Abstract
The aim of the present study was to investigate the mechanisms of long non-coding RNAs (lncRNAs) in a gastric cancer cell line treated with celecoxib. The human gastric carcinoma cell line NCI-N87 was treated with 15 µM celecoxib for 72 h (celecoxib group) and an equal volume of dimethylsulfoxide (control group), respectively. Libraries were constructed by NEBNext Ultra RNA Library Prep kit for Illumina. Paired-end RNA sequencing reads were aligned to a human hg19 reference genome using TopHat2. Differentially expressed genes (DEGs) and lncRNAs were identified using Cuffdiff. Enrichment analysis was performed using GO-function package and KEGG profile in Bioconductor. A protein-protein interaction network was constructed using STRING database and module analysis was performed using ClusterONE plugin of Cytoscape. ATP5G1, ATP5G3, COX8A, CYC1, NDUFS3, UQCRC1, UQCRC2 and UQCRFS1 were enriched in the oxidative phosphorylation pathway. CXCL1, CXCL3, CXCL5 and CXCL8 were enriched in the chemokine signaling and cytokine-cytokine receptor interaction pathways. ITGA3, ITGA6, ITGB4, ITGB5, ITGB6 and ITGB8 were enriched in the integrin-mediated signaling pathway. DEGs co-expressed with lnc-SCD-1:13, lnc-LRR1-1:2, lnc-PTMS-1:3, lnc-S100P-3:1, lnc-AP000974.1-1:1 and lnc-RAB3IL1-2:1 were enriched in the pathways associated with cancer, such as the basal cell carcinoma pathway in cancer. In conclusion, these DEGs and differentially expressed lncRNAs may be important in the celecoxib treatment of gastric cancer.
Keywords: celecoxib, gastric cancer, differentially expressed genes, enrichment analysis
Introduction
Despite the mortality rate for gastric carcinoma reducing 3.1% annually and the overall 5-year relative survival rate increasing to 28% over the past 10 years, the mortality rate for gastric carcinoma remains >50% worldwide (1). The most effective treatment for resectable gastric cancer is surgery, which presents good survival rates. The majority of cases of gastric cancer are diagnosed at an advanced stage or as a relapse after surgery (2). Therefore, a further understanding of the molecular mechanisms of gastric cancer is of clinical importance and it is required in order to improve the early diagnosis and therapeutic strategies of gastric cancer.
Over the last decade, the majority of the potential therapeutic targets reported and the diagnostic markers for gastric cancer are protein-coding genes identified from large-scale DNA microarray analysis, including the novel genes KLF5, FAT4, KMT2C, GATA4, MLL and GATA6 (3–6). The majority of studies on non-coding RNAs (ncRNAs) are focused on short ncRNAs called microRNAs, while alterations in the structure, expression levels and cognate RNA-binding proteins of long ncRNAs (lncRNAs) with a length of >200 nucleotides (nt) have been associated with cancer, and appear to be gaining prominence as further studies are conducted (7). In addition, growing evidence has confirmed that lncRNAs that are capable of regulating tumor suppression or that exhibit oncogenic effects may be considered as novel biomarkers and therapeutic targets for cancer (8,9). Furthermore, it has been demonstrated that differentially expressed long non-coding RNAs (DE-lncRNAs), including H19 and uc001lsz, may present potential roles in the development and occurrence of gastric cancer (10). In a study by Hu et al (11), a novel lncRNA GAPLINC (924 bp) was highly expressed in gastric cancer specimens and it was capable of controlling the expression levels of CD44 to regulate cell invasion by competing for miR211-3p.
A previous study demonstrated that celecoxib induced apoptosis and autophagy of gastric cancer SGC-7901 cells via the PI3K/Akt signaling pathway (12). According to a study by Lan et al (13), celecoxib inhibited Helicobacter pylori-induced invasion in gastric cancer via the adenine nucleotide translocator-dependent pathways. Furthermore, the activated Notch1 signaling pathway may contribute to the pathogenesis of gastric cancer, at least partly through COX-2 (14). Treatment with celecoxib, a COX-2 inhibitor, can significantly reduce the incidence of gastric cancer in rats (15). In addition, an elevated COX-2 expression level is an independent prognostic factor indicative of poor prognosis and it is associated with reduced survival in patients with gastric cancer (16). Pang et al (17) reported that the Akt/GSK3β/NAG-1 signaling pathway may be considered as the major mechanism of the COX-2-independent effects of celecoxib on gastric cancer cells. COX-2 has been indicated to regulate E-cadherin expression via the NF-κB and Snail signaling pathway in gastric cancer (18). It has also been reported that celecoxib has the potential for clinical use in gastric cancer treatment by the mechanism of activating miR-29c (19). Although various advances have been made in the study of mechanisms of lncRNAs in gastric cancer, the understanding of the expression patterns and functional roles of lncRNAs in gastric cancer treated with celecoxib requires further investigation.
In the present study, the RNA sequencing data of NCI-N87 human gastric carcinoma cells treated with or without celecoxib were prepared and analyzed using bioinformatics methods. Briefly, differentially expressed genes (DEGs) and lncRNAs were identified for pathway enrichment analysis. A protein-protein interaction (PPI) network for DEGs was constructed and module analysis was performed. Finally, co-expression analysis of DEGs and lncRNAs was performed. The results of the data in the present study may provide novel insight into the roles of celecoxib in gastric cancer.
Materials and methods
Cell culture and celecoxib treatment
The human gastric carcinoma cell line NCI-N87 was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin (Thermo Fisher Scientific, Inc.) in a humidified air incubator (Thermo Fisher Scientific, Inc.) at 37°C and with 5% CO2. The cells were passaged at 80–90% confluence with 0.25% trypsin (Thermo Fisher Scientific, Inc.).
Cells at the exponential growth phase with a density of 1×106 were seeded in a cell culture dish (Corning Inc., NY, USA) with a diameter of 6 cm and incubated in 5 ml serum-free Dulbecco's modified Eagle medium (Thermo Fisher Scientific, Inc.) overnight. Celecoxib (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in dimethylsulfoxide (DMSO; Sigma-Aldrich), and the cells were treated with 15 µM celecoxib for 72 h (celecoxib group). Cells treated with an equal volume of DMSO were used as a control group.
RNA sequencing data
The total RNA was extracted using TRIzol (Thermo Fisher Scientific, Inc.) following the manufacturer's protocol, and were quantified with a 721 spectrophotometer (Shanghai Precision Instrument Co., Ltd., Shanghai, China). Next, libraries were prepared by the NEBNext Ultra RNA Library Prep kit for Illumina (#E7530; New England BioLabs, Inc., Ipswich, MA, USA) according to the manufacturer's instructions. Briefly, RNA fragments ~200 nt in length were generated and then double-stranded cDNA was synthesized and end-repaired. Following the adaptor ligation, PCR amplification was performed as follows: A library was added with 10 µl 5X HF Buffer, 1 µl 10 µM reverse PCR primer 2–1: 5′-CAAGCAGAAGACGGCATACGAGATCGTGATGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT-3′ and primer 2–2: 5′-CAAGCAGAAGACGGCATACGAGATACATCGGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT-3′, primer 2–3: 5′-CAAGCAGAAGACGGCATACGAGATGCCTAAGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT-3′, primer 2–4: 5′-CAAGCAGAAGACGGCATACGAGATTGGTCAGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT-3′, 1.5 µl dNTP, 0.5 µl Phusion High-Fidelity DNA Polymerase (2 U/µl) and 5 µl ddH2O, and then incubated at 98°C for 40 sec, 65°C for 30 sec and 72°C for 30 sec. Next, 1 µl of 10 µM forward PCR primer (5′-AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCT-3′) was added and incubated at 98°C for 10 sec, 10 cycles at 65°C for 30 sec, 72°C for 30 sec, and 72°C for 3 min. Finally, the library was dissolved in 20 µl ddH2O after being purified by 50 µl AMPure XP magnetic beads. A 1 µg input for 15 cycles and a 5 µg input for 12 cycles was used and the library quality was assessed on a 2100 Electrophoresis Bioanalyzer instrument (Agilent Technologies, Inc., Santa Clara, CA, USA). Finally, sequencing was conducted on a HiSeq 2500 System (Illumina, Inc., San Diego, CA, USA).
Data preprocessing and sequence alignment
Quality control (QC) of obtained next generation sequencing (NGS) data was conducted with an NGS QC Toolkit (version 2.3.3; www.nipgr.res.in/ngsqctoolkit.html) in order to remove low quality reads with default parameters (20). Reads with ≥10% low quality bases (Phred quality score <20) were filtered.
