Abstract
Cancer- associated fibroblasts (CAFs) are actively involved in breast carcinoma. Our previous study demonstrated that the majority of these CAFs were smooth muscle actin (SMA) positive and were therefore termed peritumoral myofibroblast (PMY). Glucocorticoid, linked or not with its receptor (GR), has been postulated to serve a major role in normal breast and breast carcinoma; however, their role in CAFs remains poorly understood. The aim of the present study was to assess the presence of GR in breast CAFs and particularly in PMY in 56 cases of invasive breast carcinoma in correlation with clinicopathological parameters, by immunohistochemistry. GR was observed in CAFs in 51 cases (91%) and were more frequent in luminal A subtype (19/19 cases; 100%). The stromal expression was statistically correlated with the tumor grade (P=0.03), the Ki-67 index (P=0.003) and the presence of GR in the epithelial component (P=0.01). The demonstration of a frequent expression of GR in breast CAFs may serve as an interesting target for future therapeutics for the regulation of the tumoral breast microenvironment.
Keywords: cancer associated fibroblast, peritumoral myofibroblast, glucocorticoid, glucocorticoid receptor, breast carcinoma, stroma
Introduction
Glucocorticoids (GCs) are essential for survival and serve a major role in embryonic development, tissue homeostasis and in the regulation of the inflammatory response (1,2). In breast, the functions of GCs are complex and depend, in part, if they are linked to their receptor (GR) and consist of the control of milk secretion, differentiation and apoptosis (3). Morphologically, previous studies have demonstrated that GR nuclear expression is observed both in normal breast, in situ carcinoma and less frequently in invasive carcinoma (4–7). In invasive tumors, their expression is limited in tumors with a small size, low grade, good prognosis and expressing estrogen receptor (ER) (4,7). In addition, our previous study clearly demonstrated that dexamethasone has an antiproliferative effect in the MCF-7 breast cells that express GR (8).
Over previous years, the tumor-associated stroma and, in particular, the cancer associated fibroblasts (CAFs) have been demonstrated to serve a crucial role in cancer pathogenesis (9,10).
Our previous study clearly demonstrated that the majority of these CAFs were smooth muscle actin (SMA)-positive with a myofibroblastic-like phenotype and that the presence of these peritumoral myofibroblasts (PMY) is important both in situ and in invasive breast carcinoma of no special type (11). This is also important in metastatic disease by promoting tumor invasion, growth and angiogenesis through paracrine factors and/or direct cell-cell crosstalk (11–13).
Our previous study demonstrated the presence of ER or progesterone receptors (PR); however, the presence and potential role of GR is poorly understood in breast carcinoma PMYs (4).
Therefore, the present study aimed to assess, by immunohistochemistry, the presence or absence of GR in breast CAFs and in CAFs smooth muscle positive/PMY in correlation with clinicopathological variables. Investigating this may assist with elucidating the role of GR in breast carcinoma.
Materials and methods
Patient selection
Breast tissue samples were retrieved from the Departments of Pathology at the Erasme Hospital and IRIS South Hospital (Brussels, Belgium), and consisted of 56 cases of invasive carcinoma. The present study was approved by the Ethics Committee from Erasme University Hospital (no. P2014/418).
Immunohistochemistry
The immunohistochemical assessment of ER, PR, Ki-67 and human epidermal growth factor receptor (HER)2 was routinely performed using an antigen retrieval method using the Leica BOND-III fully automated system (Leica Microsystems, Ltd., Newcastle, UK), as previously described (4). According to these parameters, carcinoma were divided into five groups, as previously described (14): Luminal A (n=19), Luminal B (n=12), HER2+/ER+ (n=7), HER2+/ER- (n=9) and triple negative (n=9). In addition, the following parameters were also included for each patient: Age, stage, tumoral size and lymph node status. All parameters are shown in the Table I.
Table I.
