison group of children with orthopedic injury would help to control for factors, such as preinjury child and family characteristics, which might predispose children to being hospitalized for an injury. The sole use of preinjury estimates of family resources as a predictor of outcomes is a limitation.
Research including children with abusive injuries faces unique challenges. Estimates of preinjury functioning are less likely to be available in children with putative abusive injuries. Nonrandom missing data may bias assessment of the relation of preinjury variables, such as responsive parenting, with outcomes. Outcomes may also be influenced by decisions regarding guardianship. For example, biological and social outcomes may differ, and may sometimes be more favorable, in maltreated children removed from parental care relative to children remaining with birth parents (Bernard, Butzin-Dozier, Rittenhouse, & Dozier, 2010).
Strengths of the study include the longitudinal design that allowed examination of variables that impacted subsequent cognitive and social development in a large sample of young children with different external causes of brain injury. Direct measures of infant social relatedness in a naturalistic setting were used to predict later developmental outcomes. The combination of direct assessment of social communication and ratings based on caregiver interview minimizes collinearity given the variability in methods.
This study contributes to the nascent literature showing relations between early markers of social interaction and the quality of later social and cognitive development in children with CNS disorders. Longitudinal studies following young children with TBI into middle childhood and adolescence are essential to characterize how JA during early stages of outcome is related to long-term social, academic, and adaptive behavior outcomes. The child’s engagement of other people and readiness to be engaged in interactions with others may be candidate markers of later outcomes that may be used to target children in need of different intervention services.
Acknowledgments
This publication was supported by Grant Number R01 NS029462 from the National Institute of Neurological Disorders and Stroke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. The participation of the children and families, as well as the assistance of the Texas Department of Protective and Regulatory Services, is gratefully acknowledged. The authors report no financial or other conflict of interest.
REFERENCES
- Anderson VA, Catroppa C, Dudgeon P, Morse SA, Haritou F, Rosenfeld JV. Understanding predictors of functional recovery and outcome 30 months following early childhood head injury. Neuropsychology. 2006;20(1):42–57. doi: 10.1037/0894-4105.20.1.42. [DOI] [PubMed] [Google Scholar]
- Anderson VA, Morse SA, Klug GL, Catroppa C, Haritou F, Rosenfeld J, Pentland L. Predicting recovery from head injury in young children: A prospective analysis. Journal of the International Neuropsychological Society. 1997;3:568–580. [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant Development. Second. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- Beauchamp MH, Anderson VA, Catroppa C, Maller JJ, Godfrey C, Rosenfeld JV, Kean M. Implications of reduced callosal area for social skills after severe traumatic brain injury in children. Journal of Neurotrauma. 2009;26(10):1645–1654. doi: 10.1089/neu.2009.0916. [DOI] [PubMed] [Google Scholar]
- Bernard K, Butzin-Dozier Z, Rittenhouse J, Dozier M. Cortisol production patterns in young children living with birth parents vs children placed in foster care following involvement of Children’s Protective Services. Archives of Pediatric and Adolescent Medicine. 2010;164(5):438–443. doi: 10.1001/archpediatrics.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce DA, Zimmerman RA. Shaken impact syndrome. Pediatric Annals. 1989;18:482–489. doi: 10.3928/0090-4481-19890801-07. [DOI] [PubMed] [Google Scholar]
- Butterworth G. Origins of mind in perception and action. In: Moore C, Dunham PJ, editors. Joint attention: Its origins and role in development. Hillsdale, NJ: Erlbaum; 1995. pp. 29–40. [Google Scholar]
- Carpenter M, Nagell K, Tomasello M. Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monographs of the Society for Research in Child Development. 1998;63(4):i–vi. 1–143. [PubMed] [Google Scholar]
- Carpenter M, Pennington BF, Rogers SJ. Interrelations among social-cognitive skills in young children with autism. Journal of Autism and Developmental Disorders. 2002;32(2):91–106. doi: 10.1023/a:1014836521114. [DOI] [PubMed] [Google Scholar]
- Chapman LA, Taylor HG, Wade SL, Walz NC, Stancin T, Yeates KO. Clinically significant behavior problems during the initial 18 months following early childhood traumatic brain injury. Rehabilitation Psychology. 2010;55(1):48–57. doi: 10.1037/a0018418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman T, Baron-Cohen S, Swettenham J, Baird G, Cox A, Drew A. Testing joint attention, imitation, and play as infancy precursors to language and theory of mind. Cognitive Development. 2000;15:481–498. [Google Scholar]