The paired-end RNA sequencing reads were aligned to the human hg19 reference genome using TopHat2 (ccb.jhu.edu/software/tophat) (21), and the human hg19 reference genome and its annotation files were obtained from the University of California Santa Cruz Genome Browser (genome.ucsc.edu) (22). The ‘-no-mixed’ option was handled and other parameters were set to default.
Identification of DEGs and lncRNAs
Following sequence alignment and refseq annotation, Cuffdiff (23) was applied to screen DEGs with a cut-off criteria of q<0.05. DE-lncRNAs were identified with the combination of lncRNA annotation by LNCipedia 3.0 (www.lncipedia.org) (24). q<0.05 was considered as the threshold value.
Functional and pathway enrichment analysis for DEGs
Gene ontology (GO) terms in the biological process (BP), cellular component (CC) and molecular function (MF) categories were enriched for DEGs using the GO-function package in Bioconductor (www.bioconductor.org) (25). KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway enrichment analysis was also conducted by the KEGG profile in Bioconductor. The enrichment thresholds were P<0.05 and the gene counts ≥2.
Construction of the PPI network and module analysis
The Search Tool for the Retrieval of Interacting Genes (STRING; www.string-db.org) database not only provides uniquely comprehensive coverage but also contains predicted, experimental, transferred and text-mined interactions (26). The PPIs for DEGs were predicted using version 9.1 of the STRING database with a combined score >0.7 (26). Cytoscape software version 2.8 (27) was used to visualize the PPI network (www.cytoscape.org).
The ClusterONE plugin of Cytoscape (28) was used to perform module analysis for the PPI network with default parameters. In addition, functional and pathway enrichment analysis of DEGs in the two modules with the highest significance was performed with the cut-off criteria of P<0.05 and gene counts ≥2.
Co-expression analysis of DEGs and lncRNAs
Pearson correlation coefficients between DEGs and lncRNAs were calculated. The co-expressed genes and lncRNA pairs were selected with a Pearson correlation coefficient >0.98. Pathway enrichment analysis was conducted for the DEGs co-expressed with each DE-lncRNA, with thresholds of P<0.05 and gene counts ≥2.
Results
DEGs and lncRNAs
A total of 490 DEGs, of which 302 were upregulated and 188 downregulated genes, were identified in the celecoxib and the control groups. A total of 37 DE-lncRNAs, of which 19 were upregulated and 18 downregulated, were screened (Table I).
Table I.
Differentially expressed lncRNAs in the celecoxib and the control groups.
| lncRNAs ID | Celecoxib | Control | Fold change | q value |
|---|---|---|---|---|
| Upregulated | ||||
| lnc-IGFL3-2:1 | 18.43 | 44.60 | 1.27 | 1.07×10−7 |
| lnc-PTMS-1:3 | 427.83 | 613.56 | 0.52 | 1.71×10−6 |
| lnc-SCD-1:13 | 105.06 | 152.80 | 0.54 | 1.71×10−6 |
| lnc-TNS4-2:1 | 6.28 | 15.94 | 1.34 | 1.71×10−6 |
| lnc-TTLL10-3:1 | 7.71 | 11.67 | 0.60 | 2.91×10−5 |
| lnc-CKMT1A-1:1 | 50.37 | 97.92 | 0.96 | 5.80×10−5 |
| lnc-LRR1-1:2 | 4422.95 | 5914.51 | 0.42 | 6.36×10−5 |
| lnc-RAB3IL1-2:1 | 2279.25 | 3231.60 | 0.50 | 7.18×10−4 |
| lnc-JUNB-1:1 | 372.34 | 562.08 | 0.59 | 2.00×10−3 |
| lnc-RP11-259P6.1.1–2:1 | 29.78 | 39.57 | 0.41 | 3.20×10−3 |
| lnc-IGFL2-2:1 | 105.84 | 167.35 | 0.66 | 5.76×10−3 |
| lnc-S100P-3:1 | 142.85 | 240.91 | 0.75 | 6.65×10−3 |
| lnc-SRGAP3-1:29 | 0 | 1.73 | 1.80e+308 | 1.19×10−2 |
| lnc-RAB44-3:1 | 8.43 | 14.88 | 0.82 | 1.25×10−2 |
| lnc-GLTSCR2-2:7 | 18.58 | 26.75 | 0.53 | 1.26×10−2 |
| lnc-PDZD7-3:2 | 0 | 3.41 | 1.80e+308 | 2.41×10−2 |
| lnc-CEACAM6-1:1 | 33.20 | 57.59 | 0.79 | 2.51×10−2 |
| lnc-SPNS3-1:3 | 18.58 | 27.11 | 0.54 | 4.89×10−2 |
| lnc-UNC5B-1:1 | 12.62 | 18.20 | 0.53 | 4.89×10−2 |
| Downregulated | ||||
| lnc-C9orf16-2:1 | 875.46 | 425.36 | −1.04 | 0 |
| lnc-C9orf16-3:1 | 352.54 | 156.14 | −1.17 | 0 |
| lnc-TRIM31-1:2 | 47.17 | 21.45 | −1.14 | 4.10×10−8 |
| lnc-DDX47-3:1 | 211.38 | 145.19 | −0.54 | 1.46×10−7 |
| lnc-PCK1-3:1 | 13.92 | 5.48 | −1.35 | 8.52×10−7 |
| lnc-MYO16-7:1 | 349.12 | 200.98 | −0.80 | 1.71×10−6 |
| lnc-YPEL5-5:1 | 71.82 | 44.54 | −0.69 | 1.71×10−6 |
| lnc-TNK2-8:1 | 16.60 | 2.33 | −2.83 | 4.54×10−6 |
| lnc-AC069257.9.1-4:73 | 124.40 | 66.92 | −0.90 | 6.69×10−5 |
| lnc-CCDC80-1:4 | 18.79 | 3.17 | −2.57 | 1.74×10−3 |
| lnc-AC069257.9.1-4:72 | 151.58 | 81.26 | −0.90 | 5.89×10−3 |
| lnc-KRT36-1:1 | 45.00 | 18.61 | −1.27 | 6.65×10−3 |
| lnc-CCDC33-1:1 | 32.45 | 19.88 | −0.71 | 8.64×10−3 |
| lnc-CXCL3-1:1 | 5.06 | 1.51 | −1.74 | 2.51×10−2 |
| lnc-PDZK1IP1-3:1 | 25.26 | 11.57 | −1.13 | 3.28×10−2 |
| lnc-SUSD3-4:2 | 19.48 | 9.17 | −1.09 | 3.37×10−2 |
| lnc-AC069257.9.1-4:53 | 99.18 | 57.43 | −0.79 | 3.59×10−2 |
| lnc-AP000974.1-1:1 | 36.09 | 16.13 | −1.16 | 4.79×10−2 |
Celecoxib and control columns indicate the average expression values of the lncRNAs in the celecoxib and the control group, respectively. lncRNA, long non-coding ribonucleic acids.
Functional and pathway enrichment analysis for DEGs
GO enrichment analysis demonstrated that 672, 108 and 120 terms in the BP, CC and MF categories, respectively, were identified as upregulated genes (Table II), and 453, 45 and 67 terms were identified for downregulated genes (Table III). The most enriched GO terms in the categories for upregulated genes were as follows: BP, CC and MF categories for upregulated genes were small molecule metabolic processes (P=1.87×10−9), extracellular region (P=3.64×10−23) and protein binding (P=7.34×10−7), respectively (Table II). The most enriched GO terms in the BP, CC and MF categories for downregulated genes were tissue development (P=4.66×10−8); extracellular region (P=1.02×10−10) and protein kinase C binding (P=1.31×10−3), respectively (Table III).
Table II.