Association of clinicopathological characteristics with immunohistochemical levels of GR in the peritumoral stroma.
| GT expression in the stroma | |||||
|---|---|---|---|---|---|
| Characteristic | No. cases (%) | Strong | Weak | Negative | P-value |
| Age, years | 0.56 | ||||
| ≤50 | 24 (43) | 18 | 5 | 1 | |
| >50 | 32 (57) | 23 | 5 | 4 | |
| Tumor size, mm | 1 | ||||
| <20 | 28 (50) | 21 | 5 | 2 | |
| ≥20 | 28 (50) | 20 | 5 | 3 | |
| Stage | 0.81 | ||||
| T1 | 28 (50) | 21 | 5 | 2 | |
| T2 | 21 (37.5) | 15 | 3 | 3 | |
| T3 | 7 (12.5) | 5 | 2 | 0 | |
| Tumor grade | 0.03 | ||||
| Grade 1 | 10 (17.9) | 10 | 0 | 0 | |
| Grade 2 | 22 (39.2) | 12 | 8 | 2 | |
| Grade 3 | 24 (42.9) | 19 | 2 | 3 | |
| Lymph node status | 0.19 | ||||
| Negative | 29 (51.8) | 24 | 4 | 1 | |
| Positive | 27 (48.2) | 17 | 6 | 4 | |
| ER status | 0.64 | ||||
| Negative | 18 (32.1) | 14 | 2 | 2 | |
| Positive | 38 (67.9) | 27 | 8 | 3 | |
| PR status | 0.39 | ||||
| Negative | 23 (41.1) | 15 | 6 | 2 | |
| Positive | 33 (58.9) | 26 | 4 | 3 | |
| Ki-67 index, % | 0.003 | ||||
| ≤14 | 19 (33.9) | 19 | 0 | 0 | |
| >14 | 37 (66.1) | 22 | 10 | 5 | |
| HER 2 status | 0.2 | ||||
| Negative | 40 (71.4) | 31 | 7 | 2 | |
| Positive | 16 (28.6) | 10 | 3 | 3 | |
| Intrinsic subtype | 0.189 | ||||
| Luminal A | 19 (33.9) | 19 | 0 | 0 | |
| Luminal B/HER2− | 12 (21.4) | 5 | 5 | 2 | |
| Luminal B/HER2+ | 7 (12.5) | 4 | 2 | 1 | |
| HER2+ | 9 (16.1) | 6 | 1 | 2 | |
| Triple negative | 9 (16.1) | 7 | 2 | 0 | |
| GR status in glands | 0.01 | ||||
| Negative | 26 (46.4) | 15 | 6 | 5 | |
| Positive | 30 (53.6) | 26 | 4 | 0 | |
P-values in bold are statistically significant (P<0.05). ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor; GR, glucocorticoid receptor.
For the demonstration of GR, a manual technique was applied. Tissue sections (4 µm) were cut sequentially and mounted onto superfrost-treated slides (Menzel-Gläser, Braunschweig, Germany). The slides were dried overnight at 37°C prior to deparaffinization in xylene and rehydration through graded ethanols. For epitope retrieval, the slides were immersed in a waterbath at 95–99°C for 90 min with an ethylenediamine tetraacetic acid buffer (pH 9.0; S236; Dako Corp., Glostrup, Denmark). The slides were subsequently cooled in the buffer for 20 min at room temperature. H2O2 (0.3%) was subsequently added to the slides and incubated for 30 min. The tissues were then incubated for 1 h with a monoclonal antibody against the N-terminus of the GR (clone 4H2; cat. no. NCL-GCR; 1:25; Novocastra Laboratories, Newcastle, UK) (4).
Double immunostaining
In addition, for the specific visualization of the expression of ER/PR and GR in SMA-positive CAFs, a double stain was also performed by using the EnVision G/2 double stain system (Dako Corp.), as previously described (15). The same monoclonal antibodies (ER, PR and GR) described above were applied to rehydrated paraffin tissue sections and allowed to incubate for 1 h at room temperature. Endogenous peroxidase was inhibited and 3,3′-diaminobenzidine was used to visualize the binding of these primary antibodies. The sections were subsequently incubated for 1 h with a secondary antibody against SMA (clone αSM-1; 1:50; Novocastra Laboratories, Newcastle, UK). Alkaline phosphatase-conjugated secondary antibody and fuchsin as substrate chromogen system were used to complete the secondary immunostain. Negative controls used the replacement of the different primary antibodies with the corresponding isotypes. In addition, to ensure the absence of PMY in the normal breast, 10 cases of normal breast tissue from patients who underwent plastic surgery were also included.