- D’Entremont B, Seamans E. Do infants need social cognition to act socially? An alternative look at infant pointing. Child Development. 2007;78(3):723–728. doi: 10.1111/j.1467-8624.2007.01026.x. [DOI] [PubMed] [Google Scholar]
- Duhaime AC, Christian CW, Rorke LB, Zimmerman RA. Nonaccidental head injury in infants-the “shaken-baby syndrome.”. The New England Journal of Medicine. 1998;338:1822–1829. doi: 10.1056/NEJM199806183382507. [DOI] [PubMed] [Google Scholar]
- Duhaime AC, Gennarelli TA, Thibault LE, Bruce DA, Margulies SS, Wiser R. The shaken baby syndrome. A clinical, pathological, and biomechanical study. Journal of Neurosurgery. 1987;66:409–415. doi: 10.3171/jns.1987.66.3.0409. [DOI] [PubMed] [Google Scholar]
- Dunst KL, Leet HE. Measuring the adequacy of resources in households with young children. Childcare, Health, and Human Development. 1987;13:111–129. doi: 10.1111/j.1365-2214.1987.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Fletcher JM, Levin HS, Francis DJ, Davidson K, Miner ME. Longitudinal neuropsychological outcome in infants and preschoolers with traumatic brain injury. Journal of the International Neuropsychological Society. 1997;3:581–591. [PubMed] [Google Scholar]
- Ewing-Cobbs L, Kramer L, Prasad M, Canales DN, Louis PT, Fletcher JM, y Cheung K. Neuroimaging, physical, and developmental findings after inflicted and noninflicted traumatic brain injury in young children. Pediatrics. 1998;102:300–307. doi: 10.1542/peds.102.2.300. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad M, Kramer L, Louis PT, Baumgart-ner J, Fletcher JM, Alpert B. Acute neuroradiologic findings in young children with inflicted or noninflicted traumatic brain injury. Child’s Nervous System. 2000;16:25–34. doi: 10.1007/s003810050006. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad MR, Swank P, Kramer L, Mendez D, Treble A, y Bachevalier J. Social communication in young children with traumatic brain injury: Relations with corpus callosum morphometry. International Journal of Developmental Neuroscience. 2012;30(3):247–254. doi: 10.1016/j.ijdevneu.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischen A, Bayliss AP, Tipper SP. Gaze cueing of attention: Visual attention, social cognition, and individual differences. Psychological Bulletin. 2007;133(4):694–724. doi: 10.1037/0033-2909.133.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesalingam K, Yeates KO, Taylor HG, Walz NC, Stancin T, Wade S. Executive functions and social competence in young children 6 months following traumatic brain injury. Neuropsychology. 2011;25(4):466–476. doi: 10.1037/a0022768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstrohm SL, Arffa S. Preschool children with mild to moderate traumatic brain injury: An exploration of immediate and post-acute morbidity. Archives of Clinical Neuropsychology. 2005;20:675–695. doi: 10.1016/j.acn.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Hahn YS, Chyung C, Barthel M, Bailes J, Flannery A, McLone DG. Head injuries in children under 36 months of age. Child’s Nervous System. 1988;4:34–40. doi: 10.1007/BF00274081. [DOI] [PubMed] [Google Scholar]
- Hirotani M, Stets M, Striano T, Friederici AD. Joint attention helps infants learn new words: Event-related potential evidence. Neuroreport. 2009;20(6):600–605. doi: 10.1097/WNR.0b013e32832a0a7c. [DOI] [PubMed] [Google Scholar]
- Hoehl S, Reid VM, Parise E, Handl A, Palumbo L, Striano T. Looking at eye gaze processing and its neural correlates in infancy-implications for social development and autism spectrum disorder. Child Development. 2009;80(4):968–985. doi: 10.1111/j.1467-8624.2009.01311.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four factor index of social status. New Haven: Yale University Press; 1975. [Google Scholar]
- Hymel KP, Rumack CM, Hay TC, Strain JD, Jenny C. Comparison of intracranial computed tomographic (CT) findings in pediatric abusive and accidental head trauma. Pediatric Radiology. 1997;27:743–747. doi: 10.1007/s002470050215. [DOI] [PubMed] [Google Scholar]
- Keenan HT, Hooper SR, Wetherington CE, Nocera M, Runyan DK. Neurodevelopmental consequences of early traumatic brain injury in 3-year-old children. Pediatrics. 2007;119(3):e616–e623. doi: 10.1542/peds.2006-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan HT, Runyan DK, Nocera MA. Child outcomes and family characteristics 1 year after severe inflicted or noninflicted traumatic brain injury. Pediatrics. 2006;117(2):317–324. doi: 10.1542/peds.2005-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinicmi M, Kyyka T, Nybo T, Jarho L. Long-term outcome after severe brain injury in preschoolers is worse than expected. Archives of Pediatric and Adolescent Medicine. 1995;149:249–254. doi: 10.1001/archpedi.1995.02170150029004. [DOI] [PubMed] [Google Scholar]