Top five enriched gene ontology terms in biological process, cellular component and molecular function categories for upregulated DEGs.
| A, Biological process | ||||
|---|---|---|---|---|
| GO_ID | Term | Count | P-value | DEGs |
| GO:0044281 | Small molecule metabolic process | 99 | 1.87×10−9 | ABCC3, ACAA1, B3GNT3, CD320, DDX11, ECHS1, FA2H, GAPDH, UQCRFS1, WNT11a |
| GO:0055114 | Oxidation-reduction process | 44 | 9.79×10−9 | ACAA1, ACSS2, COX8A, ECHS1, FA2H, HMOX1, UQCRC1, UQCRC2, UQCRFS1, VAT1a |
| GO:0044710 | Single-organism metabolic process | 137 | 3.01×10−8 | ABCC3, ACAA1, B3GNT3, BMP4, PCBD1, PSMD8, RHOB, VAT1, WNT11, XRCC6a |
| GO:0043436 | Oxoacid metabolic process | 44 | 6.07×10−8 | ABCC3, ACAA1, B3GNT3, CKMT1A, ECHS1, SOD1, SULT2B1, TPI1, TST, UGT1A6a |
| GO:0006082 | Organic acid metabolic process | 44 | 9.50×10−8 | ABCC3, ACAA1, B3GNT3, CKMT1A, GOT1, SERINC2, SLC2A1, TPI1, TST, UGT1A6a |
| B, Cellular component | ||||
| GO_ID | Term | Count | P-value | DEGs |
| GO:0005576 | Extracellular region | 138 | 3.64×10−23 | ADIRF, BMP4, CAPG, IL1RN, ITGA3, ITGA6, ITGB4, ITGB5, VAT1, VDAC1a |
| GO:0031982 | Vesicle | 129 | 5.68×10−20 | ADIRF, AHNAK2, ENO1, ITGA3, ITGB4, ITGB5, SFN, UQCRC2, VASP, VAT1a |
| GO:0031988 | Membrane-bounded vesicle | 126 | 4.25×10−18 | ADIRF, ATP6AP1, BAIAP2L2, CAPG, EPS8L1, FTH1, FURIN, GAPDH, UQCRC2, VASPa |
| GO:0043230 | Extracellular organelle | 118 | 2.21×10−18 | ADIRF, GOT1, ITGA3, ITGB4, ITGB5, KLK14, UGT1A6, UPK3B, UQCRC2, VASPa |
| GO:0044421 | Extracellular region | 131 | 8.27×10−13 | ADIRF, HMOX1, IL1RN, ITGA3, ITGA6, ITGB4, ITGB5, KLK14, TXN, WNT11a |
| C, Molecular function | ||||
| GO_ID | Term | Count | P-value | DEGs |
| GO:0005515 | Protein binding | 182 | 7.34×10−7 | AATK, HSP90AA1, IRF2BP1, ITGA3, ITGA6, ITGB4, ITGB5, UQCRFS1, VASP, VDAC1a |
| GO:0016491 | Oxidoreductase activity | 31 | 9.29×10−7 | ACAA1, GAPDH, HMOX1, HPDL, HR, LDHA, MAOB, NDUFS3, PCBD1, PIRa |
| GO:0043236 | Laminin binding | 6 | 7.96×10−6 | ECM1, GPC1, ITGA3, ITGA6, LGALS1, LYPD3 |
| GO:0050840 | Extracellular matrix binding | 7 | 2.07×10−5 | ECM1, GPC1, GPR56, ITGA3, ITGA6, LGALS1, LYPD3 |
| GO:0008106 | Alcohol dehydrogenase (NADP+) activity | 4 | 3.68×10−5 | AKR1B1, AKR1C2, AKR1C3, ALDH3A1 |
GO, gene ontology; DEGs, differentially expressed genes; NADP, nicotinamide adenine dinucleotide phosphate.
Not all of the gene names were included in the table.
Table III.
Top five enriched gene ontology terms in the biological process, cellular component and molecular function categories for downregulated DEGs.
| A, Biological process | ||||
|---|---|---|---|---|
| GO_ID | Term | Count | P-value | DEGs |
| GO:0009888 | Tissue development | 42 | 4.66×10−8 | ADAM9, ALDH1A3, FNDC3B, NTN4, PKP2, RIPK4, TNFRSF19, TRIM16, TSC22D3, WNT7Ba |
| GO:0048513 | Organ development | 58 | 1.90×10−7 | ADAM9, EGLN1, LTBP3, MAP3K1, MDK, NRIP1, TNFRSF19, TNS3, TRIM16, TSC22D3a |
| GO:0048731 | System development | 70 | 6.10×10−7 | ADAM9, SGPL1, TNFAIP2, TNFRSF19, TNS3, TRIM16, TRIO, TSC22D3, WNT7B, ZSWIM6a |
| GO:0048518 | Positive regulation of biological process | 74 | 6.85×10−7 | ADAM9, GLIS3, HSPB1, IGFBP3, IRF1, ITGB8, KLK6, TRIM16, TRIO, WNT7Ba |
| GO:0009653 | Anatomical structure morphogenesis | 49 | 7.77×10−7 | ADAM9, MAP1B, MAP2, NTN4, PKP2, PTPRJ, RIPK4, SAT1, SEMA7A, SGPL1a |
| B, Cellular component | ||||
| GO_ID | Term | Count | P-value | DEGs |
| GO:0044421 | Extracellular region | 73 | 1.02×10−10 | ADAM9, CCDC80, CLIC5, FRAS1, SNX18, SOSTDC1, ST6GAL1, SULF2, TNFAIP2, VWA2a |
| GO:0005615 | Extracellular space | 35 | 1.02×10−8 | ADAM9, HSPG2, IGFBP3, MUC4, PLAT, POTEF, SERPINA3, TNFAIP2, VWA2, WNT7Ba |
| GO:0005576 | Extracellular region | 77 | 1.78×10−8 | ADAM9, KRT15, LCN2, SLC7A5, SNX18, SOSTDC1, ST6GAL1, SULF2, TACSTD2, TGM2a |
| GO:0043230 | Extracellular organelle | 56 | 1.92×10−8 | ADAM9, IVL, KRT13, MYOF, PLAT, POTEF, SLC7A5, SNX18, ST6GAL1, TACSTD2a |
| GO:0065010 | Extracellular organelle, membrane-bound | 56 | 1.92×10−8 | ADAM9, IGFBP3, LTBP3, MARCKS, SELENBP1, SNX18, ST6GAL1, TGM2, THSD4, VWA2a |
| C, Molecular function | ||||
| GO_ID | Term | Count | P-value | DEGs |
| GO:0005080 | Protein kinase C binding | 4 | 1.31×10−3 | ADAM9, HSPB1, MARCKS, PKP2 |
| GO:0008009 | Chemokine activity | 4 | 1.42×10−3 | CXCL1, CXCL3, CXCL5, CXCL8 |
| GO:0019838 | Growth factor binding | 6 | 1.43×10−3 | BMPR2, CTGF, IGFBP3, IGFBP6, LTBP3, TRIM16 |
| GO:0031994 | Insulin-like growth factor I binding | 2 | 2.28×10−3 | IGFBP3, IGFBP6 |
| GO:0055106 | Ubiquitin-protein transferase regulator activity | 2 | 2.28×10−3 | CDKN2A, TRIB1 |
GO, gene ontology; DEGs, differentially expressed genes.
Not all of the gene names were included in the table.
According to the pathway enrichment analysis, 28 and 7 pathways were identified for the upregulated and downregulated genes, respectively (Table IV). The upregulated genes were significantly enriched in the glycolysis/gluconeogenesis (P=1.03×10−6), metabolic pathways (P=6.04×10−5), phenylalanine metabolism (P=4.00×10−4), oxidative phosphorylation (P=2.11×10−2) and the metabolism of xenobiotics by cytochrome P450 (P=4.14×10−3) (Table IV).
Table IV.
Top ten enriched pathways for upregulated differentially expressed genes and seven enriched pathways for downregulated DEGs.