Immunohistochemical evaluation
All the slides were examined by two independent observers (Xavier Catteau and Jean-Christophe Noël) and the evaluation of ER, PR and GR was made independently by the two pathologists using the Allred score (16), and estimated the proportion of positive CAFs (0, no positive cells; 1, ≤1; 2, 1–10; 3, 11–33; 4, 34–66; 5, 67–100% positive cells) and the average staining intensity (0, negative; 1, weak; 2, Intermediate; 3, strong). The proportion score and the intensity score were added to obtain a total score ranging from 0–8. Subsequently, three grades of immunoreactivity were established: Score 0–2, negative; score 3–4, weak positivity; score 5–8, strong positivity.
Statistical analysis
The correlation analysis was performed. χ2-test and Fisher's exact test were used. All statistical analyses were performed using XLSTAT software (Addinsoft, Paris, France). P<0.05 was considered to indicate a statistically significant difference.
Results
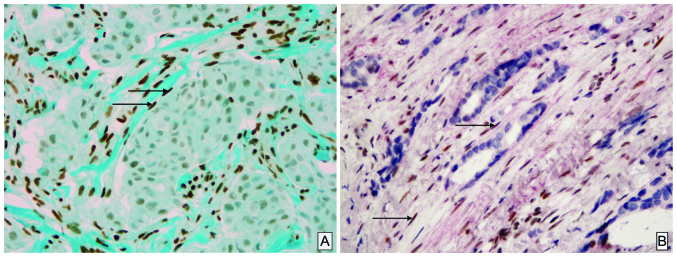
Weak or strong expression of GR in CAFs was observed in 10 cases (18%) and 41 cases (73%), respectively. A total of 5 cases were negative (5%; Table I). The stromal expression was frequent in luminal A tumor (100% of cases; Fig. 1A) and is statistically correlated with the tumor grade (P=0.03), the Ki-67 index (P=0.003) and GR status in glandular/carcinomatous component (P=0.01); however, was not correlated with age, tumor size, lymph node status and the expression of ER or PR, at least with a positive status for the latest as ≥1% in accordance with the World Health Organization recommendations (Table I). The double stain immunohistochemistry confirming unequivocally that among these CAFs, SMA-positive PMY clearly showed a nuclear staining of GR (Fig. 1B).
Figure 1.
Immunohistochemical staining of CAFs. (A) A typical example of glucocorticoid receptor in CAFs (arrows). Note the strong immunoreactivity score of these cells by comparison with the epithelial carcinomatous component, which is negative (magnification, ×10). (B) The double staining immunohistochemistry confirmed the positivity of these nuclear receptors (brown) in α-smooth muscle actin positive CAFs/peritumoral myofibroblats stained in red (arrow) (magnification, ×40). The immunoreactivity for glucocorticoid receptor is moderate. CAF, cancer associated fibroblast.
Discussion
For numerous years, the majority of studies in breast carcinoma have been focused predominantly on the epithelial component; however, recently CAFs and in particular CAFs SMA-positive PMY have been demonstrated to serve an important role in cancer pathogenesis as a result of paracrine cross-interaction between these and epithelial cancer cells. Indeed CAFs/PMY are able to secrete various factors implicated in invasion, matrix remodeling, cell proliferation, differentiation and apoptosis. In breast carcinoma, the hormonal regulation of epithelial cells is well documented and is the result of interaction between estrogen and progesterone, and their respective receptors, at least in hormone-dependent tumor types (16,17). The role of GCs in normal breast is more controversial and likely depends on the balance between targets of linked and non-linked GR with opposing functions: linked GR being involved in maintaining functional differentiation and non-linked GR appearing to be proapoptotic (3,6). In breast carcinoma, the GCs acting through their nuclear receptors are considered as a potential tumor suppressor promoting accurate chromosome segregation during mitosis occurring tumoral cell division (6,18,19). Indeed, according to these data, our previous study demonstrated an antiproliferative effect of dexamethosone in the MCF-7 breast cancer cells line that contains nuclear GR (8). The underlying mechanisms of hormonal regulation of CAFs/PMY remain to be determined; however, our previous study and other previous studies have clearly demonstrated that ER and PR were not present in these cells.