- Kurowski BG, Taylor HG, Yeates KO, Walz NC, Stancin T, Wade SL. Caregiver ratings of long-term executive dysfunction and attention problems after early childhood traumatic brain injury: Family functioning is important. Physical Medicine and Rehabilitation. 2011;3(9):836–845. doi: 10.1016/j.pmrj.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry SH, Miller-Loncar CL, Smith KE, Swank PR. The role of early parenting in children’s development of executive processes. Developmental Neuropsychology. 2002;21(1):15–41. doi: 10.1207/S15326942DN2101_2. [DOI] [PubMed] [Google Scholar]
- Landry SH, Swank PR, Steubing K, Prasad M, Ewing-Cobbs L. Social competence in young children with inflicted traumatic brain injury. Developmental Neuropsychology. 2004;26(3):707–733. doi: 10.1207/s15326942dn2603_4. [DOI] [PubMed] [Google Scholar]
- Leung EHL, Rheingold H. Development of pointing as a social gesture. Developmental Psychology. 1981;17:215–220. [Google Scholar]
- Levin HS, Hanten G, Roberson G, Li X, Ewing-Cobbs L, Dennis M, y Swank P. Prediction of cognitive sequelae based on abnormal computed tomography findings in children following mild traumatic brain injury. Journal of Neurosurgery: Pediatrics. 2008;1(6):461–470. doi: 10.3171/PED/2008/1/6/461. [DOI] [PubMed] [Google Scholar]
- Mundy P. Annotation: The neural basis of social impairments in autism: The role of the dorsal medial-frontal cortex and anterior cingulate system. Journal of Child Psychology and Psychiatry. 2003;44(6):793–809. doi: 10.1111/1469-7610.00165. [DOI] [PubMed] [Google Scholar]
- Mundy P, Jarrold W. Infant joint attention, neural networks and social cognition. Neural Networks. 2010;23(8–9):985–997. doi: 10.1016/j.neunet.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybo T, Sainio M, Muller K. Stability of vocational outcome in adulthood after moderate to severe preschool brain injury. Journal of the International Neuropsychological Society. 2004;5:719–723. doi: 10.1017/S1355617704105109. [DOI] [PubMed] [Google Scholar]
- Rosema S, Crowe L, Anderson V. Social function in children and adolescents after traumatic brain injury: a systematic review 1989–2011. Journal of Neurotrauma. 2012;29(7):1277–1291. doi: 10.1089/neu.2011.2144. [DOI] [PubMed] [Google Scholar]
- Scaife M, Bruner JA. The capacity for joint visual attention in the infant. Nature. 1975;253:265–266. doi: 10.1038/253265a0. [DOI] [PubMed] [Google Scholar]
- Sheinkopf SJ, Mundy P, Claussen AH, Willoughby J. Infant joint attention skill and preschool behavioral outcomes in at-risk children. Developmental Psychopathology. 2004;16(2):273–291. doi: 10.1017/s0954579404044517. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- St. James-Roberts I. Neurological plasticity, recovery from brain insult, and child development. Advances in Child Development and Behavior. 1979;14:253–318. doi: 10.1016/s0065-2407(08)60116-0. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness: A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding and sharing intentions: The origins of cultural cognition. Behavioral and Brain Sciences. 2005;28(5):675–691. doi: 10.1017/S0140525X05000129. [DOI] [PubMed] [Google Scholar]
- Vaughan Van Hecke A, Mundy P, Block JJ, Delgado CE, Parlade MV, Pomares YB, Hobson JA. Infant responding to joint attention, executive processes, and self-regulation in preschool children. Infant Behavior and Development. 2012;35(2):303–311. doi: 10.1016/j.infbeh.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan Van Hecke A, Mundy PC, Acra CF, Block JJ, Delgado CE, Parlade MV, Pomares YB. Infant joint attention, temperament, and social competence in preschool children. Child Development. 2007;78(1):53–69. doi: 10.1111/j.1467-8624.2007.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vygotsky L. Mind in society. Cambridge: Harvard University Press; 1978. [Google Scholar]
- Walz NC, Yeates KO, Taylor HG, Stancin T, Wade S. Theory of mind skills 1 year after traumatic brain injury in 6- to 8-year-old children. Journal of Neuropsychology. 2010;4(Pt2):181–195. doi: 10.1348/174866410X488788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz NC, Yeates KO, Wade SL, Mark E. Social information processing skills in adolescents with traumatic brain injury: Relationship with social competence and behavior problems. Journal of Pediatric Rehabilitation Medicine. 2009;2(4):285–295. doi: 10.3233/PRM-2009-0094. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Dennis M, Rubin KH, Taylor HG, Bigler ED, Gerhardt CA, Vannatta K. Social outcomes in childhood brain disorder: A heuristic integration of social neuroscience and developmental psychology. Psychological Bulletin. 2007;133(3):535–556. doi: 10.1037/0033-2909.133.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Walz NC, Stancin T, Wade SL. The family environment as a moderator of psychosocial outcomes following traumatic brain injury in young children. Neuropsychology. 2010;24(3):345–356. doi: 10.1037/a0018387. [DOI] [PMC free article] [PubMed] [Google Scholar]