| Pathway | Count | P-value | Gene symbol |
|---|---|---|---|
| Upregulated | |||
| Glycolysis/gluconeogenesis | 10 | 1.03×10−6 | ACSS2, ALDH3A1, ALDOA, ENO1, ENO2, GAPDH, LDHA, PGM1, PKM, TPI1 |
| Metabolic pathways | 43 | 6.04×10−5 | ACAA1, ACSL5, ACSS2, AGPAT2, AK1, AKR1B1, ALDH1A1, ALDH3A1, ALDOA, ALPP, ALPPL2, ATP5G1, ATP5G3, ATP6AP1, B3GNT3, CKMT1A, CKMT1B, COX8A, CYC1, ECHS1, ENO1, ENO2, GAPDH, GOT1, ITPK1, LDHA, MAOB, MGAT3, NDUFS3, NT5E, PGM1, PGP, PIK3C2B, PKM, PLA2G4B, PLCE1, PRDX6, TPI1, TST, UGT1A6, UQCRC1, UQCRC2, UQCRFS1 |
| Phenylalanine metabolism | 4 | 4.00×10−4 | ALDH3A1, GOT1, MAOB, PRDX6 |
| Parkinson's disease | 10 | 4.67×10−4 | ATP5G1, ATP5G3, COX8A, CYC1, NDUFS3, SLC25A5, UQCRC1, UQCRC2, UQCRFS1, VDAC1 |
| Huntington's disease | 12 | 5.52×10−4 | ATP5G1, ATP5G3, CLTB, COX8A, CYC1, NDUFS3, SLC25A5, SOD1, UQCRC1, UQCRC2, UQCRFS1, VDAC1 |
| Prion diseases | 5 | 8.46×10−4 | EGR1, HSPA1A, MAPK3, SOD1, STIP1 |
| Oxidative phosphorylation | 9 | 2.11×10−3 | ATP5G1, ATP5G3, ATP6AP1, COX8A, CYC1, NDUFS3, UQCRC1, UQCRC2, UQCRFS1 |
| Alzheimer's disease | 10 | 3.16×10−3 | ATP5G1, ATP5G3, COX8A, CYC1, GAPDH, MAPK3, NDUFS3, UQCRC1, UQCRC2, UQCRFS1 |
| Metabolism of xenobiotics by cytochrome P450 | 6 | 4.14×10−3 | AKR1C2, AKR1C3, ALDH3A1, CYP1B1, EPHX1, UGT1A6 |
| Cardiac muscle contraction | 6 | 6.17×10−3 | ATP1A1, COX8A, CYC1, UQCRC1, UQCRC2, UQCRFS1 |
| Downregulated | |||
| Epithelial cell signaling in H. pylori infection | 3 | 2.92×10−2 | CXCL1, CXCL8, MAP3K14 |
| Complement and coagulation cascades | 3 | 3.03×10−2 | C3, PLAT, SERPINA1 |
| Histidine metabolism | 2 | 3.29×10−2 | ALDH1A3, AOC1 |
| Arrhythmogenic right ventricular cardiomyopathy | 3 | 3.62×10−2 | ITGB6, ITGB8, PKP2 |
| Axon guidance | 4 | 3.80×10−2 | EFNB2, NFAT5, NTN4, SEMA7A |
| Chemokine signaling pathway | 5 | 3.80×10−2 | BCAR1, CXCL1, CXCL3, CXCL5, CXCL8 |
| Cytokine-cytokine receptor interaction | 6 | 4.55×10−2 | BMPR2, CXCL1, CXCL3, CXCL5, CXCL8, TNFRSF19 |
DEGs, differentially expressed genes.
The downregulated genes were enriched in epithelial cell signaling in Helicobacter pylori infection (involving, CXCL1 and CXCL8; P=2.92×10−2), complement and coagulation cascades (P=3.03×10−2), arrhythmogenic right ventricular cardiomyopathy (P=3.62×10−2), chemokine signaling pathway (involving CXCL1, CXCL3, CXCL5 and CXCL8; P=3.80×10−2) and cytokine-cytokine receptor interaction (involving CXCL1, CXCL3, CXCL5 and CXCL8; P=4.55×10−2) (Table IV).
PPI network and module analysis
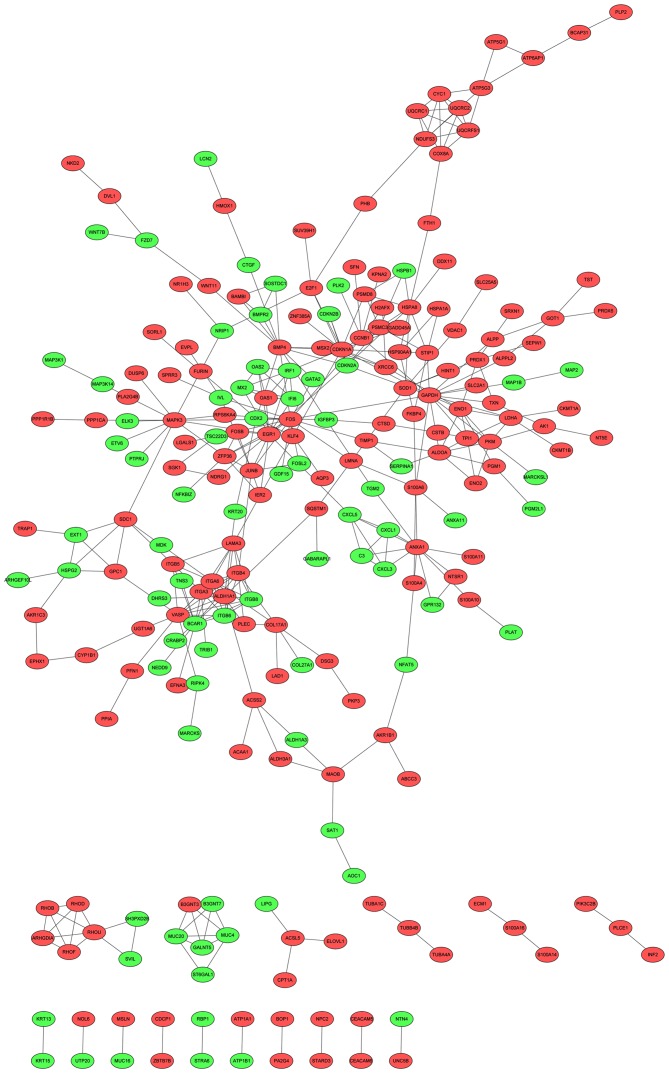
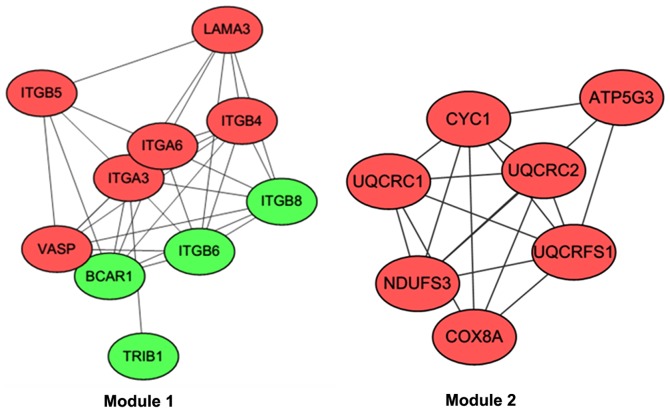
After the PPIs of DEGs were predicted using the STRING database, the PPI network was visualized (Fig. 1). Based on the ClusterONE plugin, two modules with the highest significance (module 1, P=9.96×10−5, nodes=10; module 2, P=8.98×10−4, nodes=7) were selected (Fig. 2).
Figure 1.
Constructed protein-protein interaction network for the differentially expressed genes. Red and green nodes indicate the up- and downregulated differentially expressed genes, respectively.
Figure 2.
Two modules selected from the protein-protein interaction network. Red and green nodes indicate the up- and downregulated differentially expressed genes, respectively.
The DEGs in module 1 (including, ITGB6, ITGA6, ITGB4, ITGB5, ITGA3 and ITGB8) were most significantly associated with functions of the integrin complex (CC, P=3.33×10−15), the protein complex involved in cell adhesion (CC, P=3.33×10−15) and the integrin-mediated signaling pathway (BP, P=1.34×10−14) (Table V). In module 2, DEGs were involved in the respiratory electron transport chain (BP, P=4.60×10−13) and the electron transport chain (BP, P=5.17×10−13) (Table VI).
Table V.
Top five enriched gene ontology terms in biological process, cellular component and molecular function categories for DEGs in module 1.