By contrast, the present study clearly demonstrated the presence of a marked GC nuclear immunoreactivity of CAFs in 73% of cases. In addition, this strong immunoreactivity was demonstrated by double labeling in CAFs SMA-positive PMY (Fig. 1) for the first time, to the best of our knowledge. This strong CAFs immunoreactivity was typically more frequent in luminal A (100%) compared with in other subtypes. In addition, it appeared to be correlated with different conventional clinicopathological parameters, including the grade (P=0.03) and Ki-67 index (P=0.003). The CR expression in CAFs was also more frequent when these receptors were present in the carcinomatous counterpart (P=0.01). These data suggested that, as previously shown for matrix metalloproteinase-2, the characteristics and properties of CAFs present in breast carcinoma microenvironment are probably complex and different from one subtype to another (10,20–22).
GCs are considered as agents capable of regulating the proliferation of myofibroblasts in different pathologies where they serve a major role as wound healing or asthma (23,24). Previously, in a myofibroblast cell line associated with colonic carcinoma, dexamethasone inhibited the expression of different classical procarcinogenic factors, including tenascin C, hepatocyte growth factor and transforming growth factor-β, in a receptor-dependent manner (25).
The present data are interesting since over the last few years it appears that in addition to the development concerning the classical therapies (hormone therapies, chemotherapies and immunotherapies), the peritumoral stroma served as potential target therapy in various carcinomas (9,24,25). Finally, even if it remains hypothetical, it has been postulated that the stress through GCs can be a promoting agent in breast cancer (26,27). From this point of view, demonstrating the presence of GR in the CAFs may be important.
The demonstration of a frequent expression of GR in breast CAFs may serve as an interesting target for future therapy in the regulation of the tumoral breast microenvironment. Naturally, future research is required, firstly to establish with larger cohorts the assocaition between the presence of GR in CAFs and the overall survival, and to understand how a therapy may influence the CAF associated with breast carcinoma. Such investigations are in progress.
Acknowledgements
The authors would like to thank Mrs. Isabelle Fayt and Mrs. Nadège De Kindt for their excellent technical work. The present study was supported by the Institut de Recherche Scientifique de Pathologie et de Génétique.
References
- 1.Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ArangoLievano M, Lambert WM, Jeanneteau F. Molecular biology of glucocorticoid signaling. Adv Exp Med Biol. 2015;872:33–57. doi: 10.1007/978-1-4939-2895-8_2. [DOI] [PubMed] [Google Scholar]
- 3.Ritter HD, Mueller CR. Expression microarray identifies the unliganded glucocorticoid receptor as a regulator of gene expression in mammary epithelial cells. BMC Cancer. 2014;14:275. doi: 10.1186/1471-2407-14-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buxant F, EngohanAloghe C, Noël JC. Estrogen receptor, progesterone receptor, and glucocorticoid receptor expression in normal breast tissue, breast in situ carcinoma, and invasive breast cancer. Appl Immunohistochem Mol Morphol. 2010;18:254–257. doi: 10.1097/PAI.0b013e3181c10180. [DOI] [PubMed] [Google Scholar]
- 5.Lien HC, Lu YS, Cheng AL, Chang WC, Jeng YM, Kuo YH, Huang CS, Chang KJ, Yao YT. Differential expression of glucocorticoid receptor in human breast tissues and related neoplasms. J Pathol. 2006;209:317–327. doi: 10.1002/path.1982. [DOI] [PubMed] [Google Scholar]
- 6.Vilasco M, Communal L, Mourra N, Courtin A, Forgez P, Gompel A. Glucocorticoid receptor and breast cancer. Breast Cancer Res Treat. 2011;130:1–10. doi: 10.1007/s10549-011-1689-6. [DOI] [PubMed] [Google Scholar]
- 7.Abduljabbar R, Negm OH, Lai CF, Jerjees DA, AlKaabi M, Hamed MR, Tighe PJ, Buluwela L, Mukherjee A, Green AR, Ali S, et al. Clinical and biological significance of glucocorticoid receptor (GR) expression in breast cancer. Breast Cancer Res Treat. 2015;150:335–346. doi: 10.1007/s10549-015-3335-1. [DOI] [PubMed] [Google Scholar]