| A, Biological process | ||||
|---|---|---|---|---|
| GO_ID | Term | Count | P-value | DEG |
| GO:0007229 | Integrin-mediated signaling pathway | 7 | 1.34×10−14 | ITGB6, BCAR1, ITGA6, ITGB4, ITGB5, ITGA3, ITGB8 |
| GO:0030198 | Extracellular matrix organization | 7 | 3.66×10−10 | ITGB6, LAMA3, ITGA6, ITGB4, ITGB5, ITGA3, ITGB8 |
| GO:0043062 | Extracellular structure organization | 7 | 3.73×10−10 | ITGB6, LAMA3, ITGA6, ITGB4, ITGB5, ITGA3, ITGB8 |
| GO:0007155 | Cell adhesion | 8 | 1.30×10−8 | ITGB6, BCAR1, LAMA3, ITGA6, ITGB4, ITGB5, ITGA3, ITGB8 |
| GO:0022610 | Biological adhesion | 8 | 1.35×10−8 | ITGB6, BCAR1, LAMA3, ITGA6, ITGB4, ITGB5, ITGA3, ITGB8 |
| B, Cellular component | ||||
| GO_ID | Term | Count | P-value | DEG |
| GO:0008305 | Integrin complex | 6 | 3.33×10−15 | ITGB6, ITGA6, ITGB4, ITGB5, ITGA3, ITGB8 |
| GO:0098636 | Protein complex involved in cell adhesion | 6 | 3.33×10−15 | ITGB6, ITGA6, ITGB4, ITGB5, ITGA3, ITGB8 |
| GO:0043235 | Receptor complex | 6 | 2.87×10−9 | ITGB6, ITGA6, ITGB4, ITGB5, ITGA3, ITGB8 |
| GO:0030055 | Cell-substrate junction | 6 | 2.02×10−8 | BCAR1, VASP, ITGA6, ITGB4, ITGB5, ITGA3 |
| GO:0009986 | Cell surface | 6 | 4.88×10−7 | ITGB6, ITGA6, ITGB4, ITGB5, ITGA3, ITGB8 |
| C, Molecular function | ||||
| GO_ID | Term | Count | P-value | DEG |
| GO:0005178 | Integrin binding | 4 | 3.15×10−7 | ITGB6, ITGA6, ITGB5, ITGA3 |
| GO:0050839 | Cell adhesion molecule binding | 4 | 2.35×10−6 | ITGB6, ITGA6, ITGB5, ITGA3 |
| GO:0005102 | Receptor binding | 7 | 2.36×10−6 | ITGB6, LAMA3, ITGA6, ITGB4, ITGB5, ITGA3, ITGB8 |
| GO:0043236 | Laminin binding | 2 | 1.47×10−4 | ITGA6, ITGA3 |
| GO:0050840 | Extracellular matrix binding | 2 | 4.39×10−4 | ITGA6, ITGA3 |
GO, gene ontology; DEGs, differentially expressed genes.
Table VI.
Top five enriched gene ontology terms in biological process, cellular component and molecular function categories for DEGs in module 2.
| A, Biological process | ||||
|---|---|---|---|---|
| GO_ID | Term | Count | P-value | DEGs |
| GO:0022904 | Respiratory electron transport chain | 6 | 4.60×10−13 | CYC1, COX8A, UQCRC1, NDUFS3, UQCRC2, UQCRFS1 |
| GO:0022900 | Electron transport chain | 6 | 5.17×10−13 | CYC1, COX8A, UQCRC1, NDUFS3, UQCRC2, UQCRFS1 |
| GO:0045333 | Cellular respiration | 6 | 6.05×10−12 | CYC1, COX8A, UQCRC1, NDUFS3, UQCRC2, UQCRFS1 |
| GO:0015980 | Energy derivation by oxidation of organic compounds | 6 | 5.80×10−10 | CYC1, COX8A, UQCRC1, NDUFS3, UQCRC2, UQCRFS1 |
| GO:0006091 | Generation of precursor metabolites and energy | 6 | 2.32×10−9 | CYC1, COX8A, UQCRC1, NDUFS3, UQCRC2, UQCRFS1 |
| B, Cellular component | ||||
| GO_ID | Term | Count | P-value | DEGs |
| GO:0005743 | Mitochondrial inner membrane | 7 | 1.67×10−12 | ATP5G3, CYC1, COX8A, UQCRC1, NDUFS3, UQCRC2, UQCRFS1 |
| GO:0019866 | Organelle inner membrane | 7 | 3.57×10−12 | ATP5G3, CYC1, COX8A, UQCRC1, NDUFS3, UQCRC2, UQCRFS1 |
| GO:0070469 | Respiratory chain | 5 | 2.12×10−11 | CYC1, UQCRC1, NDUFS3, UQCRC2, UQCRFS1 |
| GO:0031966 | Mitochondrial membrane | 7 | 2.30×10−11 | ATP5G3, CYC1, COX8A, UQCRC1, NDUFS3, UQCRC2, UQCRFS1 |
| GO:0005740 | Mitochondrial envelope | 7 | 3.57×10−11 | ATP5G3, CYC1, COX8A, UQCRC1, NDUFS3, UQCRC2, UQCRFS1 |
| C, Molecular function | ||||
| GO_ID | Term | Count | P-value | DEGs |
| GO:0015078 | Hydrogen ion transmembrane transporter activity | 4 | 4.15×10−8 | ATP5G3, COX8A, UQCRC1, UQCRFS1 |
| GO:0008121 | Ubiquinol-cytochrome-c reductase activity | 2 | 3.57×10−6 | UQCRC1, UQCRFS1 |
| GO:0016681 | Oxidoreductase activity, acting on diphenols and related substances as donors, cytochrome as acceptor | 2 | 3.57×10−6 | UQCRC1, UQCRFS1 |
| GO:0016679 | Oxidoreductase activity, acting on diphenols and related substances as donors | 2 | 4.76×10−6 | UQCRC1, UQCRFS1 |
| GO:0015077 | Monovalent inorganic cation transmembrane transporter activity | 4 | 6.29×10−6 | ATP5G3, COX8A, UQCRC1, UQCRFS1 |
DEG, differentially expressed genes; GO, gene ontology; BP, biological process; CC, cellular component; MF, molecular function.
The DEGs in module 1 were most significantly enriched in the focal adhesion pathway (P=5.20×10−14) and the extracellular matrix (ECM)-receptor interaction pathway (P=3.66×10−12) (Table VII). In addition, the DEGs in module 2 were enriched in Parkinson's disease (P=2.23×10−12), oxidative phosphorylation (P=2.49×10−12), Alzheimer's disease (P=1.33×10−11), Huntington's disease (P=2.56×10−11) and metabolic pathways (P=9.66×10−6) (Table VII).
Table VII.
The 13 and 6 enriched pathways for differentially expressed genes in modules 1 and 2, respectively.
| Pathway | Count | P-value | Gene symbol |
|---|---|---|---|
| A, Module 1 | |||
| Focal adhesion | 9 | 5.20×10−14 | ITGB6, BCAR1, VASP, LAMA3, ITGA6, ITGB4, ITGB5, ITGA3, ITGB8 |
| ECM-receptor interaction | 7s | 3.66×10−12 | ITGB6, LAMA3, ITGA6, ITGB4, ITGB5, ITGA3, ITGB8 |
| Arrhythmogenic right ventricular cardiomyopathy | 6 | 2.67×10−10 | ITGB6, ITGA6, ITGB4, ITGB5, ITGA3, ITGB8 |
| Hypertrophic cardiomyopathy | 6 | 5.41×10−10 | ITGB6, ITGA6, ITGB4, ITGB5, ITGA3, ITGB8 |
| Dilated cardiomyopathy | 6 | 8.90×10−10 | ITGB6, ITGA6, ITGB4, ITGB5, ITGA3, ITGB8 |
| Regulation of actin cytoskeleton | 7 | 2.55×10−9 | ITGB6, BCAR1, ITGA6, ITGB4, ITGB5, ITGA3, ITGB8 |
| Small cell lung cancer | 3 | 2.31×10−4 | LAMA3, ITGA6, ITGA3 |
| Hematopoietic cell lineage | 2 | 7.47×10−3 | ITGA6, ITGA3 |
| Pathways in cancer | 3 | 1.11×10−2 | LAMA3, ITGA6, ITGA3 |
| Leukocyte transendothelial migration | 2 | 1.27×10−2 | BCAR1, VASP |
| Toxoplasmosis | 2 | 1.63×10−2 | LAMA3, ITGA6 |
| Cell adhesion molecules | 2 | 1.65×10−2 | ITGA6, ITGB8 |
| B, Module 2 | |||
| Parkinson's disease | 7 | 2.23×10−12 | ATP5G3, CYC1, COX8A, UQCRC1, NDUFS3, UQCRC2, UQCRFS1 |
| Oxidative phosphorylation | 7 | 2.49×10−12 | ATP5G3, CYC1, COX8A, UQCRC1, NDUFS3, UQCRC2, UQCRFS1 |
| Alzheimer's disease | 7 | 1.33×10−11 | ATP5G3, CYC1, COX8A, UQCRC1, NDUFS3, UQCRC2, UQCRFS1 |
| Huntington's disease | 7 | 2.56×10−11 | ATP5G3, CYC1, COX8A, UQCRC1, NDUFS3, UQCRC2, UQCRFS1 |
| Cardiac muscle contraction | 5 | 7.02×10−9 | CYC1, COX8A, UQCRC1, UQCRC2, UQCRFS1 |
| Metabolic pathways | 7 | 9.66×10−6 | ATP5G3, CYC1, COX8A, UQCRC1, NDUFS3, UQCRC2, UQCRFS1 |
ECM, extracellular matrix.
Co-expression analysis of DEGs and DE-lncRNAs
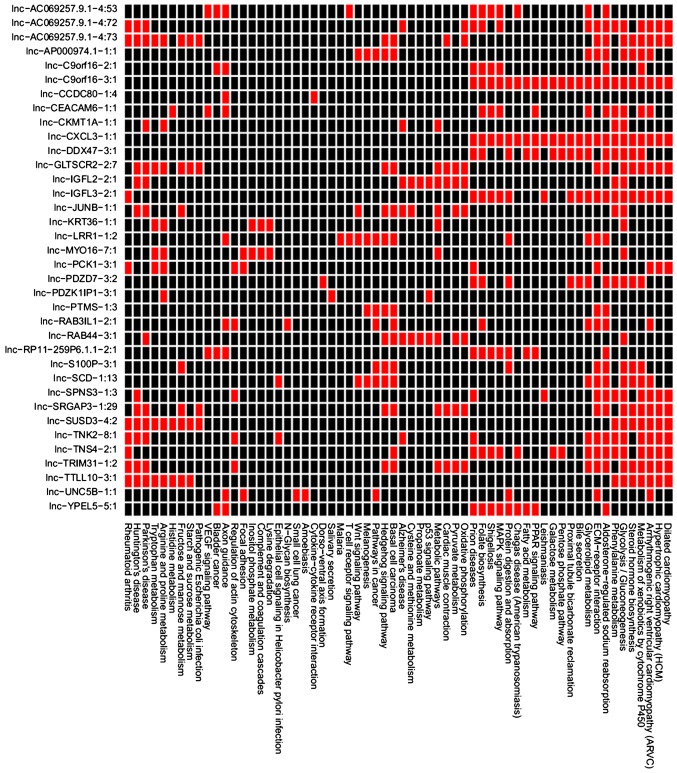
The pairs of co-expressed genes and lncRNAs were obtained and the enriched pathways for the DEGs co-expressed with each DE-lncRNAs are presented in Fig. 3. The DEGs co-expressed with lnc-SCD-1:13, lnc-LRR1-1:2, lnc-PTMS-1:3, lnc-S100P-3:1, lnc-AP000974.1-1:1 and lnc-RAB3IL1-2:1 were enriched in the pathways associated with cancer, such as basal cell carcinoma, pathways in cancer and ECM-receptor interaction (Table VIII). The DEGs co-expressed with lnc-SCD-1:13, lnc-LRR1-1:2 and lnc-S100P-3:1 were enriched in the Wnt signaling pathway (Table VIII). The DEGs co-expressed with lnc-SCD-1:13, lnc-LRR1-1:2, lnc-PTMS-1:3, lnc-S100P-3:1 and lnc-AP000974.1-1:1 were enriched in the Hedgehog signaling pathway (Table VIII).
Figure 3.
Enriched pathways for the differentially expressed genes co-expressed with each differentially expressed lncRNAs. Horizontal and vertical axis respectively indicate the enriched pathways and differentially expressed lncRNAs. lncRNAs, long non-coding RNAs.
Table VIII.
The DEGs co-expressed with differentially expressed lncRNAs associated with pathways in cancer.
| lncRNA/pathway | DEG |
|---|---|
| lnc-SCD-1:13 | |
| Wnt signaling pathway | DVL1, FZD7, NFAT5, WNT11, WNT7B |
| Hedgehog signaling pathway | BMP4, WNT11, WNT7B |
| Basal cell carcinoma | BMP4, DVL1, FZD7, WNT11, WNT7B |
| ECM-receptor interaction | HSPG2, ITGA3, ITGB4, LAMA3, SDC1 |
| Glycolysis/gluconeogenesis | ALDH1A3, ALDOA, PKM |
| Aldosterone-regulated sodium reabsorption | ATP1A1, SFN, SGK1 |
| Glycerolipid metabolism | AGPAT2, AKR1B1, LIPG |
| Metabolism of xenobiotics by cytochrome P450 | AKR1C2, ALDH1A3, CYP1B1 |
| Steroid hormone biosynthesis | AKR1C2, CYP1B1, SULT2B1 |
| Epithelial cell signaling in H. pylori infection | ATP6AP1, CXCL8, MAP3K14 |
| Pathways in cancer | BMP4, CXCL8, DVL1, FOS, FZD7, ITGA3, LAMA3, WNT11, WNT7B |
| Arrhythmogenic right ventricular cardiomyopathy | ITGA3, ITGB4, PKP2 |
| Melanogenesis | DVL1, FZD7, WNT11, WNT7B |
| lnc-LRR1-1:2 | |
| Wnt signaling pathway | DVL1, NFAT5, WNT11, WNT7B |
| Axon guidance | EFNA3, NFAT5, RHOD, UNC5B |
| ECM-receptor interaction | ITGA3, LAMA3, SDC1 |
| Basal cell carcinoma | BMP4, DVL1, WNT11, WNT7B |
| Aldosterone-regulated sodium reabsorption | ATP1A1, SGK1 |
| Hedgehog signaling pathway | BMP4, WNT11, WNT7B |
| Malaria | CXCL8, SDC1 |
| Glycerolipid metabolism | AGPAT2, AKR1B1 |
| T cell receptor signaling pathway | FOS, MAP3K14, NFAT5 |
| Pathways in cancer | BMP4, CXCL8, DVL1, FOS, ITGA3, LAMA3, SLC2A1, WNT11, WNT7B |
| Melanogenesis | DVL1, WNT11, WNT7B |
| Protein digestion and absorption | ATP1A1, KCNE3, SLC1A5 |
| lnc-PTMS-1:3 | |
| Basal cell carcinoma | BMP4, DVL1, WNT11, WNT7B |
| Aldosterone-regulated sodium reabsorption | SFN, SGK1 |
| Hedgehog signaling pathway | BMP4, WNT11, WNT7B |
| ECM-receptor interaction | ITGA3, LAMA3, SDC1 |
| Pathways in cancer | BMP4, DVL1, FOS, ITGA3, LAMA3, SLC2A1, WNT11, WNT7B |
| Melanogenesis | DVL1, WNT11, WNT7B |
| lnc-S100P-3:1 | |
| ECM-receptor interaction | ITGA3, LAMA3, SDC1 |
| Basal cell carcinoma | BMP4, DVL1, WNT11, WNT7B |
| Glycolysis/gluconeogenesis | ACSS2, ALDH1A3, ALDOA, ENO2 |
| Aldosterone-regulated sodium reabsorption | ATP1A1, SFN, SGK1 |
| Hedgehog signaling pathway | BMP4, WNT11, WNT7B |
| Metabolism of xenobiotics by cytochrome P450 | AKR1C2, ALDH1A3, CYP1B1 |
| Steroid hormone biosynthesis | AKR1C2, CYP1B1, SULT2B1 |
| Pathways in cancer | BMP4, CXCL8, DVL1, FOS, ITGA3, LAMA3, SLC2A1, WNT11, WNT7B |
| Fructose and mannose metabolism | AKR1B1, ALDOA |
| Protein digestion and absorption | ATP1A1, KCNE3, SLC1A5 |
| lnc-AP000974.1-1:1 | |
| Wnt signaling pathway | DVL1, FZD7, NFAT5, WNT11, WNT7B |
| Hedgehog signaling pathway | BMP4, WNT11, WNT7B |
| Basal cell carcinoma | BMP4, DVL1, FZD7, WNT11, WNT7B |
| ECM-receptor interaction | HSPG2, ITGA3, ITGB4, LAMA3, SDC1 |
| Glycolysis/gluconeogenesis | ALDH1A3, ALDOA, ENO2, PKM |
| Aldosterone-regulated sodium reabsorption | ATP1A1, SFN, SGK1 |
| Glycerolipid metabolism | AGPAT2, AKR1B1, LIPG |
| Metabolism of xenobiotics by cytochrome P450 | AKR1C2, ALDH1A3, CYP1B1 |
| Steroid hormone biosynthesis | AKR1C2, CYP1B1, SULT2B1 |
| Pathways in cancer | BMP4, CXCL8, DVL1, FOS, FZD7, ITGA3, LAMA3, WNT11, WNT7B |
| Arrhythmogenic right ventricular cardiomyopathy | ITGA3, ITGB4, PKP2 |
| Melanogenesis | DVL1, FZD7, WNT11, WNT7B |
| lnc-RAB3IL1-2:1 | |
| Axon guidance | NFAT5, SEMA7A, UNC5B |
| Basal cell carcinoma | DVL1, FZD7 |
| Extracellular matrix-receptor interaction | HSPG2, ITGA3, ITGA6 |
| Aldosterone-regulated sodium reabsorption | ATP1A1, SGK1 |
| Folate biosynthesis | ALPP, ALPPL2 |
| Glycerolipid metabolism | AGPAT2, LIPG |
| N-Glycan biosynthesis | MGAT3, ST6GAL1 |
| Regulation of actin cytoskeleton | FGD3, ITGA3, ITGA6, PFN1 |
| Pathways in cancer | CXCL8, DVL1, FZD7, ITGA3, ITGA6 |
| Arrhythmogenic right ventricular cardiomyopathy | ITGA3, ITGA6, PKP2 |
lncRNA, long non-coding ribonucleic acid; DEGs, differentially expressed genes.