- 8.Buxant F, Kindt N, Laurent G, Noël JC, Saussez S. Antiproliferative effect of dexamethasone in the MCF-7 breast cancer cell line. Mol Med Rep. 2015;12:4051–4054. doi: 10.3892/mmr.2015.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otranto M, Sarrazy V, Bonté F, Hinz B, Gabbiani G, Desmoulière A. The role of the myofibroblast in tumor stroma remodeling. Cell Adh Migr. 2012;6:203–219. doi: 10.4161/cam.20377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandellini P, Andriani F, Merlino G, D'Aiuto F, Roz L, Callari M. Complexity in the tumour microenvironment: Cancer associated fibroblast gene expression patterns identify both common and unique features of tumour-stroma crosstalk across cancer types. Semin Cancer Biol. 2015;35:96–106. doi: 10.1016/j.semcancer.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Catteau X, Simon P, Vanhaeverbeek M, Noël JC. Variable stromal periductular expression of CD34 and smooth muscle actin (SMA) in intraductal carcinoma of the breast. PLoS One. 2013;8:e57773. doi: 10.1371/journal.pone.0057773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catteau X, Simon P, Noël JC. Myofibroblastic stromal reaction and lymph node status in invasive breast carcinoma: Possible role of the TGF-β1/TGF-βR1 pathway. BMC Cancer. 2014;14:499. doi: 10.1186/1471-2407-14-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catteau X, Simon P, Noël JC. Myofibroblastic reaction is a common event in metastatic disease of breast carcinoma: A descriptive study. Diagn Pathol. 2014;9:196. doi: 10.1186/s13000-014-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preat F, Simon P, Noel JC. Differences in breast carcinoma immunohistochemical subtypes between immigrant Arab and European women. Diagn Pathol. 2014;9:26. doi: 10.1186/1746-1596-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noel JC, Fayt I, Buxant F. Proliferating activity in paget disease of the nipple. Pathol Oncol Res. 2010;16:7–10. doi: 10.1007/s12253-009-9179-4. [DOI] [PubMed] [Google Scholar]
- 16.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 17.Senkus E, Kyriakides S, Ohno S, PenaultLlorca F, Poortmans P, Rutgers E, Zackrisson S, Cardoso F. ESMO Guidelines Committee: Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v8–v30. doi: 10.1093/annonc/mdv298. [DOI] [PubMed] [Google Scholar]
- 18.MitreAguilar IB, CabreraQuintero AJ, Zentella-Dehesa A. Genomic and non-genomic effects of glucocorticoids: Implications for breast cancer. Int J Clin Exp Pathol. 2015;8:1–10. [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews LC, Berry AA, Morgan DJ, Poolman TM, Bauer K, Kramer F, Spiller DG, Richardson RV, Chapman KE, Farrow SN, et al. Glucocorticoid receptor regulates accurate chromosome segregation and is associated with malignancy. Proc Natl Acad Sci USA. 2015;112:5479–5484. doi: 10.1073/pnas.1411356112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rønnov-Jessen L, Bissell MJ. Breast cancer by proxy: Can the microenvironment be both the cause and consequence? Trends Mol Med. 2009;15:5–13. doi: 10.1016/j.molmed.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol Cancer Res. 2012;10:1403–1418. doi: 10.1158/1541-7786.MCR-12-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell DW. Myofibroblasts: Paracrine cells important in health and disease. Trans Am Clin Climatol Assoc. 2000;111:271–292. discussion 292-293. [PMC free article] [PubMed] [Google Scholar]
- 23.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: Paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grose R, Werner S, Kessler D, Tuckermann J, Huggel K, Durka S, Reichardt HM, Werner S. A role for endogenous glucocorticoids in wound repair. EMBO Rep. 2002;3:575–582. doi: 10.1093/embo-reports/kvf119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drebert Z, Bracke M, Beck IM. Glucocorticoids and the non-steroidal selective glucocorticoid receptor modulator, compound A, differentially affect colon cancer-derived myofibroblasts. J Steroid Biochem Mol Biol. 2015;149:92–105. doi: 10.1016/j.jsbmb.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Michael YL, Carlson NE, Chlebowski RT, Aickin M, Weihs KL, Ockene JK, Bowen DJ, Ritenbaugh C. Influence of stressors on breast cancer incidence in the Women's Health Initiative. Health Psychol. 2009;28:137–146. doi: 10.1037/a0012982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonova L, Aronson K, Mueller CR. Stress and breast cancer: From epidemiology to molecular biology. Breast Cancer Res. 2011;13:208. doi: 10.1186/bcr2836. [DOI] [PMC free article] [PubMed] [Google Scholar]