Discussion
In the present study, the RNA sequencing data between gastric cancer cells treated with celecoxib and those treated with DMSO was used to explore the mechanism of celecoxib treatment in gastric cancer cells. It has been previously demonstrated that altered patterns of DNA methylation associated with Helicobacter pylori infection of gastric epithelial cells may contribute to the risk of gastric cancer (29). Following Helicobacter pylori infection, the significant expression of CXCL5 and CXCL8 was observed in primary human gastric epithelial cells (30). Verbeke et al (31) also reported that CXC chemokines may contribute to the transition of chronic inflammation in esophageal and gastric cancer. In addition, CXC chemokines (CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7 and CXCL8) could promote the migration and proliferation of endothelial cells by interacting with CXCR2 (32). Furthermore, the overexpression of CXCL1 and CXCR2 may be involved in the tumor invasion in gastric cancer (33). The study by Park et al (34) demonstrated that the overexpression of CXCL5 may contribute to the pathogenesis of gastric cancer.
The results of the present study revealed that some DEGs (CXCL1 and CXCL8) were enriched in the epithelial cell signaling pathway in Helicobacter pylori infection whereas other DEGs (CXCL1, CXCL3, CXCL5 and CXCL8) were enriched in both the chemokine signaling and cytokine-cytokine receptor interaction pathways, which were consistent with the previous reports. Based on these results, CXCL1, CXCL3, CXCL5 and CXCL8 were suggested to contribute to the development of gastric cancer through multiple pathways.
ITGA3 is known to be involved in the development of gastric cancer (35). The MPS-1/ITGB4 signaling axis mediates cell migration and invasiveness, which may be used as targets during the therapy of gastric cancer (36). Song et al (35) revealed that the polymorphisms of microRNA-binding sites in the 3′UTR region of the integrin genes (ITGA3, ITGA6, ITGB3, ITGB4 and ITGB5) were associated with the susceptibility of gastric cancer. Pathway enrichment analysis revealed that integrin genes (ITGA3, ITGA6, ITGB4, ITGB5, ITGB6 and ITGB8) in module 1 were enriched in the integrin-mediated signaling pathway. Altogether, we could speculate that these integrin genes may participate in the celecoxib treatment of gastric cancer via the integrin-mediated signaling pathway.
Co-expression analysis revealed that the DEGs co-expressed with lnc-SCD-1:13, lnc-LRR1-1:2, lnc-PTMS-1:3, lnc-S100P-3:1, lnc-AP000974.1-1:1 or lnc-RAB3IL1-2:1 were enriched in a number of pathways, including ECM-receptor interaction, Wnt signaling and Hedgehog signaling pathways. A number of studies reported that lncRNAs are important in the pathogenesis of gastric cancer (37–39). Chang et al (40) revealed that the genes in the ECM-receptor interaction pathway were involved in the metastasis and aggression of gastric cancer. In addition, Tang et al (41) demonstrated that miR-200b and miR-22 could synergistically inhibit the growth of gastric cancer through the Wnt-1 signaling pathway. Furthermore, Yan et al (42) reported that the activated Hedgehog signaling pathway was involved in the progression of gastric cancer. These results implied that lnc-SCD-1:13, lnc-LRR1-1:2, lnc-PTMS-1:3, lnc-S100P-3:1, lnc-AP000974.1-1:1 and lnc-RAB3IL1-2:1 may be important in the celecoxib treatment of gastric cancer via different pathways. However, the correlation between COX-2 and DEGs or DE-lncRNAs remains unclear, and needs to be confirmed by further experiments.
In conclusion, a total of 490 DEGs and 37 DE-lncRNAs were identified in the celecoxib group. Several DEGs (including CXCL1, CXCL3, CXCL5, CXCL8 and integrin genes) and DE-lncRNAs (including lnc-SCD-1:13, lnc-LRR1-1:2, lnc-PTMS-1:3, lnc-S100P-3:1, lnc-AP000974.1-1:1 and lnc-RAB3IL1-2:1) may affect celecoxib treatment of gastric cancer through different pathways. However, these results were obtained by bioinformatics analysis and require further validation.
Glossary
Abbreviations
- lncRNAs
long non-coding RNAs
- DEGs
differentially expressed genes
- PPI
protein-protein interaction
- ncRNAs
non-coding RNAs
- miRNAs
microRNAs
- DMSO
dimethylsulfoxide
- QC
quality control
- NGS
next generation sequencing
- GO
gene ontology
- BP
biological process
- CC
cellular component
- MF
molecular function
- mPTP
mitochondrial permeability transition pore
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Akagi H, Higuchi H, Sumimoto H, Igarashi T, Kabashima A, Mizuguchi H, Izumiya M, Sakai G, Adachi M, Funakoshi S, et al. Suppression of myeloid cell leukemia-1 (Mcl-1) enhances chemotherapy-associated apoptosis in gastric cancer cells. Gastric Cancer. 2013;16:100–110. doi: 10.1007/s10120-012-0153-6. [DOI] [PubMed] [Google Scholar]
- 3.Hippo Y, Taniguchi H, Tsutsumi S, Machida N, Chong JM, Fukayama M, Kodama T, Aburatani H. Global gene expression analysis of gastric cancer by oligonucleotide microarrays. Cancer Res. 2002;62:233–240. [PubMed] [Google Scholar]
- 4.Lee CH, Bang SH, Lee SK, Song KY, Lee IC. Gene expression profiling reveals sequential changes in gastric tubular adenoma and carcinoma in situ. World J Gastroenterol. 2005;11:1937–1945. doi: 10.3748/wjg.v11.i13.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chia NY, Deng N, Das K, Huang D, Hu L, Zhu Y, Lim KH, Lee MH, Wu J, Sam XX, et al. Regulatory crosstalk between lineage-survival oncogenes KLF5, GATA4 and GATA6 cooperatively promotes gastric cancer development. Gut. 2015;64:707–719. doi: 10.1136/gutjnl-2013-306596. [DOI] [PubMed] [Google Scholar]
- 6.Wadhwa R, Song S, Lee JS, Yao Y, Wei Q, Ajani JA. Gastric cancer-molecular and clinical dimensions. Nat Rev Clin Oncol. 2013;10:643–655. doi: 10.1038/nrclinonc.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Du Z, Fei T, Verhaak RG, Su Z, Zhang Y, Brown M, Chen Y, Liu XS. Integrative genomic analyses reveal clinically relevant long noncoding RNAs in human cancer. Nat Struct Mol Biol. 2013;20:908–913. doi: 10.1038/nsmb.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Passon DM, Lee M, Rackham O, Stanley WA, Sadowska A, Filipovska A, Fox AH, Bond CS. Structure of the heterodimer of human NONO and paraspeckle protein component 1 and analysis of its role in subnuclear body formation. Proc Natl Acad Sci USA. 2012;109:4846–4850. doi: 10.1073/pnas.1120792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song H, Sun W, Ye G, Ding X, Liu Z, Zhang S, Xia T, Xiao B, Xi Y, Guo J. Long non-coding RNA expression profile in human gastric cancer and its clinical significances. J Transl Med. 2013;11:225. doi: 10.1186/1479-5876-11-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Y, Wang J, Qian J, Kong X, Tang J, Wang Y, Chen H, Hong J, Zou W, Chen Y, et al. Long noncoding RNA GAPLINC regulates CD44-dependent cell invasiveness and associates with poor prognosis of gastric cancer. Cancer Res. 2014;74:6890–6902. doi: 10.1158/0008-5472.CAN-14-0686. [DOI] [PubMed] [Google Scholar]
- 12.Liu M, Li CM, Chen ZF, Ji R, Guo QH, Li Q, Zhang HL, Zhou YN. Celecoxib regulates apoptosis and autophagy via the PI3K/Akt signaling pathway in SGC-7901 gastric cancer cells. Int J Mol Med. 2014;33:1451–1458. doi: 10.3892/ijmm.2014.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lan C, Yang L, Fan L, Zhang Y, Wang J, Guo GJ, Wan S, Yang S, Wang R, Fang D. Celecoxib inhibits helicobacter pylori-induced invasion of gastric cancer cells through an adenine nucleotide translocator-dependent mechanism. Anticancer Agents Med Chem. 2013;13:1267–1272. doi: 10.2174/18715206113139990324. [DOI] [PubMed] [Google Scholar]
- 14.Yeh TS, Wu CW, Hsu KW, Liao WJ, Yang MC, Li AF, Wang AM, Kuo ML, Chi CW. The activated Notch1 signal pathway is associated with gastric cancer progression through cyclooxygenase-2. Cancer Res. 2009;69:5039–5048. doi: 10.1158/0008-5472.CAN-08-4021. [DOI] [PubMed] [Google Scholar]
- 15.Hu PJ, Yu J, Zeng ZR, Leung WK, Lin HL, Tang BD, Bai AH, Sung JJ. Chemoprevention of gastric cancer by celecoxib in rats. Gut. 2004;53:195–200. doi: 10.1136/gut.2003.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thiel A, Mrena J, Ristimäki A. Cyclooxygenase-2 and gastric cancer. Cancer Metastasis Rev. 2011;30:387–395. doi: 10.1007/s10555-011-9312-1. [DOI] [PubMed] [Google Scholar]
- 17.Pang RP, Zhou JG, Zeng ZR, Li XY, Chen W, Chen MH, Hu PJ. Celecoxib induces apoptosis in COX-2 deficient human gastric cancer cells through Akt/GSK3β/NAG-1 pathway. Cancer Lett. 2007;251:268–277. doi: 10.1016/j.canlet.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, Liu M, Liu X, Huang S, Li L, Song B, Li H, Ren Q, Hu Z, Zhou Y, Qiao L. COX-2 regulates E-cadherin expression through the NF-κB/Snail signaling pathway in gastric cancer. Int J Mol Med. 2013;32:93–100. doi: 10.3892/ijmm.2013.1376. [DOI] [PubMed] [Google Scholar]
- 19.Saito Y, Suzuki H, Imaeda H, Matsuzaki J, Hirata K, Tsugawa H, Hibino S, Kanai Y, Saito H, Hibi T. The tumor suppressor microRNA-29c is downregulated and restored by celecoxib in human gastric cancer cells. Int J Cancer. 2013;132:1751–1760. doi: 10.1002/ijc.27862. [DOI] [PubMed] [Google Scholar]
- 20.Patel RK, Jain M. NGS QC toolkit: A platform for quality control of next-generation sequencing data. Enc Metagenomics. 2013:1–5. doi: 10.1007/978-1-4614-6418-1_348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenbloom KR, Armstrong J, Barber GP, Casper J, Clawson H, Diekhans M, Dreszer TR, Fujita PA, Guruvadoo L, Haeussler M, et al. The UCSC genome browser database: 2015 update. Nucleic Acids Res. 2015;43:D670–D681. doi: 10.1093/nar/gku1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with tophat and cufflinks. Nat Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volders PJ, Helsens K, Wang X, Menten B, Martens L, Gevaert K, Vandesompele J, Mestdagh P. LNCipedia: A database for annotated human lncRNA transcript sequences and structures. Nucleic Acids Res. 2013;41:D246–D251. doi: 10.1093/nar/gks915. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C, Jensen LJ. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–D815. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohl M, Wiese S, Warscheid B. Cytoscape: Software for visualization and analysis of biological networks. Methods Mol Biol. 2011;696:291–303. doi: 10.1007/978-1-60761-987-1_18. [DOI] [PubMed] [Google Scholar]
- 28.Nepusz T, Yu H, Paccanaro A. Detecting overlapping protein complexes in protein-protein interaction networks. Nat Methods. 2012;9:471–472. doi: 10.1038/nmeth.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niwa T, Tsukamoto T, Toyoda T, Mori A, Tanaka H, Maekita T, Ichinose M, Tatematsu M, Ushijima T. Inflammatory processes triggered by Helicobacter pylori infection cause aberrant DNA methylation in gastric epithelial cells. Cancer Res. 2010;70:1430–1440. doi: 10.1158/0008-5472.CAN-09-2755. [DOI] [PubMed] [Google Scholar]
- 30.Mustapha P, Paris I, Garcia M, Tran CT, Cremniter J, Garnier M, Faure JP, Barthes T, Boneca IG, Morel F, et al. Chemokines and antimicrobial peptides cag-dependent early response to helicobacter pylori infection in primary human gastric epithelial cells. Infect Immun. 2014;82:2881–2889. doi: 10.1128/IAI.01517-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verbeke H, Geboes K, Van Damme J, Struyf S. The role of CXC chemokines in the transition of chronic inflammation to esophageal and gastric cancer. Biochim Biophys Acta. 2012;1825:117–129. doi: 10.1016/j.bbcan.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Mukaida N, Sasaki S, Baba T. Chemokines in cancer development and progression and their potential as targeting molecules for cancer treatment. Mediators Inflamm. 2014;2014:170381. doi: 10.1155/2014/170381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng WL, Wang CS, Huang YH, Tsai MM, Liang Y, Lin KH. Overexpression of CXCL1 and its receptor CXCR2 promote tumor invasion in gastric cancer. Ann Oncol. 2011;22:2267–2276. doi: 10.1093/annonc/mdq739. [DOI] [PubMed] [Google Scholar]
- 34.Park JY, Park KH, Bang S, Kim MH, Lee JE, Gang J, Koh SS, Song SY. CXCL5 overexpression is associated with late stage gastric cancer. J Cancer Res Clin Oncol. 2007;133:835–840. doi: 10.1007/s00432-007-0225-x. [DOI] [PubMed] [Google Scholar]
- 35.Song X, Zhong H, Zhou J, Hu X, Zhou Y, Ye Y, Lu X, Wang J, Ying B, Wang L. Association between polymorphisms of microRNA-binding sites in integrin genes and gastric cancer in Chinese han population. Tumor Biol. 2015;36:2785–2792. doi: 10.1007/s13277-014-2903-z. [DOI] [PubMed] [Google Scholar]
- 36.Yang ZY, Jiang H, Qu Y, Wei M, Yan M, Zhu ZG, Liu BY, Chen GQ, Wu YL, Gu QL. Metallopanstimulin-1 regulates invasion and migration of gastric cancer cells partially through integrin β4. Carcinogenesis. 2013;34:2851–2860. doi: 10.1093/carcin/bgt226. [DOI] [PubMed] [Google Scholar]
- 37.Lin XC, Zhu Y, Chen WB, Lin LW, Chen DH, Huang JR, Pan K, Lin Y, Wu BT, Dai Y, Tu ZG. Integrated analysis of long non-coding RNAs and mRNA expression profiles reveals the potential role of lncRNAs in gastric cancer pathogenesis. Int J Oncol. 2014;45:619–628. doi: 10.3892/ijo.2014.2431. [DOI] [PubMed] [Google Scholar]
- 38.Chen S, Li P, Xiao B, Guo J. Long noncoding RNA HMlincRNA717 and AC130710 have been officially named as gastric cancer associated transcript 2 (GACAT2) and GACAT3, respectively. Tumor Biol. 2014;35:8351–8352. doi: 10.1007/s13277-014-2378-y. [DOI] [PubMed] [Google Scholar]
- 39.Okugawa Y, Toiyama Y, Hur K, Toden S, Saigusa S, Tanaka K, Inoue Y, Mohri Y, Kusunoki M, Boland CR, et al. Metastasis-associated long non-coding RNA drives cancer development and promotes peritoneal metastasis. Carcinogenesis. 2014;35:2731–2739. doi: 10.1093/carcin/bgu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang W, Ma L, Lin L, Gu L, Liu X, Cai H, Yu Y, Tan X, Zhai Y, Xu X, et al. Identification of novel hub genes associated with liver metastasis of gastric cancer. Int J Cancer. 2009;125:2844–2853. doi: 10.1002/ijc.24699. [DOI] [PubMed] [Google Scholar]
- 41.Tang H, Kong Y, Guo J, Tang Y, Xie X, Yang L, Su Q, Xie X. Diallyl disulfide suppresses proliferation and induces apoptosis in human gastric cancer through Wnt-1 signaling pathway by up-regulation of miR-200b and miR-22. Cancer Lett. 2013;340:72–81. doi: 10.1016/j.canlet.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 42.Yan R, Peng X, Yuan X, Huang D, Chen J, Lu Q, Lv N, Luo S. Suppression of growth and migration by blocking the hedgehog signaling pathway in gastric cancer cells. Cell Oncol (Dordr) 2013;36:421–435. doi: 10.1007/s13402-013-0149-1. [DOI] [PubMed] [Google Scholar]